Abstract
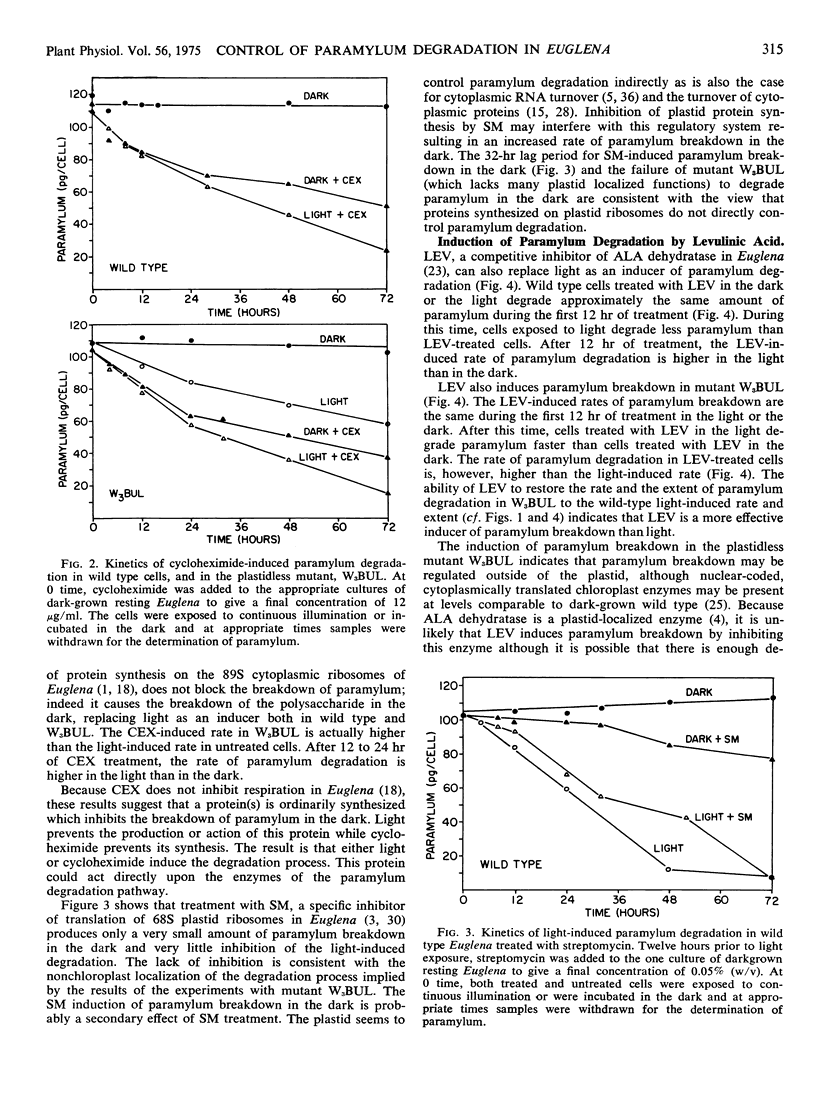
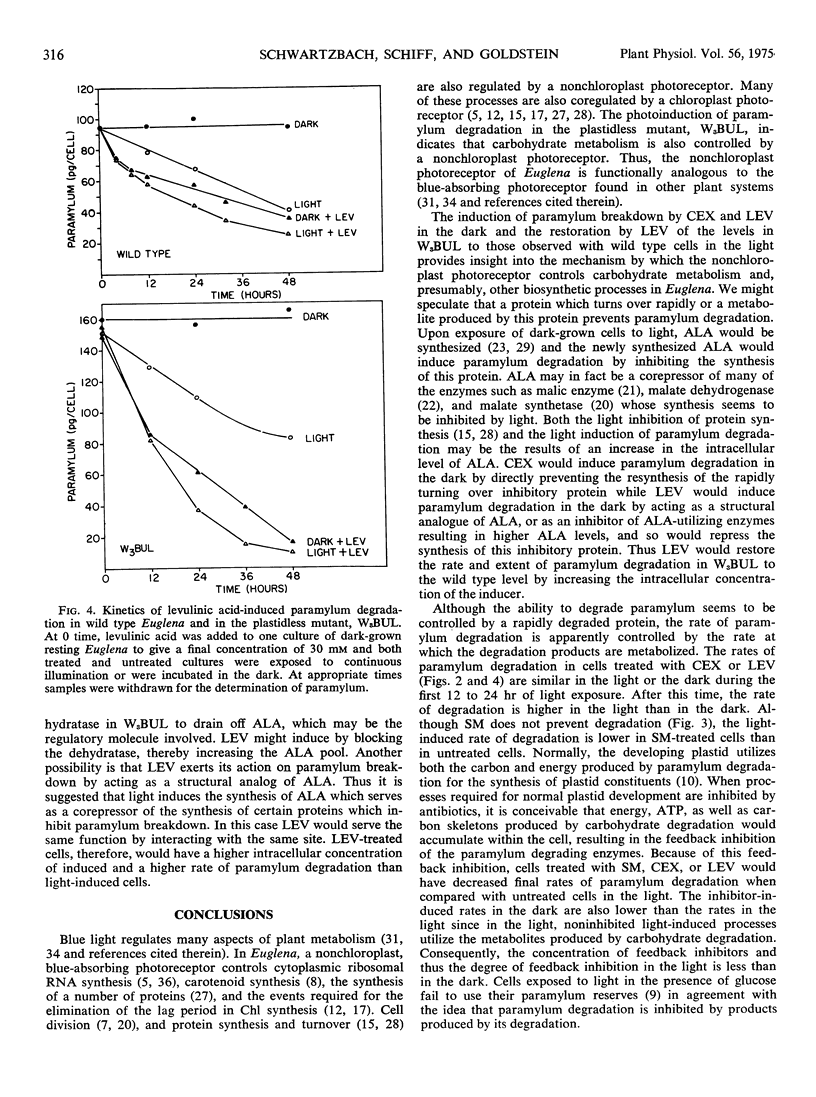
The degradation of the storage carbohydrate, paramylum, is induced by light in wild-type Euglena gracilis Klebs var. bacillaris Pringsheim and in a mutant, W3BUL, which lacks detectable plastid DNA. Treatment of wild type with cycloheximide in the dark produces 60% as much paramylum breakdown as light, whereas treatment with levulinic acid in the dark yields a slightly greater response than light. Both cycloheximide and levulinic acid produce a greater paramylum breakdown in the light than they do in the dark. Treatment of W3BUL with levulinic acid in darkness produces a larger paramylum degradation than light, with values similar to wild type in the light. Treatment of W3BUL with cycloheximide induces paramylum degradation in darkness, and as with wild type, light is slightly stimulatory in the presence of both cycloheximide or levulinic acid. Streptomycin brings about only a very small amount of paramylum breakdown in the dark and only slightly inhibits breakdown in the light. Thus paramylum breakdown induced by light does not require the synthesis of proteins on cytoplasmic or plastid ribosomes. A model which explains these results postulates the existence of a protein which inhibits paramylum breakdown. When the synthesis of this protein is prevented either by light, cycloheximide, or by levulinic acid acting as a regulatory analog of delta amino levulinic acid, paramylum breakdown takes place. Because levulinic acid is a better inducer than light in W3BUL, W3BUL may not be able to form as much delta amino levulinic acid in light as wild type. The small amount of induction by streptomycin is viewed as a secondary regulatory effect attributable to interference with plastid protein synthesis which affects regulatory signals from the plastid to the rest of the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- CARELL E. F., KAHN J. S. SYNTHESIS OF PORPHYRINS BY ISOLATED CHLOROPLASTS OF EUGLENA. Arch Biochem Biophys. 1964 Oct;108:1–6. doi: 10.1016/0003-9861(64)90347-9. [DOI] [PubMed] [Google Scholar]
- Cook J. R. Photo-inhibition of cell division and growth in euglenoid flagellates. J Cell Physiol. 1968 Apr;71(2):177–184. doi: 10.1002/jcp.1040710209. [DOI] [PubMed] [Google Scholar]
- Dolphin W. D. Photoinduced Carotenogenesis in Chlorotic Euglena gracilis. Plant Physiol. 1970 Nov;46(5):685–691. doi: 10.1104/pp.46.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M. R., Smillie R. M. A light-induced beta-1,3-glucan breakdown associated with the differentiation of chloroplasts in Euglena gracilis. Biochim Biophys Acta. 1970 Sep 1;216(2):392–401. doi: 10.1016/0005-2728(70)90231-8. [DOI] [PubMed] [Google Scholar]
- Dwyer M. R., Smillie R. M. Beta-1,3-glucan: a source of carbon and energy for chloroplast development in Euglena gracilis. Aust J Biol Sci. 1971 Feb;24(1):15–22. doi: 10.1071/bi9710015. [DOI] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Egan J. M., Dorsky D., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: VI. Action Spectra for the Formation of Chlorophyll, Lag Elimination in Chlorophyll Synthesis, and Appearance of TPN-dependent Triose Phosphate Dehydrogenase and Alkaline DNase Activities. Plant Physiol. 1975 Aug;56(2):318–323. doi: 10.1104/pp.56.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet G., Schiff J. A. The Chloroplast and Cytoplasmic Ribosomes of Euglena: II. Characterization of Ribosomal Proteins. Plant Physiol. 1974 Apr;53(4):543–554. doi: 10.1104/pp.53.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. Failure to detect effects of cycloheximide on energy metabolism in Euglena gracilis. Nature. 1970 Apr 11;226(5241):182–182. doi: 10.1038/226182a0. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Nagabhushan N., Gulyas A., Zalik S. Comparison of plant cytoplasmic ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis. Plant Physiol. 1974 Mar;53(3):516–518. doi: 10.1104/pp.53.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Ting I. P. Isoenzymes of malate dehydrogenase and their regulation in Euglena gracilis Z. Biochim Biophys Acta. 1972 Sep 19;284(1):1–15. doi: 10.1016/0005-2744(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Ting I. P. Light-induced reduction in specific activity of malate enzyme in Euglena gracilis Z. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1074–1078. doi: 10.1016/0006-291x(72)90818-2. [DOI] [PubMed] [Google Scholar]
- Richard F., Nigon V. La syntheèse de l'acide delta-aminolévulinique et de la chlorophylle lors de l'éclairement d'Euglena gracilis étiolées. Biochim Biophys Acta. 1973 Jun 20;313(1):130–149. [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Schiff J. A. The development, inheritance, and origin of the plastid in Euglena. Adv Morphog. 1973;10:265–312. doi: 10.1016/b978-0-12-028610-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Epstein H. T., Schiff J. A. Studies of Chloroplast Development in Euglena. VI. Light Intensity as a Controlling Factor in Development. Plant Physiol. 1964 Mar;39(2):226–231. doi: 10.1104/pp.39.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin M. H., Schiff J. A. RNA metabolism during light-induced chloroplast development in euglena. Plant Physiol. 1967 Jul;42(7):922–932. doi: 10.1104/pp.42.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]


