Abstract
Sarcomas differ from carcinomas in their mesenchymal origin. Therapeutic advancements have come slowly so alternative drugs and models are urgently needed. These studies report a new drug for sarcomas that simultaneously targets both tumor and tumor neovasculature. eBAT is a bispecific angiotoxin consisting of truncated, deimmunized Pseudomonas exotoxin fused to epidermal growth factor (EGF) and the amino terminal fragment (ATF) of urokinase. Here, we study the drug in an in vivo “ontarget” companion dog trial since eBAT effectively kills canine hemangiosarcoma (HSA) and human sarcoma cells in vitro. We reasoned the model has value due to the common occurrence of spontaneous sarcomas in dogs and a limited lifespan allowing for rapid accrual and data collection. Splenectomized dogs with minimal residual disease were given one cycle of eBAT followed by adjuvant doxorubicin in an adaptive dose-finding, phase I–II study of 23 dogs with spontaneous, stage I–II, splenic HSA. eBAT improved 6-month survival from <40% in a comparison population to ~70% in dogs treated at a biologically active dose (50 μg/kg). Six dogs were long-term survivors, living >450 days. eBAT abated expected toxicity associated with EGFR-targeting, a finding supported by mouse studies. Urokinase plasminogen activator receptor (uPAR) and EGFR are targets for human sarcomas, so thorough evaluation is crucial for validation of the dog model. Thus, we validated these markers for human sarcoma targeting in the study of 212 human and 97 canine sarcoma samples. Our results support further translation of eBAT for human patients with sarcomas and perhaps other EGFR-expressing malignancies.
Keywords: targeted toxin, epidermal growth factor receptor, urokinase plasminogen activator receptor, sarcoma, adaptive clinical trial, canine
Introduction
Unlike carcinomas derived from epithelial tissues, sarcomas comprise a heterogeneous group of malignancies of mesenchymal origin [1, 2]. There are 15,000 new sarcoma cases per year in the United States, consisting of 12,000 cases of soft tissue sarcoma and 3,000 cases of bone sarcomas [1]. The 5-year overall survival rate is approximately 50–80% for sarcomas [2, 3]. Development of new targeted therapies for therapy-resistant sarcoma has suffered from the lack of widely-expressed mutations or overexpressed proteins that can be targeted therapeutically without risk of severe adverse events [2, 4–7].
eBAT, a bispecific EGF-urokinase angiotoxin, was developed as a targeted, second generation bispecific biologic drug consisting of human EGF (targeting EGFR), human amino terminal transferase (ATF is the high affinity binding moiety of human urokinase, targeting uPAR), and genetically modified Pseudomonas exotoxin, mutated to reduce immunogenicity and facilitate ER retention. This drug was highly efficacious in treatment of established glioma in rodent xenograft models [8]. Xenograft models are informative, but targeting human cells in “non-target” immunosuppressed mice (that do not bind human EGF and ATF) does not yield the same clinical investigative information as studies in a large animal “ontarget” models where the drug cross-reacts with native EGFR and uPAR. Thus, we chose to undertake an “ontarget” clinical trial in companion dogs with hemangiosarcoma (HSA).
Canine HSA is a common, aggressive, incurable spontaneous sarcoma that appears to have a similar ontogenetic origin as human angiosarcoma [9, 10–12]. Canine hemangiosarcoma and human angiosarcoma are both vasoformative sarcomas with similar microscopic appearance [13] that have often metastasized by the time they are diagnosed. Humans with angiosarcoma have an expected median survival of approximately 16 months [14]; dogs with HSA have a comparable, short median survival of 4 to 6 months when treated with the standard-of-care of surgery and adjuvant chemotherapy [15, 16]. Morbidity and mortality are usually caused by metastatic spread and/or acute internal hemorrhage secondary to tumor rupture. We hypothesized that since HSA is a vascular cancer, eBAT simultaneously targeting the tumor and its vasculature rendered it an excellent therapy choice.
Expression of EGFR and PLAUR/uPAR was previously characterized in human sarcomas using conventional PCR-based assays, gene expression microarrays, and immunohistochemistry [17–20]. In this study, we confirm such expression in a variety of human sarcomas and report on EGFR and uPAR expression on canine HSA.
We showed that canine HSA tumor-initiating cells express EGFR and uPAR, and that these cells are highly sensitive to eBAT [8,21–23]. Here, we used a large “ontarget” animal study that closely parallels what could be a human clinical trial to show feasibility, safety, and efficacy of eBAT to treat sarcomas in a clinically translatable setting using spontaneous canine HSA as model, in both naive disease and minimal residual disease settings. We report on the impact of bispecific targeting on the toxicity risks associated with targeting of EGFR. Our results show that eBAT is safe and potentially effective at biologically active doses despite EGFR targeting, supporting further translation for patients with sarcomas and other EGFR-expressing malignancies. Furthermore, our findings support our belief that bispecificity reduces overall toxicity risks associated with EGFR targeting.
Materials and Methods
Assessment of EGFR and PLAUR/uPAR expression in human and canine tumors
EGFR and PLAUR mRNA expression was evaluated from data for 212 human sarcomas obtained through the TCGA (The Cancer Genome Atlas) Research Network (http://cancergenome.nih.gov/). The federal project was begun in 2005 to catalog genetic mutations responsible for cancer using genome sequencing and bioinformatics. To perform a similar analysis in dogs, we used next generation RNA sequencing (RNAseq) data from canine hemangiosarcoma and canine lymphoma samples that were reported previously [24, 25]. RNAseq for 31 canine osteosarcoma samples was performed as described [24, 26, 27]. EGFR and uPAR protein expression were evaluated in a human synovial sarcoma tissue microarray (TMA) [28]; the same methods were used to build a study-specific TMA that included tumors from 15 dogs as well as normal canine spleen, liver and kidney and spleens with nodular lymphoid hyperplasia and associated hematomas as controls. A total of 97 canine sarcoma samples were analyzed (51 HSAs and 31 osteosarcomas from independent datasets, and 15 HSAs from dogs enrolled in our clinical study). IHC methods are provided (Supplementary Methods).
Cell lines
Hemangiosarcoma cell line Emma was derived by the Modiano laboratory in 2008 and authenticated in 2015 by the Modiano laboratory using short tandem repeat (STR) testing (DNA Diagnostic Center, Inc., Fairfield, OH). It was cultured in hemangiosarcoma medium as described [22, 29]. Human angiosarcoma cell line AS5 was obtained from Dr. Gary K. Schwartz, Columbia University Medical Center, in 2013 and was cultured in hemangiosarcoma medium. Human RD rhabdomyosarcoma cell line was obtained from The Global Bioresource Center (ATCC) in January 2015. Human U2OS osteosarcoma cell line was obtained from ATCC in June 2015. Human HPB-MLT T-cell lymphoma cell line was obtained from the Cell Resource Center for Biomedical Research, Cell Bank in October 2014. These cell lines were grown in Dulbecco’s modified Eagle’s Medium (DMEM) as described [30–32]. RD, U2OS and HPB-MLT were authenticated using STR profiles (DNA Diagnostics Center, Inc., Fairfield, OH) in 2016.
eBAT production
eBAT was produced at the University of Minnesota cGMP Molecular and Cellular Therapeutics (MCT) Facility as described [8]. The construction of eBAT is illustrated in Figure 1A. Release assays were done by Pace Analytical Life Sciences, LLC (Minneapolis, MN) and/or at the MCT. Release criteria were established regarding drug purity (>95%), endotoxin (<50 Eu/mg), stability, selectivity, potency (IC50<1.0 nM), sterility, and concentration. The drug was vialed and re-tested to meet critical FDA specifications.
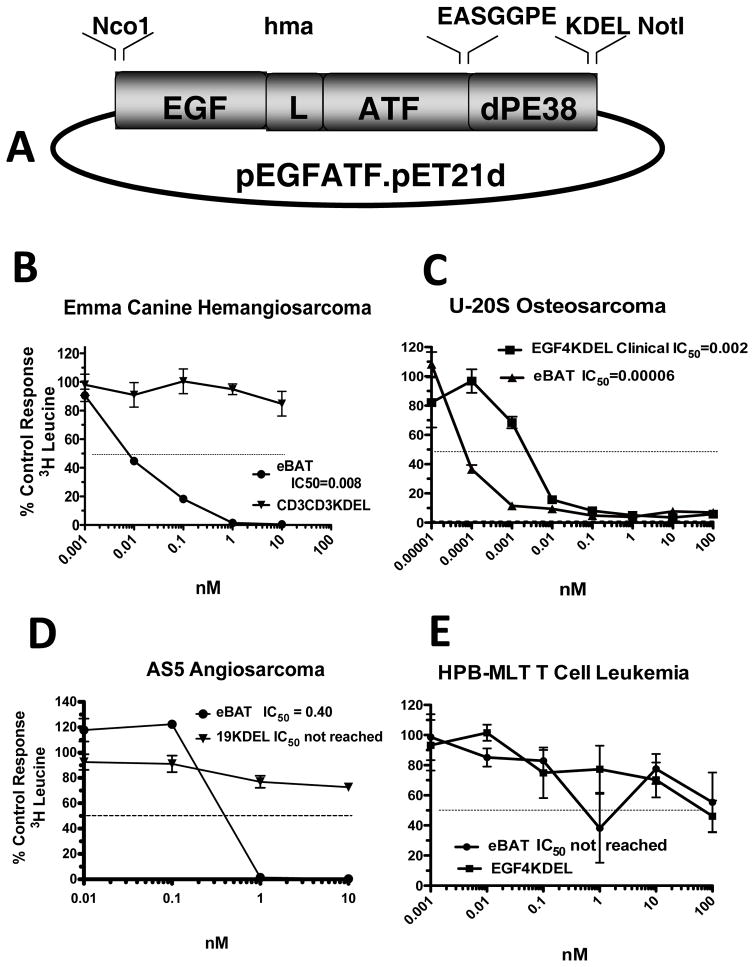
Figure 1. Construction and in vitro activity of eBAT.
Bispecific eBAT was studied for its activity against canine and human sarcoma cells. A) Expression vector for eBAT, human EGF and the high affinity amino terminal fragment of urokinase linked to a deimmunized PE38KDEL molecule. The fusion gene (from 5′ end to 3′ end) consisted of an NcoI restriction site, the genes for human EGF, an ATG initiation codon, the downstream 135-amino terminal fragment (ATF) from uPA linked by a 20 amino-acid segment of human muscle aldolase (HMA), the 7 amino-acid EASGGPE linker, the first 362 amino acids of the pseudomonas exotoxin (PE) molecule with KDEL at the C terminus, and a NotI restriction site at the 3′ end of the construct. B) Canine EMMA cells were treated with various concentrations of eBAT and control CD3CD3KDEL and then protein synthesis was measured 3 days later using a tritiated leucine uptake assay. Experimental variability is shown as quadruplicate samples ± SED. C) Human U-20S Osteosarcoma cells were treated with various concentrations of eBAT tested against EGF4KDEL and then leucine incorporation was measured. D) Human AS5 angiosarcoma cells were treated with various concentrations of eBAT tested against CD19KDEL as negative control. Leucine incorporation was measured. E) eBAT was tested against HPB-MLT cells to test specificity. eBAT, EGF4KDEL and 2219KDEL showed no significant cytotoxicity.
Laboratory Assays
Protein synthesis assays and proliferation assays measuring [3H]leucine incorporation were used to determine the effect of eBAT on cell lines. Briefly, cells were plated in 96-well flat-bottomed plates and allowed to adhere overnight. The targeted toxins were added in triplicate at 10-fold serial dilutions and incubated for 48 hours. Wells were then pulsed with [3H]leucine with 1 μCi per well and allowed to incubate for another 24 hours. Plates were then frozen to detach the cells, harvested onto glass fiber filters, washed, dried, and counted using standard scintillation methods. [3H]leucine assays were performed using Leucine-free medium. Data are reported as the percentage of control counts.
To evaluate safety, C57BL/6 mice were administered eBAT by the intraperitoneal route twice, two days apart on days 1 and day 3, and then were observed for adverse events for 3 weeks.
Canine clinical study
Safety and efficacy of adjuvant eBAT were assessed using a Bayesian adaptive Phase I–II trial design with pre-defined criteria of acceptable toxicity (no dose-limiting adverse events) and efficacy (>50% survival at 6 months) to guide dose finding [33]. eBAT was administered to dogs with spontaneous HSA after splenectomy and before the first of five cycles of doxorubicin chemotherapy. Eligibility was restricted to dogs with stage-1 or stage-2 splenic HSA with no evidence of gross metastatic disease. Adverse events (AEs) were graded according to VCOG-CTCAE criteria [34]. Survival time was measured from the date of diagnosis to the time of death and was censored at the time of last contact for dogs surviving at the time of analysis.
The clinical study, called SRCBST-1 (sarcoma bispecific toxin trial-1), was conducted with approval of the University of Minnesota (UMN) Institutional Animal Care and Use Committee (IACUC Protocols 1110A06186 and 1507-32804A). Study design and implementation conformed to Consolidated Standards of Reporting Trails (CONSORT) guidelines as they apply to studies in companion animals [35]. eBAT pharmacokinetics and neutralizing antibody assays were performed for all dogs. Detailed descriptions of the comparison group, eligibility criteria and protocols for the SRCBST-1 study, pharmacokinetics and neutralizing antibody assays are provided in the Supplementary Methods.
Statistical analysis
Univariate associations between time-to-death and gene expression, patient characteristics and tumor characteristics for the TCGA samples were assessed by cox proportional hazard regression and summarized by Kaplan-Meier curves. Associations between time-to-death and expression of EGFR or uPAR were assessed by multivariate cox regression analysis, and adjusted for each other and for patient and tumor characteristics. Associations between EGFR and uPAR expression in human and canine tumor samples were evaluated using Pearson’s correlation coefficient. Cases from TCGA were censored from analysis if they had no information on survival or if they were listed as “alive” at the end of follow up (or on the date data were analyzed) in the TCGA database.
Dogs and disease characteristics were summarized using descriptive statistics. The biologically active dose was identified as specified by the design [33]. Model-based estimates of the probability of AEs and 6-month survival were obtained from the parametric model used to guide dose finding. The probability of AEs was estimated using a logistic regression model with a linear term for dose; the probability of 6-month survival was modeled using a logistic regression model with linear and quadratic terms for dose. The probability of AEs for each dose was estimated by the sample proportion with exact confidence intervals. Kaplan-Meier curves for overall survival were fit for the entire study population and only for dogs treated at the biologically active dose to obtain a non-parametric estimate of 6-month survival and median time-to-death. Dogs were censored if died of causes other than HSA or if they were alive at the time of the analysis. Associations between AEs and baseline covariates of age, weight and body condition score (BCS) were assessed using the unpaired, two-sample t-test assuming unequal variances between groups. All p-values were two-sided. All analyses were performed using R version 3.0.1 [36].
Results
eBAT kills canine and human sarcoma cells
To assess activity, eBAT was added to Emma cells and leucine incorporation was measured as an indication of protein synthesis activity and cell viability (Figure 1B). Emma was chosen as positive control since detectable cell surface expression of EGFR and uPAR was previously reported [22]. Emma cells were killed in a dose-dependent manner and cytotoxicity was specific since a control anti-human CD3 targeted toxin, CD3CD3KDEL, recognizing the epsilon chain of the T cell receptor did not have activity. RD human rhabdomyosarcoma cells were also killed by eBAT in a dose-dependent manner whereas BIC3, a recombinant anti-human CD3 immunotoxin had no activity. The IC50 (50% inhibitory concentration for protein synthesis) for RD cells was 0.02 nM. Figure 1C shows that U-2OS human osteosarcoma cells that express high levels of EGFR and uPAR were also sensitive to eBAT and interestingly, that a bispecific targeted toxin EGF4KDEL [37, 38] that simultaneously targets EGFR and the human IL-4 receptor did not kill the human cell line as effectively as eBAT. The IC50 for these cell lines was in the subnanomolar range (0.06 pM – 0.08 nM). Figure 1D shows that eBAT effectively targeted the human angiosarcoma line AS5, originating from a histologically similar tumor as canine HSA. eBAT was also tested against human HPB-MLT T-cells, which do not express EGFR or uPAR and it showed no significant cytotoxicity as expected (Figure 1E). Together, these findings indicate that eBAT is extremely potent and inhibits both protein synthesis and DNA synthesis in a highly specific manner in vitro.
Human sarcomas express epidermal growth factor receptor and urokinase receptor
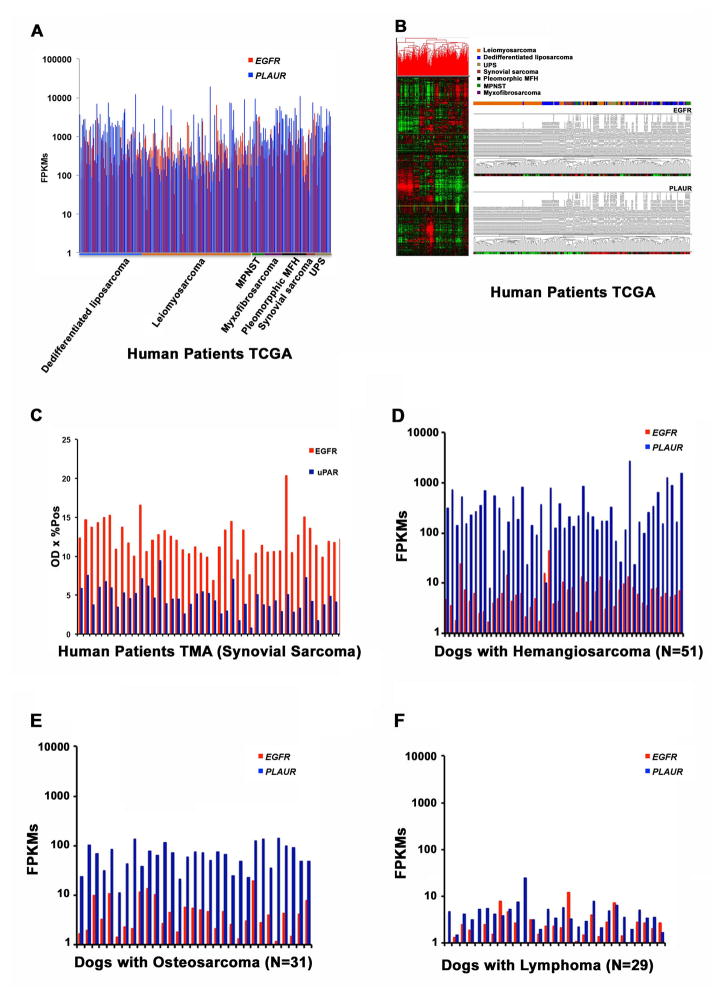
The most current bioinformatics TCGA database was used to explore the expression on of EGFR and PLAUR on 212 human sarcomas (Figure 2). Figure 2A shows that EGFR and PLAUR gene expression were detectable in 100% of samples regardless of sarcoma type with a variation in intensity. Supplementary Figure 1 shows Kaplan-Meier curves for time-to-death by EGFR expression for all subjects without subsetting. Subjects with EGFR expression above the median had shorter time-to-death than subjects with lower levels of EGFR (HR = 1.69, 95% CI: 1.02, 2.81). EGFR expression showed no correlation with metastasis, age, gender, sarcoma histological classification, or anatomic location. Figure 2B shows that PLAUR expression significantly correlated with histological classification: levels were below the median in leiomyosarcomas, synovial sarcomas, and dedifferentiated liposarcomas, whereas they were above the median in pleomorphic malignant fibrous histiocytomas (MFH), undifferentiated pleomorphic sarcomas (UPS), and myxofibrosarcomas. Expression of EGFR was not correlated with expression of PLAUR (R2=0.006). Yet, EGFR expression (p=0.043) and PLAUR expression (p=0.058) were both associated with time-to-death (Supplementary Table 1). Age, tumor volume, and presence of metastasis also were correlated with time-to-death.
Figure 2. EGFR and PLAUR gene expression analysis in human sarcomas and spontaneous canine tumors.
(A) EGFR and PLAUR gene expression analysis was done in 212 tumor tissue samples extracted from the TCGA database. The X-axis represents the patients supervised by tumor type and the Y-axis is the expression intensity as fragments per kilobase of transcript per million (FPKM) mapped reads. (B) Unsupervised hierarchical cluster and heat map highlighting EGFR and PLAUR expression in the human TCGA dataset. (C) EGFR and uPAR protein expression is shown in TMAs constructed from human synovial sarcoma tissue samples. The X-axis represents patient TMAs and the Y-axis represents optical density of EGFR and uPAR on immunohistochemistry. D) EGFR and PLAUR gene expression analysis in an independent data set of canine hemangiosarcoma samples. (E) EGFR and PLAUR gene expression analysis in canine osteosarcoma samples. (F) EGFR and PLAUR gene expression analysis in canine lymphoma samples. Tumor-bearing dogs are on the X-axis and fragments per kilobase of transcript per million mapped reads on the Y-axis, illustrating the levels of EGFR and PLAUR expression from the individual tumors. The following detailed values pertain to gene expression in TCGA samples of EGFR and PLAUR, respectively: Count: 212, 212; Mean (FPKM):653.4, 1,713; Mean (FPKM) lower confidence limit: 548.7, 1,387; Mean (FPKM) upper confidence limit: 758.0, 2,040; Variance: 600,273, 5,844,287; Standard Deviation: 774.8, 2,418; Mean Standard Error: 53.1, 165; Coefficient of Variation: 1.2, 1.4; Minimum (FPKM): 3.1, 40.9; Minimum (FPKM): 6,575.1, 19,171.7; Median (FPKM): 410.0, 757.9; Median Error: 4.56, 14.2; Percentile 25%(Q1): 215.4, 250.4; Percentile 75% (Q3): 752.9, 2,149.
Figure 2C shows expression of EGFR and uPAR proteins in human synovial sarcoma TMA. Both proteins were detectable in each of the 54 synovial sarcomas. Supplementary Table 2 shows more detailed characteristics of these patients and treatments. Neither gene was associated with survival when assessed independently, together, or with other covariates (Supplementary Table 3).
Expression of epidermal growth factor receptor and urokinase receptor is conserved in canine HSAs
In order to thoroughly evaluate EGFR and PLAUR expression in canine sarcomas, we evaluated mRNA expression in an independent dataset of 51 canine HSAs by RNAseq [24] and two additional datasets consisting of 31 canine osteosarcomas and 29 canine lymphoma tissue samples (Figures 2D, 2E, 2F) [25]. Results were similar to those in human sarcomas: expression of both EGFR and PLAUR genes was detectable in all canine sarcomas, with HSA having higher levels of PLAUR mRNA, and HSA and osteosarcomas having approximately equivalent levels of EGFR mRNA. As expected, expression of both genes was significantly lower (p<2X10−5) in canine lymphoma samples as compared to canine sarcomas (Figure 2F).
eBAT is safe and potentially effective in dogs with spontaneous HSA in a clinical setting
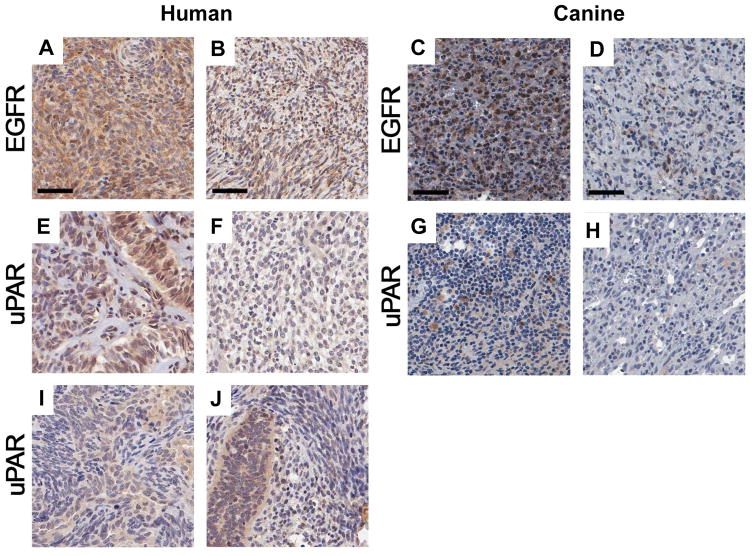
HSA was chosen as a target disease based on its extremely poor prognosis in dogs. Immunostaining of tumor tissues from 15 dogs enrolled in the SRCBST-1 study confirmed that both eBAT targets were expressed at the protein level in all dogs examined replicating the results of immunohistochemical studies in the human synovial sarcoma TMA where both proteins were expressed almost exclusively by tumor cells. Figure 3 shows representative photomicrographs of EGFR and uPAR staining in the canine and human TMAs. Expression of both proteins was variable in non-malignant tissues. Supplementary Figure 2 shows graphical data summaries.
Figure 3. EGFR and uPAR expression in human synovial sarcomas and canine HSA TMA from 15 dogs in the SRCBST study.
Synovial cell sarcoma TMA spots immunohistochemically stained for EGFR and uPAR. Representative highly and lowly stained spots for EGFR are shown (A–B human) (C–D canine). Representative highly and lowly stained spots for uPAR are shown (E–F human) (G–H canine). An example of heterogeneous expression of uPAR is shown in the human synovial TMA where uPAR expression is much higher in the glandular cells staining darkly brown and forming elongated glands, sometimes with compressed slit-like spaces between the gland cells (I). An admixture of spindled and glandular cells imparting a marbled-like appearance is also shown (J).
Table 1A summarizes baseline characteristics for all dogs by dose and Table 1B illustrates a treatment timeline for the canine study. The first dog accepted into the study was determined to have metastatic lesions to its liver upon enrollment in the trial, but it was decided to continue treatment and report results as part of the study. A CONSORT diagram showing the flow of study participants is provided in Supplementary Figure 3.
Table 1.
| A. Baseline characteristics for all dogs and by dose summarized by N (%) or mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Level/Unit | All Dogs | Dose 1 | Dose 2 | Dose 3 | Control | p-value1 | p-value2 |
| Age | Years | 9.4 (1.7) | 9.2 (1.6) | 9.5 (1.8) | 8.6 (1.7) | 10.5 (2.2) | 0.054 | 0.135 |
| Sex | M | 11 (47.8) | 1 (33.3) | 9 (52.9) | 1 (33.3) | 13 (46.4) | 1 | 0.763 |
| F | 12 (52.2) | 2 (66.7) | 8 (47.1) | 2 (66.7) | 15 (53.6) | |||
| BCS | 5.7 (1) | 5.7 (0.6) | 5.5 (1.1) | 6.3 (0.6) | 5.2 (1)3 | 0.159 | 0.385 | |
| Hemoabdomen | Y | 20 (87) | 2 (66.7) | 15 (88.2) | 3 (100) | 22 (78.6) | 0.487 | 0.69 |
| N | 3 (13) | 1 (33.3) | 2 (11.8) | 0 (0) | 6 (21.4) | |||
| Stage | 1 | 2 (8.7) | 0 (0) | 2 (11.8) | 0 (0) | 5 (17.9) | ||
| 2 | 20 (87) | 2 (66.7) | 15 (88.2) | 3 (100) | 23 (82.1) | |||
| 3 | 1 (4.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0.324 | 0.693 | |
| Time from surgery to treatment | Days | 22.9 (10.9) | 15 (5.2) | 25.2 (11.7) | 18 (2) | |||
| Weight | kg | 24.6 (11.7) | 30.1 (4.6) | 22.1 (12.1) | 33.3 (9.1) | 27.1 (11.3) | 0.445 | 0.178 |
| Time to Initiation of Chemotherapy | Days | 43.7 (11.3) | 35.3 (5.5) | 46.2 (12.2) | 38.7 (3.1) | 20.8 (6.5) | 0 | 0 |
| Doxorubicin | Doses | 4.3 (1.4) | 4.3 (1.2) | 4.4 (1.5) | 4 (1.7) | 4.1 (1.2) | 0.669 | 0.628 |
| B. Study Protocol Timeline | ||||||
|---|---|---|---|---|---|---|
| Splenectomy | eBAT | eBAT | eBAT | Recheck | Doxorubicin | Recheck, Doxorubicin |
|
|
|
|
|
|
|
|
| −10–0 | 1 | 3 | 5 | 8 | 21 | 22–108 |
|
|
|
|
|
|
|
|
| Blood | Blood | Blood | Blood | Blood | Blood | Blood |
| UA | PK, NA | PK | NA | NA | Staging | |
| PE, Staging | PE | PE | PE | PE | PE | PE |
p-value for comparison group vs. all dogs;
p-value for comparison group vs. dose level 2;
BCS was missing for two control dogs;
breeds enrolled in the study included 5 Labrador retrievers, 3 mixed breed, 2 English springer spaniels, and 1 each English setter, Brittany spaniel, Airedale terrier, Bichon Frise, Newfoundland, Vizsla, Goldendoodle, Cairn terrier, papillon, dachshund, golden retriever, rat terrier, German Shepherd Dog.
PE: Physical Examination
Blood: Complete Blood Count (CBC), Serum Biochemical Profile, Prothrombin Time (PT), Partial Thromboplastin Time (PTT)
UA: Urinalysis
eBAT: EGF Bispecific Angiotoxin
Doxorubicin: Adriamycin chemotherapy (30 mg/m2) intravenously every 3 weeks
PK: Pharmacokinetics
NA: Neutralizing Antibody
eBAT was safe and well tolerated in all dogs. When dog #23 reached the 6-month milestone, interim analysis showed that the study had reached stability at the biologically active dose of 50μg/kg (dose level 2 in the escalation scheme) and was unlikely to change with additional subjects so enrollment was stopped. Based on the favorable trade-off between efficacy and toxicity observed at 50μg/kg, this dose was identified as the biologically active dose, and was used for all subsequent cohorts.
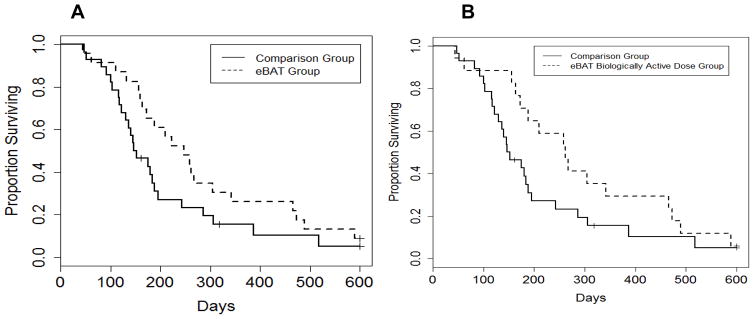
Median survival for the 23 dogs treated with adjuvant eBAT (eBAT group) was 8.1 months (Figure 4A) compared to 4.9 months for the Comparison group of dogs treated with standard of care alone. Median survival was 8.6 months for the 17 dogs treated at the biologically active dose (Figure 4B). Overall, six-month survival rates were 65.2%, and 70.6%, and 38.7%, for the eBAT group, the group treated at the biologically active dose, and the Comparison group, respectively.
Figure 4. Effect of eBAT on survival of dogs with splenic HSA treated with adjuvant doxorubicin chemotherapy.
(A) Kaplan-Meier Curve for all 23 dogs in the SRCBST-1 study versus the comparison dogs. (B) Kaplan-Meier Curve for the 17 dogs treated at the biologically active dose versus the comparison dogs. Curves illustrate prolongation of survival in dogs treated with eBAT compared to the comparison group.
Average time from splenectomy to initiation of chemotherapy was shorter in the Comparison group (20.8 days) than in the eBAT group (43.7 days) or the group treated at the biologically active dose (46.2 days). Six (26%) of 23 dogs and five of 17 (29%) treated at the biologically active dose (dose level 2) survived one year; all six dogs surviving one year had survival of at least 450 days, and two dogs are still alive at 1245 and 963 days. Detectable levels of eBAT were achieved in the systemic circulation of dogs treated by intravenous infusion (not shown).
eBAT shows limited toxicity in vivo
For our companion canine study, the estimated probabilities of AEs by dose are shown in Table 2A, and specific information regarding AEs is shown in Table 2B. No adverse events were observed at 25μg/kg (dose level 1). Reversible liver toxicity was noted in two dogs treated at dose level 2, reversible hypotensive events were observed in two dogs treated at dose level 2 and two dogs treated at dose level 3. Grade 1–3 toxicities associated with subsequent doxorubicin chemotherapy were predictable and limited to 12 dogs in total. No dogs experienced cutaneous, ocular, gastrointestinal toxicity or laboratory abnormalities that have been previously associated with EGFR targeted therapies in humans [6]. Necropsy was performed in 2 of 23 dogs and showed no evidence of chronic changes attributable to eBAT. Both of these dogs died due to progressive HSA.
Table 2.
Adverse Events (AEs) for Dogs in the SRCBST Study and Mice Treated with eBAT
| A. Summary of AEs including the empirical and model-based estimated rate by treatment group | ||||
|---|---|---|---|---|
| Dose Level | N | AEs* | AE Rate – empirical (95% CI) | AE Rate – from model (95% CI) |
| 1 (25 ug/kg) | 3 | 0 | 0% (0%, 70.8%) | 10.1% (0.3%, 31.9%) |
| 2 (50 ug/kg) | 17 | 3 | 17.6% (3.8%, 43.4%) | 19.5% (6.6%, 37.7%) |
| 3 (100 ug/kg) | 3 | 2 | 66.7% (9.4%, 99.2%) | 44.4% (10.3%, 90.6%) |
| B. Description of AEs in individual dogs, management, and outcome | ||||
|---|---|---|---|---|
| Dog ID and Breed | Dose Level | AEs | Management | Outcome |
| MN* 11 Cairn terrier |
2 | Grade 3 ALT elevation after 1st infusion Hypotensive event* during 2nd infusion |
Second eBAT infusion delayed one week IV fluid bolus 3rd eBAT infusion not administered |
Full recovery Full recovery |
| MN 17 Labrador retriever |
2 | Hypotensive event followed by a seizure during 1st infusion | IV fluid bolus, infusion restated 45 minutes later with no complications | Full recovery |
| MN 22 rat terrier |
2 | Grade 2 ALT elevation after 1st infusion | Monitoring | Full recovery |
| MN 07 Newfoundland |
3 | Hypotensive event at the end of 3rd infusion | IV fluid bolus | Full recovery |
| MN 09 Goldendoodle |
3 | Hypotensive event during second infusion | IV fluid bolus, infusion not restarted | Full recovery |
| C. Summary of death events in normal mice treated with ligand specific toxins | |||||
|---|---|---|---|---|---|
| Observed Deaths (%) | |||||
| Treatment | Dose (μg/kg) | ||||
| 10 | 20 | 40 | 80 | 160 | |
| Monospecific EGF-toxin | 0/8 (0) | 2/8 (25) | 6/8 (75) | 8/8 (100) | 8/8 (100) |
| Monospecific uPA-toxin, | 0/8 (0) | 0/8 (0) | 2/8 (25) | 8/8 (100) | 8/8 (100) |
| Monospecific EGF-toxin + monospecific uPA-toxin | 0/7 (0) | 1/7 (14) | 2/7 (29) | 7/7 (100) | 7/7 (100) |
| eBAT | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
Total count of dogs experiencing AEs (not total number of AEs)
MN = Minnesota (institutional assignment); dogs were coded using MN followed by a number assigned sequentially based on order of enrollment
Hypotensive events noted in 4 dogs were characterized by mean arterial pressure <60 mmHg, hind limb weakness, pale mucous membranes, weak femoral pulses, and a single vomiting episode in one dog. All other dogs had no adverse events.
Groups of eight C57BL/6 mice were administered monospecific EGF-toxin, monospecific uPA-toxin, monospecific EGF-toxin and monospecific uPA-toxin, and eBAT intraperitoneally twice, two days apart, on days 1 and 3 and were subsequently monitored for the occurrence of adverse events for three weeks.
Since other studies have shown that EGFR-targeted therapies are associated with significant dose-limiting cutaneous and gastrointestinal toxicities [6, 7], we further examined the safety of eBAT versus EGF-toxin alone in normal C57BL/6 mice. Maximum tolerated doses were established for monospecific EGF-toxin given alone (20 μg/kg), monospecific uPA-toxin given alone (40 μg/kg) and both drugs administered jointly (40 μg/kg); most deaths occurred within 7 days post-treatment. There were no deaths or gross toxicities in mice receiving up to 160 μg/kg of eBAT (Table 2C).
Anti-eBAT antibody responses are sporadic and do not interfere with outcome
eBAT contains a bacterial toxin, so immunogenicity was expected and considered as a potential barrier to bioactivity. Samples for NA measurement were available for all dogs at baseline, 19/23 dogs on Day 8, and 7/23 dogs on Day 21.
Dogs in which we could detect drug in the circulation on Day 1 had significantly better survival (p = 0.002) than dogs in which drug was undetectable (404 days versus 172 days; hazard ratio = 0.20 (95% confidence interval = 0.07, 0.63)). Drug was detectable on Day 1 in 4 of 9 dogs with no evidence of antibody at baseline or following eBAT administration, 7 of 8 dogs with antibody formation after eBAT treatment, and 1 of 4 dogs with pre-existing antibody (this dog was treated at the highest dose). No associations were found between survival and detectable drug at days 5 or 6 (p = 0.542), AUC at day 1 (p = 0.96), AUC at day 5 or 6 (p = 0.82) or the presence of neutralizing antibodies (p = 0.654).
Discussion
The major contributions of this study were the following: 1) first-time evaluation of a potent bispecific, anti-angiogenic targeted toxin in an “ontarget” large animal sarcoma model demonstrating potential anti-sarcoma activity and long-term survival, 2) description of an EGFR-targeted therapy that is surprisingly well-tolerated, and 3) findings supporting our belief that bispecific targeting reduces toxicity risks associated with EGFR targeting.
We tested eBAT in a model of canine HSA, using an adaptive study design in the minimal residual disease setting. We identified a biologically active dose that was safe and potentially effective. The cause of the reversible hypotensive events noted in four dogs remains unclear. Hypotension was reported in a previous study investigating treatment of advanced solid tumors with immunotoxin LMB-1, occurring in some patients treated at doses greater than 75 ug/kg. Similar to our findings, these events were transient and did not require fluids or pressor agents [39]. None of the treated dogs experienced signs of capillary leak syndrome, the toxicity of greatest concern for immunotoxins [40, 41]. Furthermore, the lack of adverse events similar to those caused by EGFR-targeted therapies [6, 7] suggests that the addition of the uPAR-directed ligand enhances targeting specificity to tumors, leading to diminished toxicity, consistent with our mouse data. However, we are aware that humans are physiologically different and may provide a greater challenge.
Bispecificity is one unique aspect of eBAT, as this may permit reactivity with a wider range of cell surface markers, enhancing the ability to kill resistant tumor cell outliers. In the case of eBAT, studies showed an ability to simultaneously target uPAR on human vascular endothelial cells (HUVEC cells) and EGFR on tumor cells [8]. We believe that bispecificity contributed to the notable clinical effect. Our results are further strengthened by the design that allowed dose finding to be guided by safety and 6-month survival [33], in turn allowing us to identify a biologically active dose without having to establish a maximum tolerated dose (MTD). Dog owners participating in companion dog studies do not abide unnecessary pet mortality risk. That being said, the data suggest the biologically active dose is lower than the MTD. The favorable clinical results could be also due to testing of the drug in the minimal residual disease setting, which is a unique opportunity afforded by the canine model and is in contrast to other studies of immunotoxins in humans, where bulky, refractory, heavily pre-treated tumor loads exceed the capabilities of the test article. Canine HSA provided a setting where we could test eBAT on a targetable disease with a high probability of detecting an efficacy signal in addition to evaluating safety. This was not done with the single intent to develop a treatment specifically for hemangio/angiosarcomas, but rather provide a proof of concept to inform and optimize the design of future clinical trials in humans with a variety of targetable cancers.
Six of seven dogs had NAs on day 21, suggesting that the use of a deimmunized toxin was justified [42]. Nonetheless, the presence of NAs was not associated with survival outcomes, and there was no correlation between NAs and the dose of eBAT received or the drug PK. These findings were similar to other studies with targeted toxin where antitoxin antibody titers did not correlate with antitumor activity [43]. Our results exceeded expectations for outcome of dogs with stage-I or stage-II HSA based on our historical data and on other published data from comparable populations treated with the standard of care [44, 45]. In fact, dogs receiving eBAT had longer survival times than dogs treated with any other contemporary experimental therapy [44–47]. The most recent detection of an efficacy signal in the treatment of canine HSA prior to our study dates back to 1995 when liposome-encapsulated muramyl tripeptide phosphatidylethanolamine ](L-MTP-PE) was used as an adjuvant to standard of care therapy [48]. The one-year survival for dogs treated with eBAT at the biologically active dose was almost 40% and the proportion of dogs living 6 months or longer nearly doubled compared to our comparison population. Five dogs were considered long-term survivors, having lived more than −450 days.
It is intriguing that time to initiation of chemotherapy was longer in dogs treated with eBAT than in the Comparison group. It is generally assumed that a shorter time to initiation of chemotherapy would produce more favorable outcomes, but survival was longer in dogs treated with eBAT even though chemotherapy was delayed. It is unlikely that the variability in chemotherapy protocols used in the Comparison group had an impact on survival since, historically, single agent doxorubicin and combination protocols are equally effective [44, 45]. Furthermore, we found no significant difference between number of doxorubicin doses in the Comparison group versus all dogs receiving eBAT or dogs treated at the biologically active dose. The use of a Comparison group enabled us to implement a novel adaptive clinical trial design and identify an efficacy signal of eBAT, but it is important to acknowledge the potential bias associated with the lack of a contemporary control group with blinding and randomization, which would more accurately predict efficacy. Our dosing and dose schedule was chosen partly on the basis of a previous study by our group in humans with an anti-B cell cancer targeted toxin [40], and partly on laboratory animal safety data. Still, metastatic disease occurred in about half of the dogs in this eBAT study. Pharmacokinetic studies show that eBAT is metabolized quickly within a few hours. We intend to use this information to optimize dose schedule in the future. Repeat cycles could prolong remissions as has been shown in studies with targeted Pseudomonas exotoxin in humans [40, 49], re-treatment at relapse could prolong survival, and even the delivery methods could be improved.
The mechanism of action of eBAT remains to be fully elucidated. In this study, both eBAT targets were expressed in human sarcoma samples. Thus, our findings from the TCGA and from the synovial sarcoma TMA analysis support other reports in the literature [17–20] regarding EGFR and PLAUR expression. A recent study confirmed that uPAR was expressed in 100% (57/57) of canine HSAs tested, but only in 30% (8/26) of hemangioma samples [50]. Here, we demonstrated expression of both targets in canine HSA samples and expression was present in the tumor cells and/or in the tumor microenvironment, but they also were present in normal tissues. Taken together, our expression data indicate that these markers are excellent targets and eBAT may be highly effective in sarcoma intervention. Furthermore, our data suggest that the excellent safety profile could be due to a unique reactivity with tumor cells, although it also could be due to the extremely low dose required to control or ablate the mass of malignant cells present in the minimal residual disease setting. However, we cannot exclude the possibility that eBAT makes the microenvironment inhospitable for tumor formation. The apparent high expression of uPAR in tumor-associated mononuclear inflammatory cells, in addition to tumor cells also raises the possibility that eBAT acts through a primary immune mechanism by eliminating or attenuating this cellular compartment, which in turn removes a strong impetus for tumor formation and/or tumor progression [24, 51, 52]. The fact that EGF4KDEL was not as effective as EGFATFKDEL (eBAT) in vitro suggests that simultaneously targeting EGFR and uPAR may be essential for optimal efficacy of this drug. Further studies are needed to understand how the bispecific nature of eBAT confers enhanced specificity even in an “on-target” animal model. Future imaging studies in companion animal models and humans will be required to elucidate the biodistribution of eBAT and identify sites of accumulation in tumor and non-tumor areas.
In conclusion, we demonstrated that eBAT is safe, and that the addition of a uPAR directed ligand to the EGFR targeting molecule abrogated the dose-limiting cutaneous, ocular, and gastrointestinal toxicities, or hypomagnesemia generally associated with EGFR targeting. We also showed that eBAT has biological activity in a highly metastatic, incurable canine sarcoma that carries many similarities with its human counterpart. In fact, in vitro testing of eBAT on the human angiosarcoma cell line AS5 showed that the drug was selective and highly effective. The strategy is not aimed at modulating EGF or uPA-dependent pathways, since neither EGFR nor uPAR appear to act as drivers of tumor progression. Rather the proteins act as “bait” for a ligand-targeted cytotoxic therapy. Given that the targets are invariably expressed in human sarcomas, our data provides a strong rationale for translation of eBAT in the treatment of human sarcomas and potentially other EGFR and uPAR-expressing tumors.
Supplementary Material
Acknowledgments
Funding
This work was supported by grant K01OD017242 (AB) from the Office of The Director, National Institutes of Health, grant AB15MN-002 from the National Canine Cancer Foundation (AB), a grant from the Masonic Cancer Center, University of Minnesota Sarcoma Translational Working Group (JFM, DAV, AB, JSK), grant 1889-G from the AKC Canine Health Foundation (JFM, MB, KLT), the US Public Health Service Grant R01 CA36725 awarded by the NCI and the NIAID, DHHS (DV), the Randy Shaver Cancer Research and Community Foundation (DV), Hyundai Scholar Senior Research Award, Hyundai Hope on Wheels (DV), a CETI Translational Award from the University of Minnesota Masonic Cancer Center (DV), and a grant from GREYlong (JFM). The NIH Comprehensive Cancer Center Support Grant to the Masonic Cancer Center (P30 CA077598) provided support for bioinformatics and comparative pathology services. The NIH Clinical and Translational Science Award to the University of Minnesota (UL1 TR000114) provided support for immunohistology services. RNA sequencing for osteosarcoma samples was supported by the Sobiech Osteosarcoma Fund Award from the Children’s Cancer Research Fund. The authors gratefully acknowledge generous support from the Angiosarcoma Awareness Foundation and donations to the Animal Cancer Care and Research Program of the University of Minnesota that helped support this project. JFM is supported in part by the Alvin S. and June Perlman Chair in Animal Oncology at the University of Minnesota.
We thank Dr. Brenda Weigel for helpful discussions, Elizabeth Taras for laboratory assistance, Dr. David Largaespada and his laboratory for providing the synovial sarcoma TMAs, Dr. Gary K. Schwartz, Columbia University Medical Center, for generously providing the AS5 human angiosarcoma cell line, Drs. M. Gerard O’Sullivan, Ingrid Cornax, Ramesh Kovi, and Jill Schappa for assistance with pathological analyses; Mitzi Lewellen for sample archiving and management; the oncology clinicians and staff of the University of Minnesota Veterinary Medical Center for assistance with management of dogs participating in the study, and importantly, clients who allowed their pets to enroll in the study, and the dogs who made the study possible.
Footnotes
Conflict of Interest: The authors declare that patent “Reduction of EGFR therapeutic toxicity,” related to this work and listing JFM, DV, and AB as inventors, has been filed by the University of Minnesota Office of Technology Commercialization.
Data and materials availability
RNAseq data for canine tumors that have not yet been published will be made available through GEO prior to publication.
References
- 1.Cassier PA, Polivka V, Judson I, Soria JC, Penel N, Marsoni S, et al. Outcome of patients with sarcoma and other mesenchymal tumours participating in phase I trials: a subset analysis of a European Phase I database. Ann Oncol. 2014;25:1222–8. doi: 10.1093/annonc/mdu108. [DOI] [PubMed] [Google Scholar]
- 2.Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187–202. doi: 10.1038/nrclinonc.2014.26. [DOI] [PubMed] [Google Scholar]
- 3.Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597–2604. [PubMed] [Google Scholar]
- 4.Borden EC, Baker LH, Bell RS, Bramwell V, Demetri VGD, Eisenberg BL, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- 5.Frith AE, Hirbe AC, Van Tine BA. Novel pathways and molecular targets for the treatment of sarcoma. Curr Oncol Rep. 2013;15(4):378–85. doi: 10.1007/s11912-013-0319-3. [DOI] [PubMed] [Google Scholar]
- 6.Funakoshi T, Latif A, Galsky MD. Safety and efficacy of addition of VEGFR and EGFR-family oral small-molecule tyrosine kinase inhibitors to cytotoxic chemotherapy in solid cancers: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. 2014;40(5):636–47. doi: 10.1016/j.ctrv.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Launay-Vacher V, Aapro M, De Castro G, Jr, Cohen E, Deray G, Dooley M, et al. Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN) Ann Oncol. 2015;26(8):1677–84. doi: 10.1093/annonc/mdv136. [DOI] [PubMed] [Google Scholar]
- 8.Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR, Vallera DA. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J Neurooncol. 2011;103(2):255–66. doi: 10.1007/s11060-010-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fosmire SP, Dickerson EB, Scott AM, Bianco SR, Pettengill MJ, Meylemans H, et al. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Invest. 2004;84(5):562–72. doi: 10.1038/labinvest.3700080. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Graef AJ, Dickerson EB, Modiano JF. Pathobiology of hemangiosarcoma in dogs: research advances and future perspectives. Vet Sci. 2015;2:388–405. doi: 10.3390/vetsci2040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Experimental hematology. 2006;34:870–878. doi: 10.1016/j.exphem.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Kakiuchi-Kiyota S, Arnold LL, Johansson SL, Wert D, Cohen SM. Pathogenesis of human hemangiosarcomas and hemangiomas. Human pathology. 2013;44:2302–2311. doi: 10.1016/j.humpath.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Andersen NJ, Froman RE, Kitchell BE, Duesbery NS. Clinical and Molecular Biology of Angiosarcoma. In: Derbel PF, editor. Soft Tissue Tumors. InTech; 2011. [Google Scholar]
- 14.Buehler D, Rice SR, Moody JS, Rush P, Hafez GR, Attia S, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37(5):473–9. doi: 10.1097/COC.0b013e31827e4e7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendelburg KM, Price LL, Burgess KE, Lyons JA, Lew FH, Berg J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012) J Am Vet Med Assoc. 2015;247(4):393–403. doi: 10.2460/javma.247.4.393. [DOI] [PubMed] [Google Scholar]
- 16.Gardner HL, London CA, Portela RA, Nguyen S, Rosenberg MP, Klein MK, et al. Maintenance therapy with toceranib following doxorubicin-based chemotherapy for canine splenic hemangiosarcoma. BMC Vet Res. 2015;11:131. doi: 10.1186/s12917-015-0446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albritton KH, Randall RL. Prospects for targeted therapy of synovial sarcoma. J Pediatr Hematol Oncol. 2005;27(4):219–22. doi: 10.1097/01.mph.0000163713.46762.72. [DOI] [PubMed] [Google Scholar]
- 18.Yang JL, Hannan MT, Russell PJ, Crowe PJ. Expression of HER1/EGFR protein in human soft tissue sarcomas. Eur J Surg Oncol. 2006;32(4):466–8. doi: 10.1016/j.ejso.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Tschoep K, Kohlmann A, Schlemmer M, Haferlach T, Issels RD. Gene expression profiling in sarcomas. Crit Rev Oncol Hematol. 2007;63(2):111–24. doi: 10.1016/j.critrevonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Benassi MS, Ponticelli F, Azzoni E, Gamberi G, Pazzaglia L, Chiechi A, et al. Altered expression of urokinase-type plasminogen activator and plasminogen activator inhibitor in high-risk soft tissue sarcomas. Histol Histopathol. 2007;22(9):1017–24. doi: 10.14670/HH-22.1017. [DOI] [PubMed] [Google Scholar]
- 21.Mazar AP, Ahn RW, O’Halloran TV. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des. 2011;17(19):1970–8. doi: 10.2174/138161211796718152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schappa JT, Frantz AM, Gorden BH, Dickerson EB, Vallera DA, Modiano JF. Hemangiosarcoma and its cancer stem cell subpopulation are effectively killed by a toxin targeted through epidermal growth factor and urokinase receptors. Int J Cancer. 2013;133(8):1936–44. doi: 10.1002/ijc.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldron NN, Oh S, Vallera DA. Bispecific targeting of EGFR and uPAR in a mouse model of head and neck squamous cell carcinoma. Oral Oncol. 2012;48(12):1202–7. doi: 10.1016/j.oraloncology.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorden BH, Kim JH, Sarver AL, Frantz AM, Breen M, Lindblad-Toh K, et al. Identification of three molecular and functional subtypes in canine hemangiosarcoma through gene expression profiling and progenitor cell characterization. Am J Pathol. 2014;184(4):985–95. doi: 10.1016/j.ajpath.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonomura N, Elvers I, Thomas R, Megquier K, Turner-Maier J, Howald C, et al. Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers. PLoS Genet. 2015;11(2):e1004922. doi: 10.1371/journal.pgen.1004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temiz NA, Moriarity BS, Wolf NK, Riordan JD, Dupuy AJ, Largaespada DA, et al. RNA sequencing of Sleeping Beauty transposon-induced tumors detects transposon-RNA fusions in forward genetic cancer screens. Genome Res. 2016;26(1):119–29. doi: 10.1101/gr.188649.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarver AE, Sarver AL, Thayanithy V, Subramanian S. Identification, by systematic RNA sequencing, of novel candidate biomarkers and therapeutic targets in human soft tissue tumors. Lab Invest. 2015;95(9):1077–88. doi: 10.1038/labinvest.2015.80. [DOI] [PubMed] [Google Scholar]
- 28.Charbonneau B, Vogel RI, Manivel JC, Rizzardi A, Schmechel SC, Ognjanovic S, et al. Expression of FGFR3 and FGFR4 and clinical risk factors associated with progression-free survival in synovial sarcoma. Hum Pathol. 2013;44(9):1918–26. doi: 10.1016/j.humpath.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamburini BA, Trapp S, Phang TL, Schappa JT, Hunter LE, Modiano JF. Gene expression profiles of sporadic canine hemangiosarcoma are uniquely associated with breed. PLoS One. 2009;4(5):e5549. doi: 10.1371/journal.pone.0005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Sarver AL, Alamgir S, Subramanian S. Downregulation of microRNAs miR-1, -206 and -29 stabilizes PAX3 and CCND2 expression in rhabdomyosarcoma. Lab Invest. 2012;92(4):571–83. doi: 10.1038/labinvest.2012.10. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Park H, Greene J, Pao J, Mulvey E, Zhou SX, et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS One. 2015;10(7):e0133152. doi: 10.1371/journal.pone.0133152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott MC, Sarver AL, Tomiyasu H, Cornax I, Van Etten J, Varshney J, et al. Aberrant Retinoblastoma (RB)-E2F Transcriptional Regulation Defines Molecular Phenotypes of Osteosarcoma. J Biol Chem. 2015;290(47):28070–83. doi: 10.1074/jbc.M115.679696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopmeiners JS, Modiano J. A Bayesian adaptive Phase I-II clinical trial for evaluating efficacy and toxicity with delayed outcomes. Clin Trials. 2014;11(1):38–48. doi: 10.1177/1740774513500589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vail DM. Veterinary Co-operative Oncology Group - Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1. 0. Vet Comp Oncol. 2004;2(4):195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 35.Hinchcliff KW, DiBartola SP. Quality matters: publishing in the era of CONSORT, REFLECT, and EBM. J Vet Intern Med. 2010;24(1):8–9. doi: 10.1111/j.1939-1676.2009.0435.x. [DOI] [PubMed] [Google Scholar]
- 36.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 37.Stish BJ, Oh S, Chen H, Dudek AZ, Kratzke RA, Vallera DA. Design and modification of EGF4KDEL 7Mut, a novel bispecific ligand-directed toxin, with decreased immunogenicity and potent anti-mesothelioma activity. British Journal of Cancer. 2009;101:1114–1123. doi: 10.1038/sj.bjc.6605297. www.bjcancer.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh S, Stish BJ, Sachdev D, Chen H, Dudek AZ, Vallera DA. A novel “reduced immunogenicity” bispecific targeted toxin simultaneously recognizing human EGF and IL-4 receptors in a mouse model of metastatic breast carcinoma. Clin Cancer Res. 2009 Oct 1;15(19):6137–6147. doi: 10.1158/1078-0432.CCR-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai LH, Wittes R, Setser A, Willingham MC, Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nature Medicine. 1996;2(3):350–353. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- 40.Bachanova V, Frankel AE, Cao Q, Lewis D, Grzywacz B, Verneris MR, et al. Phase I study of a bispecific ligand-directed toxin targeting CD22 and CD19 (DT2219) for refractory B-cell malignancies. Clin Cancer Res. 2015;21(6):1267–72. doi: 10.1158/1078-0432.CCR-14-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21(4):387–91. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 42.Onda M, Nagata S, FitzGerald DJ, Beers R, Fisher RJ, Vincent JJ, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177(12):8822–34. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 43.Frankel AE, Woo JH, Ahn C, Foss FM, Duvic M, Neville PH, et al. Resimmune, an anti-CD3ε recombinant immunotoxin, induces durable remissions in patients with cutaneous T-cell lymphoma. Haematologica. 2015;100(6):794–800. doi: 10.3324/haematol.2015.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thamm DH. Miscellaneous tumors. In: Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen’s Small Animal Clinical Oncology. 5. St. Louis: Elsevier; 2013. pp. 679–688. [Google Scholar]
- 45.Clifford CA, Mackin AJ, Henry CJ. Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern Med. 2000;14(5):479–85. doi: 10.1892/0891-6640(2000)014<0479:tochab>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Lana S, U’Ren L, Plaza S, Elmslie R, Gustafson D, Morley P, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007;21(4):764–9. doi: 10.1892/0891-6640(2007)21[764:clocfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.U’Ren LW, Biller BJ, Elmslie RE, Thamm DH, Dow SW. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. 2007;21(1):113–20. doi: 10.1892/0891-6640(2007)21[113:eoantv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Vail DM, MacEwen EG, Kurzman ID, Dubielziq RR, Helfand SC, Kisseberth WC, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. 1995;1(10):1165–70. [PubMed] [Google Scholar]
- 49.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30(15):1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anwar S, Yanai T, Sakai H. Immunohistochemical Detection of Urokinase Plasminogen Activator and Urokinase Plasminogen Activator Receptor in Canine Vascular Endothelial Tumours. J Comp Pathol. 2015;153(4):278–82. doi: 10.1016/j.jcpa.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Frantz AM, Anderson KL, Graef AJ, Scott MC, Robinson S, et al. Interleukin-8 promotes canine hemangiosarcoma growth by regulating the tumor microenvironment. Exp Cell Res. 2014;323(1):155–64. doi: 10.1016/j.yexcr.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamburini BA, Phang TL, Fosmire SP, Scott MC, Trapp SC, Duckett MM, et al. Gene expression profiling identifies inflammation and angiogenesis as distinguishing features of canine hemangiosarcoma. BMC Cancer. 2010;10:619. doi: 10.1186/1471-2407-10-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.