Graphical abstract
Following on from Jim Putney's original demonstration that agonist-induced calcium entry (particularly in the “non-excitable” cells that do not express voltage-gated calcium channels) was a consequence of the release of calcium from the endoplasmic reticulum (ER) stores - a process defined as store-operated calcium entry (SOCE) or capacitative calcium entry [1-2] - several studies demonstrated that such a pathway existed in a diverse variety of cell types. However, examination of this pathway revealed features that we felt raised some questions. For example, the absolute dependence of SOCE on the depletion of ER calcium stores could potentially be considered “dangerous”, given the importance of such ER calcium levels in the processing and “quality control” of cellular protein generation. Moreover, there was no clear indication of how such depletion of the ER calcium store could be translated into an appropriate signal for activation of a channel in the plasma membrane (PM). Of course, it was certainly possible to propose feasible models that could negate such concerns. For example, if the depletion involved only a minor subset of the total ER store, perhaps in a pool located close to the PM, then that could provide a valid explanation for the effectiveness of the SOCE process. Nevertheless, such “concerns” led us to explore the possibility of the presence of an alternative distinct pathway for agonist-induced calcium entry [3-6].
The first evidence suggesting the presence of such an alternative pathway came from an unexpected source, namely studies indicating the existence of a calcium entry pathway activated by the receptor-mediated generation of low levels of intracellular arachidonic acid (AA) [7-10]. Subsequent electrophysiological studies revealed that this AA-activated calcium entry pathway involved a conductance whose properties closely mirrored those of the previously identified store-operated CRAC channels. Specifically, small, inwardly-rectifying, highly calcium-selective currents with markedly positive reversal potentials (~+80 mV), that were blocked by low concentrations of lanthanum and gadolinium. Critically however, currents through these two channels were strictly additive in the same cell, and activation of the ARC channels was entirely independent of ER calcium store-depletion [11-14].
Despite the early identification and characterization of the store-operated CRAC channels, identification of the key molecular “players” in this pathway remained unknown until 2005/6 which saw the discovery of STIM1 as the sensor of calcium-store (ER) depletion [15, 16], and the three Orai proteins (specifically Orai1) that formed the actual store-operated CRAC channel [17,18]. Based on the finding that STIM1 was the agent that sensed the depletion of the ER calcium store, we assumed that STIM1 would likely have no role in the activation of the store-independent ARC channels. Surprisingly however, siRNA-knockdown of endogenous STIM1 resulted in the complete loss of ARC channel activity. This unexpected finding was resolved when examination of the literature revealed that STIM1 had actually been originally identified some five years earlier, where it was described as a plasma membrane protein [19-20]. Consistent with this, we showed that some15-25% of the total cellular STIM1 was typically resident in the plasma membrane (PM), and that preventing the delivery of STIM1 to the PM by introducing a glycosylation-mutant version, resulted in the specific loss of ARC channel currents without affecting the activity of co-existing CRAC channels [21]. Subsequent studies showed that, like the CRAC channels, the ARC channels were also formed by Orai proteins but, whilst Orai1 formed the CRAC channel, the ARC channel was a heteromeric assembly of Orai1 and Orai3 proteins [22-23].
A key puzzle resulting from these findings was that the essential initial step in the activation of STIM1, namely the sensing of depletion of ER free calcium levels (typically in the range of 100 to 600 μM), involved the loss of calcium from a calcium-binding EF-hand domain located within the N-terminal portion of the protein that lay within the lumen of the ER [24]. For STIM1 located in the PM, this domain would be extracellular where it would be exposed to high, relatively stable calcium concentrations of around 2-3 mM. Consequently, for PM-located STIM1, this domain would never lose its bound calcium, indicating a clear distinction between the function of STIM1 in the activation of CRAC channels versus the ARC channels. To examine this we expressed a STIM1 construct lacking both the entire N-terminal and the transmembrane portions of the protein, leaving just the extensive cytosolic domain (~435 residues), to which we attached an N-terminal Lck sequence to enable its attachment to the inner cytosolic face of the PM. Expression of this construct (Lck-STIM1) resulted in AA-activated ARC channel currents that were indistinguishable from those seen with normal full-length STIM1, whilst the corresponding store-operated CRAC currents were essentially eliminated [25]. In contrast however, deletion of the so-called CAD (CRAC Activation Domain) or SOAR (STIM-Orai-Activating Region) domain (residues ~342-448) within the cytosolic portion of the STIM1 protein and which had been shown to be essential for CRAC channel activation, was also shown to be essential for ARC channel activation [26]. Further studies showed that many of the domains within the cytosolic portions of STIM1 identified as essential for CRAC channel activation were similarly essential for activation of the ARC channels (Fig. 1). These included the C2A domain (W430, I433, L436) essential for STIM1 dimerization, and the so-called Basic Region (BR) domain (K382/384-386) essential for CRAC activation [26].
Figure 1.
Diagram of the different domains within the STIM1molecule (top), with the key regions responsible for Orai channel interaction and activation (below).
Relatively early in our studies of the AA-activated ARC channel currents we became aware that they were significantly inhibited by elevated levels of cytosolic calcium - specifically within the range of ~250-400 nM [27]. Subsequent studies showed that this was a result of a calcineurin-mediated dephosphorylation, and could be readily reversed by a PKA-mediated phosphorylation [28]. To examine the molecular basis for this response, we examined potential PKA phosphorylation sites using an in silico analysis of the STIM1 cytosolic domain [29]. Although this revealed several such potential sites, we could eliminate most of these as they lay beyond residue H448, and we had shown that termination of STIM1 at this site had no effect on ARC channel activity. The only clear potential PKA-site in the remaining STIM1 cytosolic sequence was a threonine at position 389, and in vitro hyperphosphorylation analysis confirmed that this residue was a genuine PKA target site [29] (Fig. 2A). To examine the physiological effect of phosphorylation of the T389 residue, we expressed STIM1 constructs in which the T389 residue was replaced by either a “phospho-mutant” T389A residue, or a “phosphomimetic” T389E residue. Expression of these constructs revealed that, whilst the T389A phospho-mutant displayed normal CRAC channel currents, ARC channel currents were reduced to negligible levels. Correspondingly, expression of the T389E phosphomimetic mutant showed normal ARC channel currents, whilst CRAC channel currents were essentially eliminated [29] (Fig. 2B). This PKA-mediated phosphorylation-dependent “switch”, was shown to involve an AKAP, namely AKAP79, as expression of an AKAP79 construct specifically lacking the PKA binding site resulted in the loss of ARC channel currents - an effect that could be completely restored by expression of the T389E phospho-mutant STIM1 in the presence of the same PKA-mutant AKAP79 [27]. In addition, the previously demonstrated calcineurin-dependent inhibition of ARC channel currents at elevated cytosolic calcium levels could be abolished by expression of the T389E phospho-mutant STIM1. Finally, we were able to demonstrate significant FRET signals between STIM1 and AKAP79, STIM1 and Orai3, and AKAP79 and Orai3. Critically, these signals were seen under resting conditions, suggesting that AKAP79, plasma membrane STIM1, and the ARC channel exist in a constitutive complex [29].
Figure 2.
A) Diagram illustrating the currently proposed conformation of the CC2 and CC3 domains of a STIM1 dimer, and indicating the corresponding predicted location of the critical T389 residue.
B) CRAC (grey) and ARC (red) whole-cell currents measured at −80 mV in cells treated with an siRNA against endogenous STIM1, and transfected with either the wild-type STIM1 (WT), the phospho-mutant STIM1/T389A construct, or the phosphomimetic STIM1/T389E construct.
In summary, these findings indicate that phosphorylation of the T389 residue in STIM1 is an essential feature determining the activity of store-independent ARC channels, and is mediated by an AKAP79/PKA-dependent process (Fig. 3). Moreover, because phosphorylation of T389 in STIM1 “inhibits” CRAC channel activation, this AKAP/PKA mediated STIM1-dependent process can act to modulate the relative activities of these two co-existing calcium entry pathways in a manner appropriate to induce their distinct roles in cellular calcium signaling.
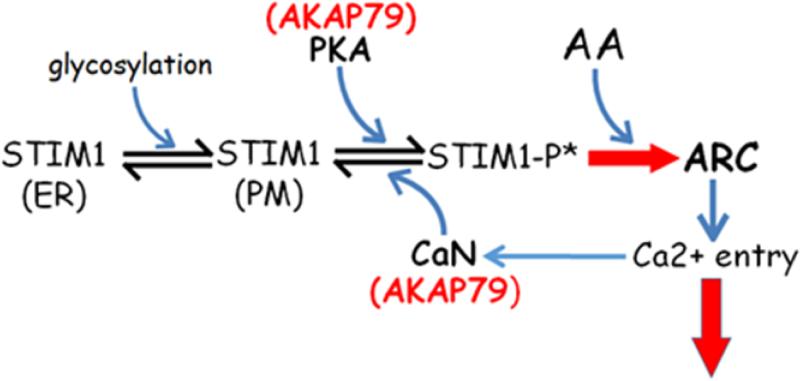
Figure 3.
A model illustrating the sequence of events leading to the activation of ARC currents and their subsequent deactivation. Shown is the constitutive translocation of a pool of STIM1 to the plasma membrane (PM), where it undergoes an AKAP79-mediated, PKA-induced phosphorylation of the T389 residue (STIM1-P*). The presence of an appropriate agonist results in the local generation of arachidonic acid (AA), and the STIM1-P*-mediated activation of the ARC channels (RED ARROW), and calcium entry. Also shown is the subsequent deactivation of the ARC channels as a result of an AKAP79-mediated calcineurin-induced dephosphorylation of the T389 residue of STIM1.
Highlights.
CRAC and ARC channels are distinct calcium entry channels formed by Orai proteins
Activation of both these channels involves distinct pools of STIM1 (ER versus PM)
Selective activation of these two channels is determined by the phosphorylation status of a single threonine reside within the STIM1 cytosolic domain
Acknowledgements
The work performed in the laboratory of the author was supported by National Institutes of Health grant GM040456 to T.J.S. The excellent technical assistance of Ms. Jill Thompson is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW., Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 3.Shuttleworth TJ. Receptor-activated calcium entry in exocrine cells does not occur via agonist-sensitive intracellular pools. Biochem. J. 1990;266:719–726. doi: 10.1042/bj2660719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuttleworth TJ. Fluoroaluminate activation of different components of the calcium signal in an exocrine cell. Biochem. J. 1990;269:417–422. doi: 10.1042/bj2690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuttleworth TJ, Thompson JL. Evidence for a non-capacitative Ca2+ entry during [Ca2+] oscillations. Biochem. J. 1996;316:819–824. doi: 10.1042/bj3160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuttleworth TJ. What drives calcium entry during [Ca2+]i oscillations?--challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- 7.Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem. 1996;271:21720–21726. doi: 10.1074/jbc.271.36.21720. [DOI] [PubMed] [Google Scholar]
- 8.Shuttleworth TJ, Thompson JL. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J. Biol. Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- 9.Shuttleworth TJ, Thompson JL. Discriminating between capacitative and arachidonate-activated Ca(2+) entry pathways in HEK293 cells. J. Biol. Chem. 1999;274:31174–31178. doi: 10.1074/jbc.274.44.31174. [DOI] [PubMed] [Google Scholar]
- 10.Osterhout JL, Shuttleworth TJ. A Ca(2+)-independent activation of a type IV cytosolic phospholipase A(2) underlies the receptor stimulation of arachidonic acid-dependent noncapacitative calcium entry. J. Biol. Chem. 2000;275:8248–8254. doi: 10.1074/jbc.275.11.8248. [DOI] [PubMed] [Google Scholar]
- 11.Mignen O, Shuttleworth TJ. I(ARC), a novel arachidonate-regulated, noncapacitative Ca(2+) entry channel. J. Biol. Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 12.Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the noncapacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J. Biol. Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- 13.Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 14.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 15.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Sarina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 19.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochem. Biophys. Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 20.Williams RT, Senior PV, van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Biochem. Biophys. Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 21.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J. Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mignen O, Thompson JL, Shuttleworth TJ. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J. Physiol. 2009;587:4181–4197. doi: 10.1113/jphysiol.2009.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JL, Shuttleworth TJ. A plasma membrane-targeted cytosolic domain of STIM1 selectively activates ARC channels, an arachidonate-regulated store-independent Orai channel. Channels. 2012;6:370–378. doi: 10.4161/chan.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JL, Shuttleworth TJ. Molecular basis of activation of the arachidonate-regulated Ca2+ (ARC) channel, a store-independent Orai channel, by plasma membrane STIM1. J. Physiol. 2013;591:3507–3523. doi: 10.1113/jphysiol.2013.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mignen O, Thompson JL, Shuttleworth TJ. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]
- 28.Mignen O, Thompson JL, Shuttleworth TJ. Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein. J. Physiol. 2005;567:787–798. doi: 10.1113/jphysiol.2005.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson JL, Shuttleworth TJ. Anchoring protein AKAP79-mediated PKA phosphorylation of STIM1 determines selective activation of the ARC channel, a store-independent Orai channel. J. Physiol. 2015;593:559–572. doi: 10.1113/jphysiol.2014.284182. [DOI] [PMC free article] [PubMed] [Google Scholar]