Abstract
Eradication of leukemia stem cells (LSCs) is the ultimate goal of treating acute myeloid leukemia (AML). We recently showed that the combined loss of Runx1/Cbfb inhibited the development of MLL-AF9-induced AML. However, c-Kit+/Gr-1− cells remained viable in Runx1/Cbfb-deleted cells, indicating that suppressing RUNX activity may not eradicate the most immature LSCs. In this study, we found upregulation of several hemostasis-related genes, including the thrombin-activatable receptor PAR-1 (protease-activated receptor-1), in Runx1/Cbfb-deleted MLL-AF9 cells. Similar to the effect of Runx1/Cbfb deletion, PAR-1 overexpression induced CDKN1A/p21 expression and attenuated proliferation in MLL-AF9 cells. To our surprise, PAR-1 deficiency also prevented leukemia development induced by a small number of MLL-AF9 leukemia stem cells (LSCs) in vivo. PAR-1 deficiency also reduced leukemogenicity of AML1-ETO-induced leukemia. Re-expression of PAR-1 in PAR-1-deficient cells combined with a limiting-dilution transplantation assay demonstrated the cell-dose-dependent role of PAR-1 in MLL-AF9 leukemia: PAR-1 inhibited rapid leukemic proliferation when there were a large number of LSCs, while a small number of LSCs required PAR-1 for their efficient growth. Mechanistically, PAR-1 increased the adherence properties of MLL-AF9 cells and promoted their engraftment to bone marrow. Taken together, these data revealed a multifaceted role for PAR-1 in leukemogenesis, and highlight this receptor as a potential target to eradicate primitive LSCs in AML.
INTRODUCTION
Leukemia stem cells (LSCs) are capable of limitless self-renewal and indefinitely propagating acute myeloid leukemia (AML). Early pioneering xenograft studies using NOD/SCID (nonobese diabetic/severe-combined immunodeficiency) mice suggested that LSCs in AML are exclusively CD34+CD38−, similar to the cell-surface phenotype of primitive hematopoietic stem cells (HSCs). However, it has become increasingly evident that LSCs are not necessarily primitive cells (reviewed in Goyama et al.1). Studies using mouse AML models for MLL-fusion leukemia have shown that some LSCs express mature myeloid markers,2,3 and recent work showed that myeloid differentiation is a prerequisite for LSC formation.4 These findings challenge the original concept that LSCs are the most primitive cells, but instead indicate that slightly differentiated LSCs can be responsible for the development of AML. On the other hand, several studies identified primitive HSC-like cells that have acquired leukemia-related mutations in AML patients. These cells, termed ‘preleukemic HSCs’, retain multilineage differentiation capacity but show a repopulation advantage over normal HSCs.5–7 Taken together, it appears that there are multiple types of stem cells associated with AML development, including the slightly differentiated ‘active’ LSCs and the primitive stem cells possessing leukemia-initiating mutations that remain relatively ‘dormant’. The factors that differentiate these types of LCSs remain poorly defined.
Recently, we showed that RUNX function is necessary to sustain the leukemogenic cell phenotype in AML. Suppression of RUNX1 function in human AML1-ETO- and MLL-AF9-expressing cord blood cells induced cell-cycle arrest and apoptosis in these cells. In a mouse transplantation model of MLL-AF9 leukemia, combined deletion of Runx1/Cbfb substantially inhibited leukemogenesis. However, we also observed that LSC-enriched cells with an immature surface phenotype (c-Kit+Gr-1−)8 remained viable in Runx1/Cbfb-deleted MLL-AF9 cells.9 These findings suggest that RUNX targeting inhibits leukemic proliferation, but may not eradicate the primitive and dormant LSCs. The data also imply that experimentally targeting RUNX may provide a means of identifying important modulators of LSC biology in AML. In the current study, we found that the expression of multiple hemostasis-related genes, including PAR-1, were significantly unregulated in LSC-enriched Runx1/Cbfb-deleted MLL-AF9 cells, suggesting a role for this receptor in LSC pathobiology. PAR-1, encoded by F2R, is expressed in many types of cells related to hematopoiesis, including HSCs,10–12 bone marrow stromal cells such as osteoblasts11,13,14 and hemogenic endothelium.15 PAR-1 activation has been implicated in the progression of multiple solid tumors, and has been shown to promote cellular proliferation, survival and invasion in these contexts.16 A role for PAR-1 in the pathogenesis of AML is suggested by flow cytometry analyses showing that cell-surface expression of PAR-1 in French–American–British subtype M4/M5 AML cells is higher compared with monocytes or granulocytes from healthy donors.17 Notably, MLL rearrangements are most frequently found in M4/M5 AML,18 suggesting that PAR-1 is likely to be highly expressed in MLL-fusion leukemia. A few recent studies examined the functions of PAR-1 in hematopoiesis and leukemogenesis. These reports showed that PAR-1 inhibits endothelial-to-hematopoietic transition,15 regulates the retention and recruitment of HSCs in bone marrow11,19 and suppresses the development of MLL-AF9-induced leukemia.12 However, the precise role of PAR-1 in HSCs and LSCs is not yet completely characterized.
The studies presented here reveal a novel, cell-dose-dependent role of PAR-1 in MLL-AF9 leukemia. Consistent with a previous report,12 PAR-1 acts as an antioncogenic factor to inhibit rapid leukemic proliferation when there are a large number of LSCs to induce leukemia. However, PAR-1 also acts as a pro-oncogenic factor to support the growth and/or survival of LSCs in bone marrow when these cells are present in small numbers. The crucial importance of PAR-1 in the maintenance of a small LSC populations suggests that PAR-1 may have a key role in early stages of leukemogenesis, or in contexts where a minimal residual disease state is achieved. PAR-1 deficiency also reduced clonogenicity and leukemogenicity of AML1-ETO-induced AML. Taken together, these data imply that targeting PAR-1 may represent a means of eradicating minimal residual disease, thereby preventing relapse in AML patients.
RESULTS
Expression of multiple hemostasis-related genes is upregulated in Runx1/Cbfb-deleted MLL-AF9 cells
We previously showed that Runx1/Cbfb deletion in MLL-AF9 cells resulted in the increased frequency of c-Kit+Gr-1− LSC-enriched population.9 To identify the pathways that are active in these primitive LSCs, we performed gene set enrichment analysis using expression profiles of control and Runx1/Cbfb-deleted MLL-AF9 cells (Goyama et al.9 and Table S3, GSE47350). This analysis revealed that genes related to blood coagulation were significantly upregulated upon Runx1/Cbfb depletion in MLL-AF9 cells (Figure 1a). The upregulated genes are all either thrombin targets or directly related to a thrombin target (Figure 1b). Among these genes, we focused on PAR-1 (encoded by the F2R gene), which has a central role in thrombin signaling. Upregulation of PAR-1 in Runx1/Cbfb-deleted cells was confirmed by quantitative PCR (qPCR) (Figure 1c). Furthermore, previous reports showed that: (1) RUNX1 binds to the promoter region of F2R (Figure 1d),20 (2) thrombin as well as PAR-1 pathway genes are upregulated in RUNX1-mutated AML21 and (3) PAR-1 has the opposite function to Runx1 in fetal hematopoietic development.15 We also found that PAR-1 expression in RUNX1-altered AML is significantly higher compared with that in RUNX1-intact AML (Figure 1e). These data suggest that PAR-1 is likely a transcriptional target of RUNX1 and has important roles in the regulation of normal and malignant hematopoiesis.
Figure 1.
Multiple hemostatic system-related genes are upregulated in Runx1/Cbfb-deleted MLL-AF9 cells. (a). Gene set enrichment analyses using MSigDB (http://software.broadinstitute.org/gsea/msigdb) revealed upregulation of genes related to hemostatic functions in Runx1/Cbfb-deleted MLL-AF9 cells. (b) Protein interaction among these genes upregulated in Runx1/Cbfb-deleted MLL-AF9 cells (F2r (PAR-1), F2rl3, Thbd, F13a1, Hnf4a, Itgb3, red circle) was investigated using STRING (v.9.0). Note that all of the proteins encoded by these genes are either directly or indirectly influenced by the central hemostatic protease, thrombin (F2). (c) Murine bone marrow progenitors derived from wild-type mice, mice with Runx1-floxed alleles (Runx1-f/f) or mice with Runx1/Cbfb-floxed alleles (Runx1/Cbfb-f/f) were transduced with MLL-AF9. Immortalized cells from the third to fifth round of in vitro plating were subsequently transduced with CreER. Cells were treated with ethanol (EtOH) or 4-hydroxytamoxifen (4-OHT) for 4 days, and relative mRNA levels of PAR-1 in 4-OHT-treated Runx1-f/f and Runx1/Cbfb-f/f MLL-AF9/CreER cells were examined. Results were normalized to Gapdh (glyceraldehyde 3-phosphate dehydrogenase), with the relative mRNA level in EtOH-treated cells set to 1. Data are shown as mean ± s.d. of triplicates. (d) Runx1 binds to the promoter region of PAR-1 in Runx1+CD41+ early hematopoietic cells.20 (e) A box plot showing PAR-1 expression in RUNX1-altered AML (including AMLs with RUNX1-RUNX1T1 translocation) and RUNX1-intact AML cases was drawn using cBioPortal.
PAR-1 activation inhibits cell-cycle progression and leukemogenesis of MLL-AF9 cells
We first assessed the effect of PAR-1 overexpression on the growth of human CB cells expressing MLL-AF9. MLL-AF9 immortalizes CB cells in vitro and produces human leukemia in immunodeficient mice.22 We transduced vector control, human PAR-1, and an arginine-to-alanine mutant form of PAR-1 (R41A) into MLL-AF9-expressing CB cells. The R41A mutation results in loss of the thrombin cleavage site, making this mutant PAR-1 insensitive to activation by thrombin and other proteases. These human PAR-1 constructs contain an amino-terminal FLAG sequence, providing a means to detect the expression of either the wild-type or R41A mutant proteins on the cell surface (green fluorescent protein-positive (GFP+) cells). As expected, thrombin-mediated cleavage of PAR-1 at R41 resulted in loss of cell surface FLAG expression in cells expressing wild-type PAR-1, but not in cells expressing the R41A mutant (Figure 2a), indicating that thrombin cannot activate the R41A PAR-1 mutant. Functionally, expression of PAR-1, but not the R41A mutant, inhibited the growth of MLL-AF9 cells in the presence of thrombin (Figure 2b). Thrombin-mediated PAR-1 activation resulted in cell-cycle arrest without inducing apoptosis (Figure 2c and Supplementary Figures S1A–C). As a mechanism for PAR-1-mediated cell-cycle arrest, we found upregulation of CDKN1A/p21 in PAR-1-expressing MLL-AF9 cells stimulated by thrombin (Figure 2c). Thus, similar to the effect of RUNX1 depletion,9 thrombin-induced PAR-1 activation leads to CDKN1A/p21 upregulation and inhibits cell-cycle progression in human MLL-AF9 cells.
Figure 2.
Thrombin-mediated PAR-1 activation inhibits proliferation and leukemogenesis induced by MLL-AF9. (a) Human CB cells expressing MLL-AF9 were transduced with a vector control, human PAR-1 and a human PAR-1-R41A mutant (an inactive form of PAR-1). All these constructs coexpress GFP and contain an amino-terminal Flag sequence that is cleaved by thrombin. Flag expression on GFP− (untransduced) and GFP+ (transduced) cells was assessed in the presence/absence of thrombin. Note that the addition of thrombin to PAR-1-expressing cells induced loss of Flag expression in GFP+ fraction, which was not seen for the R41A mutant. (b) Human MLL-AF9 cells transduced with PAR-1 constructs as described in (a) were cultured in cytokine containing media with/without thrombin. The mixed transduction culture containing both transduced GFP(+) and untransduced GFP(− ) cells were passaged to score the frequency of GFP(+) cell by flow cytometric analysis as a measure of the impact of the transduced gene on cellular proliferation rate. The initial frequency of GFP(+) cells immediately after transduction was set as 1. Wild-type PAR-1, but not the R41A mutant, showed a growth-inhibitory effect on human MLL-AF9 cells in the presence of thrombin. (c) Human CB cells expressing MLL-AF9 cells were transduced with vector/PAR-1/R41A, and were cultured in cytokine containing media with/without thrombin. Cell-cycle status and the levels of CDKN1A/p21 and tubulin were assessed after 24 h of culture. Thrombin-mediated PAR-1 activation decreased the frequency of S/G2/M-phase cells (left) and induced upregulation of CDKN1A/p21 (right). See also Supplementary Figure S1A. (d) Mouse bone marrow c-Kit+ cells were retrovirally transduced with MLL-AF9 together with vector, PAR-1 or PAR-1-R41A (coexpressing GFP), and the cells were transplanted into mice. Frequencies of the GFP+ (vector/PAR-1/R41A-transduced) fraction in bone marrow cells before transplantation and in leukemic cells after transplantation are shown. PAR-1-expressing GFP+ cells were not detected in leukemia cells, whereas the frequency of vector- and R41A-transduced GFP+ cells were increased in leukemia cells (N = 3 for each group).
Next, we assessed the role of PAR-1 in leukemogenesis using mouse models for MLL-AF9 leukemia. The function of mouse PAR-1 and R41A constructs was confirmed using NIH3T3 cells. Reflecting its focus-forming ability,23 PAR-1 promoted the growth of NIH3T3 cells in a culture with high cellular density, whereas PAR-1-expressing NIH3T3 cells grew normally in a culture with optimal cellular density (Supplementary Figure S2). We retrovirally transduced a vector control, mouse PAR-1 or the R41A mutant (coexpressing GFP) together with MLL-AF9 into mouse bone marrow progenitors, and transplanted these cells into recipient mice. PAR-1 overexpression substantially inhibited MLL-AF9-induced leukemia development in vivo, as indicated by the depletion of GFP+ cells in leukemic mice. The R41A mutant did not show a similar negative effect (Figure 2d). Thus, PAR-1 over-expression inhibited cell-cycle progression and in vivo leukemic growth of MLL-AF9 cells.
PAR-1 deficiency impairs leukemia development induced by a small number of LSCs
PAR-1 is highly expressed in HSCs and was shown to regulate HSC retention/recruitment in bone marrow,10,11,19 but a recent report showed that absence of PAR-1 did not change HSC function.12 We found that PAR-1-deficiency did not impact colony-forming ability and serial transplantation potential, suggesting that PAR-1 is dispensable for normal HSCs (Supplementary Figures S3A–C). However, we observed a modest but statistically significant diminution in the engraftment potential of PAR-1-deficient cells relative to control cells in the bone marrow, but not in the spleen (Supplementary Figure S3D). These data probably indicate a role for PAR-1 in regulating the interplay between HSCs and the bone marrow niche, as suggested by previous reports.11,19 To determine the role of endogenous PAR-1 in leukemogenesis, we transduced MLL-AF9 into bone marrow progenitors derived from wild-type or PAR-1-deficient mice. These cells were serially replated up to five times, and were then transplanted into sublethally irradiated recipient mice (Figure 3a). PAR-1 deficiency showed little effect on the colony-replating ability of MLL-AF9 cells (Supplementary Figure S4A). However, we observed impaired engraftment and significantly delayed MLL-AF9 leukemia development in mice receiving PAR-1-deficient cells relative to those receiving control cells (Figure 3b and Supplementary Figure S4B). Of note, about half the recipient mice transplanted with PAR-1-deficient cells never developed leukemia, suggesting a significant reduction of LSCs in PAR-1-deficient MLL-AF9 cells. This observation was in contrast to a previous report, in which Baumer et al.12 showed that PAR-1 deficiency did not alter initial leukemia development induced by MLL-AF9.12 We speculated that differences in the relative numbers of LSCs injected into recipient mice could explain these differing results. To test this hypothesis, we performed limiting-dilution transplantation assays using wild-type or PAR-1-deficient bone marrow progenitors transduced with MLL-AF9. This assay clearly demonstrated that PAR-1 expression is important for leukemogenesis only when relatively small numbers of cells (that is, 1 × 104 cells) were transplanted, whereas PAR-1 is dispensable in mice transplanted with larger cell doses (that is, more than 1 × 105 cells Figure 3c). These data suggest that PAR-1 has an important supporting role to produce full-blown AML in contexts where LSCs are relatively few in number, whereas a much larger number of LSCs can produce AML in the absence of PAR-1 functions.
Figure 3.
PAR-1 is required to produce MLL-AF9 leukemia in vivo in contexts where LSCs are limited in number. (a) Experimental scheme used in (b). Bone marrow c-Kit+ cells derived from wild-type or PAR-1-deficient mice were retrovirally transduced with MLL-AF9, and the cells were serially replated in vitro. After three to five rounds of plating, MLL-AF9-expressing colony-forming cells (1 ×106 cells per mouse) were injected into sublethally irradiated (700 cGy) recipient mice. (b) Shown are the frequency of CD45.2+ donor cells in peripheral blood collected 4 and 8 weeks post-transplant from recipient mice (left), and survival curves of the mice transplanted with 1 × 106 wild-type or PAR-1-deficient MLL-AF9-expressing colony-forming cells (right). PAR-1-deficient MLL-AF9 showed impaired engraftment compared with wild-type MLL-AF9 cells at 8 weeks, and mice transplanted with PAR-1-deficient cells showed significantly improved survival relative to mice transplanted with wild-type cells. N = 12 per group. Two independent experiments were performed with similar outcomes. See also Supplementary Figure S4B. (c) Bone marrow c-Kit+ cells derived from wild-type or PAR-1-deficient mice were transduced with MLL-AF9, and the indicated number of cells were directly injected into sublethally irradiated (700 cGy) recipient mice. Shown are survival curves of mice transplanted with the indicated number of wild-type or PAR-1-deficient c-Kit+ cells transduced with MLL-AF9. N = 4 for each group. Note that transplantation with PAR-1-deficient cells only resulted in a significant survival advantage relative to wild-type cells when the recipient mice received a lower cells dose.
PAR-1 mediates thrombin-induced adhesion and promotes engraftment of MLL-AF9 cells into the bone marrow
Several reports have shown that thrombin induces adhesion of many types of cells.24–26 We therefore examined the role of PAR-1 in thrombin-induced adhesion of MLL-AF9 cells to a recombinant human fibronectin fragment (Retronectin). Treatment with thrombin significantly increased the adhesion of wild-type, but not PAR-1-deficient MLL-AF9 cells to Retronectin (Figure 4a). Because adherence properties are related to the homing/engraftment capacity of leukemia cells, we then assessed the role of PAR-1 in the homing and retention of MLL-AF9 cells in bone marrow in vivo. Here, equal numbers of wild-type or PAR-1-deficient MLL-AF9 colony-forming cells were intravenously coinjected into sublethally irradiated recipient mice to assess the percentage of donor cells in the bone marrow and spleen over time. The relative frequencies of wild-type and PAR-1-deficient MLL-AF9 cells were similarly low in both the bone marrow and spleen 16 h after injection. However, by 96 h after injection, there was a substantial increase in the frequency of wild-type, but not PAR-1-deficient MLL-AF9 cells in the bone marrow. This genotype-dependent increase in donor cells was not observed in the spleen (Figure 4b). Thus, PAR-1 promotes the early growth, retention and/or survival of MLL-AF9 cells in bone marrow immediately after transplantation.
Figure 4.
PAR-1 promotes the adhesive properties and bone marrow homing/engraftment of MLL-AF9 cells. (a) Wild-type and PAR-1-deficient MLL-AF9 colony-forming cells were seeded into Retronectin-precoated wells with 0, 0.5 or 1 U/ml thrombin. The number of adherent cells was estimated using WST-1 after the removal of non-adherent cells. Data are shown as mean ± s.d. from two independent experiments. Note that the addition of thrombin significantly increased cell adhesion in PAR-1-expressing MLL-AF9 cells, but not in PAR-1-deficient cells. (b) Engraftment of MLL-AF9-expressing wild-type or PAR-1-deficient colony-forming cells (CD45.2+GFP+ cells) at 16 or 96 h post-transplant in the bone marrow and spleen of recipient mice. PAR-1 deficiency significantly limited bone marrow engraftment 96 h post-transplant. Data are show as mean ± s.d. N = 6 for each group.
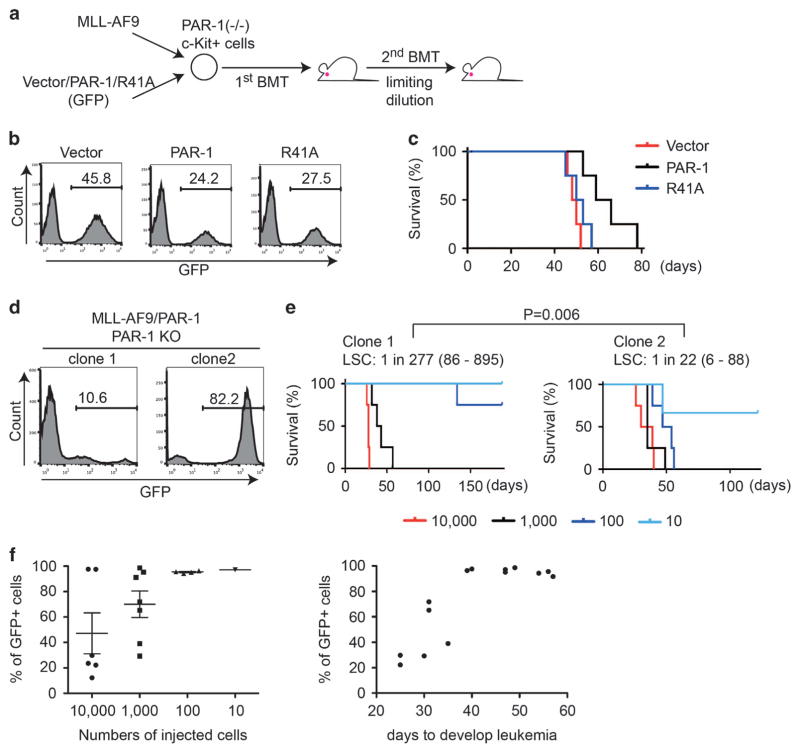
Re-expression of PAR-1 into PAR-1-deficient cells increases the frequency of MLL-AF9 LSCs
To determine the precise role of PAR-1 in MLL-AF9-induced leukemia, we cotransduced MLL-AF9 with a vector control, wild-type PAR-1 or the R41A mutant (each coexpressing GFP) into PAR-1-deficient BM progenitors, and transplanted the cells into recipient mice (Figure 5a). Similar transduction efficiency of wild-type PAR-1 and the R41A mutant was confirmed before transplantation (Figure 5b). When we injected sufficient numbers of MLL-AF9 cells, re-expression of PAR-1 into PAR-1-deficient cells slowed leukemia development (Figure 5c). We then assessed LSC frequency in two MLL-AF9 leukemia clones with different proportions of PAR-1+ cells; PAR-1-deficient (GFP−) or PAR-1-expressing (GFP+) cells were dominant in clone 1 or clone 2, respectively (Figure 5d). The limiting-dilution transplantation assay revealed that clone 2 contained more LSCs than clone 1 (Figure 5e), confirming a role for PAR-1 in supporting LSCs. The proportion of c-Kit+Gr-1− LSCs was not substantially different between the clones (data not shown), indicating that PAR-1 does not directly regulate the cell fate to be LSC or not. Rather, it appears that PAR-1 functionally supports the growth and survival of LSCs.
Figure 5.
Cell-dose-dependent role of PAR-1 in the development of MLL-AF9 leukemia. (a) Experimental scheme used in (b–f). PAR-1-deficient bone marrow c-Kit+ cells were transduced with MLL-AF9 together with vector/PAR-1/R41A coexpressing GFP, and were transplanted into recipient mice. MLL-AF9 leukemia cells were isolated from spleens of moribund primary recipients, and secondary transplantation was performed using the indicated number (10–10 000 cells) of leukemia cells. (b) Initial transduction of vector control, PAR-1 or PAR-1-R41A in c-Kit+ BM cells was evaluated by the expression of GFP. Wild-type PAR-1 and the R41A mutant showed similar transduction efficiency before transplantation. (c) Shown are survival curves of mice transplanted with 1 × 106 PAR-1-deficient bone marrow progenitors transduced with MLL-AF9 in combination with vector/PAR-1/R41A. N = 4 for each group. Reintroduction of wild-type PAR-1, but not the R41A mutant, into PAR-1-deficient progenitors slowed leukemia development. (d) Shown are histograms of two independent PAR-1-deficient MLL-AF9 leukemia clones with significantly different degrees of PAR-1 expression based on GFP positivity in cells. Clone 1 was composed mostly of GFP− (PAR-1-deficient) cells, whereas clone 2 was composed mostly of GFP+ (PAR-1-expressing) cells. (e) A limiting-dilution transplantation assay was performed using the two MLL-AF9 leukemia clones. The survival data indicates that clone 2 contains more LSCs than clone 1. P = 0.006. (f) MLL-AF9 leukemia cells from clone 2 were transplanted into secondary recipient mice, and the frequency of GFP+ cells was assessed in leukemia cells from the spleens of moribund mice. The relative number of GFP+ (that is, PAR-1 expressing) cells in the spleens at this late time point in disease progression was inversely dependent on the number of cells injected, and also correlated with the length of the period to onset of leukemia. Note the higher frequency of GFP+ (PAR-1-expressing) cells in mice injected with a small number of cells that develop leukemia after a long latency.
We next examined the changes in GFP frequency in recipient mice that were injected with various numbers of the same leukemic clone (clone 2) after they became moribund. The relative percentage of GFP+ (that is, PAR-1-expressing) cells in the spleens of these animals with advanced disease varied depending on the cell numbers injected into recipient mice. GFP+ cells always became the major population when a small number of cells (that is, 10 or 100) were transplanted. However, GFP− cells (that is, PAR-1-deficient cells) often became dominant when the recipient mouse received more than 1000 cells. There was also an expected inverse correlation between the relative number of PAR-1-expressing leukemia cells, as indicated by GFP frequency, and the time to evidence of leukemia onset (Figure 5f). Taken together, these results support the conclusion that PAR-1 has a key role in supporting LSCs, and hence leukemogenesis, in contexts where AML develops slowly and originates from a small number of LSCs.
PAR-1 deficiency impairs the clonogenicity and leukemogenicity of AML1-ETO-induced leukemia
To investigate the role of PAR-1 in another subtype of AML, we transduced AML1-ETO into wild-type or PAR-1-deficient bone marrow progenitors and serially replated the cells (Figure 6a). PAR-1-deficient AML1-ETO cells contained a smaller c-Kit+ population and showed substantially reduced colony-forming activity after fourth or fifth rounds of replating compared with wild-type AML1-ETO cells (Figure 6b). We then transduced AML1-ETO9a, a shorter isoform of AML1-ETO with more potent leukemogenic activity, into wild-type and PAR-1-deficient fetal liver cells, and transplanted a small number (1 × 104/mouse) of AML1-ETO9a-expressing cells into sublethally irradiated recipient mice. Similar to the results for MLL-AF9-induced leukemia, we observed significantly delayed leukemia development in mice receiving PAR-1-deficient AML1-ETO9a cells relative to those receiving control cells. Two out of seven recipient mice never developed leukemia, suggesting a reduction of LSCs in PAR-1-deficient AML1-ETO cells (Figure 6c). These data indicate that PAR-1 also has a role in LSC activity in AML1-ETO-induced leukemia.
Figure 6.
PAR-1 deficiency inhibits leukemogenesis induced by AML1-ETO. (a) Experimental scheme used in (b). Bone marrow c-Kit+ cells derived from wild-type or PAR-1-deficient mice were transduced with AML1-ETO, and the cells were serially replated in vitro. (b) Colony numbers of AML-ETO-expressing wild-type or PAR-1-deficient bone marrow progenitors. Shown are weekly colony counts per 104 replated cells (mean ± s.d.) from duplicate plates (upper) after third round (R3), and Gr-1 and c-Kit expression in wild-type or PAR-1-deficient AML1-ETO cells at fifth round (lower). Two independent experiments were performed. The percentages of Gr-1+ and c-Kit+ cells are indicated. PAR-1-deficient AML-ETO cells showed reduced colony-forming activity and contain fewer c-Kit+ cells compared with wild-type AML1-ETO cells. (c) c-Kit+ cells derived from wild-type or PAR-1-deficient fetal liver were transduced with AML1-ETO9a (a truncated form of AML1-ETO with stronger leukemogenic activity), and were transplanted into recipient mice. Shown are survival curves of the mice transplanted with 1 × 104 wild-type or PAR-1-deficient fetal liver cells transduced with AML1-ETO9a. N = 7 for each group. P = 0.0071, log-rank test.
DISCUSSION
PAR-1 has been shown to have oncogenic roles in several solid tumors, including melanoma,27 breast cancer28,29 and glioma.30 In hematopoietic neoplasms, a recent study showed an unexpected antioncogenic role of PAR-1 in MLL-AF9 leukemia.12 In agreement with this report, we found that PAR-1 overexpression induced cell-cycle arrest and inhibited the growth of MLL-AF9 cells in several experimental assays. Interestingly, we also found that PAR-1 has an important role in enhancing LSC activity in MLL-AF9 leukemia, which becomes apparent in experimental contexts where relatively small numbers of LSCs are used to induce leukemia. These observations are in contrast to the previous report, in which Baumer et al.12 suggested that the loss of PAR-1 enhanced LSC function. Given the cell-dose-dependent function of PAR-1 in LSC maintenance, differences in LSC numbers injected into recipient mice could explain this discrepancy. Baumer et al.12 injected 90 000 MLL-AF9-transduced bone marrow progenitors into primary recipients, while we injected 10 000 MLL-AF9-transduced progenitors or MLL-AF9 colony-forming cells cultured in vitro. For secondary transplantation, Baumer et al.12 injected 100 c-Kit+ cells, while we only injected 10 or 100 whole MLL-AF9 cells. Taken together with the data presented here, the fact that we injected significantly fewer LSCs provides an explanation for the apparent differences in observed PAR-1 functions in LSC activity between these studies and the studies of Baumer et al.12 In addition to the cell-dose-dependent role of PAR-1, our data suggest that PAR-1 may have the dosage-dependent function to promote leukemogenesis, which should be investigated in future studies.
The mechanisms underlying PAR-1-mediated LSC regulation remain to be fully elucidated. We found that PAR-1 deficiency in MLL-AF9 leukemia results in impaired cell adhesion and attenuated homing/engraftment to the bone marrow compartment. These data suggest the involvement of PAR-1 in the regulation of interactions between LSCs and the bone marrow niche. This view is consistent with a recent report showing that PAR-1 regulates the retention and recruitment of bone marrow HSCs.19 The study suggested that direct thrombin-mediated PAR-1 signaling events led to HSC mobilization. Conversely, thrombin bound to thrombomodulin converts protein C to its activated form (aPC) that stimulates endothelial protein C receptor (EPCR)-PAR-1 signaling, which supported retention of HSCs in the bone marrow microenvironment.19 Given that thrombomodulin is also upregulated in the LSC-enriched Runx1/Cbfb-deleted MLL-AF9 cells (Figures 1a and b), the aPC–EPCR–PAR-1 axis may contribute to the development of MLL-AF9 leukemia by supporting LSC retention in the bone marrow. In addition to thrombin and aPC, it is recognized that PAR-1 can be activated by the TF/fVIIa/fXa complex and matrix metalloproteases.31 It is also notable that matrix metalloproteases were shown to promote leukemogenesis.32 The key protease(s) driving PAR-1 activation in the context of AML progression remain to be determined. In addition, how PAR-1 activation upregulates CDKN1A/p21 and whether CDKN1A/p21 regulates LSC dormancy need to be clarified in future studies. Interestingly, a previous report showed that thrombin-mediated activation of PAR-1 induced CDKN1A/p21 upregulation via the JAK/STAT pathway in various types of cells.33 The possible interaction among thrombin/PAR-1, JAK/STAT and CDKN1A/p21 in LSCs warrants further investigation. Furthermore, mechanisms by which PAR-1 increases the colony formation of AML1-ETO cells remain to be elucidated. Given that cells were cultured in semisolid media, adherence properties are probably not related to the decreased colony-forming activity of PAR-1-deficient AML1-ETO cells. Therefore, PAR-1 appears to have other functions to promote leukemogenesis.
Accumulating evidence suggests that there are multiple subtypes of LSCs with distinct genetic alterations and epigenetic modifications. We previously showed that a certain level of Runx activity is required to sustain LSC proliferation in MLL-AF9 leukemia, but the cells with an immature phenotype remained viable even in Runx1/Cbfb-deleted cells.9 Here, we found a key role for PAR-1 in these primitive cells and showed the indispensable role of PAR-1 in enhancing LSC activity in MLL-AF9 and AML1-ETO leukemia. Based on these findings, we propose a revised model of LSCs in AML (Figure 7). Runx/Cbfb complex is required for actively proliferating LSCs to produce AML in vivo. PAR-1 activation inhibits rapid proliferation of the active LSCs partly through CDKN1A/p21 upregulation, but supports the growth of dormant LSCs with an immature phenotype. Sequential targeting of RUNX and PAR-1 could be a promising approach to eradicate all types of LSCs, thereby providing a means to eradicate minimal residual disease in patients with AML.
Figure 7.

Proposed model of ‘dormant’ and ‘active’ LSCs in AML. Active LSCs are slightly differentiated and require the Runx1/Cbfb complex for efficient proliferation to develop AML. PAR-1, which is downregulated by Runx1, inhibits the proliferation of active LSCs partly through the upregulation of CDKN1A/p21. In contrast, PAR-1 is required for the growth of dormant LSCs with a primitive phenotype.
MATERIALS AND METHODS
Vectors and viral transduction
Human PAR-1 and PAR-1-R41A containing an amino-terminal FLAG sequence (DYKDDDD)34 were provided by Dr JoAnn Trejo, and we cloned them into a retroviral vector pGCDNsam-IRES-GFP (a gift by Dr M Onodera). Murine PAR-1 was cloned into the pMYs-IRES-GFP vector,35 and we introduced a mutation to generate mouse PAR-1-R41A using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). For MLL-AF9 expression, we used pMSCV-MLL-AF9-pgk-EGFP or pMSCV-MLL-AF9-pgk-puro. For AML1-ETO expression, we used HA-tagged AML1-ETO or HA-tagged AML1-ETO9a in a pMSCV-IRES-Thy1.1 retroviral vector. Viral production was performed by transfecting viral plasmids along with gag, pol, env-expressing plasmids into 293T cells, as described previously.36,37
Human CB cell culture
Human umbilical CB cells were obtained from Translational Trials Development and Support Laboratory at Cincinnati Children’s Hospital Medical Center according to an institutional review board-approved protocol. Informed consent was obtained in accordance with the Declaration of Helsinki. CD34+ cells were separated using EasySep CD34 Selection Kit (STEMCELL Technologies, Vancouver, BC, Canada). We engineered human MLL-AF9- and AML-ETO-expressing cells by transducing MLL-AF9 and AML1-ETO into CB cells using retrovirus, as described previously.22,38–40 Cells were cultured in IMDM media containing 20% BIT9500 (STEMCELL Technologies) and 10 ng/ml human stem cell factor, thrombopoietin, FLT3 ligand, interleukin-3 and interleukin-6, as described previously.41,42
Flow cytometry
Cells were analyzed on a FACSCanto and were sorted with a FACSAria (BD Biosciences, San Jose, CA, USA). Cells were stained with fluorochrome-conjugated antibodies (Mouse c-Kit (CD117)-PE (2B8; BD Biosciences), mouse Gr-1 (Ly-6G/C)-APC (RB6–8C5; BD Biosciences), DYKDDDDK Tag (D6W5B) rabbit mAb (Cell Signaling Technology, Danvers, MA, USA; no. 14793)), were incubated for 30 min at 4 °C and were washed with 2% fetal bovine serum in phosphate-buffered saline before analysis. Cell-cycle analysis (Vybrant DyeCycle Violet Stain; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and apoptosis analysis (AnnexinV-APC Kit; BD Biosciences) were performed according to the manufacturer’s recommendations.
Western blotting
Cells were lysed directly in 1× Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA; no. 161-0737). Whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Bio-Rad). The blot was incubated with anti-p21 Waf1/Cip1 (12D1) rabbit mAb (Cell Signaling Technology; no. 2947) and anti-tubulin (D-66) antibody (Sigma-Aldrich, St Louis, MO, USA; no. T0198). Signals were detected with SuperSignalWest Pico (Pierce, Thermo Fisher Scientific).
Mice
PAR-1 (F2r)-deficient mice (CD45.2) were described previously.43 B6.SJL (CD45.1) mice were obtained from the CCHMC/CBDI mouse core. All animal studies were conducted according to an approved Institutional Animal Care and Use Committee protocol and federal regulations.
Myeloid colony assay and bone marrow transplantation assay
For MLL-AF9-induced leukemia, bone marrow progenitors (c-Kit+ cells) were transduced with MLL-AF9 and were plated in M3434 cytokine-enriched methylcellulose according to the manufacturer’s instructions (STEMCELL Technologies). A total of 1 × 103 cells were plated for each round of plating. Colony counting and replating were performed every 4 or 5 days. After three to five rounds of plating, MLL-AF9-expressing colony-forming cells (1 × 106 cells per mouse) were injected into sublethally irradiated (700 cGy) recipient mice (N = 12 per group) via the tail vein. Secondary transplantation was performed using the indicated numbers (10–10 000 cells) of spleen cells isolated from leukemic mice (N = 4 per group). In some experiments, c-Kit+ bone marrow progenitors were transduced with MLL-AF9 and were directly transplanted into mice.
For AML1-ETO-induced colony-replating assay, c-Kit+ bone marrow cells were transduced with full-length AML1-ETO and were plated in M3434 as described above. A total of 1 × 104 cells were plated for each round of plating. Colony counting and replating were performed every 7 days. For AML1-ETO-induced leukemia in vivo, c-Kit+ fetal liver cells were transduced with AML1-ETO9a (a shorter form of AML1-ETO with strong leukemogenic activity), and 1 × 104 AML1-ETO9a-expressing fetal liver cells were transplanted into sublethally irradiated (700 cGy) recipient mice (N = 7 per group). Randomization and blinding were not performed in this study.
In vivo homing and engraftment assay
MLL-AF9-expressing wild-type or Par-1-deficient colony-forming cells (2 × 106 cells per mouse) were injected into sublethally irradiated (700 cGy) B6.SJL (CD45.1) recipient mouse. Mice were killed 16 or 96 h after the transplantation, and the percentage of donor cells (CD45.2+GFP+ cells) in the bone marrow and spleen was determined using flow cytometry.
Adhesion assay
MLL-AF9-expressing wild-type or Par-1-deficient colony-forming cells were resuspended in Iscove’s modified Dulbecco’s media containing 20% BIT9500 (STEMCELL Technologies) with 0, 0.5 or 1 U/ml thrombin, and were seeded into 96-well plates (1 × 105 cells per well in 200 μl medium) precoated with 8 μg/cm2 RetroNectine (Takara-bio, Shiga, Japan). After 90 min of incubation, media and non-adherent cells were carefully removed, and the plates were washed two times with phosphate-buffered saline. After the addition of 100 μl fresh medium to each well, the number of adhesive cells was estimated by incubation with 10 μl WST-1 (Roche, Indianapolis, IN, USA) at 37 °C for 4 h.
Gene expression analysis
We assessed overlaps between up/downregulated gene lists in Runx1/Cbfb-deleted MLL-AF9 cells (Goyama et al.9 and Table S3) and gene sets in MSigDB (http://software.broadinstitute.org/gsea/msigdb). We also used cBioPortal (http://www.cbioportal.org/public-portal/)44,45 to examine PAR-1 expression in AML cases with/without RUNX1 alterations.
Statistics
Unpaired and two-tailed t-test was used to evaluate differences between groups (Figures 3b and 4b and Supplementary Figure S3D). Error bars representing s.d. indicate variation for each group and variance was found to be similar between compared groups. The survival distributions (Figures 3b, c and 6c and Supplementary Figure S4B) were compared using a log-rank test. Results for the limiting-dilution transplantation assays (Figure 5e) were compared using ELDA (http://bioinf.wehi.edu.au/software/elda/).46
Supplementary Material
Acknowledgments
We thank Dr J Trejo and Dr M Onodera for plasmids. We also thank the Flow Cytometry Core and the Mouse Core at Cincinnati Children’s Hospital Medical Center for their help. This work was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 1UL1RR026314-01, Translational Trials Development and Support Laboratory award (USPHS Grant Number MO1 RR 08084), CA176907 (JCM) and a Center of Excellence in Molecular Hematology P30 award (DK090971). JCM is a Leukemia and Lymphoma Society Scholar.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
AUTHOR CONTRIBUTIONS
SG conceived the project, designed and performed the research, analyzed the data and wrote the paper. MS, JS, LR, WM, EO assisted with experiments. BM and TK participated in analyzing the data, JSP and JCM conceived the project, secured funding, designed the research, analyzed the data and wrote the paper.
References
- 1.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood. 2015;125:2630–2640. doi: 10.1182/blood-2014-11-570218. [DOI] [PubMed] [Google Scholar]
- 2.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 4.Ye M, Zhang H, Yang H, Koche R, Staber PB, Cusan M, et al. Hematopoietic differentiation is required for initiation of acute myeloid leukemia. Cell Stem Cell. 2015;17:611–623. doi: 10.1016/j.stem.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012;4:149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Pre-leukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. 2014;111:2548–2553. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyama S, Schibler J, Cunningham L, Zhang Y, Rao Y, Nishimoto N, et al. Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J Clin Invest. 2013;123:3876–3888. doi: 10.1172/JCI68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong JF, Zhao Y, Sutton S, Su A, Zhan Y, Zhu L, et al. Gene expression profile of murine long-term reconstituting vs. short-term reconstituting hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:2448–2453. doi: 10.1073/pnas.0409459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronovich A, Nur Y, Shezen E, Rosen C, Zlotnikov Klionsky Y, Milman I, et al. A novel role for factor VIII and thrombin/PAR1 in regulating hematopoiesis and its interplay with the bone structure. Blood. 2013;122:2562–2571. doi: 10.1182/blood-2012-08-447458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumer N, Krause A, Kohler G, Lettermann S, Evers G, Hascher A, et al. Proteinase-activated receptor 1 (PAR1) regulates leukemic stem cell functions. PLoS One. 2014;9:e94993. doi: 10.1371/journal.pone.0094993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song SJ, Pagel CN, Campbell TM, Pike RN, Mackie EJ. The role of protease-activated receptor-1 in bone healing. Am J Pathol. 2005;166:857–868. doi: 10.1016/S0002-9440(10)62306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagel CN, Song SJ, Loh LH, Tudor EM, Murray-Rust TA, Pike RN, et al. Thrombin-stimulated growth factor and cytokine expression in osteoblasts is mediated by protease-activated receptor-1 and prostanoids. Bone. 2009;44:813–821. doi: 10.1016/j.bone.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Yue R, Li H, Liu H, Li Y, Wei B, Gao G, et al. Thrombin receptor regulates hematopoiesis and endothelial-to-hematopoietic transition. Dev Cell. 2012;22:1092–1100. doi: 10.1016/j.devcel.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Degen JL, Palumbo JS. Hemostatic factors, innate immunity and malignancy. Thromb Res. 2012;129(Suppl 1):S1–S5. doi: 10.1016/S0049-3848(12)70143-3. [DOI] [PubMed] [Google Scholar]
- 17.de Veiga CS, Carneiro-Lobo TC, Coelho CJ, Carvalho SM, Maia RC, Vasconcelos FC, et al. Increased expression of protease-activated receptor 1 (PAR-1) in human leukemias. Blood Cells Mol Dis. 2011;46:230–234. doi: 10.1016/j.bcmd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Bower M, Parry P, Carter M, Lillington DM, Amess J, Lister TA, et al. Prevalence and clinical correlations of MLL gene rearrangements in AML-M4/5. Blood. 1994;84:3776–3780. [PubMed] [Google Scholar]
- 19.Cohen SG, Itkin T, Chakrabarty S, Graf C, Kollet O, Ludin A, et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med. 2015;21:1307–1317. doi: 10.1038/nm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Joshi A, Wilson NK, Kinston S, Nishikawa S, Gottgens B. The transcriptional programme controlled by Runx1 during early embryonic blood development. Dev Biol. 2012;366:404–419. doi: 10.1016/j.ydbio.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendler JH, Maharry K, Radmacher MD, Mrozek K, Becker H, Metzeler KH, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and MicroRNA expression signatures. J Clin Oncol. 2012;30:3109–3118. doi: 10.1200/JCO.2011.40.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Micro-environment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CB, Mahon GM, Klinger MB, Kay RJ, Symons M, Der CJ, et al. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20:1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 24.Nierodzik ML, Plotkin A, Kajumo F, Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991;87:229–236. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heider I, Schulze B, Oswald E, Henklein P, Scheele J, Kaufmann R. PAR1-type thrombin receptor stimulates migration and matrix adhesion of human colon carcinoma cells by a PKCepsilon-dependent mechanism. Oncol Res. 2004;14:475–482. doi: 10.3727/0965040042380496. [DOI] [PubMed] [Google Scholar]
- 26.Kanemaru M, Maehara N, Iwamura T, Chijiiwa K. Thrombin stimulates integrin beta1-dependent adhesion of human pancreatic cancer cells to vitronectin through protease-activated receptor (PAR)-1. Hepatogastroenterology. 2012;59:1614–1620. doi: 10.5754/hge10036. [DOI] [PubMed] [Google Scholar]
- 27.Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–3137. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- 28.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 29.Yang E, Cisowski J, Nguyen N, O’Callaghan K, Xu J, Agarwal A, et al. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene. 2016;35:1529–1540. doi: 10.1038/onc.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auvergne R, Wu C, Connell A, Au S, Cornwell A, Osipovitch M, et al. PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene. 2016;35:3817–3828. doi: 10.1038/onc.2015.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121:431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara F, Kitaura J, Uchida T, Nishida C, Togami K, Inoue D, et al. Hes1 promotes blast crisis in chronic myelogenous leukemia through MMP-9 upregulation in leukemic cells. Blood. 2014;123:3932–3942. doi: 10.1182/blood-2013-01-476747. [DOI] [PubMed] [Google Scholar]
- 33.Huang YQ, Li JJ, Karpatkin S. Thrombin inhibits tumor cell growth in association with up-regulation of p21(waf/cip1) and caspases via a p53-independent, STAT-1-dependent pathway. J Biol Chem. 2000;275:6462–6468. doi: 10.1074/jbc.275.9.6462. [DOI] [PubMed] [Google Scholar]
- 34.Ishii K, Hein L, Kobilka B, Coughlin SR. Kinetics of thrombin receptor cleavage on intact cells. Relation to signaling. J Biol Chem. 1993;268:9780–9786. [PubMed] [Google Scholar]
- 35.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Therapy. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 36.Wunderlich M, Krejci O, Wei J, Mulloy JC. Human CD34+ cells expressing the inv (16) fusion protein exhibit a myelomonocytic phenotype with greatly enhanced proliferative ability. Blood. 2006;108:1690–1697. doi: 10.1182/blood-2005-12-012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou FS, Griesinger A, Wunderlich M, Lin S, Link KA, Shrestha M, et al. The THPO/MPL/Bcl-xL pathway is essential for survival and self-renewal in human pre-leukemia induced by AML1-ETO. Blood. 2012;120:709–719. doi: 10.1182/blood-2012-01-403212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulloy JC, Cammenga J, MacKenzie KL, Berguido FJ, Moore MA, Nimer SD. The AML1-ETO fusion protein promotes the expansion of human hematopoietic stem cells. Blood. 2002;99:15–23. doi: 10.1182/blood.v99.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Mulloy JC, Cammenga J, Berguido FJ, Wu K, Zhou P, Comenzo RL, et al. Maintaining the self-renewal and differentiation potential of human CD34+ hematopoietic cells using a single genetic element. Blood. 2003;102:4369–4376. doi: 10.1182/blood-2003-05-1762. [DOI] [PubMed] [Google Scholar]
- 40.Goyama S, Schibler J, Gasilina A, Shrestha M, Lin S, Link KA, et al. UBASH3B/Sts-1-CBL axis regulates myeloid proliferation in human preleukemia induced by AML1-ETO. Leukemia. 2016;30:728–739. doi: 10.1038/leu.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wunderlich M, Mulloy JC. Model systems for examining effects of leukemia-associated oncogenes in primary human CD34+ cells via retroviral transduction. Methods Mol Biol. 2009;538:263–285. doi: 10.1007/978-1-59745-418-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulloy JC, Wunderlich M, Zheng Y, Wei J. Transforming human blood stem and progenitor cells: a new way forward in leukemia modeling. Cell Cycle. 2008;7:3314–3319. doi: 10.4161/cc.7.21.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darrow AL, Fung-Leung WP, Ye RD, Santulli RJ, Cheung WM, Derian CK, et al. Biological consequences of thrombin receptor deficiency in mice. Thromb Hae-most. 1996;76:860–866. [PubMed] [Google Scholar]
- 44.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.