Abstract
Empathy is a phenomenon often considered dependent on higher-order emotional control and an ability to relate to the emotional state of others. It is, by many, attributed only to species having well-developed cortical circuits capable of performing such complex tasks. However, over the years, a wealth of data has been accumulated showing that rodents are capable not only of sharing emotional states of their conspecifics, but also of prosocial behavior driven by such shared experiences. The study of rodent empathic behaviors is only now becoming an independent research field. Relevant animal models allow precise manipulation of neural networks, thereby offering insight into the foundations of empathy in the mammalian brains. Here we review the data on empathic behaviors in rat and mouse models, their neurobiological and neurophysiological correlates, and the factors influencing these behaviors. We discuss how simple rodent models of empathy enhance our understanding of how brain controls empathic behaviors.
Keywords: neuronal correlates, animal empathy, emotional contagion, vicarious experience, rodent, rat, mouse
1. Introduction
By motivating prosocial behavior, inhibiting aggression and providing a basis for moral development empathy plays a fundamental role in human life and society. Human empathy is a very complex social phenomenon, which has been defined in different ways. Most definitions, however, have common elements including the ability to experience and share feelings of others and to respond with care to the distress in others (de Waal, 2008). For a long time, empathy received much attention from philosophers and psychologists rather than neuroscientists. More recently, vigorous development of human brain imaging techniques (especially fMRI) encouraged systematic neuropsychological studies of empathy that provided many correlates of higher psychological functions (Bernhardt and Singer, 2012; Stanley and Adolphs, 2013). These studies identified brain regions activated during processing of complex social stimuli involved in advanced forms of empathic behaviors observed in humans. However, since neuroimaging studies are correlative in nature and their resolution is limited, neural mechanisms of empathy are largely unknown. Studies employing animal models provide mechanistic insights into the exquisite organization of the neuronal circuitry underlying emotional behaviors such as fear (Tovote at al., 2015), suggesting that these complex neural mechanisms may also control social emotions. To date, however, methods allowing detailed (at the level of neuronal circuits) insight into the mechanism of such control have not been developed for human studies.
Empathy is considered by many to be a uniquely human trait. The possibility of emphatic behaviors in non-human animals has been largely ignored. However, accumulating data show pro-social behaviors in multiple species including primates and rodents, suggesting that some forms of empathy are phylogenetically older than humans. Such findings strongly support the hypothesis about evolutionary continuity of empathic behaviors. Taking such a perspective offers an experimental insight into simpler forms of empathy and gives a chance to understand neuronal processes underlying empathic behaviors. Several theories of empathy that adopted an evolutionary perspective and widened the scope of research have been proposed. One of the most influential ones is de Waal’s multi-level conceptualization of empathy. It puts the simplest forms of empathy, involving adoption of another’s emotional state (emotional contagion) at the core of all empathic behaviors, followed by more complex level of the continuum involving concern about another’s state and attempts to ameliorate this state by, e.g., consolation (sympathetic concern), and the most elaborate level - attributing emotional state to another instead of self (empathetic perspective taking) (de Waal, 2008, also see Preston and de Waal, 2002). Emotional contagion has been observed in many animal species (Darwin, 1871; Panksepp and Panksepp, 2013), sympathetic concern and consolation has been described in non-human primates (de Wall and Aureli, 1997) and canines (Custance and Mayer, 2012), whereas the highest level of empathy in the de Waal’s model, including targeted helping coming from cognitive appreciation of the other animal’s situation (perspective taking), characterize mainly humans and apes (Hare et al., 2006, 2001; Hirata, 2009). Some levels of cognitive empathy, however, involving an understanding of what caused the distress in another animal (and subsequent active inhibition of that behavior in order to minimize the distress of another individual) was also observed in Rhesus monkeys and rats (Church et al., 1959, Masserman et al., 1964).
The evolutionary roots of empathy, and thus the existence of many levels of empathic complexity, has been also acknowledged by Decety and Lamm, who postulated that empathy encompasses both emotion sharing and cognitive control. According to their definition, affective representations in the brain are automatically activated by perceptual input, whereas cognitive control is mediated by cortical structures, mainly the prefrontal cortex (Decety and Lamm, 2006).
More recently, another model emphasizing the complex, multilayered character of empathy based on brain circuits involved in the control of empathic behaviors has been proposed (Panksepp and Panksepp, 2013). It recognizes three levels: the deeply subcortical primary level responsible for emotional contagion; the secondary level based on basal ganglia and limbic structures involved in learning and memory; and the tertiary process governed largely by cortical and limbic structures required for cognitive empathy. Several of these brain circuits may be crucial for more than one level of empathy and their specific actions are dependent on the given emotion. At the primary level, emotions such as seeking, rage, fear, lust, care, panic or play (Panksepp, 1997), could theoretically be shared through so-called emotional empathy. Top-down control exerted by cortical and limbic structures is required for both the formation of conditioned reflexes based on information from other conspecifics (i.e., secondary empathy: the learning and memory formation), and for cognitive regulation of behavioral responses to these stimuli (i.e., tertiary process). This elegant definition is uniform for many species, including humans.
The theories proposed by de Waal and Panksepp and Panksepp form a frame for studying primal emotional foundations of empathy in mammalian brains. Acknowledging the existence of empathy in other animals allows the design of relevant animal models. Here, we will describe the relevant rodent models and review the data, gathered with the use of such models, on the neurobiological and neurophysiological correlates and the factors influencing empathic behaviors. In order to gain insight into animal empathic behaviors, in line with Tinbergen’s four questions (Tinbergen, 1963), besides the mechanisms we will also discuss their ontogeny, phylogeny and adaptative value. The reviewed models involve different levels of empathy (emotional contagion, social modulation of learning and empathic concern), thus providing an opportunity to model complex human disorders characterized with impairments of different aspects of empathy. Such models may shed some light on neural mechanisms of empathy, which are still largely unknown. One of the most interesting hypotheses on neural basis of emotional sharing proposed so far is mirroring mechanism, which we discuss in the next section.
2. Do mirror neurons control social emotions?
The discovery of mirror neurons, originally found in macaque premotor cortex (Gallese et al., 1996; Rizzolatti et al., 1996; Umiltà et al., 2001), fueled speculations about neuronal mechanisms of imitation and mimicry. Their involvement was hypothesized in the wide range of abilities and diagnoses, including empathy and autism spectrum disorder (Baird et al., 2011). In the social domain, mirroring occurs when the same neurons are activated by the emotions experienced directly and by observing/interacting with others who are experiencing emotions. Such vicarious activation would give us an insight into the feelings of others. Vicarious activations in the anterior insula and, to a lesser extent, in the anterior cingulate cortex have been shown in people feeling disgust and observing faces expressing disgust (Wicker et al., 2003). Similarly, Singer and colleagues (Singer et al., 2004) showed that experiencing pain and empathizing with pain of others evoked overlapping neural activations in cingulate and insular cortices. However, despite years of studies we still do not fully understand the function of mirror neurons and whether they play a role in these vicarious activations (Hickok, 2009).
Most of the results supporting the mirror mechanism in humans were obtained with transcranial magnetic stimulation (TMS), EEG, MEG, and brain imaging techniques (PET, fMRI). None of the abovementioned methods allows for identification of single cell activity. Imaging techniques used in human studies do not have single cell resolution necessary to understand such processes at the cellular level, at which, in the light of the recent discoveries in rodents, such studies should be conducted. The studies in rats and mice have shown that there are highly specialized networks of neuronal circuits that control specific behaviors and that they differ from the surrounding neurons in their connectivity with other brain structures (Tovote et al., 2015). Such circuits can be virtually physically overlapping (Knapska et al., 2012), which makes them indistinguishable with lower resolution techniques. Since the studies employing single-cell activity recordings showed that mirror neurons constitute less than 17% of all recorded cells (Gallese et al., 1996, Mukamel et al., 2010), techniques measuring activation of whole brain structures cannot verify whether the mirror neurons are actually involved in a given process. Vicarious emotional experience may rely on mirror neurons only, mirror neurons and accompanying cells (e.g., forming neural circuits with mirror neurons) or no mirror neurons at all. To understand which types of neurons are involved, how they are interconnected and what is their function in control of empathic behavior, adequate techniques allowing single-cell resolution and manipulation are needed.
It has been recently shown that in the basolateral amygdala of monkeys there are neurons that mirror value of rewards delivered to self and others (Chang et al., 2015), suggesting a possible role of such neurons in vicarious experience of emotions. However mirror mechanism is not the only possible explanation of vicarious emotions. Instead of some pre-wired mirror neurons one can imagine that socially induced emotions recruit some cells from a population of equivalent neurons controlling particular emotion. For instance, in case of directly acquired fear, it has been shown that neurons in the lateral amygdala are recruited randomly by fear conditioning, depending on relative neuronal excitability immediately before training (Yiu et al., 2014). Moreover, emotional events separated in time seem to be represented by non-overlapping populations of neurons in the lateral amygdala (Rashid et al., 2016). Together, these results suggest that recruitment of neurons by fear-inducing stimuli may be stochastic rather than dependent on activation of some sub-population of pre-wired fear neurons. One can imagine that similar mechanism may underlie socially transferred fear.
In summary, although we do not have convincing evidence that mirror neurons are crucial for social interactions or empathy, it still remains an interesting hypothesis deserving further studies (Ferrari and Rizzolatti, 2014). Certainly, in order to understand the mechanisms through which the brain controls vicarious emotions and resonance behaviors, we need data obtained with single-cell resolution and techniques that allow for investigating the function of identified neurons. In the next paragraphs we will discuss animal models that can be used to test the hypothesis about the role of mirroring mechanism in social sharing of emotions.
3. Emotional contagion
Emotional contagion is defined as sharing of the emotional states between individuals. Tuning one’s emotional state to that of another increases the probability of similar behavior, which thereby allows for a rapid adaptation to environmental challenges (Hatfield et al., 1994). Importantly, as proposed by Hatfield, affective contagion is transmodal, i.e., separated elements of emotional expression which are being witnessed, e.g., facial expression, induce changes not only in the same aspects of emotional expression in the witnessing subject, but result in full emotional response including, e.g., vocalizations and body language. Emotional contagion is commonly observed and evolutionarily conserved in the animal world. Most studies aimed at rodent empathic behaviors focus on the capability to share negative emotional states, such as pain or fear and anxiety (see Table. 1). Modeling of positive emotions appears less popular, mostly due to numerous difficulties with standardization of the procedures.
Table 1.
Examples of emotional contagion elicited by painful and fearful stimuli
| Study | Species (strain) | Sex | Demonstrator experience | Observer experience | Observer response | Observer Pain Experience required? | Familiarity required? | Sensory Modality | |
|---|---|---|---|---|---|---|---|---|---|
| Pain and/or restraint | Li et al. 2014 | Rat | Subcutaneous injection with bee venom | Observing the Demonstrator pain response + subcutaneous injection with bee venom | Increased mechanical sensititvity and paw flinch reflex | yes | yes | Not tested | |
| Kavaliers et al. 2003 | Mouse (CF-1) | M | Exposure to biting flies | Observing the Demonstrator getting bit and exposure to non-biting flies on the following day | Burying in the bedding while exposed to non-biting flies | no | no | Not tested | |
| Langford et al. 2006 | Mouse (CD1) | M and F | Not treated | Observing the pain response of the Demonstrator + 0.9% acetic acid | Decrease in writhing | yes | yes | Visual | |
| 0.9% acetic acid | Increase in writhing and thermal hyperalgesia | yes | yes | ||||||
| no | |||||||||
| 1% or 5% formalin injection | Observing the pain response of the Demonstrator + 1% or 5% formalin injection | Decrease in paw licking in 5% f treated mice watching 1% treated mouse | yes | Not tested | Not tested | ||||
| Watanabe 2011 | Mouse (c57BL/6J) | M | Restraint (single, group or single in the presence of cagemates | Restraint or Observation of a restrained cagemate | Step down in a fear conditioning (0.1mA every 1 sec) extinction trial | no | yes | Not tested | |
| Watanabe 2012 | Mouse (c57BL/6J) | M | Restraint or formalin injection (hind paw) | Conditioned place preference with a restrained or formalin injected cagemate in one of the chambers | While restraint did not evoke conditioned place aversion (CPA), formalin injection, after a session of enhanced interest resulted with CPA. | no | Not tested | Not tested | |
| Watanabe 2015 | Mouse (ICR) | M | Restraint | none | hyperthermia | yes | Not tested | Not tested | |
| Restraint cagemate | No hyperthermia | ||||||||
| Freely moving cagemate | Increased hyperthermia | ||||||||
| Martin et al. 2015 | Mouse (CD-1) | M | 0.9% acetic acid | Observing the pain response of the Demonstrator | Analgesia reduced by naloxone and glucocorticoid synthesis inhibitor | no | Not tested | ||
| Observing the pain response of the Demonstrator + 0.9% acetic acid | Pain response increased in cagemates only, glucocorticoid synthesis inhibitor enables similar reaction in strangers | yes | Yes (enhancement) | ||||||
| 30 min restraint prior to observing the pain response of the Demonstrator | Abolished hyperalgesia in both isolated mice and cagemates | Not tested | |||||||
| Remote fear | Knapska et al. 2006 | Rat (Wistar) | M | Fear conditioning (9 × 1 sec, 1.3mA, 55 sec ITI) | Interaction with a stressed cagemate in the home cage | Increased exploration and acoustic startle response | no | Not tested | Not tested |
| Knapska et al. 2010 | Rat (Wistar) | M | Fear conditioning (9 × 1 sec, 1.3mA, 55 sec ITI) | Interaction with a stressed cagemate in the home cage + avoidance training to 5 sec 1 mA shock (cue) | Increased exploration and allogrooming of the Demonstrator. Improved acquisition and retention of avoidance | yes | Not tested | Not tested | |
| Fear conditioning (9 × 1 sec, 1.3mA, 55 sec ITI) | Interaction with a stressed cagemate in the home cage + 1 sec 1 mA shock (context) | Increased freezing to context | yes | No | Not tested | ||||
| Mikosz et al. 2015 | Rat (Long-Evans) | M and F | Fear conditioning (10 × 1 sec, 1mA) | Home cage interaction with a stressed cagemate | Increase in social approach + better performance in two-way avoidance task | no | Not tested | Not tested | |
| Bredy and Barad 2009 | Mouse (c57BL/6J) | M | Fear conditioning (3 × 2 sec, 1 mA, 120 sec ITI) | Interaction with a stressed cagemate followed by fear conditioning | Decreased freezing to cue | yes | Not tested | Olfactory | |
| Interaction with a stressed cagemate followed by fear conditioning and extinction | Decreased freezing to cue (increased extinction) | yes | Not tested | Olfactory | |||||
| Fear conditioning (3 × 2 sec, 1 mA, 120 sec ITI) + 10 extinction trials | Interaction with a stressed cagemate followed by fear conditioning and extinction | Decreased freezing to cue (increased extinction) | yes | Not tested | Non-olfactory | ||||
| Nowak et al. 2013 | Mouse (c57BL/6J) | M | Fear conditioning (5 × 1 sec, 0.6 mA, context A) + extinction (6 × 10 CS, context B) + test (10 CS, context B) | Fear conditioning (5 × 1 sec, 0.6 mA, context A + test (10 CS, context B, tested together with the Demonstrator or separately) | Animals tested together behave identically | yes | Not tested | Not tested | |
| Observer tested separately from the Demonstrator freezes more | |||||||||
| Naïve + test (10 CS, context B, tested together with the Demonstrator) | No freezing | ||||||||
| Air puff to cue in the Intellicage learning unit, followed by extinction and rinstatement | Air puff to cue in the Intellicage learning unit, followed by extinction | Reinstatement of avoidance reaction | Yes (group testing) | ||||||
| Meyza et al. 2015 | Mouse (c57BL/6J and BTBR T+ Itpr3tf/J) | M | Fear conditioning (10 × 1 sec, 0.6 mA) | Home cage interaction with a stressed cagemate | c57: increased number and duration of social contacts, BTBR does not | Not tested | Not tested | Not tested | |
| Iminent fear | Bruchey et al. 2010 | Rat (Sprague-Dawley) | M | Fear conditioning (3 × 0.5 sec, 0.7 mA, 180 sec ITI) | Observation of conditioned response of the Demonstrator | Increased freezing to cue | no | Not tested | Not tested |
| Observation of conditioned response of the Demonstrator + Fear conditioning (3 × 0.5 sec, 0.4 mA, 180 sec ITI) | Increased freezing to cue | yes | Not tested | Not tested | |||||
| Naïve + Fear conditioning (3 × 0.5 sec, 0.4 mA, 180 sec ITI) | Freezing | yes | Not tested | Not tested | |||||
| Kim et al. 2010 | Rat (Sprague-Dawley) | M | Naïve or Fear conditioned (10 × 1 sec, 2 mA, 120 sec ITI) | Naïve or Fear conditioned (10 × 1 sec, 2 mA, 120 sec ITI) | Increased freezing to cue | Yes | Not tested | Auditory | |
| Atsak et al. 2011 | Rat (Long Evans) | F | Fear conditioned (5 × 5 sec, 0.8 mA, 240 and 360 sec ITI) | Naïve or Fear conditioned (4 × 1 sec, 0.8 mA, 240 and 360 sec ITI) + 1 × 1 sec 0.8mA prior to witnessing the conditioning of the Demonstrator | Decrease in activity, increase in freezing (in experiences Observers) | yes | Not tested | Not tested | |
| none | Naïve or Fear conditioned (4 × 1 sec, 0.8 mA, 240 and 360 sec ITI) + USVs from conditioning session | Experienced animals freeze | Auditory | ||||||
| Yusufishaq and Rosenkranz, 2013 | Rat (Sprague-Dawley) | M | Fear conditioned (8 × 1 sec, 0.5–0.8 mA, 80 sec ITI) | Observation of the fear conditioning session | Increased freezing | no | Not tested but pair housed rats froze more than single housed ones | Not tested | |
| Jones et al. 2014 | Rat (Sprague-Dawley) | F | Fear conditioning (3 × 0.5 sec, 0.7 mA, 180 sec ITI) | Observation of conditioned response of the Demonstrator + cue | Increased freezing to cue | no | Yes, familiarity enhances freezing | Not tested | |
| Carillo et al. 2015 | Rat (Long Evans) | M | Fear conditioning 6 days of 5 × 1 sec, 1.5mA, 120–180 sec ISI | Fear conditioning (4 × 1 sec, 0.8mA, 240–360 sec ISI) | Observers freezing decreases (and yawning increases) with consecutive testing days. | yes | Not tested | Not tested | |
| Jones and Monfils 2016 | Rat (Sprague-Dawley) | M | Fear conditioning (3 × 0.5 sec, 0.7 mA, 180 sec ITI) | Observation of conditioned response of the Demonstrator + cue | Subordinate individuals display stronger freezing response. Muscimol inactivation of anterior cingulate cortex disables social transfer of fear | no | Not tested | 22 kHz USV contribute to the freezing response while 50 KHz impede social transfer of information | |
| Chen et al. 2009 | Mouse (c57BL/6J and BALB/c) | M and F | Fear conditioning (10 × 2 sec, 0.5 mA, 118 sec ITI) 2 sessions | Observation of the fear conditioning session + exposure to cue | Increased orientation towards the Demonstrator and freezing to cue | Not tested but had previous shock experience | no | Auditory | |
| Jeon and Shin 2011 (Jeon et al. 2010) | Mouse (c57BL/6J) | M or F | Fear conditioning (24 × 2sec, 1mA, 10 sec ITI) | Observation of the fear conditioning of the Demonstrator | Increased freezing during the conditioning session and later for context | No | Enhanced by | Visual | |
| Sanders et al. 2013 | Mouse (c57BL/6J) | M and F | Naïve or fear conditioned (6 × 1sec, 0.7mA, 15 sec ITI) | Naïve + observation of the Demonstrator | Only the previously experienced Observers froze during observation of the fear conditioning of the Demonstrator | yes | Not tested | Not tested | |
| Fear conditioning (6 × 1sec, 0.7mA, 15 sec ITI) + observation of the Demonstrator | |||||||||
| Swim stress + observation of the fear conditioning of the Demonstrator | |||||||||
| Gonzalez-Liencres et al. 2014 | Mouse (c57BL/6J) | M | Fear conditioning (24 × 1 sec, 0.5 mA, 10 s ITI) | Direct observation of the fear conditioning of the Demonstrator | Enhanced freezing in pairs of familiar mice | no | yes | Not tested | |
| Ito et al. 2015 | Mouse (129SvEv/C57BL/6N F1 hybrid) | M | Fear conditioning (24 × 2 sec, 1 mA, 10 s ITI) | Direct observation of the fear conditioning of the Demonstrator + passive avoidance training (1sec, 0.15 mA) | enhanced passive avoidance memory | yes | Not tested | Not tested | |
| Keum et al. 2016 | Mouse (C57BL/6J, C57BL/6NTac, 129S1/SvImJ, 129S4/SvJae BTBR T(+) Itpr3(tf)/J, AKR/J, BALB/cByJ, C3H/HeJ, DBA/2J, FVB/NJ and NOD/ShiLtJ) | M | Fear conditioning (24 × 2sec, 1mA, 10 sec ITI) | Observation of the fear conditioning of a same strain Demonstrator | C57BL/6J, C57BL/6NTac, 129S1/SvImJ,
129S4/SvJae and BTBR T(+) Itpr3(tf)/J: Increased freezing during
the conditioning session and later for context, AKR/J, BALB/cByJ, C3H/HeJ, DBA/2J, FVB/NJ and NOD/ShiLtJ: no observational fear learning |
no | Not tested | Not tested | |
| B6 Observers paired with B6J, 129S1 or FVB Demonstrators | Display observational lfear learning |
3.1. Emotional contagion of pain
In their seminal study Langford et al. (Langford et al., 2006) reported social modulation of pain in mice. Mice given an identical noxious stimulus (0.9% acetic acid in the abdominal constriction test) and tested in dyads displayed higher levels of pain behavior than mice tested separately. Co-occurrence in writhing (pain) behavior was significantly higher in cagemates than in strangers, the effect dependent on visual observation. Moreover, bidirectional modulation of pain behavior in the familiar mice injected with formalin was shown; formalin-induced pain behavior was increased when the partner mouse received a more noxious stimulus and decreased when the stimulus given to the partner mouse was weaker. Consistent with these results, it was also observed that thermal hyperalgesia can be produced by either injection of acetic acid or by mere observation of a cage mate injected with acetic acid. The effect was limited to familiar mice. Since the interaction with an unfamiliar conspecific induces stress response in mice, the results suggested that the effects of familiarity depend on the level of stress associated with social interaction. In line with this reasoning, further studies have shown that emotional contagion of pain is prevented by 30-min restraint stress in cage mates but can be evoked in strangers by blocking the endocrine stress response (Martin et al., 2015). Consistently, there are also results showing that sensitivity to noxious stimulation is reduced in close proximity to a stranger mouse; however when the physical contact between mice is limited, mice show hyperalgesia rather than analgesia. The analgesic effect, which has been observed only in male-male dyads, is testosterone-dependent and accompanied by increased plasma corticosterone level (Langford et al., 2011).
3.2. Emotional contagion of fear
Fear learning is an adaptive, evolutionarily conserved process that allows animals to respond appropriately to threats in the environment. The past few years have brought a number of observations suggesting that rats and mice are able to socially share states of fear (Panksepp and Lahvis, 2011; Panksepp and Panksepp, 2013), laying the foundation for studying neural mechanisms of a primal form of empathy. Several rodent models of emotional contagion of fear that differ in threat imminence have been developed (Table 1). The animal that is a source of emotional stimulation (termed the Demonstrator) is either subjected to classical fear conditioning or fear memory retrieval in a small enclosure lacking the possibility to escape (imminent danger) or has been subjected to fear conditioning and transferred to a safe cage where social interaction with an observer animal takes place (remote danger). When the threat is inescapable, passive coping strategies, such as freezing, are usually elicited; they are accompanied by autonomic inhibition (hypotension, bradycardia) and an increase in the neuroendocrine response (activation of the hypothalamo-pituitary-adrenal axis and increased glucocorticoid secretion (Engel and Schmale, 1972; Steimer, 2002). On the other hand, active coping strategies are used when an escape from the threat is possible, and the autonomic changes associated with these active strategies are mediated predominantly by sympathetic activation (hypertension, tachycardia). This is the fight-or-flight response originally described by Cannon (Cannon, 1915; Steimer, 2002). Importantly, specific brain circuits appear to mediate distinct coping reactions to different types of stressors (Bandler et al., 2000; Keay and Bandler, 2001). Below we review the results of studies on emotional contagion of fear in immediate and remote danger models (Figure 1).
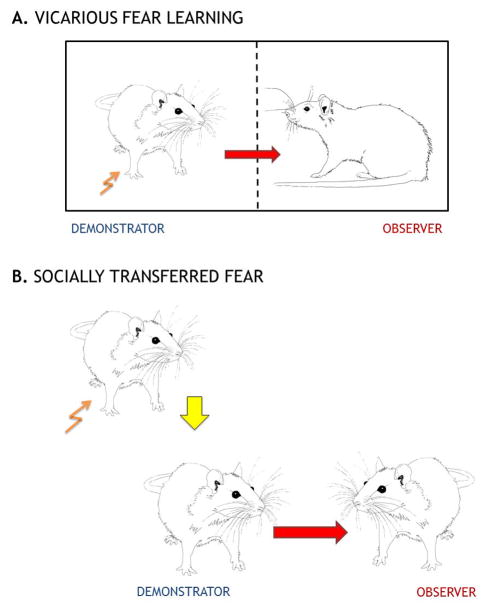
Fig. 1. Immediate (upper panel) and remote (lower panel) danger models.
In the model of vicarious fear learning the animals are put into the cage usually divided into two compartments by a perforated transparent partition allowing the rats to see, hear and smell the neighbor, but not to contact him physically. One of the animals (“demonstrator”) is subjected to either subjected to fear conditioning or exposed to the conditioned stimuli that evoke fear. The observer rats are not subjected to any training. The observer animal is conditioned for context-dependent fear by observing the behavior of the demonstrator animal receiving aversive stimuli. In the model of socially transferred fear “demonstrators” are subjected to fear conditioning alone. When the demonstrators are trained, their cohabitants (observers) are kept in the home cages in a different sound-attenuating room. Immediately after the training, the demonstrators are placed back in their home cages and allowed to interact with the observers.
3.2.1. Vicarious fear
Witnessing a partner subjected to fear conditioning exerts profound effects on the behavior of an observer animal. Staying in the same apparatus as the conspecific receiving aversive stimulation (usually behind a divider) results in an immediate fear response in both rats and mice (Atsak et al., 2011; Chen et al., 2009; Gonzalez-Liencres et al., 2014; Jeon et al., 2010). It has been shown that observing a conspecific’s distress leads to heart rate deceleration (Chen et al., 2009) and freezing responses (Atsak et al., 2011; Jeon et al., 2010). Magnitude of vicarious freezing response was modulated by previous experience with shocks (Atsak et al., 2011; Sanders et al., 2013), familiarity (Jeon et al., 2010; Gonzalez-Liencres et al., 2014), genetic background (Chen et al., 2009), and rearing conditions (Yusufishaq and Rosenkranz, 2013; Panksepp and Lahvis, 2016). Interestingly, repeated exposure to a cage mate experiencing foot shocks resulted in a gradual decrease of socially triggered freezing in the observers (Carrillo et al., 2015). It is noteworthy that vicarious fear learning is associated trait measure of empathy in humans (Kleberg et al., 2015), which indicates that the rodent models described above may tap into some fundamental features of empathy across species.
3.2.2. Fear Conditioning by Proxy
Since the behavioral procedure used in the abovementioned studies involves painful footshocks, observer animals probably respond to both pain and fear of their partners. In a different model, Fear conditioning by-proxy (FCbP), rats are allowed to freely interact with a conspecific that was previously conditioned during a fear memory retrieval (Bruchey et al., 2010; Jones et al., 2014; Jones & Monfils, 2016). As such, the previously conditioned rat is expressing a fear response, but is not in pain. Some rats display conditioned responding to a cue after interacting with a cage-mate during fear memory retrieval (Bruchey et al., 2010). The FCbP paradigm makes use of rats housed in triads, and takes place over three days. On day 1, one rat of each triad is fear conditioned to a cue paired with a foot-shock. On day 2, the fear-conditioned rat (FC rat) is returned to the fear-conditioning chamber accompanied by a cage-mate (FCbP rat) and the tone is played in the absence of the foot-shock. The third rat (No FC rat) remains in the home cage on day 2. The following day (day 3), all rats (FC, FCbP, and No FC) are placed in the chambers alone and tested for fear expression (freezing) to the CS. This paradigm is potentially advantageous in studying social fear learning in that 1) rats freely interact with each other during the social learning session and 2) behavior can be observed both as a pair, during training on day 2, and alone, during the follow up test on day 3. Testing in the absence of the demonstrator is essential to determine if learning has occurred by ruling out any motivational or social facilitatory effects that can occur when animals are present in the same chamber. FCbP is thought to engage mechanisms that possibly overlap with direct fear condition, since FCbP experienced prior to pairing the tone to a mild shock leads to increased freezing to the tone the next day compared to experiencing the CS+US pairing alone (Bruchey et al., 2010).
3.2.3. Socially transferred fear
In the vicarious fear learning protocols, animals are exposed to a partner which is in an immediate danger and therefore displays appropriate fear responses, such as freezing, emission of distress calls and physiological signs of stress such as bradycardia, urination and defecation. In contrast, in the model of socially transferred fear, the partner rat or mouse is still stressed due to the recent fear conditioning procedure but the danger is remote (Knapska et al., 2010, 2006), with a recent adaptation of the protocol for mice, (Meyza et al., 2015). In this model, the animals are housed in pairs and one member of the pair (the demonstrator) is removed and subjected to fear conditioning. After the fear-conditioning episode, the conditioned animal is allowed to interact with its naïve cage mate (the observer). In the control group the demonstrator is exposed to the experimental cage without fear conditioning. It has been shown that the demonstrator’s emotions are socially transferred to the observer, resulting in both rapid increase in exploratory behavior of the observer and increase in acoustic startle response, which is a measure of emotional arousal (Knapska et al., 2006).
In summary, in vicarious fear models the observers, placed in very similar conditions to those of the demonstrators, mirror their defensive responses (freezing). In contrast, interaction with a recently conditioned partner in a familiar environment (socially transferred fear model) results in an increase of emotional arousal, as well as exploratory and risk-assessment behaviors (Fig. 1, Table 1). The fear conditioning by proxy model combines the two approaches while relying mostly on socially transferred information. Thus, these models seem to be well-suited for studying socially transferred emotions that lead to, respectively, passive and active defensive responses. These models also encourage studies of contextual modulation of behavioral choices in animals subjected to emotional contagion. Interestingly, familiarity effect is commonly observed in models of pain contagion, whereas it is less common in models of fear contagion. It is then possible that learning about danger from conspecifics is beneficial even when the conspecifics are unfamiliar. Affective resonance in response to pain of an unfamiliar conspecific may, on the other hand, induce defensive aggression on the side of that conspecific and thus safety of interactions limit this behavior to familiar animals only. It is also worth noting that in certain conditions the presence of a partner or vicarious learning can attenuate some forms of fear and anxiety (a phenomenon called social buffering, for details see section 5).
3.3. Social modulation of fear memories
Tuning one’s emotional state to that of another increases the probability of similar behavior, which thereby allows rapid adaptation to environmental challenges (Hatfield et al., 1994). Another question is whether the state-matching in emotional contagion can exert long-lasting effects on behavior affecting learning and memory.
Although one can learn about potentially harmful stimuli by directly experiencing an aversive event, observation or an interaction with a conspecific in danger and/or in pain may also provide valuable information about environmental threats. In humans, most emotional learning probably occurs through observing other people (or through language), rather than through direct experience (Bandura, 1971).
Several works show that social learning can be also observed in rats and mice. For instance, observing a conspecific receiving aversive stimulation results in vicarious fear learning in both rats and mice (Atsak et al., 2011; Chen et al., 2009; Gonzalez-Liencres et al., 2014; Jeon et al., 2010). It has been also shown that socially transferred fear promotes aversive learning and memory in an otherwise naïve animal (Knapska et al., 2010). Knapska et al. (2010) observed that a brief social interaction with a recently fear-conditioned partner before learning session facilitates both the acquisition and memory in a shock-motivated shuttle avoidance task and increases conditioned freezing measured on the next day in contextual fear conditioning task. The observed effects were not due to a stress-induced increase in pain sensitivity or analgesia. The study of Knapska et al. (2010) was carried out in rats. On the other hand, in mice, Bredy and Barad (Bredy and Barad, 2009) showed that exposure to a recently fear-conditioned familiar animal impaired acquisition of cued conditioned fear. They obtained similar effects using an olfactory chemosignal emitted by a recently fear-conditioned familiar mouse and by the putative stress-related anxiogenic pheromone beta-phenylethylamine (beta-PEA). The discrepancy of the rats and mice experiments results may stem from different levels of stress induced by interaction with an emotionally aroused partner in these species, similarly as described earlier for emotional contagion of pain (section 2.1). This hypothesis, however, requires further studies.
In addition to affecting the rate of acquisition of conditioned fear responses, social interaction with a stressed conspecific can affect the extinction of already acquired conditioned reflexes. This phenomenon was studied extensively in mice (Bredy and Barad, 2009; Nowak et al., 2013) with several protocols, which yielded distinct results. While home cage exposure to a recently fear-conditioned mouse facilitated extinction of conditioned fear response, an exposure to recently fear-extinguished mouse (but not its urine alone) impaired fear extinction (Bredy and Barad, 2009). On the other hand, an exposure (in an adjacent chamber of a shuttle box) during extinction memory retention test to a fear conditioned but not to an extinguished mouse impaired fear extinction memory retrieval (Nowak et al., 2013). The apparent discrepancy between the results may be caused by the differences in the experimental protocols and handling procedures leading to different levels of stress during the experiments in both studies.
In summary, it has been consistently observed that social interaction with an emotionally aroused partner results in long-lasting changes of behavior. It has been also shown that such social interaction modulates subsequent learning. However, additional studies are needed to fully understand the factors through which social interaction improves or impairs learning.
3.4. Contagious yawning
Emotional contagion can also be related to non-fearful events. One of the phenomena commonly referred to as “contagious” is yawning. The effect was observed uniformly among mammals. It was reported in humans (Gallup and Church, 2015; Platek et al., 2003; Provine et al., 1987), non-human primates (Campbell and de Waal, 2011, 2010), canines (Romero et al., 2014) and rodents (Moyaho et al., 2015) alike. Contagious yawning has been shown to have no respiratory function (Provine et al., 1987). Though generally appearing in non-fearful situations, yawning has been also observed in stressful situations in different species and can serve as a possible indicator of increased stress (Aureli and de Waal, 1997; Kubota et al., 2014; Leone et al., 2014; Miller et al., 2012). In line with this hypothesis, a reduction of yawning has been observed following administration of the glucocorticoid synthesis blocker, metyrapone (Carrillo et al., 2015). However, intranasal oxytocin, believed to suppress stress and enhance empathy (Hurlemann et al., 2010), did not affect yawning per se, but increased awareness of the act in participants, which resulted in higher rate of efforts made to conceal it (Gallup and Church, 2015). Since the behavioral measure of emotional contagion (yawning) is easy to observe and quantify, contagious yawning seems to be a good model to study mirroring mechanism in the brain (Haker et al., 2013).
4. Neuronal correlates of emotional contagion
According to Panksepp and Panksepp’s theory, emotional contagion involves mainly the subcortical brain structures. However, the mechanisms by which animals share emotional states are largely unknown (see section 2). Human brain imaging studies showed activation of several brain structures, such as the amygdala, the insular and anterior cingulate cortex (ACC) in people observing others suffering from pain (Bernhardt and Singer, 2012) or experiencing fear (Olsson et al., 2007). These studies also showed that similar brain areas are activated during the first-hand experience of pain and fear. In rodents, in socially transferred fear model it has been observed that c-Fos expression (a marker of neuronal activation) in the observer’s amygdala and prefrontal cortex generally mirrors that of the shocked demonstrators; c-Fos expression was heightened in the basolateral and medial nuclei of the amygdala and the prelimbic and infralimbic parts of the prefrontal cortex of both distressed rats and the respective observers (Knapska et al., 2006, Mikosz et al. 2015). However, increased c-Fos induction in the central nucleus of the amygdala (CeA) differentiated these two groups and was heightened only in the observers. This finding suggests that particular neural circuits of the amygdala are responsive to the distress of others.
Interestingly, the pattern of brain activation by socially transferred fear seems to be species-specific (Knapska et al., 2006; Meyza et al., 2015; Mikosz et al., 2015). In the demonstrator rats increased activation has been observed in the basal, lateral and medial nuclei, but not in the CeA. In mice, c-Fos increases were observed in the basal, medial and central nuclei, but not in the lateral nucleus of the amygdala. In the observer rats, c-Fos expression was higher than in the demonstrators in the CeA. This did not hold true for mice. The increase in the number of c-Fos positive nuclei in observer mice exposed to a stressed cagemate was seen only for the basal nucleus of amygdala, while in rats it was observed for the basal, lateral and medial nuclei. The patterns of activation of the prefrontal cortex were similar in rats and mice.
It has been also observed that lidocaine inactivation of the ACC, parafascicular (Pf) or mediodorsal (MD) thalamic nuclei, which comprise the medial pain system representing the affective or emotional dimension of pain, impairs vicarious fear learning. Similarly, the ACC limited deletion of the Cav1.2 type 1 Ca2+ channels, contributing to synaptic transmission and neural excitability, impaired vicarious fear learning. On the other hand, inactivation of the thalamic nuclei that belong to the lateral, sensory pain system had no effect. Inactivation of the ACC, Pf or MD thalamic nuclei led to impaired vicarious fear learning but did not disturb fear expression, whereas inactivation of the lateral amygdala (La) resulted in impairments of both fear learning and fear expression. Activity of the ACC and La was augmented and synchronized during vicarious fear learning suggesting functional connectivity between these regions (Jeon et al., 2010). Further studies have shown that D2 dopamine receptors but not D1 dopamine receptors or serotonin receptors in the anterior cingulate cortex are required for vicarious fear, whereas increased serotonin, but not dopamine, levels impaired vicarious fear and altered the regularity of neural oscillations in the anterior cingulate cortex (Kim et al., 2014). Interestingly, in the same model, hemispheric lateralization of vicarious fear learning has been shown. Inactivation of the right but not the left ACC impaired vicarious fear learning. Such lateralization has not been observed in the thalamic nuclei (Kim et al., 2012).
The Fear conditioning by-proxy (FCbP) paradigm was recently found to involve neural pathways that overlap with those engaged in direct fear conditioning (FC), but to also require distinct regions that appear specific to FCbP and not FC (Jones and Monfils, 2016). Specifically, the neural processes underlying FCbP were evaluated in parallel with those involved in direct FC using c-Fos immunohistochemistry. The results showed that both social acquisition as well as retrieval of directly conditioned fear activated the lateral nucleus of the amygdala and the ventral CA1 region of the hippocampus. Additionally, the acquisition of FCbP uniquely activated neurons in the anterior cingulate cortex (ACC) as well as the ventral CA3 region of the hippocampus. Importantly, selective inactivation (with intracranial microinfusions of muscimol) of the ACC, but not the ventral hippocampus, was found to prevent acquisition of FCbP, and not direct FC (Jones and Monfils, 2016). Together, these findings suggest that the ACC is necessary for the acquisition of FCbP.
Moreover, a recent study showing the existence of emotional contagion, state matching, familiarity bias and self-other discrimination in prairie voles (Burkett et al. 2016) proved that intact oxytocin signaling in the anterior cingulate cortex is crucial for consolation in this monogamic species.
Taken together, these results show that brain is strongly activated by socially transferred emotions; however, it would be premature to draw definite conclusions about the precise functional roles of its different parts. The patterns of activation seem to differ depending on the social context, but the data are still scarce. The involvement of the amygdala, ACC and PFC, and thalamic and hypothalamic nuclei in socially transferred emotions has been shown; however it is not clear how they interact with one another and whether different neural circuits within these structures are involved in specific social behaviors. Little is also known about the role of the insular cortex, which seems to play a key role in human social behavior. In order to answer these questions, more advanced techniques of studying anatomy and function of neural circuits are needed. Since c-fos-dependent tools proved to be very useful in the studies on the role of neural circuits in control of non-social emotional behavior and forming memory traces (Gore et al., 2015, Ramirez et al., 2015), description of patterns of c-Fos expression in response to socially transferred emotions seems to be a good starting point for analogous studies in the field of social emotions. Moreover, reciprocal functional connectivity should be described to understand relations between different brain structures. The existing data also point toward the role of oxytocin in social behaviors (e.g., see Guzmán et al., 2014 for an example of their role in social modulation of fear learning), which opens up another promising line of research (Stoop, 2014). Oxytocin plays also an important role in social buffering, an important phenomenon that allows relief from fear by interacting with conspecifics and often parallels fear contagion.
5. Social buffering
Several human studies have suggested that company of a familiar individual helps dealing with everyday stress and recover faster from trauma (Bowen et al., 2014). Similar phenomenon was described in non-human primates (Gunnar et al., 2015; Sanchez et al., 2015). In the series of studies Kiyokawa and co-workers showed that in rodents, social interaction with a naïve individual modulates the behavior of the stressed animals (experimental details are summarized in Table 2). When fear-conditioned rats were exposed to the conditioning context along with an accompanying conspecific animal, stress-induced hyperthermia, behavioral fear responses (freezing) and c-Fos expression in the paraventricular nucleus were attenuated. The effect depended on the stress status of a partner rat, non-shocked partners were more effective than shocked partners (Kiyokawa et al., 2004). Further studies showed that the behavioral effect depends also on the type and timing of the interaction (pair-exposure to the stressor versus pair-housing after the shock). Pair-housing for 24 h with an unfamiliar rat following auditory fear conditioning resulted in a suppressed stress-induced hyperthermia, but not freezing response and increased c-Fos expression in the lateral nucleus of the amygdala and ventrolateral periaqueductal gray. On the other hand, pair-exposure reduced behavioral, but not the autonomic, response and increased c-Fos expression in the basal nucleus of the amygdala and infralimbic region of the prefrontal cortex. Rats that had been pair-housed and then pair-exposed showed no behavioral, autonomic or neural fear responses (Kiyokawa et al., 2007). The data suggest that consequences of “housing” and “exposure” social buffering differ at the behavioral, autonomic and neuronal levels.
Table 2.
Examples of prosocial behavior in rodents
| Study | Species (strain) | Sex | Demonstrator experience | Observer experience | Observer response | Observer Pain Experience required? | Familiarity required? | Sensory Modality | |
|---|---|---|---|---|---|---|---|---|---|
| Helping behavior | Church 1959 | Rat | Shocked during lever pressing | Naïve or pre-exposed to shock rats were trained to press lever for food. | Experienced rats stop to press the lever for food when the condition is changed to shocking the Demonstrator | yes | Not tested | Not tested | |
| Rice and Gainer 1962 | Rat | Hung by the tail | Pressing the lever to lower the Demonstrator | Lowering of the Demonstrator (but not Styrofoam block) | no | Not tested | Not tested | ||
| Greene 1969 | Rat (Sprague-Dawley) | M | Shocked during lever pressing | Pressing their preferred lever delivered shocks to the Demonstrator | Experienced rats change from preferred to the other lever (to obtain food), despite it requiring double the effort to produce reward | yes | Not tested | Not tested | |
| Continuously shocked | Pressing lever ceased shocks to the Demonstrator | No change in preference | |||||||
| Ben-Ami Bartal et al. 2011 | Rat (Sprague-Dawley) | M and F | Restraint | Staying in the same compartment as the restrainer | Freeing the restrained rat | no | yes | Not tested | |
| Schneeberger et al. 2012 | Rat (Norway) | F | Hungry vs. Satiated Cooperative or defective receiver of food | none | Feeds the Demonstrator | no | no | Not tested | |
| Silberberg et al. 2014 | Rat (Sprague-Dawley) | F | Restraint | Staying in the compartment adjacent to the restrainer and either releasing the Demonstrator to a distal chamber or to your own chamber | Latency of freeing the rat to the distal chamber was higher than for the release to Observers compartment | no | Not tested (yes) | Not tested | |
| Ben-Ami Bartal et al. 2014 | Rat (Sprague-Dawley and Long Evans) | M | Restraint | Staying in the same compartment as the restrainer | Freeing the restrained rat. | no | Yes | Not tested | |
| Ben-Ami Bartal et al. 2016 | Rat (Sprague-Dawley) | M | Restraint | Staying in the same compartment as the restrainer | Freeing the restrained rat. High dose of midazolam impaired this response | no | Yes | Not tested | |
| Social buffering | Davitz and Mason, 1955 | Rat | M | Fear conditionied + cue test | Cue test in the presence of a conditioned demonstrator | Increased the activity of the Demonstrator during cue test | no | Not tested | Not tested |
| Kiyokawa et al. 2004 | Rat (Wistar) | M | Fear conditionied + context test | Naïve or fear conditioned rat exposed to context together with the Demonstrator | The presence of the non-shocked partner attenuated stress induced hyperthermia, freezing and activation of PVN | yes | Not tested | Not tested | |
| Kiyokawa et al. 2007 | Rat (Wistar) | M | Fear conditioned (7 × 0.5 sec, 2 mA, 30 sec ITI) | Pair housed with the Demonstrator 24 h after training | Reduced autonomic but not behavioral manifestation of fear | no | Not tested | Not tested | |
| Pair exposed to fear conditioning training (7 × 0.5 sec, 2 mA, 30 sec ITI) | Reduced freezing but not the autonomic responses to cue | ||||||||
| Pair exposed to fear conditioning training (7 × 0.5 sec, 2 mA, 30 sec ITI) and pair housed with the Demonstrator 24 h after training | Reduced both the autonomic and behavioral manifestations of stress | ||||||||
| Kiyokawa et al. 2009 | Rat (Wistar, guinea pig) | M | Fear conditioned (7 × 0.5 sec, 0.7 mA, 30–180 sec ITI) | Observation of the Demonstrator in an adjacent cage | Social buffering occurs, freezing to cue and c-Fos in the PVN of the Demonstrator low | no | Not tested | Tactile (no), Olfactory (yes) | |
| Observation of the Demonstrator in a neighboring cage (5 cm gap) | Social buffering occurs, freezing to cue and c-Fos in the PVN of the Demonstrator low | ||||||||
| Observation of the Demonstrator in an adjacent cage through solid transparent board | Social buffering does not occur, freezing to cue and c-Fos in the PVN of the Demonstrator high | ||||||||
| Takahashi et al. 2013, | Rat (Wistar) | M | Fear conditioned (7 × 0.5 sec, 0.65 mA, 30–180 sec ITI) | Odor donation | Demonstrators exposed to the odor of unstressed rats did not freeze to cue | no | Not tested | Olfactory | |
| Kiyokawa et al. 2014 | Rat (Wistar) | M | Fear conditioned (5 × 0.5 sec, 0.7 mA, 60–240 sec ITI) | Social co-habitation (without interactions) | Social buffering occurs, hyperthermia and c-Fos in the PVN of the Demonstrator low although the Demonstrator displays freezing | no | Not tested | Tactile (not necessary) | |
| Kiyokawa et al. 2014a | Rat (Wistar) | M | Fear conditioned (7 × 0.5 sec, 0.8 mA, 30–180 sec ITI) | Observation of the Demonstrator in an adjacent cage | Social buffering occurs, freezing to cue and corticosterone of the Demonstrator low | no | Not tested | Not tested | |
| Kiyokawa et al. 2014b | Rat (Wistar) | M | Fear conditioned (7 × 0.5 sec, 0.65 mA, 60–270 sec ITI) | Observation of the Demonstrator in an neighboring cage | Social buffering highest, freezing to cue and c-Fos in the PVN, LA and CeA of the Demonstrator lowest in familiar pairs | no | yes | Not tested | |
| Iishi et al. 2016 | Rat (Wistar) | F and M | Fear conditioned (7 × 0.5 sec, 0.8 mA, 60–180 sec ITI) + cue test | Fear conditioned (7 × 0.5 sec, 0.8 mA, 60–180 sec ITI) + cue test | Estrous cycle does not affect freezing responses | yes | Not tested | Not tested | |
| 5 min observation, pairs grouped by estrous phase of each of both the Demonstrators and Associates | Neither demonstrator nor the associate’s cycle phase affects the freezing behavior | ||||||||
| Observation of the same-sex Demonstrator in an neighboring cage | Social buffering occurs in both male and female pairs | ||||||||
| Mikani et al. 2016 | Rat (Wistar) | M | Fear conditioned (7 × 0.5 sec, 0.55 mA, 60–120 sec ITI) + extinction either alone or with a naïve partner + cue test | Accompanying the Demonstrator during the extinction session | Social buffering enhanced extinction of fear conditioned response to cue | no | Not tested | Not tested | |
| Nakamura et al. 2016 | Rat (Wistar, Sprague-Dawley. Long-Evans, Lewis, F344 and Brown Norway) | M | Fear conditioned (7 × 0.5 sec, 0.75 mA, 60–180 sec ITI) + cue test | Associates (Wistar, Sprague-Dawley. Long-Evans and F344) are in the neighboring cage during cue test | Social buffering occurs with Wistar, Sprague-Dawley. Long-Evans Associated but not with F344 rats | no | Not tested | Not tested | |
| Either the Demonstrator or the Associate were Wistar or Lewis rats | No difference between non-conditioned and conditioned pairs | ||||||||
| Brown Norway rats as Associates | No social buffering occurs with Brown Norway rats as Associates | ||||||||
| Guzman et al. 2009 | Mouse (c57BL/6N) | M | Fear conditioning (2 sec, 0.7 mA) | Observation of the Demonstrator + fear conditioning | Freezing to context | yes | Not tested | Visual | |
| Burkett et al. 2016 | Prairie vole | M and F | Fear conditioning (5 × 0.5 sec, 0.8 mA) distributed over the course of 24 min | Reunited with the Demonstrator in the home cage | Increased allogrooming towards the stressed Demonstrator (with shorter onset latency and longer duration) + Increased selfgrooming and freezing to cue in both animals. Oxytocin antagonist blocks consolation | Not tested | Monogamous (not tested due to speices characteristics) | Visual |
Kiyokawa et al. also showed that social buffering during pair-exposure to the conditioned stimulus does not require a physical contact between the animals, but is mediated by the main olfactory system and its projections to the lateral and central amygdala through the posteromedial region of the olfactory peduncle (Kiyokawa et al., 2012, 2009; Takahashi et al., 2013). Similarly, “Housing” type of social buffering did not require physical contact between animals (Kiyokawa et al., 2013). In contrast to these results, physical contact was necessary to prevent an increase in anxiety in the elevated plus-maze test performed two weeks after social defeat (Nakayasu and Kato, 2011) and to reduce open field exposure stress as measured by prolactin levels (Insana and Wilson, 2008). The latter study was carried out on adolescent rats. Adolescent rats have been also shown to have reduced level of corticosterone when exposed to the novel environment with a conspecific, the effect correlated with amount of physical contact (Terranova et al., 1999). Systematic comparison of social buffering effects (measured with corticosterone levels and Zif268 immunoreactivity as a marker of neuronal activation) in adult and adolescent rats during recovery after isolation stress, showed no differences between different age groups (Hodges et al., 2014). Thus, the age does not determine the role of physical contact in social buffering. The relative importance of sensory modalities for social buffering is not clear and deserves further studies.
Social buffering was studied mainly in fear and anxiety provoking behavioral tests that employ standard stimuli, such as footshocks; however, the phenomenon has been observed also in more ethologically relevant conditions. For instance, social exposure to predatory threat promoted active responding, relative to individual exposure, and lowered c-Fos expression in the dorsomedial periaqueductal grey, medial caudate putamen and lateral habenula (Bowen et al., 2013). Furthermore, social interaction with a conspecific following a poisoning reduced conditioned taste aversion in mice (Hishimura, 2015). Most of the studies on social buffering was carried out on unfamiliar rats. Recently, however, it has been shown that a familiar conspecific is even more effective at social buffering of conditioned fear responses (Kiyokawa et al., 2014b). Similar effect was recently demonstrated for prairie voles (Burkett et al. 2016).
The brain and physiological correlates of pair-exposure effects have been studied. It has been shown that the presence of a conspecific animal significantly decreased the mean peak amplitudes of auditory evoked field potentials, gamma and high frequency oscillations in the lateral amygdala (Fuzzo et al., 2015). Social buffering was also observed at the stress hormone level. Corticosterone levels in animals tested alone were much higher than those re-exposed to the stressful stimuli in the presence of a conspecific (Burkett et al. 2016; Kiyokawa et al., 2014a; Terranova et al., 1999). Pair-exposure to the open field and fear conditioned context attenuated also prolactin-secretory response (Insana and Wilson, 2008).
Other form of social interaction that decreases response to aversive events described in the literature is pro-active social buffering. Pre-exposure to a nonfearful conspecific reduced subsequent long- but not short-term contextual fear memory in mice, leaving fear conditioning in response to a novel context or cue intact (Guzmán et al., 2009). The effect was constrained by the shock intensity (Guzmán et al., 2009) and modulated bidirectionally by the septal oxytocin system (Guzmán et al., 2014). These results suggest that under certain conditions observational (vicarious) learning can attenuate some forms of fear.
Social buffering should be distinguished from general anxiolytic effects of co-housing. For instance, pair-housing before aversive experience has been shown to reduce fear learning and enhanced active avoidance learning compared to single housing (Knapska et al., 2010b). Moreover, socially-enriched environment reduced anxiogenic effects of external stressors (footshocks, forced swimming) and improve the performance in an operant task (Huzard et al., 2015). In line with these results, group housing of mice reduced levels of anxiety and depression induced by chronic restraint stress (Liu et al., 2013).
In sum, social buffering is commonly observed during social interactions taking place before, during or after aversive events. However, further research is needed to clarify the factors modulating social buffering effects and the brain mechanisms underlying this phenomenon. In particular, it is not clear which factors determine the choice between fear contagion and social buffering during social interaction.
6. Sympathetic concern
In humans, empathy is a powerful motivator of helping behavior. Pro-social behavior can emerge when an affective response to the distress of others evokes a drive to act for their benefit. For rats, a highly social mammal species, there have been numerous accounts of pro-social acts (Table 2). For instance, rats refrain from pressing a lever that shocks a conspecific (Church, 1959), press a lever to relieve a rat dangling in midair (Rice and Gainer, 1962), reciprocate food sharing (Rutte and Taborsky, 2007), prefer mutual rewards to a selfish reward (Schuster and Perelberg, 2004; Hernandez-Lallement et al., 2014; Márquez et al., 2015), and release a cage mate trapped inside a restrainer (Ben-Ami Bartal et al., 2011).
In the latter study, rats were tested in a helping behavior paradigm, where free rats learn to open the door to a restrainer in which another rat is trapped. It takes rats a few sessions to learn how to open the restrainer. Once rats are able to release the trapped cage mate, they will repeat this behavior quickly and intentionally on following sessions, indicating that door-opening is reinforcing. Rats were motivated to release trapped cage mates, even when they were pitted against chocolate chips. Rats did not open an empty restrainer or one containing a toy rat (Ben-Ami Bartal et al., 2011). These findings were recently replicated and expanded by Sato et al. (Sato et al., 2015), who showed that rats release others trapped in a pool of water.
For rats, the motivation to release a trapped cage mate is dependent on the social context. While rats were helpful to strangers of their own strain (Ben-Ami Bartal et al., 2014), they did not release strangers of an unfamiliar strain. Yet two weeks of pair-housing with a member of the other strain were sufficient to induce door-opening for strangers of that strain. This finding suggests that the in-group bias that exists in humans is biologically rooted, and is in line with evidence showing that in humans, social experience can influence empathy for strangers (Martin et al., 2015) and out-group members (Cao et al., 2015; Telzer et al., 2013; Zuo and Han, 2013). Furthermore, the pups that were fostered at birth with litters of another strain were selectively motivated to help their adoptive strain, but not their own strain, as adults. These findings suggest that the proximal mechanism that underlies pro-social motivation in rats utilizes exclusively the individual’s social experience, rather than any innate information about genetic relatedness.
7. Evolutionary roots of empathy?
It has been proposed that empathy has deep evolutionary roots, which originated long before Homo sapiens started walking the Earth. According to de Waal’s multi-level conceptualization of empathy, more complex behaviors evolved from the simplest forms involving adoption of another’s emotional states (emotional contagion). Similarly, Panksepp et al. (1997) proposed that separation distress and pain of social loss emerged from more ancient physical pain systems. Moreover, considering the continuity in sympathetic concern across species, it has been argued that empathy is rooted in a behavior common to all mammals - the caring for offspring. As suggested by Patricia Churchland (Churchland, 2011), neural evolution inclined humans to strive not only for self-preservation but for the well-being of others - first offspring, then mates, kin, and finally strangers. The move from self-caring to caring-for-others occurred by slight adjustments to the existing neural mechanisms rather than some radical new engineering plan. According to Churchland’s theory, the key player in these modifications to the brain is oxytocin, a hormone thought to play an important role in mammalian bonding, evoking feelings of contentment and trust, and reducing defensive behaviors like fleeing or fighting. This concept of the evolutionary origins of empathy elegantly explains why separation and social exclusion cause pain, and the company of loved ones causes pleasure. However, it cannot be validated in any other way than by learning whether the same neural circuits are used by the brain in social and non-social contexts. It is also not clear how more elaborated sympathetic behaviors are related to the much simpler emotional contagion. To answer this question directly, a better understanding how brain controls social behaviors is necessary (Insel and Fernald, 2004). Future research should establish whether prosocial behavior eventually limits contagion of negative emotions by suppressing their primary source: the distress of another individual.
The current view in the field is that the evolutionary roots of empathy are based in parental care of their young. Specifically, in altricial mammals, a mother needs to be able to process signals of need from the off-spring and be motivated to respond to those needs in order to be reproductively successful (Preston, 2013). This raises an interesting question about sex differentiation of the empathic neural circuits.
8. Sex differences
A growing body of human studies suggests an existence of sex differences in empathy (Baron-Cohen and Wheelwright, 2004; Rueckert and Naybar, 2008; Schulte-Rüther et al., 2008). Studies in rodents, however, are predominantly performed on males, with only a few cases where female empathic abilities were investigated (Atsak et al., 2011; Ben-Ami Bartal et al., 2011; Ishii et al. 2015; Jones et al., 2014; Langford et al., 2011; Mikosz et al. 2015; Panksepp and Lahvis, 2016). Despite strong evidence suggesting the influence of menstrual cycle on empathy in women (Derntl et al., 2013, 2008; Guapo et al., 2009; Pearson and Lewis, 2005) only three of the aforementioned studies (Ishii et al. 2015; Jones et al., 2014; Mikosz et al. 2015) accounted for the estrous cycle of the studied females, and only the latter compared the obtained results also between the sexes. In that study, in the model of socially transferred fear, differences in active avoidance learning following the interaction with a stressed demonstrator were found between male (and diestral female) versus estral female observers. Only in male observers, interaction with a fear-conditioned demonstrator resulted in enhanced neuronal activation of the central and lateral nuclei of the amygdala and the prelimbic and infralimbic parts of the prefrontal cortex. No such effect was observed in females.
Although female rats actively engaged in social interaction, they were unresponsive to fear contagion, especially in the estrus phase of the cycle. This result is consistent with the notion that elevated level of circulating estrogens is correlated with reduced anxiety (Mora et al., 1997). Also under natural conditions the estral females are less anxious and explore beyond their home range actively seeking males (Calhoun, 1963). In line with that, ovariectomy results in increased anxiety, which can be reversed by subcutaneous administration of estrogen (Walf and Frye, 2004).
These sex differences may stem from evolutionary roots. The behavior of male and female individuals in the wild is noticeably different; males are territorial and engage in aggressive encounters with other males, while in females dominance is less pronounced and aggression is lower. Females have also smaller home ranges, explore beyond them only while actively attracting males in estrus (Barnett, 1957). Assuming that socially transferred fear is an adaptation that promotes defensive behavior to potentially dangerous situations in the environment, female rats, as less exposed to dangers in the environment, can be less sensitive to fear showed by adult conspecifics. The results suggest different behavioral and neural mechanisms of emotional contagion in females, and presumably also differences in sensitivity to various other social stimuli. Further studies are required to answer the question which stimuli are more effective to induce emotional contagion in females.
9. Ontogeny of empathy
The development of empathy in the course of human ontogeny resembles that of the evolutionary increase in the complexity of empathic behaviors observed in other animals (Light and Zahn-Waxler, 2011). While newborns display uninhibited emotional contagion (they cry when hearing other babies cry), already at the age of six months they become capable of distinguishing the distress of others from their own emotional state (Hay et al. 1981). They then move to more complex manifestations of both emotional and cognitive empathy (Roth-Hanania et al., 2011), with the age of two years serving as a key developmental milestone for empathy and prosocial development. With the development of speech and accuracy of movement, toddlers begin to more visibly display helping behaviors (such as telling or pointing to objects and fetching items that appear out of reach for other people, Dunfield et al., 2011). Their responsiveness is initially strongest to the distress of their closest caretakers (Zahn-Waxler et al., 1992), gradually shifting towards members of the same group and eventually strangers. With the gradual maturation of neuronal circuits controlling emotional behavior (including medial, ventromedial and dorsolateral prefrontal cortex, anterior cingulate cortex, amygdala and insula) children become more aware of subtle differences in emotional expression of others. This, in turn, lets them become more selective in their response to others (Decety and Lamm, 2006).
While human studies provide a wealth of data on the development of empathic responses with age (for review see Decety et al., 2016), the data concerning the ontogeny of empathy in other animals is scarce. In non-human apes, the development of empathic responses seems to follow a similar trajectory to that of human babies. Moreover, in young bonobos (Clay and de Waal, 2013a,b) consolation was more likely to be offered by juveniles as compared to adults. Similarly, young adolescent (4 weeks old) c57BL/6 mice exhibited stronger observational fear learning, than adult individuals (Keum et al., 2016), although this effect could have been a result of stronger expression of general freezing in the Pavlovian fear conditioning at that developmental stage. Social exclusion during restraint was also found to strongly affect adolescent rats, inducing elevated anxiety in these animals (Lee and Noh, 2015).
Further studies in pre-weaning pups are required to trace the trajectory of emotional contagion development in rodents. The time around weaning is a critical period for the development of social repertoire. It would therefore be most interesting to see if major changes in empathic and prosocial responses occur in rodents at that time. Especially that the shift from in-nest to out-door type of dwelling requires a major change in both social and non-social exploratory activity. E.g., while for pre-weaning pups it is vitally important to adjust the amount of ultrasound vocalization based on the familiarity of the smell (strong for a mother returning to the nest vs. inhibited towards an unfamiliar male, which poses threat to the pup (Takahashi, 1992), post-weaning, mobile animals can adopt other behavioral strategies during social encounters. The verification of empathy ontogeny in rodents is also crucial for the validation of rodent models/protocols designed to study empathy impairments otherwise characteristic of certain neurodevelopmental disorders (described in detail in section 9).
10. Empathy and psychopathology
One of the main reasons to study empathy in rodents is the possibility of developing genetic and environmental models of human disorders characterized with empathy impairments. These, however, are not all uniform; for review see (Gonzalez-Liencres et al., 2013). In 2006, a model dividing empathy impairments into functional classes was developed (Smith, 2006) to reflect that diversity. It encompasses four distinct categories: Cognitive Empathy Deficit Disorder (CEDD), Emotional Empathy Deficit Disorder (EEDD), General Empathy Deficit Disorder (GEDD) and General Empathy Surfeit Disorder (GESD). While CEDD is characteristic of neurodevelopmental disorders such as autism spectrum disorder (Smith, 2009) DSM-V), where patients do not comprehend the emotional states of others due to impaired Theory of Mind (Baron-Cohen, 2002; Sucksmith et al., 2013), but declare the ability to share others’ emotions (Dziobek et al., 2008); EEDD is comorbid with psychopathy, conduct disorder and antisocial personality disorder. Such patients are aware of the emotional states of others, but do not share them. This, combined with a lack of scruples and/or respect for socially accepted boundaries, often leads to taking advantage of other people. GEDD (the lack of both forms of empathy) is reported in schizophrenia patients, who tend to isolate themselves (Derntl et al., 2015; Green et al., 2015). The opposite phenotype is observed in the Williams syndrome (Riby and Back, 2010), although the extent of understanding of the emotional state of others is difficult to judge due to cognitive disability.
Such clear dissection may sound appealing, but it does not reflect the complexity of many of these conditions. The complex etiology of these pathologies makes them hard to model in laboratory animals. Only in a limited number of cases a monogenic cause of the given phenotype is known. There are several genetic models of autism spectrum disorder (see (Ebrahimi-Fakhari and Sahin, 2015) for a review) and Williams syndrome (Osborne, 2010). However, much less is known about the genetic causes of schizophrenia (Escudero and Johnstone, 2014) or psychopathy (for review see (Cummings, 2015).
There are, however, certain neuroanatomical features that can be modeled in rodents, which seem to be of functional relevance to empathy impairments. The size and activity of the amygdalar complex is known to affect empathic concern. It is enhanced in Williams syndrome (Capitão et al., 2011) and decreased in individuals with psychopathic tendencies (Marsh, 2015). Its connectivity and cell composition (fewer neurons and oligodendrocytes) was also reported altered in autism spectrum disorder (Morgan et al., 2014). Changes in amygdalar functions can be modeled in rodents either with the use of directed conditioned genetic modifications or by transient, temporary inactivation or overstimulation of this brain region. Studying strain differences in mouse studies of emotional contagion (Chen et al., 2009) and coping with fearful stimuli (Szklarczyk et al., 2012) also offers an insight into the role of amygdalar reactivity in the development of empathy.
Another neuroanatomical abnormality linked with impairments in recognition of certain emotions (fear and disgust) was reported for patients lacking the corpus callosum (Bridgman et al., 2014). 45% of children with agenesis of corpus callosum are co-diagnosed with autism spectrum disorder (Lau et al., 2013). An acallosal mouse model of idiopathic autism, the BTBR T+Itpr3tf/J mouse, was recently found to display impaired social transfer of fear combined with hypoactivation of amygdala in response to contact with a stressed cagemate (Meyza et al., 2015). These results encourage further studies on rodent models of human empathy disorders. Relatively easy access to genetically modified mouse lines should prove useful in assessing the efficacy of treatments aimed at improvement of empathic behaviors in narrow, specialized populations of patients and in studying their mechanism of action prior to the commencement of actual clinical tests. As an illustration, the intranasal oxytocin supplementation was found efficient only in certain autistic and schizophrenic patients (see (Gonzalez-Liencres et al., 2013) for review), while in borderline patients it worsened the social anxiety of the subjects (Bartz et al., 2011). Similar discrepancies were found in distinct mouse models of these disorders (Bales et al., 2014; Sobota et al., 2015; Teng et al., 2013).
11. Future directions, outstanding questions
The studies of the neural basis of emotional empathy receiving a surge of interest in recent years mostly employed human neuroimaging. Validation of a set of simpler animal models would pave the way for systematic, single-cell recordings and tissue-specific manipulations in the brain regions implicated in empathy. It would also allow for thorough preclinical screening of potential therapeutic agents. Moreover, animal models offer a unique opportunity to study comparative aspects of empathy.
Several questions, however, arise as a result of studying empathy in rodents. Firstly, how accurately can we model neuronal mechanisms of empathy in rodents, which, with the exception of prairie voles and the Fear conditioning by-proxy paradigm, seem to rely less on insular and anterior cingulate circuitry and more on the amygdalo-prefrontal pathway in transmitting information about the emotional state of their conspecifics? To address this concern, systematic comparisons between patterns of human and rodent brain activation in simple models of emotional contagion are required. Secondly, is there an equivalent of mirror neuron system in rodents? Are the same neuronal circuits activated by social and non-social emotions? To answer these questions employing of more advance techniques of studying anatomy and function of neuronal circuits in animal models of social contagion will be necessary. Also, does rodent empathy develop in complexity with age, as it does in humans? Answers to these questions might not be readily available, but should be continually addressed in order to validate rodent models of empathy for preclinical use.
Highlights.
Rodents are capable of emotional contagion to both painful and fearful stimuli
Rats and mice respond to unpleasant vicarious experiences with activation of amygdala, prefrontal and anterior cingulate cortices
Rats display pro-social behaviors towards conspecifics in need
Familiarity and physical similarity modulate empathic behaviors
Stress level and sex influence the ability to share emotions
Acknowledgments
E.K. was supported by the National Science Centre grant 2013/11/B/NZ3/01560.
K.M. was supported by the Foundation for Polish Science grant (Homing Plus/2012-6/6) and the National Science Centre grant 2015/18/E/NZ4/00600.
Footnotes
Review based on the symposium “Neuronal correlates of rodent empathy”, held at the 24th Annual Meeting of the International Behavioral Neuroscience Society, June 2–7, 2015 in Victoria, BC, Canada.
References
- 1.Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, Keysers C. Experience modulates vicarious freezing in rats: a model for empathy. PloS One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aureli F, de Waal FB. Inhibition of social behavior in chimpanzees under high-density conditions. Am J Primatol. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Baird AD, Scheffer IE, Wilson SJ. Mirror neuron system involvement in empathy: a critical look at the evidence. Soc Neurosci. 2011;6(4):327–35. doi: 10.1080/17470919.2010.547085. [DOI] [PubMed] [Google Scholar]
- 4.Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandler R, Price JL, Keay KA. Brain mediation of active and passive emotional coping. Prog Brain Res. 2000;122:333–349. doi: 10.1016/s0079-6123(08)62149-4. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A. Social Learning Theory. General Learning Corporation; 1971. pp. 1–46. [Google Scholar]
- 7.Barnett S. An analysis of social behaviour in wild rats. Proc Zool Soc Lond. 1957;130:107–152. [Google Scholar]
- 8.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 9.Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 10.Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. eLife. 2014;3:e01385. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Ami Bartal I, Shan H, Molasky NM, Murray TM, Williams JZ, Decety J, Mason P. Anxiolytic Treatment Impairs Helping Behavior in Rats. Front Psychol. 2016;7:850. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 15.Bowen MT, Kevin RC, May M, Staples LG, Hunt GE, McGregor IS. Defensive aggregation (huddling) in Rattus norvegicus toward predator odor: individual differences, social buffering effects and neural correlates. PLoS One. 2013;8(7):e68483. doi: 10.1371/journal.pone.0068483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen KS, Uchino BN, Birmingham W, Carlisle M, Smith TW, Light KC. The stress-buffering effects of functional social support on ambulatory bloodpressure. Health Psychol. 2014;33(11):1440–3. doi: 10.1037/hea0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem Cold Spring Harb N. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridgman MW, Brown WS, Spezio ML, Leonard MK, Adolphs R, Paul LK. Facial emotion recognition in agenesis of the corpus callosum. J Neurodev Disord. 2014;6:32. doi: 10.1186/1866-1955-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruchey AK, Jones CE, Monfils MH. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res. 2010;214:80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375–8. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calhoun JB. The ecology and sociology of the Norway rat. U.S. Dept. of Health, Education, and Welfare, Public Health Service; 1963. [Google Scholar]
- 22.Campbell MW, de Waal FBM. Ingroup-outgroup bias in contagious yawning by chimpanzees supports link to empathy. PloS One. 2011;6:e18283. doi: 10.1371/journal.pone.0018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell MW, de Waal FBM. Methodological problems in the study of contagious yawning. Front Neurol Neurosci. 2010;28:120–127. doi: 10.1159/000307090. [DOI] [PubMed] [Google Scholar]
- 24.Cannon WB. Bodily Changes in Pain, Hunger, Fear, and Rage: An Account of Recent Researches Into the Function of Emotional Excitement. D. Appleton; 1915. [Google Scholar]
- 25.Cao Y, Contreras-Huerta LS, McFadyen J, Cunnington R. Racial bias in neural response to others’ pain is reduced with other-race contact. Cortex J Devoted Study Nerv Syst Behav. 2015;70:68–78. doi: 10.1016/j.cortex.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Capitão L, Sampaio A, Sampaio C, Vasconcelos C, Férnandez M, Garayzábal E, Shenton ME, Gonçalves OF. MRI amygdala volume in Williams Syndrome. Res Dev Disabil. 2011;32:2767–2772. doi: 10.1016/j.ridd.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Carrillo M, Migliorati F, Bruls R, Han Y, Heinemans M, Pruis I, Gazzola V, Keysers C. Repeated Witnessing of Conspecifics in Pain: Effects on Emotional Contagion. PLoS ONE. 2015:10. doi: 10.1371/journal.pone.0136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang SW, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci U S A. 2015;112(52):16012–7. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PloS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 31.Churchland PS. Braintrust: What Neuroscience Tells Us about Morality. Princeton University Press; 2011. [Google Scholar]
- 32.Clay Z, de Waal FBM. Bonobos respond to distress in others: Consolation across the age spectrum. PLoS One. 2013a;8(1):e55206. doi: 10.1371/journal.pone.0055206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clay Z, de Waal FBM. Development of socio-emotional competence in bonobos. Proceedings of the National Academy of Sciences, U S A. 2013b;110(45):18121–18126. doi: 10.1073/pnas.1316449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings MA. The neurobiology of psychopathy: recent developments and new directions in research and treatment. CNS Spectr. 2015;20:200–206. doi: 10.1017/S1092852914000741. [DOI] [PubMed] [Google Scholar]
- 35.Custance D, Mayer J. Empathic-like responding by domestic dogs (Canis familiaris) to distress in humans: an exploratory study. Anim Cogn. 2012;15:851–859. doi: 10.1007/s10071-012-0510-1. [DOI] [PubMed] [Google Scholar]
- 36.Davitz JR, Mason DJ. Socially facilitated reduction of a fear response in rats. J Comp Physiol Psychol. 1955;48(3):149–51. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- 37.Darwin C. The Descent of man. D. Appleton and Company; 1871. [Google Scholar]
- 38.Decety J, Bartal IBA, Uzefovsky F, Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Phil Trans R Soc B. 2016;371:20150077. doi: 10.1098/rstb.2015.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derntl B, Hack RL, Kryspin-Exner I, Habel U. Association of menstrual cycle phase with the core components of empathy. Horm Behav. 2013;63:97–104. doi: 10.1016/j.yhbeh.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Derntl B, Michel TM, Prempeh P, Backes V, Finkelmeyer A, Schneider F, Habel U. Empathy in individuals clinically at risk for psychosis: brain and behaviour. Br J Psychiatry J Ment Sci. 2015;207:407–413. doi: 10.1192/bjp.bp.114.159004. [DOI] [PubMed] [Google Scholar]
- 43.de Waal FBM. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 44.de Wall FB, Aureli F. Conflict resolution and distress alleviation in monkeys and apes. Ann N Y Acad Sci. 1997;807:317–328. doi: 10.1111/j.1749-6632.1997.tb51929.x. [DOI] [PubMed] [Google Scholar]
- 45.Dunfield K, Kuhlmeier VA, O’Connell L, Kelley E. Examining the diversity of prosocial behavior: helping, sharing, and comforting in infancy. Infancy. 2011;16:227–247. doi: 10.1111/j.1532-7078.2010.00041.x. [DOI] [PubMed] [Google Scholar]
- 46.Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) J Autism Dev Disord. 2008;38:464–473. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- 47.Ebrahimi-Fakhari D, Sahin M. Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr Opin Neurol. 2015;28:91–102. doi: 10.1097/WCO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 48.Engel GL, Schmale AH. Conservation-withdrawal: a primary regulatory process for organismic homeostasis. Ciba Found Symp. 1972;8:57–75. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- 49.Escudero I, Johnstone M. Genetics of schizophrenia. Curr Psychiatry Rep. 2014;16:502. doi: 10.1007/s11920-014-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrari PF, Rizzolatti G. Mirror neuron research: the past and the future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130169. doi: 10.1098/rstb.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–13. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- 52.Fuzzo F, Matsumoto J, Kiyokawa Y, Takeuchi Y, Ono T, Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioral and neurophysiological evidence. Front Neurosci. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 54.Gallup AC, Church AM. The effects of intranasal oxytocin on contagious yawning. Neurosci Lett. 2015;607:13–16. doi: 10.1016/j.neulet.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M. Emotional contagion in mice: the role of familiarity. Behav Brain Res. 2014;263:16–21. doi: 10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Liencres C, Shamay-Tsoory SG, Brüne M. Towards a neuroscience of empathy: ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev. 2013;37:1537–1548. doi: 10.1016/j.neubiorev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Gore F, Schwartz EC, Salzman CD. Manipulating neural activity in physiologically classified neurons: triumphs and challenges. Philos Trans R Soc Lond B Biol Sci. 2015;370(1677):20140216. doi: 10.1098/rstb.2014.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- 59.Greene JT. Altruistic behavior in the albino rat. Psychon Sci. 1969;14:47–8. [Google Scholar]
- 60.Guapo VG, Graeff FG, Zani ACT, Labate CM, dos Reis RM, Del-Ben CM. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology. 2009;34:1087–1094. doi: 10.1016/j.psyneuen.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc Neurosci. 2015;10(5):474–8. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201(1):173–8. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzmán YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology (Berl) 2014;231:2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haker H, Kawohl W, Herwig U, Rössler W. Mirror neuron activity during contagious yawning--an fMRI study. Brain Imaging Behav. 2013;7:28–34. doi: 10.1007/s11682-012-9189-9. [DOI] [PubMed] [Google Scholar]
- 65.Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Anim Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- 67.Hatfield E, Cacioppe JT, Rapson RL. Emotional contagion. Cambridge University Press; New York: 1994. [Google Scholar]
- 68.Hay DF, Nash A, Pedersen J. Responses of sixmonth-olds to the distress of their peers. Child Dev. 1981;52:1071–1075. [PubMed] [Google Scholar]
- 69.Hernandez-Lallement J, van Wingerden M, Marx C, Srejic M, Kalenscher T. Rats prefer mutual rewards in a prosocial choice task. Front Neurosci. 2014;8:443. doi: 10.3389/fnins.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hickok G. Eight Problems for the Mirror Neuron Theory of Action Understanding in Monkeys and Humans. J Cogn Neurosci. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirata S. Chimpanzee social intelligence: selfishness, altruism, and the mother-infant bond. Primates J Primatol. 2009;50:3–11. doi: 10.1007/s10329-008-0122-1. [DOI] [PubMed] [Google Scholar]
- 72.Hishimura Y. Interactions with conspecific attenuate conditioned taste aversions in mice. Behav Processes. 2015;111:34–6. doi: 10.1016/j.beproc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Hodges TE, Green MR, Simone JJ, McCormick CM. Effects of social context on endocrine function and Zif268 expression in response to an acute stressor in adolescent and adult rats. Int J Dev Neurosci. 2014 Jun;35:25–34. doi: 10.1016/j.ijdevneu.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci Off J Soc Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huzard D, Mumby DG, Sandi C, Poirier GL, van der Kooij MA. The effects of extrinsic stress on somatic markers and behavior are dependent on animal housing conditions. Physiol Behav. 2015;151:238–45. doi: 10.1016/j.physbeh.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 76.Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Horm Behav. 2015 May;81:53–8. doi: 10.1016/j.yhbeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Insana SP, Wilson JH. Social buffering in rats: prolactin attenuation of active interaction. Psychol Rep. 2008;103(1):77–87. doi: 10.2466/pr0.103.1.77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 79.Ito W, Erisir A, Morozov A. Observation of Distressed Conspecific as a Model of Emotional Trauma Generates Silent Synapses in the Prefrontal-Amygdala Pathway and Enhances Fear Learning, but Ketamine Abolishes those Effects. Neuropsychopharmacology. 2015;40(11):2536–45. doi: 10.1038/npp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones CE, Monfils MH. Dominance status predicts social fear transmission in laboratory rats. Animal Cognition. 2016;19(6):1051–1069. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones CE, Riha PD, Gore AC, Monfils MH. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn. 2014;17:827–834. doi: 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kavaliers M, Colwell DD, Choleris E. Learning to fear and cope with a natural stressor: individually and socially acquired corticosterone and avoidance responses to biting flies. Horm Behav. 2003 Jan;43(1):99–107. doi: 10.1016/s0018-506x(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 84.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 85.Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, Shin HS. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016 Feb;15(2):231–42. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- 86.Kim BS, Lee J, Bang M, Seo BA, Khalid A, Jung MW, Jeon D. Differential regulation of observational fear and neural oscillations by serotonin and dopamine in the mouse anterior cingulate cortex. Psychopharmacology (Berl) 2014;231:4371–4381. doi: 10.1007/s00213-014-3581-7. [DOI] [PubMed] [Google Scholar]
- 87.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5(12):e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Mátyás F, Lee S, Acsády L, Shin HS. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci U S A. 2012;109:15497–15501. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiyokawa Y, Hiroshima S, Takeuchi Y, Mori Y. Social buffering reduces male rats’ behavioral and corticosterone responses to a conditioned stimulus. Horm Behav. 2014a;65(2):114–8. doi: 10.1016/j.yhbeh.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Kiyokawa Y, Honda A, Takeuchi Y, Mori Y. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav Brain Res. 2014b;267:189–93. doi: 10.1016/j.bbr.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 91.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- 92.Kiyokawa Y, Kodama Y, Takeuchi Y, Mori Y. Physical interaction is not necessary for the induction of housing-type social buffering of conditioned hyperthermia in male rats. Behav Brain Res. 2013;256:414–9. doi: 10.1016/j.bbr.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 93.Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neurosci. 2007;26:3606–3613. doi: 10.1111/j.1460-9568.2007.05969.x. [DOI] [PubMed] [Google Scholar]
- 94.Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2009;29(4):777–85. doi: 10.1111/j.1460-9568.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- 95.Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2012;36(10):3429–37. doi: 10.1111/j.1460-9568.2012.08257.x. [DOI] [PubMed] [Google Scholar]
- 96.Kleberg JL, Selbing I, Lundqvist D, Hofvander B, Olsson A. Spontaneous eye movements and trait empathy predict vicarious learning of fear. Int J Psychophysiol. 2015;98:577–583. doi: 10.1016/j.ijpsycho.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, Kaczmarek L. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A. 2012;109(42):17093–8. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem Cold Spring Harb N. 2010a;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2010b;16(8):486–93. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kubota N, Amemiya S, Yanagita S, Nishijima T, Kita I. Emotional stress evoked by classical fear conditioning induces yawning behavior in rats. Neurosci Lett. 2014;566:182–187. doi: 10.1016/j.neulet.2014.02.064. [DOI] [PubMed] [Google Scholar]
- 102.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 103.Langford DJ, Tuttle AH, Briscoe C, Harvey-Lewis C, Baran I, Gleeson P, Fischer DB, Buonora M, Sternberg WF, Mogil JS. Varying perceived social threat modulates pain behavior in male mice. J Pain Off J Am Pain Soc. 2011;12:125–132. doi: 10.1016/j.jpain.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Lau YC, Hinkley LBN, Bukshpun P, Strominger ZA, Wakahiro MLJ, Baron-Cohen S, Allison C, Auyeung B, Jeremy RJ, Nagarajan SS, Sherr EH, Marco EJ. Autism traits in individuals with agenesis of the corpus callosum. J Autism Dev Disord. 2013;43:1106–1118. doi: 10.1007/s10803-012-1653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee H, Noh J. Social exclusion intensifies anxiety-like behavior in adolescent rats. Behav Brain Res. 2015;284:112–7. doi: 10.1016/j.bbr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Leone A, Ferrari PF, Palagi E. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Sci Rep. 2014;4:4010. doi: 10.1038/srep04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Z, Lu YF, Li CL, Wang Y, Sun W, He T, Chen XF, Wang XL, Chen J. Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain. 2014;155:1253–1261. doi: 10.1016/j.pain.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 108.Light S, Zahn-Waxler C. Empathy: From bench to bedside. 2011. 7 Nature and Forms of Empathy in the First Years of Life; p. 109. [Google Scholar]
- 109.Liu X, Wu R, Tai F, Ma L, Wei B, Yang X, Zhang X, Jia R. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 2013 Mar 28;1502:71–80. doi: 10.1016/j.brainres.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 110.Márquez C, Rennie SM, Costa DF, Moita MA. Prosocial Choice in Rats Depends on Food-Seeking Behavior Displayed by Recipients. Curr Biol CB. 2015;25:1736–1745. doi: 10.1016/j.cub.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 111.Marsh AA. Understanding amygdala responsiveness to fearful expressions through the lens of psychopathy and altruism. J Neurosci Res. 2015 doi: 10.1002/jnr.23668. [DOI] [PubMed] [Google Scholar]
- 112.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, Slepian PM, Trost Z, Bartz JA, Sapolsky RM, Sternberg WF, Levitin DJ, Mogil JS. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol CB. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 113.Masserman JH, Wechkin S, Terris W. “Altruistic” behavior in rhesus monkeys. Am J Psychiatry. 1964;121:584–585. doi: 10.1176/ajp.121.6.584. [DOI] [PubMed] [Google Scholar]
- 114.Meyza K, Nikolaev T, Kondrakiewicz K, Blanchard DC, Blanchard RJ, Knapska E. Neuronal correlates of asocial behavior in a BTBR T (+) Itpr3(tf)/J mouse model of autism. Front Behav Neurosci. 2015;9:199. doi: 10.3389/fnbeh.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mikami K, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering enhances extinction of conditioned fear responses in male rats. Physiol Behav. 2016;163:123–8. doi: 10.1016/j.physbeh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 116.Mikosz M, Nowak A, Werka T, Knapska E. Sex differences in social modulation of learning in rats. Sci Rep. 2015;5:18114. doi: 10.1038/srep18114. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller ML, Gallup AC, Vogel AR, Vicario SM, Clark AB. Evidence for contagious behaviors in budgerigars (Melopsittacus undulatus): an observational study of yawning and stretching. Behav Processes. 2012;89:264–270. doi: 10.1016/j.beproc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 118.Mora S, Dussaubat N, Diaz-viz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1997;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 119.Morgan JT, Barger N, Amaral DG, Schumann CM. Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PloS One. 2014;9:e110356. doi: 10.1371/journal.pone.0110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moyaho A, Rivas-Zamudio X, Ugarte A, Eguibar JR, Valencia J. Smell facilitates auditory contagious yawning in stranger rats. Anim Cogn. 2015;18:279–290. doi: 10.1007/s10071-014-0798-0. [DOI] [PubMed] [Google Scholar]
- 121.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20(8):750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakamura K, Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y. The strain of an accompanying conspecific affects the efficacy of social buffering in male rats. Horm Behav. 2016;82:72–7. doi: 10.1016/j.yhbeh.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 123.Nakayasu T, Kato K. Is full physical contact necessary for buffering effects of pair housing on social stress in rats? Behav Processes. 2011;86(2):230–5. doi: 10.1016/j.beproc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Narvaez D. The emotional foundations of high moral intelligence. New Dir Child Adolesc Dev. 2010;2010:77–94. doi: 10.1002/cd.276. [DOI] [PubMed] [Google Scholar]
- 125.Nowak A, Werka T, Knapska E. Social modulation in extinction of aversive memories. Behav Brain Res. 2013;238:200–205. doi: 10.1016/j.bbr.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 126.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Osborne LR. Animal models of Williams syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:209–219. doi: 10.1002/ajmg.c.30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Panksepp JB, Lahvis GP. Differential influence of social versus isolate housing on vicarious learning in adolescent mice. Behav Neurosci. 2016;130(2):206–11. doi: 10.1037/bne0000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries. Ann N Y Acad Sci. 1997 Jan 15;:807, 78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- 131.Panksepp J, Panksepp JB. Toward a cross-species understanding of empathy. Trends Neurosci. 2013;36:489–496. doi: 10.1016/j.tins.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pearson R, Lewis MB. Fear recognition across the menstrual cycle. Horm Behav. 2005;47:267–271. doi: 10.1016/j.yhbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Platek SM, Critton SR, Myers TE, Gallup GG. Contagious yawning: the role of self-awareness and mental state attribution. Brain Res Cogn Brain Res. 2003;17:223–227. doi: 10.1016/s0926-6410(03)00109-5. [DOI] [PubMed] [Google Scholar]
- 134.Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 135.Preston SD. The origins of altruism in offspring care. Psychol Bull. 2013;139:1305–1341. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- 136.Provine RR, Tate BC, Geldmacher LL. Yawning: no effect of 3–5% CO2, 100% O2, and exercise. Behav Neural Biol. 1987;48:382–393. doi: 10.1016/s0163-1047(87)90944-7. [DOI] [PubMed] [Google Scholar]
- 137.Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, Tonegawa S. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522(7556):335–9. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW, Josselyn SA. Competition between engrams influences fear memory formation and recall. Science. 2016;353(6297):383–7. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Riby DM, Back E. Can individuals with Williams syndrome interpret mental states from moving faces? Neuropsychologia. 2010;48:1914–1922. doi: 10.1016/j.neuropsychologia.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 140.Rice GE, Gainer P. “Altruism” in the albino rat. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 141.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 142.Romero T, Ito M, Saito A, Hasegawa T. Social modulation of contagious yawning in wolves. PloS One. 2014;9:e105963. doi: 10.1371/journal.pone.0105963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roth-Hanania R, Davidov M, Zahn-Waxler C. Empathy development from 8 to 16 months: early signs of concern for others. Infant Behav Dev. 2011;34:447–458. doi: 10.1016/j.infbeh.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 144.Rueckert L, Naybar N. Gender differences in empathy: the role of the right hemisphere. Brain Cogn. 2008;67:162–167. doi: 10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Rutte C, Taborsky M. Generalized reciprocity in rats. PLoS Biol. 2007;5:e196. doi: 10.1371/journal.pbio.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 2015;10(5):512–26. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sanders J, Mayford M, Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS One. 2013;8:e74609. doi: 10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18:1039–1047. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- 149.Schneeberger K, Dietz M, Taborsky M. Reciprocal cooperation between unrelated rats depends on cost to donor and benefit to recipient. BMC Evol Biol. 2012;12:41. doi: 10.1186/1471-2148-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schuster R, Perelberg A. Why cooperate? An economic perspective is not enough. Behav Processes. 2004;66:261–277. doi: 10.1016/j.beproc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 151.Schulte-Rüther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42:393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- 152.Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, Slotnick B. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim Cogn. 2014;17(3):609–18. doi: 10.1007/s10071-013-0692-1. [DOI] [PubMed] [Google Scholar]
- 153.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 154.Smith A. The Empathy Imbalance Hypothesis of Autism: A Theoretical Approach to Cognitive and Emotional Empathy in Autistic Development. Psychol Rec. 2009:59. [Google Scholar]
- 155.Smith A. Cognitive Empathy and Emotional Empathy in Human Behavior and Evolution. Psychol Rec. 2006;56:3. [Google Scholar]
- 156.Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav Neurosci. 2015;129:389–398. doi: 10.1037/bne0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stanley DA, Adolphs R. Toward a neural basis for social behavior. Neuron. 2013;80:816–826. doi: 10.1016/j.neuron.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;4:231–249. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol. 2014;29:187–193. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 160.Sucksmith E, Allison C, Baron-Cohen S, Chakrabarti B, Hoekstra RA. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia. 2013;51:98–105. doi: 10.1016/j.neuropsychologia.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Szklarczyk K, Korostynski M, Golda S, Solecki W, Przewlocki R. Genotype-dependent consequences of traumatic stress in four inbred mouse strains. Genes Brain Behav. 2012;11:977–985. doi: 10.1111/j.1601-183X.2012.00850.x. [DOI] [PubMed] [Google Scholar]
- 162.Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav. 1992;52(3):493–8. doi: 10.1016/0031-9384(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 164.Telzer EH, Humphreys KL, Shapiro M, Tottenham N. Amygdala sensitivity to race is not present in childhood but emerges over adolescence. J Cogn Neurosci. 2013;25:234–244. doi: 10.1162/jocn_a_00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24(6):639–56. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 167.Tinbergen N. On aims and methods of ethology. Zeitschrift fur Tierpsychologie. 1963;20:410–433. [Google Scholar]
- 168.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317–31. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 169.Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing a neurophysiological study. Neuron. 2001;31(1):155–65. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 170.Watanabe S. Social factors modulate restraint stress induced hyperthermia in mice. Brain Res. 2015;1624:134–9. doi: 10.1016/j.brainres.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 171.Watanabe S. Distress of mice induces approach behavior but has an aversive property for conspecifics. Behav Processes. 2012;90(2):167–73. doi: 10.1016/j.beproc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 172.Watanabe S. Empathy and reversed empathy of stress in mice. PLoS One. 2011;6(8):e23357. doi: 10.1371/journal.pone.0023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 174.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83(3):722–35. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 175.Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behav Brain Res. 2013;242:142–149. doi: 10.1016/j.bbr.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zahn-Waxler C, Cole PM, Welsh JD, Fox NA. Psychophysiological correlates of empathy and prosocial behaviors in preschool children with behavior problems. Dev Psychopathol. 1995;7:27–48. [Google Scholar]
- 177.Zuo X, Han S. Cultural experiences reduce racial bias in neural responses to others’ suffering. Cult Brain. 2013;1:34–46. [Google Scholar]
- 178.Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Development of concern for others. Dev Psychol. 1992;28:126–136. [Google Scholar]