Abstract
Stress plays a key role in addiction etiology and relapse. Rodent models posit that following repeated periods of alcohol and other drug intoxication, compensatory allostatic changes occur in the central nervous system (CNS) circuits involved in behavioral and emotional response to stressors. We examine a predicted manifestation of this neuroadaptation in recently abstinent alcohol dependent humans. Participants completed a translational laboratory task that uses startle potentiation to unpredictable (vs. predictable) stressors implicated in the putative CNS mechanisms that mediate this neuroadaptation. Alcohol dependent participants displayed significantly greater startle potentiation to unpredictable than predictable stressors relative to non-alcoholic controls. The size of this effect covaried with alcohol-related problems and degree of withdrawal syndrome. This supports the rodent model thesis of a sensitized stress response in abstinent alcoholics. However, this effect could also represent pre-morbid risk or mark more severe and/or comorbid psychopathology. Regardless, pharmacotherapy and psychological interventions may target unpredictable stressor response to reduce stress-induced relapse.
Keywords: Addictive disorders, Affective neuroscience, Psychopharmacology, Stress, Substance disorders
Stress plays a key role in addiction etiology and relapse, but our understanding of the specific mechanisms remains limited (Kaye, Bradford, Magruder, & Curtin, in press). Behavioral neuroscience research in rodents has provided strong evidence to document the role of stress in alcohol and other drug (AOD) addiction (Koob & Le Moal, 2008a). Chronic AOD use in rodents causes heightened anxiety-like behavioral responses to stressors during periods of AOD deprivation (George et al., 2007; Olive, Koenig, Nannini, & Hodge, 2002). Stressors also potently instigate relapse in rodents (i.e. stress-induced reinstatement; Mantsch, Baker, Funk, Lê, & Shaham, 2016). These stress-induced behaviors are largely dependent on central nervous system (CNS) mechanisms involving corticotropin-releasing factor (CRF) and norepinephrine (NE), among other neurotransmitters, in the central extended amygdala (Koob & Le Moal, 2008a). Rodent models posit that repeated homeostatic adjustments in brain stress systems to acute periods of AOD intoxication eventually lead to long-lasting, compensatory allostatic changes in the structures and circuits involved in behavioral and emotional response to stressors (i.e., stress neuroadaptations; Koob & Le Moal, 2008a).
In humans, these stress neuroadaptations are hypothesized to result in dysregulated emotional response to stressors on cessation of use and provide the strong motivational press for further use that manifests as craving and increased risk for relapse when stressed (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Koob & Le Moal, 2008a). AOD dependent individuals report elevated negative affect (e.g., anxiety) when abstinent, particularly in response to stressors (Fox, et al., 2007; McKee et al., 2010) and at increasing levels before AOD lapses during quit attempts (Berkman, et al., 2011; Kenford et al., 2002). Furthermore, laboratory stressor-induced craving has been shown to predict shorter time to relapse among AOD patients (Higley et al., 2011; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006). The majority of biological studies of the stress response in human AOD samples have focused on measures of HPA-axis peripheral nervous system functioning (e.g., salivary cortisol; al’Absi, 2006; Sinha, 2008). However, rodent models clearly implicate neuroadaptations in extrahypothalamic CRF/NE circuits as a critical mechanism for sensitized stressor-induced behaviors in addiction. Human addiction research has not sufficiently focused on these CNS mechanisms to date. Moreover, cross-species “bench-to-bedside” research is common but often done with methods that are so divergent across species that much gets lost in translation.
Startle potentiation provides a non-invasive, psychophysiological index of heightened defensive response to stressors. It has been employed with rodents, non-human primates, and humans using highly parallel methods and measures, positioning it as an attractive, truly translational measure (Davis, Walker, Miles, & Grillon, 2010; Grillon & Baas, 2003). Rodent models of startle potentiation measured specifically during unpredictable stressors have confirmed involvement of NE and CRF sensitive pathways through the lateral divisions of the central amygdala and bed nucleus of the stria terminalis (BNST; Davis et al., 2010). These are the same CNS circuits that show sensitized stress neuroadaptations following chronic AOD use and mediate stress-induced relapse in rodents. In this study, we focus on the contrast of startle potentiation during unpredictable vs. predictable stressors to test predictions from rodent models about CNS stress neuroadaptations in human alcoholics. This explicit focus on the unpredictable (vs. predictable) startle potentiation contrast uses the predictable condition to control for overall differences in defensive reactivity across stressors to allow more precise targeting of mechanisms selectively recruited by unpredictable stressors (Davis et al., 2010). Such control is particularly important to evaluate group differences when groups are not randomly assigned (e.g. alcoholics vs. healthy controls). Startle potentiation during unpredictable (vs. predictable) stressors has proven sensitive to the stress response dampening effects of alcohol in previous related research (e.g., Bradford, Shapiro, & Curtin, 2013; Moberg & Curtin, 2009).
We predicted that alcoholics in early protracted abstinence would display sensitized response to unpredictable stressors, manifest as selectively elevated startle potentiation to unpredictable (vs. predictable) stressors. This prediction in humans rests on the substantial evidence base from rodent models reviewed here and elsewhere (Kaye et al., in press; Koob & Le Moal, 2008a). Our observations that a single acute administration of alcohol selectively reduces human startle potentiation to unpredictable (vs. predictable) stressors provides evidence that alcohol may impact these stress mechanisms and provide a press for compensatory neuroadaptation among alcoholics following chronic heavy use (Bradford et al., 2013; Hefner & Curtin, 2012; Hefner, Moberg, Hachiya, & Curtin, 2013; Moberg & Curtin, 2009). Furthermore, we have previously demonstrated that 24-hour nicotine-deprived smokers display increased response to unpredictable stressors (Hogle, Kaye, & Curtin, 2010).
We also examined four focal individual differences to guide future research into the potential causes, correlates, and consequences of the predicted sensitized response to unpredictable stressors in alcoholics. Specifically, we tested for covariation across alcoholics in the size of their unpredictable (vs. predictable) startle potentiation and: 1. Alcohol-related problems, to document the clinical consequences, 2. Presence of a withdrawal syndrome, to establish a clinical symptom correlate of this effect, 3. Duration of abstinence, to evaluate the stability of this effect across alcoholics at different points in their recovery, and 4. Quantity of alcohol use, to begin to examine potential causes of the effect.
Method
Following recommendations about research transparency (Simmons, Nelson, & Simonsohn, 2012), we have reported how we determined our sample size, all data exclusions, all manipulations, and all measures in the study. Following emerging open science guidelines (Schönbrodt, Maier, Heene, & Zehetleitner, 2015), we have made the data, analysis scripts, questionnaires, and other study materials associated with this report publicly available via Open Science Framework. These materials can be accessed at https://osf.io/ykmuh. In addition, recent high profile papers have highlighted concerns about the robustness and replicability of scientific research (Ioannidis, 2005; Open Science Collaboration, 2015; Simmons, Nelson, & Simonsohn, 2011). In particular, excessive researcher degrees of freedom have been targeted as one important contributor to these problems. To reduce concern about researcher degrees of freedom impacts on our primary results, we report robustness analyses. This allows for increased confidence that conclusions about our primary results are not dependent on selection of one specific analytic strategy.
Participants
We recruited 58 alcoholic and 57 non-alcoholic, control participants via flyers, online advertisements, and word of mouth. We required participants in the “alcoholic” group to meet DSM-IV-TR criteria for alcohol dependence. Alcoholics also had to self-report abstinence from alcohol for a minimum of one week but no more than two months at the time of their experimental session. We required participants in the non-alcoholic “control” group to report no lifetime history of alcohol dependence or current alcohol abuse. We excluded participants from both groups if they reported lifetime history of illicit substance dependence, lifetime history of any severe and persistent mental illness (e.g., bipolar disorder, schizophrenia or other psychotic disorders), current use of any medication known to affect the startle response, or any medical condition that contraindicated their safe participation. We compensated participants with $25/hour.
We determined the sample size for this experiment to provide adequate power to test the critical contrast between alcoholic vs. control participants for unpredictable vs. predictable startle potentiation. Specifically, we selected a target sample size of 128 participants (64 per group) to provide 80% power to detect a moderate effect size (d=0.5) increase in unpredictable (vs. predictable) startle potentiation among alcoholics relative to controls using a two-tailed alpha of 0.05 (Cohen, 1992). We terminated data collection early when we reached a sample size of 115 participants (58 alcoholics, 57 controls) as a result of slower recruitment rate than anticipated and a deadline for project completion for the lead author’s dissertation requirement.
General Procedure
All procedures were approved by the Social and Behavioral Sciences Institutional Review Board at the University of Wisconsin-Madison. We determined preliminary eligibility during a phone screening. At a subsequent in-person screening session participants provided informed consent after receiving information about study procedures and protections provided by the NIH Certificate of Confidentiality. A clinician conducted a Timeline Follow-back (Sobell & Sobell, 1992) to assess alcohol use (last 28 days for control participants; last 28 days prior to their most recent cessation of use for alcoholics). Finally, the clinician conducted the Structured Clinical Interview for DSM Disorders-Research Version (SCID-RV; First, Spitzer, Gibbon, & Williams, 2002) to assess for DSM-IV-TR alcohol use disorders and other psychiatric conditions relevant to inclusion/exclusion criteria. Eligible participants were scheduled to return for an experimental session.
At the experimental session, participants’ blood alcohol concentration (BAC) was assessed via breath test to confirm a BAC of 0.00% as required for participation. Alcoholics also reported their baseline alcohol craving (Love, James, & Willner, 1998), which was used to determine a safe level of craving for end of session release.
We next assessed participants’ startle reactivity during a series of 12 cues (i.e., colored squares) presented on a computer monitor. Each cue was presented for 5 seconds with a variable inter-trial interval (ITI; range 15–20 seconds). Eight startle-eliciting acoustic probes were presented during the cues. An additional 2 probes were presented during the ITIs to reduce probe predictability and 3 probes were presented at the start of this procedure to habituate the non-linear portion of the startle response. Startle reactivity was calculated as the mean startle magnitude to the 8 probes presented during the cues (see Startle Potentiation below for additional detail). Startle reactivity was included in all analyses of startle potentiation to increase power to test predicted effects as recommended by Bradford and colleagues (2014; see also, Bradford, Starr, Shackman, & Curtin, 2015; Kaye, Bradford, & Curtin, 2016).
Following this, participants reported their subjective response to a series of increasing intensity 200 millisecond duration electric shocks administered to their fingers (Hogle et al., 2010). Shock intensity during the main task was set to each participant’s subjective maximum tolerance threshold to minimize individual differences in sensitivity. Participants next completed the main task (see below). Participants then completed a self-report battery of individual difference measures. Participants next were debriefed, paid, and released. Alcoholics were released only after their alcohol craving had returned to baseline.
Unpredictable/Predictable Stressor Task
Participants completed eight blocks of trials in the unpredictable/predictable stressor task (Hefner et al., 2013). In each block, participants viewed a series of cues (i.e., colored squares) presented in one of four block types: Predictable Shock blocks, Predictable No-shock blocks, Unpredictable Shock blocks, and Unpredictable No-shock blocks. Participants were instructed about the specific cue-shock contingencies in each block prior to task start. Participants completed two blocks of each block type in one of eight between-subjects counterbalanced task block orders. A message indicating block type was presented on the monitor at the onset of each block. The color of the cues varied across the four block types to further highlight the block type. The entire procedure required approximately 30 minutes to complete.
In the Predictable Shock blocks, participants were instructed that the duration of all cues was 5 seconds, separated by an inter-trial interval (ITI; range: 10–20 seconds). They were instructed that each cue would co-terminate with an electric shock (0.25 seconds prior to cue offset) and that no shocks would be administered at any other time. Therefore, shock administration was temporally predictable and imminent following cue onset (4.75 seconds after each cue) in these blocks. A total of 10 predictable shock cues were presented.
In Unpredictable Shock blocks, participants were instructed that the duration of cues would vary from 5 seconds to 3 minutes, separated by an ITI (range: 10–20 seconds). In fact, four discrete cue durations were used (5, 20, 50, and 80 seconds). They were instructed that each cue would co-terminate with an electric shock (0.25 seconds prior to cue offset). Therefore, given that the duration of these cues was unknown, shock administration was temporally unpredictable following cue onset in these blocks. A total of 12 unpredictable cues (3x per duration) were presented1 .
We also included two Predictable and two Unpredictable No-shock blocks. All parameters (e.g., number of cues, cue duration) were identical to their matched shock blocks. However, participants were instructed that no shocks would be administered at any time during these no-shock blocks. These blocks were included as a non-aversive control condition from which to calculate startle potentiation in shock blocks.
Measures
Startle Response
We measured electromyographic startle response to acoustic probes (50 milliseconds of 102 dB white noise with near instantaneous rise time) administered 4.5 seconds after cue onset during both predictable and unpredictable cues and at later times 19.5, 49.5 and 79.5 seconds during unpredictable cues. A total of 24 probes (6x each) were presented at 4.5 seconds post cue onset during a subset of predictable and unpredictable shock and no-shock cues in the main task. Twelve probes (2x each) were presented at 19.5, 49.5 and 79.5 seconds post cue onset during a subset of the longer unpredictable shock and no-shock cues. An additional 24 probes were presented during ITIs across all blocks to decrease probe predictability. Three probes were also presented at the start of this procedure to habituate the non-linear portion of the startle response. Habituation and ITI probes were not included in any analyses. Serial position of the probes was counterbalanced within-subjects.
We recorded electromyographic response to the acoustic startle probes from two 4 mm Ag-AgCl sensors placed according to published guidelines beneath the right eye over the orbicularis oculi muscle (Blumenthal et al., 2005; van Boxtel, Boelhouwer, & Bos, 1998). We sampled electromyographic activity at 2500 Hz with an online bandpass filter (1–500 Hz) using NeuroScan SynAmps bioamplifiers and Scan 4.3 acquisition software (Compumedics USA, Charlotte, NC). We performed offline processing in Matlab using EEGLab (Delorme & Makeig, 2004) and PhysBox plugins (Curtin, 2011). This processing included epoching (−50 to 250 milliseconds surrounding probe), high pass filtering (28 Hz, 4th order Butterworth, zero phase shift), smoothing (signal rectification followed by 30 Hz, 2nd order Butterworth low pass filter, zero phase shift), and baseline correction. Startle magnitude was scored as the peak response between 20–100 milliseconds post-probe onset. We rejected trials containing artifact consistent with standard practices from our laboratory (Kaye et al., 2016). This included trials with deflections greater than ±20 μV in the 50 millisecond pre-probe baseline (i.e., unstable baseline) and trials with mean activity ≤ −10 μV between 150–250 milliseconds post probe onset (i.e., baseline over-correction due to pre-epoch artifact). Startle potentiation was calculated as the increase in startle magnitude during shock cues relative to no-shock cues in matched blocks. We tested our primary prediction using startle potentiation at 4.5 seconds to allow for a matched comparison across unpredictable and predictable cues. We evaluated startle potentiation at the later probe times during unpredictable cues to examine the stability of the primary effect2 .
Self-Report Measures
All participants completed a self-report battery including demographic information, Beck Anxiety Inventory (BAI; Beck & Steer, 1990), Beck Depression Inventory (BDI; Beck & Steer, 1987), Trait Fear-55 scale (Vizueta, et al., 2012) and the Rutgers Alcohol Problems Index (White & Labouvie, 1989). We used the SCID-RV to determine alcohol dependence diagnoses, presence of a withdrawal syndrome (absent, subthreshold, or present) and duration of abstinence. We assessed quantity of alcohol use (drinks/28 days) with the Timeline Follow-back3,4 .
Results
We accomplished data analysis and figure preparation with R (R Development Core Team, 2015) within R-Studio (RStudio, 2016) using the lmSupport (Curtin, 2015) package.
Sample Characteristics by Group
We report and test group differences for sample characteristics in Table 1. The groups were comparable on age, sex, race, ethnicity, and startle reactivity. As expected, significant group differences were observed for quantity of alcohol use and alcohol-related problems. In addition, the two groups were significantly different on trait fear, anxiety, and depression.
Table 1.
Individual Difference Measures by Group
| Control | Alcoholic | p-value | |
|---|---|---|---|
|
|
|||
| Demographics | |||
| Age | 43.5 (9.0) | 43.7 (11.6) | 0.883 |
| Sex | 1.000 | ||
| Female | 28.1% | 29.3% | |
| Male | 71.9% | 70.7% | |
| Race | 0.386 | ||
| African American | 5.3% | 15.5% | |
| American Indian | 1.8% | 1.7% | |
| Asian | 1.8% | 0.0% | |
| Caucasian | 89.5% | 81.0% | |
| Other | 1.8% | 1.7% | |
| Hispanic | 1.000 | ||
| No | 96.5% | 94.8% | |
| Yes | 3.5% | 5.2% | |
| Affective Individual Differences | |||
| Trait Fear | 48.7 (20.7) | 61.1 (25.5) | 0.005** |
| Beck Anxiety Inventory | 3.6 (5.7) | 9.2 (8.2) | <0.001*** |
| Beck Depression Inventory | 4.6 (7.5) | 10.1 (6.9) | <0.001*** |
| Startle Reactivity | 111.1 (84.1) | 128.4 (92.8) | 0.297 |
| Alcohol Use/Problems | |||
| Quantity of alcohol use (drinks/28 days)a | 11.9 (14.5) | 204.3 (134.5) | <0.001*** |
| Alcohol-related problemsb | 2.2 (4.3) | 53.5 (18.2) | <0.001*** |
| Duration of abstinence (days) | 32.4 (14.4) | ||
| Withdrawal syndrome | |||
| Absent | 15.5% | ||
| Subthreshold | 19.0% | ||
| Present | 65.5% | ||
NOTES: Internal consistency of all self-report questionnaires of affective individual differences and alcohol-related problems was excellent including Trait Fear (Cronbach’s α = 0.94), Beck Anxiety Inventory (α = 0.94), Beck Depression Inventory (α = 0.92), and Rutgers Alcohol Problem Index (α = 0.98).
N=115 (57 Control; 58 Alcoholic);
p < .05,
p < 0.01,
p < 0.001
Quantity of alcohol use was determined by Timeline Follow-Back (Sobell & Sobell, 1992) for most recent 28 days for controls and last 28 days preceding cessation for alcoholics.
Alcohol-related problems was assessed with the Rutgers Alcohol Problems Index (White & Labouvie, 1989).
Startle Potentiation during Unpredictable vs. Predictable Stressors
We analyzed startle potentiation at 4.5 seconds post-cue onset in a General Linear Model (GLM) with a between-subjects regressor for Group (alcoholic vs. control) and repeated measures for Stressor Type (unpredictable vs. predictable). These GLMs included Task Block Order and Startle Reactivity following published recommendations and our standard laboratory practices (Bradford et al., 2014). We also included measures of anxiety and depression (i.e., BAI, BDI) as covariates to increase power given their empirically verified relationship with startle potentiation in our task5 . We excluded one GLM model outlier (i.e., studentized residual with Bonferroni corrected p < .05; J. Fox, 1991) from all analyses involving startle potentiation as the dependent measure. We include partial eta2 (ηp2) and raw GLM parameter estimates (b) to document effect size. We present descriptives for startle magnitude and startle potentiation for all conditions in Table 2.
Table 2.
Startle Magnitude and Startle Potentiation by Stressor Type, Startle Probe Time, and Group
| Predictable: Early
|
Unpredictable: Early
|
Unpredictable: Late
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No-Shock | Shock | Potentiation | No-Shock | Shock | Potentiation | No-Shock | Shock | Potentiation | |
| Alcoholic | 87.9 (4.1) | 110.4 (5.8) | 22.6 (4.7) | 82.5 (3.5) | 117.0 (5.6) | 34.5 (4.7) | 80.3 (3.9) | 121.9 (5.2) | 41.5 (5.1) |
| Control | 82.5 (4.1) | 116.4 (5.8) | 33.9 (4.7) | 84.8 (3.4) | 113.3 (5.6) | 28.5 (4.7) | 78.9 (3.9) | 121.4 (5.2) | 42.5 (5.1) |
NOTE: Table presents point estimates (SE) for startle magnitude during no-shock and shock cues and startle potentiation (i.e., the difference in startle magnitude between shock vs. no-shock cues) for early predictable, early unpredictable and late unpredictable conditions from the GLM. This GLM adjusted for all covariates including Task Order, Startle Reactivity, Beck Anxiety Inventory, and Beck Depression Inventory (quantitative variables mean-centered).
As manipulation checks, we first confirmed significant overall startle potentiation, ηp2=0.61, b=29.9, 95% CI [24.5,35.2], t(80)=11.11, p<.001, indicating that the shock stressors elicited robust defensive reactivity. Startle potentiation was also significant separately for unpredictable stressors, ηp2=0.55, b=31.5, 95% CI [25.1,37.9], t(80)=9.80, p<.001, and predictable stressors, ηp2=0.50, b=28.2, 95% CI [21.9,34.5], t(80)=8.89, p<.001. There was not a significant overall effect of Stressor Type on startle potentiation, ηp2=0.01, b=3.3, 95% CI [−3.6,10.2], t(80)=0.96, p=.341.
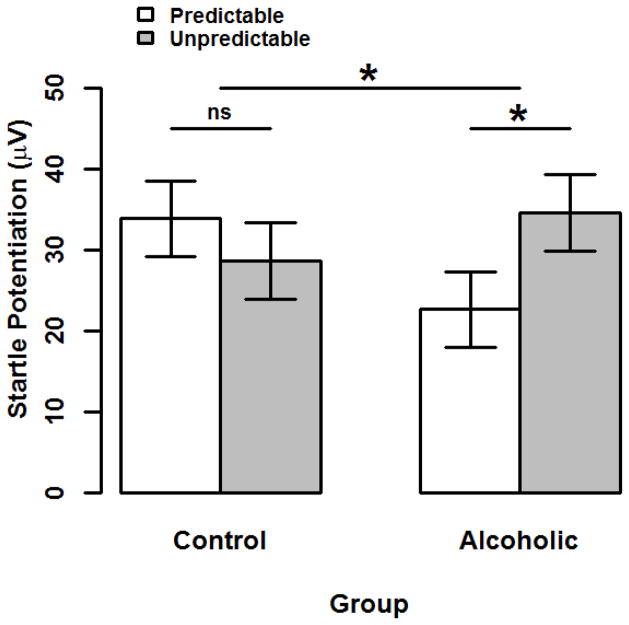
As predicted, the interaction between Group and Stressor Type was significant for startle potentiation, ηp2=0.06, b=17.3, 95% CI [2.5,32.1], t(80)=2.32, p=.023 (see Figure 1). This interaction indicates that the size of startle potentiation during unpredictable (vs. predictable) stressors was greater in the alcoholics relative to controls. Within-subject tests of Stressor Type simple effects indicated that alcoholics displayed significantly greater startle potentiation during unpredictable than predictable stressors, ηp2=0.06, b=11.9, 95% CI [1.8,22.1], t(80)=2.35, p=.021. In contrast, controls displayed comparable startle potentiation across both stressor types, ηp2=0.01, b=−5.3, 95% CI [−15.4,4.8], t(80)=1.05, p=.2976 .
Figure 1. Startle Potentiation by Group and Stressor Type.
Bars display startle potentiation to predictable (white) and unpredictable (gray) shock within each Group (alcoholic vs. control). Confidence bars represent ± one standard error for point estimates of startle potentiation from the General Linear Model (GLM). This GLM adjusted for all covariates including Task Block Order, Startle Reactivity, Beck Anxiety Inventory, and Beck Depression Inventory (quantitative variables mean-centered). The unpredictable vs. predictable startle potentiation contrast was greater among alcoholics than controls (p=.022). Moreover, this simple effect contrast was significant among alcoholics (p=.021) but not controls (p=.291).
* - p < .05. ns - Non-significant.
Figure © 2016 John Curtin, Daniel Bradford, Jesse Kaye, and Christine Moberg under Creative Commons Attribution 4.0 International Public License CC-By
We conducted and report robustness analyses to evaluate the predicted Group X Stressor Type interaction in several plausible alternative analyses that could have been conducted to test this effect. This allows for increased confidence that conclusions about this interaction are not dependent on selection of any one specific analytic strategy. First, we believe we reported the strongest test of our primary hypothesis by using only the 4.5 second startle probe time for unpredictable cues because this probe time matches the only probe time used for the predictable cues. Nonetheless, the Group X Stressor Type interaction remained significant and of comparable size in an alternative analysis that contrasted mean startle potentiation across the four probe times in unpredictable cues (4.5, 19.5, 49.5, and 79.5 seconds) vs. predictable cues, ηp2=0.05, b=13.8, 95% CI [0.3,27.3], t(80)=2.03, p=.045.
Second, covariates provide an important tool to increase statistical power and the precision of parameter estimation (Miller & Chapman, 2001). We identified and used two covariates in the analyses of startle potentiation in the primary analyses (Beck Anxiety Inventory, Beck Depression Inventory; see endnote 3). However, the Group X Stressor Type interaction remained significant and of comparable size without the Beck Anxiety Inventory, ηp2=0.05, b=15.8, 95% CI [0.9,30.7], t(81)=2.10, p=.039, or the Beck Depression Inventory, ηp2=0.06, b=17.4, 95% CI [2.8,32.0], t(81)=2.37, p=.020, in the model. Furthermore, we confirmed that neither the Beck Anxiety Inventory nor the Beck Depression Inventory moderated the Group X Stressor Type interaction (p=.481 & .886, respectively). In our primary analysis, we selected only covariates that were significant predictors of startle potentiation. Age, Race, and Trait Fear were not selected because each had only marginal (.10 < p < .05) effects. However, the Group X Stressor Type interaction remained significant and of comparable size if we included all three of these additional measures in the model, ηp2=0.05, b=15.1, 95% CI [0.0,30.1], t(74)=1.99, p=.050.
Third, we identified and removed one GLM model outlier from the primary analyses of startle potentiation. Standard practice in our laboratory is to trim (i.e., remove) model outliers from all analyses because they excessively influence the standard errors of parameter estimates and therefore negatively impact statistical power and parameter estimation precision (Bradford et al., 2013; Hefner et al., 2013; Kaye et al., 2016). However, the Group X Stressor Type interaction remains significant and of comparable size if retain this outlier in the analyses but winsorize it to reduce its influence (Keselman, Algina, Lix, Wilcox, & Deering, 2008; Wilcox & Keselman, 2003), ηp2=0.05, b=16.2, 95% CI [0.8,31.6], t(81)=2.09, p=.040.
Fourth, task order does not moderate the Group X Stressor type interaction in the primary analysis, F(7,80) =1.07, p=.391. This indicates that the magnitude of the Group X Stressor type interaction does not vary across task orders. In addition, the Group X Stressor type interaction remained significant, ηp2=0.04, b=14.2, 95% CI [0.2,28.3], t(94)=2.01, p=.048, in an alternative analysis where we modeled task order as additive with respect to all effects of group.
Finally, although not technically a robustness analysis, we also conducted separate analyses of startle magnitude during the no-shock cues to confirm that the Group X Stressor type interaction for startle potentiation did not result from group differences during the no-shock cues. There were no main effects of Group on startle magnitude during no-shock cues overall, ηp2<0.01, b=3.6, 95% CI [−19.1,26.2], t(80)=0.31, p=.756, or separately during unpredictable no-shock cues, ηp2<0.01, b=−2.2, 95% CI [−12.3,7.8], t(80)=0.44, p=.658, or predictable no-shock cues, ηp2<0.01, b=5.3, 95% CI [−6.6,17.3], t(80)=0.89, p=.378. Equally important, the Group X Stressor Type interaction was not significant for startle magnitude during the no-shock cues, ηp2<0.01, b=−3.7, 95% CI [−12.3,5.0], t(80)=0.85, p=.400.
Startle Potentiation across Time during Unpredictable Stressors
We expected that the Group effect for startle potentiation at 4.5 seconds during the unpredictable stressors would remain stable at later time points probed within the unpredictable stressors. To test this, we analyzed startle potentiation during unpredictable stressors in a GLM with a between-subjects regressor for Group (alcoholic vs. control) and repeated measures for Time (4.5 seconds vs. mean of 19.5, 49.5, 79.5 seconds). All covariates were included as described earlier. Startle potentiation during unpredictable stressors was significant at the later probe times, ηp2=0.64, b=42.0, 95% CI [35.0,48.9], t(80)=12.01, p<.001, and significantly greater at the later probe times than at 4.5 seconds, ηp2=0.11, b=10.4, 95% CI [3.9,17.0], t(80)=3.17, p=.002. However, the Group X Time interaction was not significant, ηp2=0.01, b=−6.9, 95% CI [−21.1,7.2], t(80)=0.98, p=.332, suggesting that Group effects for unpredictable stressors were comparable across the early vs. later probe times.
Potential Causes, Correlates, and Consequences of Increased Unpredictable Startle Potentiation
In separate GLMs using only alcoholics, we analyzed startle potentiation with between-subjects regressors for each target individual difference variable and repeated measures for Stressor Type (unpredictable vs. predictable). These models included all covariates as described earlier. We focused on Individual Difference X Stressor Type interactions because they indicated that the size of the selective increase in startle potentiation during unpredictable (vs. predictable) stressors varied significantly by that individual difference.
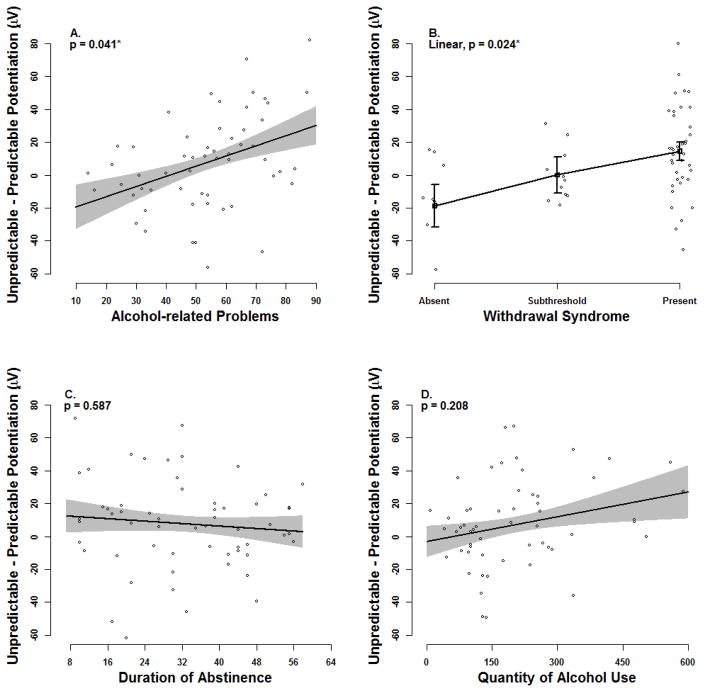
We observed a significant effect for alcohol-related problems such that the unpredictable (vs. predictable) startle potentiation contrast was greater among alcoholics who reported more alcohol-related problems, ηp2=0.10, b=0.6, 95% CI [0.0,1.2], t(39)=2.11, p=.041 (Figure 2, panel A). We also observed a significant linear effect for the withdrawal syndrome such that the selective increase in startle potentiation during unpredictable (vs. predictable) stressors increased as alcoholics reported a more substantial withdrawal syndrome (i.e. present > subthreshold > absent), ηp2=0.13, b=33.2, 95% CI [4.6,61.8], t(38)=2.35, p=.024 (Figure 2, panel B). No significant interactions were observed for duration of abstinence or quantity of alcohol use (p’s=0.587 & 0.208, respectively; see Figure 2, panels C–D)7 .
Figure 2. Individual Differences for Unpredictable (vs. Predictable) Startle Potentiation among Alcoholics.
Black lines display the relationship between the size of the unpredictable minus predictable startle potentiation difference score and each individual difference variable within the alcoholic group. Gray confidence bands (panels A, C, D) and black confidence bars (panel B) represent ± one standard error for point estimates of startle potentiation from the General Linear Model (GLM). This GLM adjusted for all covariates including Task Block Order, Startle Reactivity, Beck Anxiety Inventory, and Beck Depression Inventory (quantitative variables mean-centered).
* - p < .05
Figure © 2016 John Curtin, Daniel Bradford, Jesse Kaye, and Christine Moberg under Creative Commons Attribution 4.0 International Public License CC-By
Discussion
In this study, we observed the predicted sensitized response to unpredictable stressors in human abstinent alcoholics, which manifested as selectively elevated startle potentiation to unpredictable (vs. predictable) stressors. Equally important, the contrast between unpredictable and predictable stressor startle potentiation used here implicates a stress neuroadaptation in the same CRF and NE mechanisms in the extended amygdala proposed by rodent behavioral neuroscience research. Future research in humans can strengthen this latter claim about mechanism by direct pharmacologic manipulation of these neurotransmitter systems while measuring unpredictable stressor startle potentiation in AOD dependent users. However, research using such pharmacological manipulations must also address inherent limitations associated with systemic drug administration in humans (e.g., dose selection, blood-brain barrier penetration).
Research that pharmacologically manipulates relevant neurotransmitter systems in humans can also simultaneously document the treatment efficacy for these pharmacotherapies to ameliorate stress-induced relapse regardless of its etiologic origin. In fact, NE alpha1 antagonists and novel CRF antagonists have all generated substantial interest recently for their treatment potential (Koob & Zorrilla, 2012; Simpson et al., 2015; but see Kaye et al., in press, for recent review). Of course, more precise targeting of sources and coping strategies for unpredictable stressors may one day increase the efficacy of psychological interventions as well.
Potential Mechanisms of Stress Neuroadaptation
Koob and others have proposed that a sensitized stress response results, in part, from a between-system stress neuroadaptation where CNS stress system circuits are repeatedly recruited and strengthened to offset drug effects within the reward system following opponent-process principles (Koob & Le Moal, 2008b; Solomon & Corbit, 1973). In rodents, this mechanism is proposed to operate broadly for many addictive drugs beyond alcohol. Consistent with the cross-drug thesis from rodent models, we have now observed preliminary evidence for sensitized response to unpredictable stressors among abstinent alcoholics (in the current study), 24-hour nicotine deprived smokers (Hogle et al., 2010), and heavy daily marijuana users (Hefner, Starr, & Curtin, in prep).
Between-system neuroadaptations provide one set of etiologic mechanisms for sensitized response to unpredictable stressors in addiction. Within-system neuroadaptations, where the primary cellular response within a specific system adapts to neutralize the drug’s effects (Koob & Le Moal, 2008b) within that same system, may also contribute to a sensitized response to unpredictable stressors. While Koob et al. have discussed neuroadaptations within the reward system in depth (Koob & Le Moal, 2008b), the current study combines with other data from our laboratory to implicate a possible within-system adaptation in the stress system to repeated alcohol exposure (Kaye et al., in press). Specifically, across a programmatic series of experiments (Bradford et al., 2013; Hefner & Curtin, 2012; Hefner et al., 2013; Moberg & Curtin, 2009) we have demonstrated that acute administration of alcohol selectively reduces startle potentiation to unpredictable (vs. predictable) stressors in humans (see Bradford et al., 2013 for discussion of implications for Stress Response Dampening theory). Thus, allostatic neuroadaptations to repeated alcohol stress response dampening may also contribute to the compensatory sensitized response to unpredictable stressors observed in abstinent alcoholics in this study. We hope these preliminary observations encourage reverse translational research to search for the neural mechanisms of this potential within-stress system adaption in rodent models (Koob, Lloyd, & Mason, 2009; Sinha, Shaham, & Heilig, 2011). Cross-sectional and longitudinal studies with rodents may measure startle potentiation after both acute and chronic alcohol administration to probe these opposing compensatory effects on stress response dampening and sensitization, respectively. This behavioral neuroscience research may be most sensitive to detect stress neuroadaptations by focusing on the distinction between predictable vs. unpredictable stressors. We and others have recently made calls for an increased focus on this critical feature of stressor predictability in refining rodent models of stress neuroadaptation and stress-induced reinstatement (Kaye et al., in press; Mantsch et al., 2016).
This study was motivated to test the rodent model thesis that chronic alcohol use would cause sensitized response to unpredictable stressors via stress neuroadaptation in human alcoholics. Our results are consistent with this thesis. However, the cross-sectional measurement of startle potentiation in pre-existing groups of alcoholics and healthy controls allows for other plausible interpretations. For example, increased startle potentiation to unpredictable stressors may represent a pre-morbid risk factor for AOD use disorders rather than a consequence of chronic AOD use (Gorka, Lieberman, Phan, & Shankman, 2016; Rasmussen & Kincaid, 2015). In other research, Gorka, Nelson, & Shankman (2013) observed that participants with co-morbid panic and alcohol use disorders displayed increased startle potentiation to unpredictable stressors relative to both participants with only panic disorder and healthy controls. They suggested that elevated startle potentiation in panic disorder may motivate heavy alcohol use that contributes to development of co-morbid alcohol use disorder. Indeed some participants in the current study had co-morbid mental health disorders (e.g., depression, anxiety disorders) which increases the generalizability of our findings, but our study was not designed to examine comorbidity specifically. Clearly additional research including longitudinal designs is required to adjudicate between these and other competing interpretations.
We focused on the unpredictable vs predictable startle potentiation contrast to explicitly test for group differences selectively during unpredictable stressors over and above any possible differences in generic threat responding. However, the observed pattern of group means may represent independent contributions from both this selective increase in response to unpredictable (vs. predictable) stressors in alcoholics and other premorbid differences or neuroadaptations associated with reduced responding to predictable stressors. Whereas responses to unpredictable stressors are mediated by NE and CRF sensitive pathways through the lateral divisions of the central amygdala and BNST, responses to predictable stressors are mediated by an overlapping but separate pathway through the medial division of the central amygdala (mCeA). Of note, the BNST has inhibitory effects on the mCeA (Campeau et al., 1997; Grillon et al., 2015; Haufler, Nagy, & Pare, 2013), which can manifest as attenuated startle potentiation or other fear expression to predictable stressors (Grillon et al., 2015; Kim et al., 2013; Meloni, Jackson, Gerety, Cohen, & Carlezon, 2006; Walker, Miles, & Davis, 2009). If stress neuroadaptations lead to generally increased BNST activity in alcoholics, these individuals could display somewhat attenuated response to predictable stressors due to increased inhibitory effects of the BNST on the mCeA. Consistent with this possibility, Grillon et al. (2015) recently demonstrated that administration of a CRF antagonist to healthy participants increased their startle potentiation to predictable stressors presumably through decreased activation of the CRF sensitive BNST.
Individual Differences in Possible Stress Neuroadaptation
Our secondary analyses of alcoholics’ individual differences clarify the nature of this increased response to unpredictable stressors and highlights important next steps. To start, alcoholics who displayed greater unpredictable (vs. predictable) startle potentiation also reported more alcohol-related problems. Taken at face value, this relationship establishes unpredictable startle potentiation as clinically relevant. As such, it may serve as a marker of one dimension of addiction severity (Gorka et al., 2016). Furthermore, it may be that individuals who experience greater unpredictable startle potentiation may struggle more with stronger urges and difficulty controlling their use in key situations when problems begin to emerge. Future research should clarify the causal relationship between unpredictable startle potentiation and alcohol-related problems and measure possible mediators such as drinking urge.
Unpredictable (vs. predictable) startle potentiation was greater among alcoholics who reported a clinically significant withdrawal syndrome. This connects this effect with a cardinal diagnostic criterion for AOD use disorders (Baker et al., 2004; Heilig, Egli, Crabbe, & Becker, 2010), the withdrawal syndrome. Given that negative affect is the motivational core of the withdrawal syndrome (Baker et al., 2004), it may be that a stress neuroadaptation contributes to both sensitized response to unpredictable stressors and withdrawal-related negative affect. In our study, we found no evidence that the relative increase in unpredictable (vs. predictable) startle potentiation varied as a function of duration of abstinence among alcoholics who had abstained from between 1 week and 2 months. This is consistent with other research that suggests that stressors continue to instigate AOD relapse well into protracted abstinence in humans (Brown et al., 1990; McKay, 1999) and rodents (Mantsch et al., 2016). In contrast to the more transient physical symptoms of withdrawal, withdrawal-related negative affect may also be long-lasting and contribute to later relapse among some AOD users (Baker et al., 2004). Our study’s cross-sectional design does not allow us to examine the temporal ordering of the increased unpredictable (vs. predictable) startle potentiation, alcohol use patterns, alcohol-related problems, and the emergence of the withdrawal syndrome. Future longitudinal research in humans can clarify issues related to the relative onset, developmental course, and persistence of these key features of AOD use disorders. In particular, we believe that questions about whether an activated withdrawal syndrome from acute deprivation is sufficient or even necessary to observe increased reactivity to unpredictable stressors are important to consider immediately (for detailed review of these issues see Kaye et al., in press).
Quantity of alcohol use in the 28 days prior to cessation did not predict the size of unpredictable (vs. predictable) startle potentiation in this study. If this sensitized stress response results from chronic alcohol use it may be that a more comprehensive assessment aggregated over a longer time span may be necessary to detect the impact of drinking quantity. Alternatively, use characteristics other than quantity may be more critical to its development. For example, rodent models suggest that particular patterns of episodic drinking (e.g., repeated binging and withdrawal) rather than overall quantity may be necessary to promote allostatic changes in stress-related neurocircuitry (Griffin, Lopez, & Becker, 2009; O’Dell, Roberts, Smith, & Koob, 2004).
Of course, increased confidence in these individual differences as well as the mechanism(s) that account for increased unpredictable (vs. predictable) startle potentiation overall in abstinent alcoholics requires replication with varied research designs. We hope that such research proceeds in parallel with both humans and animal models as facilitated by the use of startle potentiation in cross-species translational research. Such a program of research holds high promise for rapid bi-directional translation between basic research on mechanism and applications in the pharmacologic and psychosocial treatment of AOD use disorders (Kaye et al., in press).
Bi-directional translation can also occur between laboratory and more naturalistic research on stressors in the “real world”, with a different set of opportunities and challenges. For example, the stressors in the current task were temporally unpredictable, but real world stressors may incorporate unpredictability in alternative, often complex ways. For this reason, we have developed alternative laboratory tasks that manipulate how predictable the stressor may be with respect to probability (Hefner & Curtin, 2012), intensity (Bradford et al., 2013), or location (Bradford, Motschman, Starr, & Curtin, 2017). These features (e.g., probabilistic and temporal uncertainty) can be combined to increase stressor unpredictability (Moberg & Curtin, 2009). This previous research suggests acute alcohol administration selectively reduces response when these stressors are unpredictable, regardless its source, but we have yet to consider the impact of chronic AOD use in these tasks in a clinical sample. Other researchers have recently noted that the effects of alcohol on response to unpredictable stressors in social drinkers also appear to extend beyond manipulations of electric shock to more real world situations that include inherent unpredictability, such as in most social interactions (e.g., Sayette, 2017; see Fairbairn & Sayette, 2014 for review).
Other important characteristics of real world stressors besides predictability can also be manipulated in the laboratory. For example, stressor intensity (Bradford et al., 2013; Moberg, Weber, & Curtin, 2011) or controllability (Bradford, Shireman, Schneck, & Curtin, n.d.; Maier, 2015) may have influences on AOD-related behavior. Stressors may become less predictable if appraisal processes are degraded by distractors and this too can be modeled in the laboratory (e.g., Curtin, Patrick, Lang, Cacioppo, & Birbaumer, 2001). Finally, naturalistic research can complement these laboratory approaches by taking advantage of rapidly developing mobile technologies that allow for real-time measurement of subjective emotional response, behavior, and physiology combined with important contextual information provided by GPS location services and indices of peer-to-peer interactions in the real world (Curtin, Zhu, Gustafson, & Alagoz, 2015; Harari et al., 2016). All of these approaches can and should be marshalled to better understand and treat the contributions of unpredictable stressors to the etiology and maintenance of AOD use disorders.
General Scientific Summary.
Stress plays a key role in addiction etiology and relapse but our understanding of specific mechanisms for these relationships remain limited. Rodent models suggest that repeated alcohol use changes the central nervous system circuits involved in behavioral and emotional response to stressors. This study provides preliminary support that indicates similar changes may occur from alcohol use by human alcoholics such that they experience an exaggerated response to unpredictable stressors when abstinent.
Acknowledgments
Research reported in this publication was supported by the National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health (NIH) under award numbers R01 AA024388 and F31 AA018608, and by dissertation research awards from the American Psychological Association (APA) and Robert Wood Johnson Foundation (RWJF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, APA, or RWJF. Portions of this research have previously been presented at the annual conferences of the Research Society on Alcoholism, Association for Psychological Science, and the Society for Psychophysiology Research and in the Journal of Studies on Alcohol and Drugs (Kaye, Bradford, Magruder & Curtin, 2017). We have made the data, analysis scripts, questionnaires, and other study materials associated with this report publicly available via Open Science Framework. These materials can be accessed at https://osf.io/ykmuh
Footnotes
The differing number of cues across predictable and unpredictable conditions followed from design decisions. Specifically, we wanted to match the number of startle probes in the two primary conditions (6 probes during predictable shock cues and 6 probes at 4.5 seconds into unpredictable shock cues). However, we also included an additional 6 probes at later time points in the unpredictable shock cues (2 probes each at 19.5, 49.5, & 79.5 seconds) to allow us to test if group differences in startle potentiation persisted during unpredictable shock cues. As such, two more unpredictable cues (12 total) were needed to allow for these additional startle probes during unpredictable cues to assess responding at later time points.
We calculated Spearman-Brown corrected split half (odd vs. even trials) internal consistency for startle magnitude in all conditions at 4.5 seconds post cue onset (unpredictable no-shock rsb = 0.96, predictable no-shock rsb = 0.93, unpredictable shock rsb = 0.95, predictable shock rsb =0.96) and later times in unpredictable cues (unpredictable no-shock rsb = 0.95, unpredictable shock rsb = 0.94). We calculated Spearman-Brown corrected split half internal consistency for startle potentiation at 4.5 seconds post cue (unpredictable rsb = 0.64, predictable rsb = 0.37) and at later times during unpredictable cues (rsb = 0.52).
All participants also completed the Short Michigan Alcohol Screening Test (Selzer, Vinokur, & van Rooijen, 1975), Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991), Externalizing Spectrum Inventory–100 (Venables & Patrick, 2012), Childhood Trauma Questionnaire—Short Form (Bernstein et al., 2003) and a report of the typical quantity and frequency of their alcohol use.
Participants also completed the Short form of the Positive and Negative Affect Schedule (Short PANAS; Mackinnon et al., 1999) during the experimental session to measure current mood, independent of the primary unpredictable stressor task. Participants completed the PANAS-X at four times during the experimental session 1) prior to initial startle reactivity assessment, 2) after shock sensitivity assessment, 3) mid-point of unpredictable stressor task, 4) after unpredictable stressor task. We analyzed the PANAS-X negative affect subscale in a GLM with a between-subjects regressor for Group (alcoholic vs. control) and repeated measures for Time (baseline vs. post-shock sensitivity vs. mid-task vs. post-task). There was a significant effect of Time, F(3,318)=4.33, p=.005, consistent with expected general increases in self-reported state negative affect after receiving electric shock. However, there was no significant main effect of Group, t(106)=0.77, p=.441, or Group X Time interaction, F(3,318)=0.79, p=.499. We did not expect to observe group differences on this measure for two reasons. First, the PANAS assesses current mood rather than phasic reactivity to the stressors included in the stressor task. Rodent affective neuroscience indicates that stress neuroadaptations in the CRF and NE mechanisms that are putatively indexed by startle potentiation to unpredictable stressors support “dynamic, active response to an acute stressor” rather than tonic, persistent negative mood states (Koob & Zorrilla, 2012, p. 309; also see Heilig, Goldman, Berrettini, & O’Brien, 2011). Second, the PANAS was not used to assess reactivity selectively to unpredictable stressors, but rather overall mood state at various points during the experimental session.
Covariates are an important tool to increase power to test focal group effects in clinical and other research. We evaluated all individual difference measures from the demographics and affect sections of Table 1 as potential covariates. We did not consider individual difference variables related to alcohol use/problems as covariates because these variables are fundamentally related to the focal Group variable and therefore their variance should not be removed from primary analyses (Miller & Chapman, 2001). Covariates were selected if we confirmed that the specific covariate significantly predicted either overall startle potentiation or the selective increase in startle potentiation to unpredictable (vs. predictable) cues in GLMs that included only the Task Order and Startle Reactivity factors. Critically, Group was not included in these covariate selection analyses to avoid biasing selection of covariates by their relationship with Group.
Tests of between group simple effects are often not appropriate to decompose an interaction, particularly when pre-existing differences in non-manipulated grouping variables may confound these simple effects (Rosnow & Rosenthal, 1995). Nonetheless, we still report these simple Group effects here for the interested reader. Specifically, the simple effect tests of Group on startle potentiation were not significant during either unpredictable stressors, ηp2<0.01, b=6.0, 95% CI [−7.8,19.8], t(80)=0.86, p=.390, or predictable stressors, ηp2=0.03, b=−11.3, 95% CI [24.9,2.3], t(80)=1.65, p=.103.
We focused our analyses of individual difference moderators on four specific individual differences that were relevant to the stress neuroadaptation model. Three (alcohol related problems, withdrawal, and quantity of alcohol use) of these four individual differences were expected to significantly moderate the unpredictable vs. predictable startle potentiation contrast. The size of this contrast was not expected to vary by duration of abstinence. Given our focus on only a few candidate moderators, we did not correct the p-values from these analyses for multiple comparisons. A false discovery rate correction for the three predicted significant moderators yields p-values of .082, .082, and .587, respectively (Benjamini & Hochberg, 1995). As such, the effects of these moderators should be interpreted cautiously pending replication in independent samples.
References
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. https://doi.org/10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory: Manual. San Antonio, TX: The Psychiatric Corporation; 1987. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Berkman ET, Dickenson J, Falk EB, Lieberman MD. Using SMS text messaging to assess moderators of smoking reduction: Validating a new tool for ecological measurement of health behaviors. Health Psychology. 2011;30(2):186–194. doi: 10.1037/a0022201. https://doi.org/10.1037/a0022201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, … Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. https://doi.org/10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. https://doi.org/10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Kaye JT, Curtin JJ. Not just noise: individual differences in general startle reactivity predict startle response to uncertain and certain threat. Psychophysiology. 2014;51(5):407–411. doi: 10.1111/psyp.12193. https://doi.org/10.1111/psyp.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science. 2013;24(12):2541–2549. doi: 10.1177/0956797613499923. https://doi.org/10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shireman J, Schneck SE, Curtin JJ. Preregistration: Alcohol, Uncontrollability and Unpredictability. n.d Retrieved December 21, 2016, from https://osf.io/q8d96/register/565fb3678c5e4a66b5582f67.
- Bradford DE, Starr MJ, Motschman CA, Curtin JJ. Alcohol's effects on emotionally motivated attention, defensive reactivity, and subjective anxiety during uncertain threat. 2017 doi: 10.1093/scan/nsx095. Manuscript in under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Starr MJ, Shackman AJ, Curtin JJ. Empirically based comparisons of the reliability and validity of common quantification approaches for eyeblink startle potentiation in humans. Psychophysiology. 2015;52(12):1669–1681. doi: 10.1111/psyp.12545. https://doi.org/10.1111/psyp.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. Journal of Abnormal Psychology. 1990;99(4):344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: Behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. http://dx.doi.org/10.1016/S0306-4522(96)00632-X. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Curtin JJ. PhysBox: The Psychophysiology toolbox. An open source toolbox for psychophysiological data reduction within EEGLab. 2011 Retrieved from http://dionysus.psych.wisc.edu/PhysBox.htm.
- Curtin JJ. lmSupport: Support for Linear Models (Version 2.9.2) 2015 Retrieved from https://cran.r-project.org/web/packages/lmSupport/index.html.
- Curtin JJ, Patrick CJ, Lang AR, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12(6):527–531. doi: 10.1111/1467-9280.00397. https://doi.org/10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Zhu X, Gustafson D, Alagoz O. NIAAA R01: Dynamic, real-time prediction of alcohol use lapse using mHealth technologies. 2015 Retrieved January 5, 2017, from https://osf.io/szjue/
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology Reviews. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. https://doi.org/doi:10.1037/npp.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. https://doi.org/10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Fairbairn CE, Sayette MA. A social-attributional analysis of alcohol response. Psychological Bulletin. 2014;140(5):1361–1382. doi: 10.1037/a0037563. https://doi.org/10.1037/a0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox HC, Bergquist KL, Kwang-Ik H, Sinha R. Stress-Induced and Alcohol Cue-Induced Craving in Recently Abstinent Alcohol-Dependent Individuals. Alcoholism: Clinical and Experimental Research. 2007;31(3):395–404. doi: 10.1111/j.1530-0277.2006.00320.x. https://doi.org/10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox J. Regression Diagnostics (Sage University Paper Series on Quantitative Application of the Social Sciences, Series No. 07–079) Newbury Park, CA: Sage; 1991. [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, … Koob GF. CRF–CRF1 system activation mediates withdrawalinduced increases in nicotine self-administration in nicotine-dependent rats. PNAS. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. https://doi.org/10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132(1–2):216–222. doi: 10.1016/j.drugalcdep.2013.02.003. https://doi.org/10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism, Clinical and Experimental Research. 2009;33(11):1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. https://doi.org/10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;144(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. https://doi.org/10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH1 Antagonist GSK561679 Increases Human Fear But Not Anxiety as Assessed by Startle. Neuropsychopharmacology. 2015;40(5):1064–1071. doi: 10.1038/npp.2014.316. https://doi.org/10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari GM, Lane ND, Wang R, Crosier BS, Campbell AT, Gosling SD. Using Smartphones to Collect Behavioral Data in Psychological Science: Opportunities, Practical Considerations, and Challenges. Perspectives on Psychological Science. 2016;11(6):838–854. doi: 10.1177/1745691616650285. https://doi.org/10.1177/1745691616650285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learning & Memory (Cold Spring Harbor, NY) 2013;20(11):633–641. doi: 10.1101/lm.031799.113. https://doi.org/10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstroem KO. The Fagerstroem Test for Nicotine Dependence: A revision of the Fagerstroem Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. Journal of Psychopharmacology. 2012;26(2):232–244. doi: 10.1177/0269881111416691. https://doi.org/10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122(3):756–769. doi: 10.1037/a0033407. https://doi.org/10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Starr MJ, Curtin JJ. Increased stress response to threat among heavy marijuana users (in prep) [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. https://doi.org/10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature Reviews Neuroscience. 2011;12(11):670–684. doi: 10.1038/nrn3110. https://doi.org/10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology. 2011;218(1):121–129. doi: 10.1007/s00213-011-2355-8. https://doi.org/10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biological Psychiatry. 2010;68(8):687–688. doi: 10.1016/j.biopsych.2010.06.003. https://doi.org/10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. PLoS Medicine. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. https://doi.org/10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53(8):1241–1255. doi: 10.1111/psyp.12663. https://doi.org/10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Magruder KP, Curtin JJ. Probing for neuroadaptations to unpredictable stressors in addiction: Translational methods and emerging evidence. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2017.78.353. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70(1):216–227. [PubMed] [Google Scholar]
- Keselman HJ, Algina J, Lix LM, Wilcox RR, Deering KN. A generally robust approach for testing hypotheses and setting confidence intervals for effect sizes. Psychological Methods. 2008;13(2):110–129. doi: 10.1037/1082-989X.13.2.110. https://doi.org/10.1037/1082-989X.13.2.110. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, … Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. https://doi.org/10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the Brain Antireward System. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. https://doi.org/10/1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc B. 2008b;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. https://doi.org/10/1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Reviews Drug Discovery. 2009;8(6):500–515. doi: 10.1038/nrd2828. https://doi.org/10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37(1):308–309. doi: 10.1038/npp.2011.213. https://doi.org/10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love A, James D, Willner P. A comparison of two alcohol craving questionnaires. Addiction. 1998;93(7):1091–1102. doi: 10.1046/j.1360-0443.1998.937109113.x. https://doi.org/10.1046/j.1360-0443.1998.937109113.x. [DOI] [PubMed] [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27(3):405–416. https://doi.org/10.1016/S0191-8869(98)00251-7. [Google Scholar]
- Maier SF. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiology of Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. https://doi.org/10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41(1):335–356. doi: 10.1038/npp.2015.142. https://doi.org/10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR. Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. Journal of Studies on Alcohol. 1999;60(4):566–576. doi: 10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2010;25 doi: 10.1177/0269881110376694. https://doi.org/10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA. Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Annals of the New York Academy of Sciences. 2006;1071:538–541. doi: 10.1196/annals.1364.059. https://doi.org/10.1196/annals.1364.059. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. Journal of Abnormal Psychology. 2009;118(2):335–347. doi: 10.1037/a0015636. https://doi.org/10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Weber S, Curtin JJ. Alcohol dose effects on stress response to cued threat vary by threat intensity. Psychopharmacology. 2011;218:217–227. doi: 10.1007/s00213-011-2304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism, Clinical and Experimental Research. 2004;28(11):1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology, Biochemistry and Behavior. 2002;72(1–2):213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Open Science Collaboration. Estimating the reproducibility of psychological science. Science. 2015;349(6251) doi: 10.1126/science.aac4716. https://doi.org/10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Retrieved from http://www.R-project.org. [Google Scholar]
- Rasmussen DD, Kincaid CL. Acoustic startle in alcohol-naïve male rats predicts subsequent voluntary alcohol intake and alcohol preference. Alcohol and Alcoholism (Oxford, Oxfordshire) 2015;50(1):56–61. doi: 10.1093/alcalc/agu065. https://doi.org/10.1093/alcalc/agu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. “Some things you learn aren’t so”: Cohen’s paradox, Asch’s paradigm, and the interpretation of interaction. Psychological Science. 1995;6(1):3–9. https://doi.org/doi:10.1111/j.1467-9280.1995.tb00297.x. [Google Scholar]
- RStudio: Integrated development environment for R. Boston, MA: RStudio; 2016. (Version 0.97.449) Retrieved from http://www.rstudio.org/ [Google Scholar]
- Sayette MA. The effects of alcohol on emotion in social drinkers. Behaviour Research and Therapy. 2017;88:76–89. doi: 10.1016/j.brat.2016.06.005. https://doi.org/10.1016/j.brat.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbrodt FD, Maier M, Heene M, Zehetleitner M. Voluntary commitment to research transparency. 2015 Retrieved from http://www.researchtransparency.org.
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36(1):117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22(11):1359–1366. doi: 10.1177/0956797611417632. https://doi.org/10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. A 21 Word Solution [SSRN Scholarly Paper] 2012 Oct 14; Retrieved March 21, 2016, from http://dx.doi.org/10.2139/ssrn.2160588.
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, … Saxon AJ. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcoholism, Clinical and Experimental Research. 2015;39(5):808–817. doi: 10.1111/acer.12703. https://doi.org/10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. https://doi.org/10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. https://doi.org/10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218(1):69–82. doi: 10.1007/s00213-011-2263-y. https://doi.org/10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale (ADS) User’s Guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: II. Cigarette addiction. Journal of Abnormal Psychology. 1973;81(2):158–171. doi: 10.1037/h0034534. https://doi.org/10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous and photic blink reflexes. Psychophysiology. 1998;35(6):690–697. [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment. 2012;24(1):88–100. doi: 10.1037/a0024703. https://doi.org/10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizueta N, Patrick CJ, Jiang Y, Thomas KM, He S. Dispositional fear, negative affectivity, and neuroimaging response to visually suppressed emotional faces. NeuroImage. 2012;59(1):761–771. doi: 10.1016/j.neuroimage.2011.07.015. https://doi.org/10.1016/j.neuroimage.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. https://doi.org/10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50(1):30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: measures of central tendency. Psychological Methods. 2003;8(3):254–274. doi: 10.1037/1082-989X.8.3.254. https://doi.org/10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]