Abstract
What an animal needs to learn to survive is altered dramatically as they change from dependence on the parent for protection to independence and reliance on self-defense. This transition occurs in most altricial animals, but our understanding of the behavioral neurobiology has mostly relied on the infant rat. The transformation from dependence to independence occurs over three weeks in pups and is accompanied by complex changes in responses to both natural and learned threats and the supporting neural circuitry. Overall, in early life, the threat system is quiescent and learning is biased towards acquiring attachment related behaviors to support attachment to the caregiver and proximity seeking. Caregiver-associated cues learned in infancy have the ability to provide a sense of safety throughout lifetime. This attachment/safety system is activated by learning involving presumably pleasurable stimuli (food, warmth) but also painful stimuli (tailpinch, moderate shock). At about the midway point to independence, pups begin to have access to the adult-like amygdala-dependent threat system and amygdala-dependent responses to natural dangers such as predator odors. However, pups have the ability to switch between the infant and adult-like system, which is controlled by maternal presence and modification of stress hormones. Specifically, if the pup is alone, it will learn fear but if with the mother it will learn attachment (10–15 days of age). As pups begin to approach weaning, pups lose access to the attachment system and rely only on the amygdala-dependent threat system. However, pups learning system is complex and exhibits flexibility that enables the mother to override the control of the attachment circuit, since newborn pups may acquire threat responses from the mother expressing fear in their presence. Together, these data suggest that the development of pups’ threat learning system is not only dependent upon maturation of the amygdala, but it is also exquisitely controlled by the environment. Most notably the mother can switch pup learning between attachment to threat learning in a moment’s notice. This enables the mother to navigate pup’s learning about the world and what is threatening and what is safe.
Keywords: attachment, threat, fear, trauma, sensitive period, critical period, amygdala, development, stress, infant, maternal care, brain development
Graphical Abstract
Introduction
As we assess the development of learning it is critical to understand that the young brain is not an immature version of the adult brain. Rather it is specifically constructed to ensure learning is age-specific to accommodate the changing ecological niche typically associated with a temporally constrained learning period referred to as a sensitive period. A child learns about the world from the caregiver, including the fundamentally important ability to navigate the complex world by safety and threat signals. However, what is safe and what is threatening changes during development, suggesting the supporting learning circuitry must also change. For example, altricial infants such as humans and rodents might perceive separation from the caregiver as a danger signal and proximity to the caregiver as safety. This would require a learning system where learning the characteristics of the caregiver and expressing approach responses to the caregiver would be beneficial, while removal of caregiver cues might be defined as a threat. With maturation, and the ability of the infant to crawl away from the caregiver for brief time periods might require adding a more sophisticated learning network and the emergence of an ability to identify proximal dangers but evoke a behavioral response of return to the nest and engagement of the parent’s defense system. This would require retention of the caregiver as a safety signal. Finally, as the preparation for independence progresses, one might expect a system where dependence on the parent for protection would wane combined with the emergence of behavioral expression that ensures a flexible self-defense system to navigate a complex environment. Indeed, emerging evidence indicates that the learning neural network of the young brain is not an immature version of the adult brain: it morphs to ensure learning is age-specific to accommodate the changing ecological niche as age-specific demands change during the transition to independence.
This major transition from complete dependence on the caregiver to complete independence occurs in most altricial species, including humans, nonhuman primates and rodents. This transition occurs in three short weeks in rat pups and is accompanied by complex changes in responses to both natural and learned threats, and safety, with corresponding changes in the supporting neural circuitry. Development continues after weaning and considerable rapid learning must occur to further prepare for the emergence of adulthood and reproduction in a little more than one month later. These transition and periods of rapid learning all occur as the brain continues to develop and be shaped by environmental experiences.
One key concept for understanding infant learning is that learning in early life is biased towards attachment learning. While attachment has been considered innate, evidence now indicates a high degree of learning that activates a biologically predisposed attachment circuit used to initiate and maintain this bond. The importance of learning within attachment in humans is indicated by the rapid attachment between young babies and nonbiological parents of either gender. This is further supported by situations where atypical caregiver behaviors (i.e. neglect, abuse by the caregiver) within biological and nonbiological parent-infant dyads emerge, yet attachment occurs in the offspring, suggesting a learning system with unique features that support learning bond formation even with trauma. Here we focus on threat and safety learning within an age-specific behavioral neurobiology from the infant’s perspective, which must begin with the biological demands of infancy – to attach and remain attached to the caregiver until independent self-defense emerges.
Historical perspective: Early life safety learning within attachment and later emerging threat learning
An attachment neural circuitry for humans was first proposed in Bowlby’s Attachment Theory, which relied heavily on integration of research on early learning about the caregiver in avian imprinting (Ainsworth, 1969; Bowlby, 1977, 1978, 1984). In imprinting, hatchling birds have a temporally limited access to learning about the caregiver (a sensitive period for imprinting). During the sensitive period for imprinting, the chicks will learn to follow a moving object encountered in its immediate environment of the nest, which is typically the caregiver. However, the caregiver can be replaced by a human, box, or a stuffed bird and imprinting still occurs, which indicates the importance of learning and the wide range of stimuli that can be identified and learned as the caregiver. Imprinting also provides an illustration of how this early learning produces unique learned responses, which includes proximity seeking. The unique learning and unique expression of proximity seeking ends as the sensitive period for imprinting ends (Bolhuis & Honey, 1998). Overall, this work suggested that learning to attach to the caregiver is learned, but once learned, it engaged a biologically predetermined learned action of following the caregiver. Considerable behavioral neurobiological research has documented this imprinting learning circuit (Bateson, 2015; Solomonia & McCabe, 2015; Bolhius et al., 2015; Bolhuis & Honey, 1998; Gottlieb, 1965; Hoffman & DePaulo, 1977). Importantly, what happens to the chick during this imprinting process does not follow the traditional learning rules: providing these chicks with electric shock during imprinting still results in imprinting supporting a strong attachment (Rajecki et al., 1978; Kovach & Hess, 1963). As will be reviewed below, this phenomenon is a characteristic of attachment learning seen in other species, including rodents, nonhuman primates and abused children (see: Fig. 1 for a rodent model of a dominance of attachment learning and an emergence of threat learning). This paradoxical feature of attachment learning was noted by Bowlby, where poor quality of care, including an abusive caregiver, still supports attachment formation (Walden & Ogan, 1988).
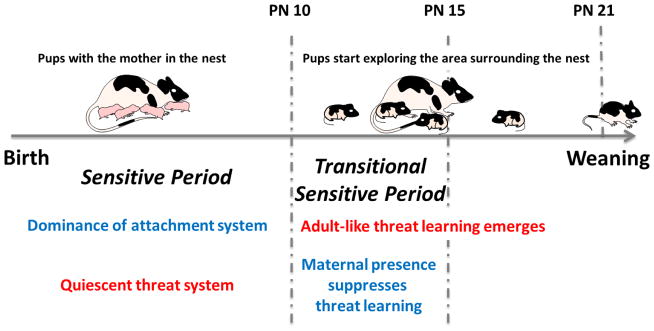
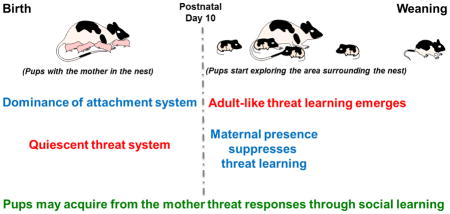
Fig. 1. Transition from the dominance of attachment learning to adult-like threat learning.
Until around postnatal day (PN) 10 the threat system is quiescent and learning is biased towards learning attachment (sensitive period). Around PN 10, adult-like fear learning emerges. However, during a transient period between PN10 and PN 15 referred to as the Transitional Sensitive Period, pups can switch between attachment learning and threat learning based on maternal presence: if the mother is present odor-pain produces attachment learning and if pups are alone, amygdala-dependent threat is learned. At PN16 and older, pups no longer have access to the attachment learning system, even if the mother is present.
Neurobiology of attachment learning: odor learning and safety
Infants must learn to identify and remember the caregiver by learning about the caregiver’s sights, voice, touch and odor. Previous work provides some insight into the mechanisms that support this learning in humans. For example, in humans and rodents, the mother’s natural odors and food odors experienced in utero are preferred after birth (Mennella 2014; Cooke & Fildes, 2011; Abate et al., 2008; Mennella et al., 2001; Varendi et al., 1996; Pedersen & Blass, 1982), although lack of subsequent exposure to these odors decrease this preference, and novel postnatal odors (natural, perfume) of the mother and other nonrelated caregivers are quickly learned (Al Ain et al., 2016; Schaal, 2015; Mennella et al., 2011; Schaal et al., 2009; Mennella & Beauchamp, 1999). Indeed, in newly born human infants a few minutes of simply pairing a novel odor with tactile stimulation on the first day of life causes that odor to be preferred but also elicits head turns towards that odor and mouthing (Sullivan et al., 1991). The mother’s odor and other characteristics of the mother (face, touch, and voice) can take on qualities that help regulate the infant’s behavior and physiology (Hostinar et al., 2014). The caregiver also acquires the ability to signal safety for the infant, or serve as a safe haven, which is well-documented by human and animal studies demonstrating that under threat infants rapidly return to the caregiver (Ainsworth, 1969; Raineki et al., 2010d; Sanchez et al., 2015), whose presence, in turn, decreases the infant’s stress hormone levels (Hostinar et al., 2014; Gunnar et al., 2015) and the amygdala activity (Gee, et al. 2014).
A similar phenomenon exists in infant rodents, which permits the exploration of neural mechanisms for this learning and provides insights for these processes in humans. In infant rat pups, pairing a novel odor with a reward quickly produces a learned preference for that odor, but this odor also becomes a characteristic of the mother and takes on the ability to regulate the infant’s physiology and behavior similarly to the natural maternal odor (Hostinar et al., 2014; Gunnar et al., 2015; Sanchez et al., 2015; Sullivan and Perry, 2015; Hennessy et al., 2009; Hennessy et al., 2015}. This learning, while similar to classical conditioning in many ways, seems to have some unique features, including the brain areas involved and a unique dependence upon the neurotransmitter norepinephrine (NE) (Sullivan et al., 1991, 1992, 2010; Yuan et al., 2000; Bordner and Spear, 2006). In an incredibly simplistic circuit, maternal stimulation of pups, including a gentle touch or stepping on pups, or an experimentally applied squirt of milk, a tailpinch or an electric shock, all have the common feature of activating the locus coeruleus (LC) (Moriceau et al. 2009; Nakamura et al. 1987; Sullivan et al., 2000b). The LC is a major source of NE within the brain and the sole source of NE to the olfactory bulb (see: Fig. 2).
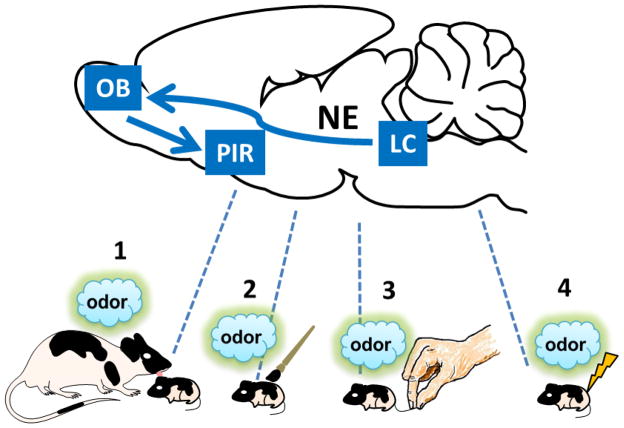
Fig. 2. Rapid Attachment Learning in Early Infancy.
Newborn rat pups rapidly learn a new maternal odor. This learning is mostly activated by presumably pleasurable stimuli, such as maternal licking (1) or gentle tactile stimulation with a paintbrush (2) but also by painful stimuli, such as tailpinch (3) or moderate electric shock (4). Attachment learning in pups is supported by a simplistic neural circuit that requires the pairing of the novel (maternal) odor with increased locus coeruleus (LC) norepinephrine (NE) release into the olfactory bulb (OB). Attachment learning-induced synaptic plasticity has been identified in the OB and the piriform cortex (PIR). The functional emergence of recurrent collaterals in the LC around postnatal day (PND) 10 allows stimulation of α2 presynaptic receptors, which downregulates the LC noradrenergic release and results in insufficient NE levels in the OB to support the synaptic plasticity necessary for learning about maternal odor.
Importantly, the young infant LC is different from the older infant and adult LC: stimulation of the infant LC releases large amounts of NE, and the LC fails to habituate to repeated stimulation and shows extensive electrical coupling to synchronize LC activity (Christie, 1997; Christie & Jelinek, 1993; Christie, Williams, & North, 1989; Nakamura et al., 1987; Williams & Marshall, 1987; Winzer-Serhan et al., 1996). This unique characteristic of the infant’s LC results in copious amounts of NE release that continue each time the pup is repeatedly stimulated by the mother. The olfactory bulb target of the LC, which is critical for learning the maternal odor, also shows responses to NE that facilitate neural plasticity (Pandipati & Schoppa, 2012; Wilson & Leon, 1988; Yuan et al., 2000, 2002; Okutani et al., 1999). The LC appears to take on adult-like features at 10 days old, just as the sensitive period for attachment ends (Nakamura et al., 1987; Winzer-Serhan et al., 1996). This olfactory bulb NE has been shown to be critical in memory formation during a sensitive period in rat pups {Pandipati & Schoppa, 2012; Yuan et al., 2014; Sullivan et al., 2000b); Todrank et al., 2011; Sullivan et al., 1992). Although we are unsure if the mechanism supporting this learning in human infants is the same as in rodents, there is some suggestion that NE’s role in attachment is a phylogenetically conserved system. NE has been shown to play a critical role in social bond formation throughout development in numerous species (Nelson & Panksepp, 1998; Numan & Young, 2016; Kaba & Huang, 2005; Corona & Levy, 2015; Calamandrei et al., 1992), as well as in modulation of learning throughout the lifespan (McGaugh, 2015; Maity et al., 2015; Shea et al., 2008; Gold, 2015). While children have high levels of NE during the first two years of life, the role of NE or any other hormone or neurotransmitter in early life attachment in humans is yet to be demonstrated, as it has been established by the rodent literature (Slotkin & Langercrantz, 1986).
Another feature of young pup learning that is unique is that the odor memory is limited to a few key structures within the brain: primarily the olfactory bulb and the piriform (olfactory) cortex (Modaresi et al., 2016; Raineki et al., 2009; Roth & Sullivan, 2006; Sullivan et al., 1990; Yuan et al., 2002). These regions are also important in the adult brain and play a role in assigning the hedonic value to a learned odor (Haberly, 2001; Schwob & Price, 1984; Swanson & Petrovich, 1998; Wilson & Stevenson, 2003). These brain sites appear to play a similar role in infancy (Moriceau & Sullivan, 2006b; Moriceau et al., 2006; Roth & Sullivan, 2005b). It is important to note that both natural maternal odor and a learned artificial maternal odor generate the same responses from the olfactory bulb (Raineki et al., 2010d; Roth & Sullivan, 2005b).
Sensitive period neurobiology of pain related/abusive attachment: Why attachment learning occurs with pain
During the sensitive period for attachment, even painful interactions with the mother or pain delivered by the experimenter still support odor preference learning {Roth et al., 2005; Raineki et al., 2015; Raineki et al., 2010b; Sullivan et al., 2000). The mechanism underlying attachment learning with pain is relatively simple: the amygdala, the brain region supporting fear learning (Maren & Fanselow, 1996; Phelps & LeDoux, 2005, Debiec & LeDoux, 2009; Janak & Tye, 2015) is not mature enough to reinforce this learning until pups are 10 days old. Thus, when pups experience odor-shock or odor-abusive mother pairing, the locus coeruleus NE attachment learning system is activated but it does not engage the amygdala. Specifically, pups feel pain (Barr, 1995; Collier & Bolles, 1980; Emerich et al., 1985; Stehouwer & Campbell, 1978), and this information arrives in the amygdala, yet the amygdala fails to show learning induced plasticity until pups reach 10 days of age (Barr et al., 2009; Moriceau et al., 2006; Sullivan et al., 2000). Abuse-related attachment formation is phylogenetically conserved and occurs in a number of species, including chicks that form attachments after being shocked during imprinting (Hess, 1962; Rajecki et al., 1978; Salzen, 1970), dogs (Stanley, 1962) and monkeys raised with a wire surrogate that inflict pain (Harlow & Harlow, 1965). More recently, this abuse-related attachment has been modeled in nonhuman primates and again shows that infants learn and retain strong preference for the abusive caregiver (Maestripieri et al., 1999; O’Connor & Cameron, 2006; Sanchez et al. 2001; Suomi, 2003). This broad species representation of abuse-related attachment was noted by Bowlby in his Attachment Theory (Bowlby, 1977, 1984; Harlow & Harlow, 1965; Harmon et al. 1984). Findings from human studies in adults suggest that abuser-related bonding depends on a number of psychosocial factors including a history of abuse in childhood, type and duration of abuse, gender, relationship with the perpetrator, social isolation, as well as accompanying cognitive patterns and distortions (Karantzas et al., 2016; Canton-Cortes et al., 2015; McCloskey, 2013; Nicholas, 2013; Julich, 2005), and thus likely involves a number of neural mechanisms outside of the amygdala threat processing circuitry. While it is unclear whether humans’ and other species’ failure to learn aversions to their abusive caregiver is also caused by lack of amygdala functioning, amygdala maturation appears delayed in many species, including humans (Gee et al., 2013; Goksan et al., 2015).
Pups during the sensitive period (<PN10) also lack responses to natural stimuli that elicit defense responses in adults, such as predator odors {Moriceau et al., 2004; Takahashi et al., 1991; Takahashi & Rubin, 1993; Wiedenmayer et al., 2003). Yet, it is important to note that adult-like threat responses are delayed in many species until they are ecologically relevant when the animal emerges from the nest or borrow (Pongrácz & Altbäcker, 2000; Putman et al., 2015). However, this does not mean that the very young animal has no defensive responses, rather it means that the adult system has not emerged. Indeed, responses that increase survival within a threatening context do exist in very young animals across species, but they typically involve age-specific stimulus-responses uniquely adapted to the early life niche of the nest or borrow. For example, some birds will be quieted (become still) by vibrations similar to that which would occur if a predator landed near the nest and rat pups will decrease activity in response to intrusion of an unfamiliar adult. These quieting responses typically involve neural circuitry distinct from adult defensive behaviors.
Maternal Control: Turning on and off the sensitive period for attachment
While the sensitive period for attachment was initially thought to end at PN10, additional research indicated that the mother is capable of rapidly turning on and off pups sensitive period. Indeed, pups’ attachment sensitive period was showing considerable flexibility, and it’s remarkable control by maternal presence and the mother’s emotional state provided a rapid method for further defining what pups learn. This is counter to our traditional view of sensitive period as strict temporally defined developmental windows of learning. While the environment is capable of changing the duration of the sensitive period, (Raineki et al., 2010b; Callaghan et al., 2013), there was no previous demonstration of any event or social partner rapidly switching on and off any sensitive period during development. Here we review two distinct situations where the mother can control switching on/off the sensitive period for attachment: one involves the mother turning on the sensitive period for attachment in older pups and suppressing threat learning (illustrated in Fig. 3) and the other involves the mother rapidly turning on the threat learning in very young sensitive period pups and suppressing the attachment system (illustrated in Fig. 5).
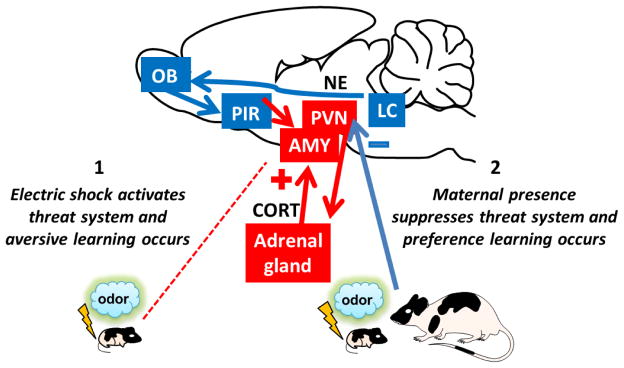
Fig. 3. Transitional Sensitive Period for Attachment Learning (PN 10–15).
As pups mature (starting around PN 10) and begin exploring the area surrounding the nest, threat learning emerges. Away from the mother, when exposed to an innately noxious event, such as mild electric shock, pup’s threat processing system is activated (1). A response to threat involves the amygdala (AMY) and the hypothalamus-pituitary-adrenal gland (HPA) axis activation resulting in the release of the stress hormone corticosterone (CORT), which modulates synaptic plasticity and supports fear/aversion learning in the AMY. However, until around PN 15, maternal presence suppresses threat-induced HPA axis activation by blocking NE action in the paraventricular nucleus of the hypothalamus (PVN) and prevents threat/aversion learning enabling attachment/preference learning (2). Although, maternal stress buffering effects continue, from PN 16 on, maternal presence loses the ability to completely abolish amygdala-dependent fear learning.
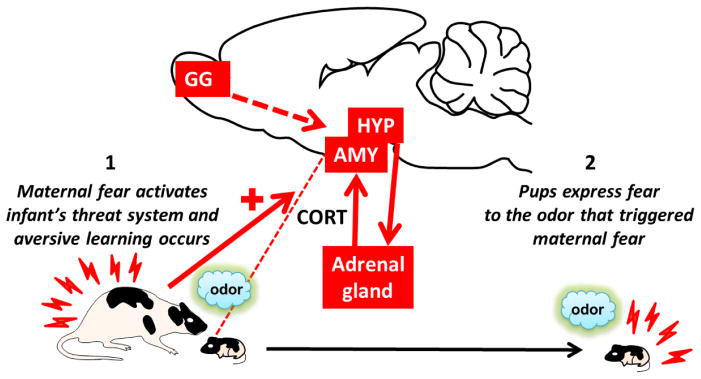
Fig. 5. Social Transmission of Fear from Mother to Infant.
A frightened mother activates in pups a number of areas involved in processing threat and stress, including the amygdala (AMY) and hypothalamus (HYP), and leads to the rise of CORT levels (1). Pups with a mother expressing fear to the previously conditioned odor, acquire fear responses to this odor, a fear transmission that involves alarm chemosignaling processing areas (including Grueneberg ganglion, GG) (2).
Here we present an example of the mother turning on the sensitive period for attachment and suppresses threat learning in older pups (see Fig. 3). Specifically, our experiments indicated that the mother was opening and closing this extended (transitional) sensitive period by manipulating pup’s stress hormone, corticosterone (CORT) levels (Moriceau & Sullivan, 2006; Shionoya et al., 2007). The clue to the role of CORT in controlling sensitive period termination came from a diverse literature. The first clue was that increasing systemic CORT levels in very young pups induced precocious expression of fear to predator odor, which is typically delayed until 10 days old (Takahashi & Rubin 1993). The second clue was provided by the literature documenting that pups younger than 10 days old have a stress hyporesponsive period (SHRP), which means that the hypothalamic-pituitary-adrenal (HPA) axis does not respond to most stressors (including shock, tailpinch) (Grino et al., 1994; Henning, 1978; Levine, 1962; Rosenfeld et al. 1992; Walker et al., 1986; Dallman, 2000; Stanton & Levine, 1985). The final clue came from the social buffering literature. Social buffering is a phenomenon where a significant social figure blocks activation of the HPA axis, a process that occurs across species and development. As pups leave the developmental period of the SHRP, shock and other painful stimuli increase CORT, social buffering of stress and fear emerges (Levine et al., 1985; Ditzen & Heinrichs, 2014; Gee et al., 2014; Hennessy et al., 2009; Hennessy et al., 2015; Hostinar & Gunnar, 2013; Kikusui et al., 2006; Moriceau & Sullivan, 2006; Nachmias et al., 1996; Sanchez, 2006; Sanchez et al., 2015; Shionoya et al., 2007; Stanton & Levine, 1990; Takahashi et al., 2013). One of the most powerful effects of social buffering is the mother’s social buffering of offspring (Gee et al., 2014; Hennessy et al., 2009; Hostinar et al., 2014; Kikusui et al., 2006; Levine, 2001; McCormack et al., 2009; Sanchez, 2006; Sanchez et al., 2015; Stanton & Levine, 1990; Suchecki et al., 1995; Sullivan & Perry, 2015; van Oers et al., 1998). In social buffering, a significant social figure typically attenuates stress hormone release throughout development, although in early life the mother can completely block release of CORT (Shionoya et al., 2007; Suchecki et al., 1993; Stanton et al., 1987; Moriceau et al., 2006) (Fig. 3). Animal models suggest the mother blocks the hypothalamic-pituitary-adrenal (HPA) axis at the level of the hypothalamic paraventricular nucleus (PVN) NE (Moriceau et al., 2006; Shionoya et al., 2007). Specifically, previous work from our group showed that maternal presence during odor-shock conditioning on PND 12 attenuated both infant’s PVN neural activity and PVN NE levels, and intra-PVN infusions of the α1-adrenergic receptor antagonist prazosin blocked fear conditioning with maternal absence, while intra-PVN α1-adrenergic receptor agonist phenylephrine permitted fear conditioning with maternal presence (Shionoya et al., 2007).
CORT control of the sensitive period learning produces profound effects on pup learning about fear but can also turn off the attachment learning circuit. Specifically, increasing CORT during the sensitive period, either naturally via mother’s milk (stressed mother), early separation from the mother or by rearing pups with an abusive caregiver, or pharmacologically (systemic injections or by intra-amygdala microinfusions), can prematurely end sensitive period learning and enable pups to learn amygdala-dependent fear. This enables the amygdala to undergo learning-related plasticity and allows pups to learn fear and avoidance (Moriceau et al., 2004; Moriceau et al., 2009; Moriceau & Sullivan, 2004, 2006; Callaghan & Richardson, 2011, 2012; Cowan et al., 2013). This indicates the young amygdala receives the shock and odor information and is capable of supporting fear learning, but it requires CORT to engage the mechanisms of learning plasticity (Thompson et al., 2008).
Conversely, a reduction of either systemic CORT (injection or pharmacological intervention at the level of the hypothalamus) or blockade of CORT limited to the amygdala disrupts learned fear/avoidance but also reinstates the sensitive period learning to induce a learned preference (Sullivan & Holman, 2000; Moriceau et al., 2006; Moriceau & Sullivan, 2006; Shionoya et al., 2007). The ability to turn on and off fear learning mirrors the ability of CORT to turn on and off fear expression to predator odor in infant rats, a threat behavior that also emerges at PN10 (Wiedenmayer et al., 2003; Moriceau et al., 2004; Takahashi & Rubin, 1993; Takahashi et al., 1991). In terms of fear and safety learning, between PN10 and PN15, the “transitional sensitive period”, maternal presence can act as a switch to turn off the fear circuitry and turn on the attachment circuitry during odor-shock conditioning simply by blocking CORT (Moriceau & Sullivan, 2006). The ability of CORT to switch between pup’s fear and attachment safety learning ends at PN15 (Upton & Sullivan, 2010).
Together, these data indicate that learning mechanisms in early infancy favor attachment/preference learning and threat/aversive learning in newborns remains quiescent (Fig. 1). This suppression of threat system, notable during the transitional sensitive period is controlled by a caregiver whose presence, at default, buffers infant’s stress and threat responses and prevents fear learning (Fig. 3).
Development of associative threat learning
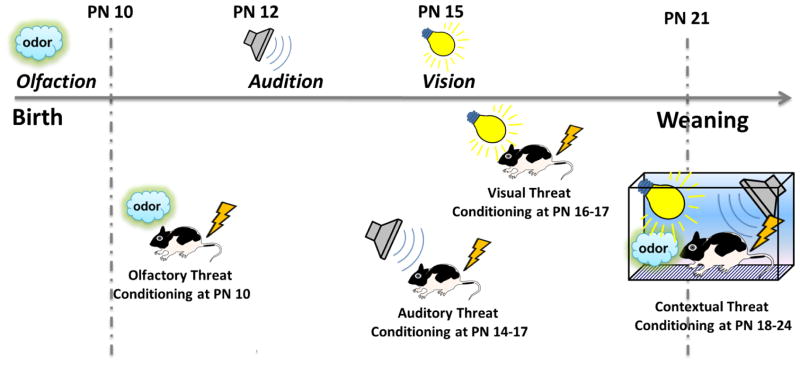
As pups transition towards weaning, their trips outside the nest become longer in distance and duration. During these first days of out-of-the-nest explorations, pups are exposed to expeditiously increasing number and variety of environmental cues which poses for them a demand to process their new experiences, including encounters with novel environmental threats. Rapid neural development and the emergence of new sensory, motor and cognitive functions advance pups’ ability to learn. The development of associative learning depends on the emergence and maturation of sensory systems, output sites directly controlling threat responses, and the connections of both with the threat system, including the main player in threat processing, the amygdala (Deal et al., 2016; Debiec & LeDoux, 2009; Hunt & Campbell, 1997; Rudy, 1993). Previous studies in rodents established that associative learning emerges in a sensory-specific sequence and that an emergence of a sensory modality precedes the occurrence of learning which involves this modality (Richardson et al., 2000; Hunt & Campbell, 1997; Moye & Rudy; 1985; Hyson & Rudy, 1984; Alberts, 1984). From birth on, pups have functional olfactory and tactile modalities which during the first postnatal days support mainly attachment learning (Fig. 2), and, starting on PN 10, also support threat learning (Fig. 3). By the end of the second postnatal week, pups are able to reflexively respond to acoustic stimuli (Hyson & Rudy, 1984). Auditory associative learning begins to emerge at PN 14 (Hyson & Rudy, 1984) and auditory fear conditioning becomes adult-like by PN 17–18 (Deal et al., 2016; Landers & Sullivan, 2012; Hunt et al., 1994; Rudy, 1993) (Fig. 4). At PN 15, pups are capable of detecting visual stimuli, yet visual fear conditioning starts emerging as early as on PN 16–17 (Hunt & Richardson, 2010; Moye & Rudy, 1985) (Fig. 4).
Fig. 4. Sensory Development and the Emergence of Threat Conditioning.
Olfactory system is functional at birth and olfactory fear conditioning emerges at PN 10. At PN 12 auditory system becomes functional and auditory fear conditioning emerges between PN 14–17. At PN 15, pups can effectively process visual stimuli and visual fear conditioning emerges at PN 16–17. Functional contextual fear conditioning emerges during the periweaning period (PN 18–24).
As weaning approaches at PN 21, pups are able to discriminate and learn a variety of stimuli from distinct modalities: olfactory, tactile, gustatory, auditory and visual (Deal et al., 2016; Hunt et al., 1994; Moye & Rudy, 1985; Rudy & Hyson, 1984; Hyson & Rudy, 1984; Vogt & Rudy, 1984). During the periweaning period, the development of the hippocampus allows the processing and encoding of more complex configurations of stimuli which make the learning context (Debiec et al., 2013; Pearce & Bouton, 2001; Fanselow, 2000), and by PN 24 contextual threat conditioning emerges (Raineki et al., 2010; Rudy, 1993) (Fig. 4). Although, contextual fear may occur even before weaning at PN 18, this learning is short-lived, a phenomenon known as infantile amnesia (Rudy & Morledge, 1994). Recent studies in mice suggest that infantile amnesia may be at least in part due to high levels of neurogenesis in the young pup’s hippocampus (Akers et al., 2012, 2014).
The developmental trajectory of associative threat learning outlined above is sensitive and may be modified by environmental factors. Previous work in rats has shown that early-life atrocities, such as maternal deprivation may accelerate the emergence of threat learning (Cowan et al, 2013). Indeed, a recent human brain imaging study suggests that early maternal deprivation accelerates the development of the threat processing system, involving the amygdala (Gee et al., 2013).
Social transfer of fear from the mother to infant
It is also well documented the caregiver can facilitate fear learning in the offspring learning through a process known as Social referencing (de Rosnay et al., 2006; Murray et al., 2008; Aktar et al., 2013, 2014), which we have recently demonstrated in rodents (Debiec & Sullivan, 2014). In social referencing, a child uses caregiver’s emotional signaling to appraise and respond to uncertain situations, such as when approached by a stranger (Walden & Ogan, 1988; Frith, 2008). Social referencing studies show that infants are especially sensitive to caregiver’s fear and anxiety (de Rosnay et al., 2006; Murray et al., 2008; Aktar et al., 2013, 2014). Consistent with these reports, we found in preweaning rat pups that an exposure to a frightened mother increases activity of a number of brain sites involved in processing threat, stress and pain, including the amygdala and hypothalamic nuclei (Debiec & Sullivan, 2014; Chang & Debiec, 2016). This activation of the threat system in the infant brain was accompanied by the elevation of plasma CORT levels (Debiec & Sullivan, 2014). Thus, although the caregiver’s presence at default buffers stress and fear, the presence of the frightened caregiver induces fear in pups. We also found that newborn pups exposed to their mother expressing fear to the previously conditioned olfactory cue acquire threat responses to this cue from their mother (Debiec & Sullivan, 2014, Chang & Debiec, 2016) (Fig. 5).
This social transmission of fear was present during the sensitive period (as early as on the day of birth) (Chang & Debiec, 2016) and required functional amygdala and intact HPA axis response (Debiec & Sullivan, 2014). Pharmacological inactivation on the pups’s amygdala or blocking of CORT synthesis prevented acquisition of socially transmitted maternal fear (Debiec & Sullivan, 2014). The early social transfer of fear was mediated by the olfactory modality and engaged areas involved in alarm chemosignaling, such as Grueneberg ganglion (GG) (Debiec & Sullivan, 2014; Brechbuhl 2008), although later in life other sensory modalities are also likely to be involved (Kim et al., 2010; Jeon et al., 2010). Maternal fear signaling may thus override natural mechanisms preventing aversive learning in infancy and serves as an unconditioned stimulus reinforcing fear learning (Debiec et al., 2010). This social fear learning occurs throughout development and has been well-documented in children and in adults (Aktar et al., 2013, 2014; Murray et al., 2008; Askew & Field, 2007; Olsson et al., 2007).
Concluding remarks
Learning occurs throughout life but what we learn and how we learn it changes to fit each developmental epoch, typically using transient “sensitive periods” that filter what is learned by enhancing some aspects of learning and inhibiting others. These defined periods of altered learning provide streamlined learning that ensures survival. The traditional view of sensitive period learning has been that it gradually or abruptly provides access and then restriction to the specialized learning circuitry. However, it is now clear that these specialized periods of learning can be turned on and off naturalistically in rat pups by the mother’s presence and emotional affect. This can be mimicked through a direct manipulation of the stress response by pharmacologically altering stress hormone CORT levels or indirectly, through changing maternal emotional states. Understanding how the caregiver’s presence alters the neurobiology of infant’s safety and threat learning may provide insight into mechanisms of adaptive and maladaptive fears, such as in anxiety disorders.
Highlights.
In newborn rat pups attachment learning is dominant and threat learning is quiescent.
Around postnatal day 10 classical threat conditioning emerges.
Maternal presence may prevent threat conditioning in pups until postnatal day 10.
From birth pups can acquire threat responses from the mother through social learning.
Acknowledgments
This work was supported NIH DC009910, MH091451, HD083217 (RMS); K08 MH014743-01A1, NARSAD Young Investigator Award from the Brain & Behavior Research Foundation, Todd Ouida Clinical Scholar Award in Childhood Anxiety & Depression (JD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med (Maywood) 2008;233(2):139–54. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MD. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn Mem. 2012;19:598–604. doi: 10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, … Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Aktar E, Majdandzic M, de Vente W, Bogels SM. The interplay between expressed parental anxiety and infant behavioural inhibition predicts infant avoidance in a social referencing paradigm. J Child Psychol Psychiatry. 2013;54(2):144–56. doi: 10.1111/j.1469-7610.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- Aktar E, Majdandzic M, de Vente W, Bogels SM. Parental social anxiety disorder prospectively predicts toddlers’ fear/avoidance in a social referencing paradigm. J Child Psychol Psychiatry. 2014;55(1):77–87. doi: 10.1111/jcpp.12121. [DOI] [PubMed] [Google Scholar]
- Al Ain S, Perry RE, Nunez B, Kayser K, Hochman C, Brehman E, … Sullivan RM. Neurobehavioral assessment of maternal odor in developing rat pups: implications for social buffering. Soc Neurosci. 2016 Mar 22;:1–18. doi: 10.1080/17470919.2016.1159605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JA. Sensory-perceptual development in the Norway rat: A view toward comparative studies. In: Kail R, Spear NE, editors. Comparative perspectives on the development of memory. Hillsdale, NJ: Erlbaum; 1984. pp. 65–101. [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex. Ann N Y Acad Sci. 2001;935(1):107–117. [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, … Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Askew C, Field AP. Vicarious learning and the development of fears in childhood. Behav Res Ther. 2007;45:2616–2627. doi: 10.1016/j.brat.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92(3):292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Barr G. Ontogeny of nociception and antinociception. NIDA Research Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, Sullivan RM. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P. Thirty years of collaboration with Gabriel Horn. Neurosci Biobehav Rev. 2015;50:4–11. doi: 10.1016/j.neubiorev.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Res Dev Brain Res. 1991;63(1–2):245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15(6):755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Brown MW, Johnson MH. Brain, memory and development: the imprint of Gabriel Horn. Neurosci Biobehav Rev. 2015;50:1–3. doi: 10.1016/j.neubiorev.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Honey RC. Imprinting, learning and development: from behaviour to brain and back. Trends Neurosci. 1998;21(7):306–311. doi: 10.1016/s0166-2236(98)01258-2. [DOI] [PubMed] [Google Scholar]
- Bordner KA, Spear NE. Olfactory learning in the one-day old rat: reinforcing effects of isoproterenol. Neurobiol Learn Mem. 2006;86(1):19–27. doi: 10.1016/j.nlm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bowlby J. The making and breaking of affectional bonds. I. Aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. Br J Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment theory and its therapeutic implications. Adolesc Psychiatry. 1978;6:5–33. [PubMed] [Google Scholar]
- Bowlby J. Violence in the family as a disorder of the attachment and caregiving systems. Am J Psychoanal. 1984;44(1):9–27. 29–31. doi: 10.1007/BF01255416. [DOI] [PubMed] [Google Scholar]
- Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321(5892):1092–5. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Teuchert-Noodt G. Postnatal development of dopamine innervation in the amygdala and the entorhinal cortex of the gerbil (Meriones unguiculatus) Brain Res. 2006;1125(1):9–16. doi: 10.1016/j.brainres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Calamandrei G, Wilkinson LS, Keverne EB. Olfactory recognition of infants in laboratory mice: role of noradrenergic mechanisms. Physiol Behav. 1992;52(5):901–7. doi: 10.1016/0031-9384(92)90369-d. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, et al. From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front Psychiatry. 2013;4:90. doi: 10.3389/fpsyt.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125(1):20–8. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Transl Psychiatry. 2012;2012:2, e138. doi: 10.1038/tp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Canton-Cortes D, Cortes MR, Canton J. Child sexual abuse, attachment style, and depression: the role of the characteristics of abuse. J Interpers Violence. 2015;30(3):420–36. doi: 10.1177/0886260514535101. [DOI] [PubMed] [Google Scholar]
- Carlson EA, Hostinar CE, Mliner SB, Gunnar MR. The emergence of attachment following early social deprivation. Dev Psychopathol. 2014;26(2):479–489. doi: 10.1017/S0954579414000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron EB, Weston-Lee P, Haggerty D, Dozier M. Community implementation outcomes of Attachment and Biobehavioral Catch-up. Child Abuse Negl. 2015 doi: 10.1016/j.chiabu.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, … Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Debiec J. Neural correlates of the mother-to-infant social transmission of fear. J Neurosci Res. 2016;94(6):526–34. doi: 10.1002/jnr.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol. 2012;520(9):1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol. 2012;520(16):3745–3763. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behav Neurosci. 2000;114(3):484–495. [PubMed] [Google Scholar]
- Christie MJ. Generators of synchronous activity of the locus coeruleus during development. Semin Cell Dev Biol. 1997;8(1):29–34. doi: 10.1006/scdb.1996.0118. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Jelinek HF. Dye-coupling among neurons of the rat locus coeruleus during postnatal development. Neuroscience. 1993;56(1):129–137. doi: 10.1016/0306-4522(93)90568-z. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J Neurosci. 1989;9(10):3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A, Bolles R. The ontogensis of defensive reactions to shock in preweanling rats. Developmental Psychobiology. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Cooke L, Fildes A. The impact of flavour exposure in utero and during milk feeding on food acceptance at weaning and beyond. Appetite. 2011;57(3):808–11. doi: 10.1016/j.appet.2011.05.317. [DOI] [PubMed] [Google Scholar]
- Corona R, Levy F. Chemical olfactory signals and parenthood in mammals. Horm Behav. 2015;68:77–90. doi: 10.1016/j.yhbeh.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, Richardson R. Acute early-life stress results in premature emergence of adult-like fear retention and extinction relapse in infant rats. Behav Neurosci. 2013;127(5):703–11. doi: 10.1037/a0034118. [DOI] [PubMed] [Google Scholar]
- Crittenden PM. Children’s strategies for coping with adverse home environments: an interpretation using attachment theory. Child Abuse Negl. 1992;16(3):329–343. doi: 10.1016/0145-2134(92)90043-q. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Moments in time--the neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141(5):1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Rosnay M, Cooper PJ, Tsigaras N, Murray L. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav Res Ther. 2006;44(8):1165–75. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Deal AL, Erickson KJ, Shiers SI, Burman MA. Limbic system development underlies the emergence of classical fear conditioning during the third and fourth weeks of life in the rat. Behav Neurosci. 2016;130:212–230. doi: 10.1037/bne0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13(5):536–7. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, LeDoux JE. The selectivity of aversive memory reconsolidation and extinction processes depends on the initial encoding of the Pavlovian association. Learn Mem. 2013;20:695–699. doi: 10.1101/lm.031609.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. The Amygdala Networks of Fear: From Animal Models to Human Psychopathology. In: McKay D, Abramowitz JS, Taylor S, Asmundson GJG, editors. Current Perspectives on the Anxiety Disorders: Implications for DSM-V and Beyond. New York: Springer; 2009. pp. 107–126. [Google Scholar]
- Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A. 2014;111(33):12222–7. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg V. Early experience and emotional development. Scientific American. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32(1):149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1(5):179–184. [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590(Pt 19):4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28(9):1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Emerich D, Scalzo F, Enters E, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18(3):215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: preliminary findings. J Am Acad Child Adolesc Psychiatry. 1992;31(5):863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol Sin. 2000;21(6):481–493. [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M. Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Soc Cogn Affect Neurosci. 2016;11(2):327–336. doi: 10.1093/scan/nsv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Social cognition. Philos Trans R Soc Lond B Biol Sci. 2008;363(1499):2033–9. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, … Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goksan S, Hartley C, Emery F, Cockrill N, Poorun R, Moultrie F, … Slater R. fMRI reveals neural activity overlap between adult and infant pain. Elife. 2015;2015:4. doi: 10.7554/eLife.06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Balancing the contributions of multiple neural systems during learning and memory. In: Jackson PA, Chiba AA, Berman RF, Ragozzino ME, editors. The Neurobiological Basis of Memory. New York: Springer; 2015. pp. 261–280. [Google Scholar]
- Gomez RL, Edgin JO. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev Cogn Neurosci. 2015;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22(24):10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Imprinting in Relation to Parental and Species Identification by Avian Neonates. J Comp Physiol Psychol. 1965;59:345–356. doi: 10.1037/h0022045. [DOI] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham YP, Heim C, Goodman SH, Miller AH, Nemeroff CB. The effects of neonatal stress on brain development: implications for psychopathology. Dev Psychopathol. 1999;11(3):545–565. doi: 10.1017/s0954579499002205. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135(6):2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog Brain Res. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc Neurosci. 2015;10(5):474–8. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chemical Senses. 2001;26(5):551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Harlow H, Harlow M. The affectional systems. In: Schrier A, Harlow H, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. New York: Academic Press; 1965. pp. 287–334. [Google Scholar]
- Harmon RJ, Morgan GA, Glicken AD. Continuities and discontinuities in affective and cognitive-motivational development. Child Abuse Negl. 1984;8(2):157–167. doi: 10.1016/0145-2134(84)90005-x. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Age differences in a circadian influence on hippocampal LTP. Brain Res. 1983;261(1):69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin-releasing factor. Ann N Y Acad Sci. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml PA, Willen R, Watanasriyakul W, Johnson J, Garrett T. Selective social buffering of behavioral and endocrine responses and Fos induction in the prelimbic cortex of infants exposed to a novel environment. Dev Psychobiol. 2015;57(1):50–62. doi: 10.1002/dev.21256. [DOI] [PubMed] [Google Scholar]
- Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. The American Journal of Physiology. 1978;235(5):E451–456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Cleland J. Developmental aspects of kin recognition. Genetica. 1998;104(3):199–205. doi: 10.1023/a:1026477724836. [DOI] [PubMed] [Google Scholar]
- Hess E. Ethology: an approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess E, Mendler G, editors. New directions in psychology. New York: Holt, Rinehart and Winston; 1962. pp. 159–199. [Google Scholar]
- Hofer MA. Relationships as regulators: a psychobiologic perspective on bereavement. Psychosom Med. 1984;46(3):183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, DePaulo P. Behavioral control by an imprinting stimulus. Am Sci. 1977;65(1):58–66. [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. J Clin Child Adolesc Psychol. 2013;42(4):564–575. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Campbell BA. Develipmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. The functional behaviorism of Robert C. Bolles: Learning, motivation, and cognition. Washington DC: American Psychological Association; 1997. pp. 53–74. [Google Scholar]
- Hunt PS, Richardson R, Campbell BA. Delayed development of fear-potentiated startle in rats. Behav Neurosci. 1994;108:69–80. doi: 10.1037//0735-7044.108.1.69. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Richardson R, Hess MF, Campbell BA. Emergence of conditioned cardiac responses to an olfactory CS paired with an acoustic startle UCS during development: form and autonomic origins. Dev Psychobiol. 1997;30:151–163. [PubMed] [Google Scholar]
- Hyson RL, Rudy JW. Ontogenesis of learning: II. Variation in the rat’s reflexive and learned responses to acoustic stimulation. Dev Psychobiol. 1984;17:263–283. doi: 10.1002/dev.420170307. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagalska-Majewska H, Wojcik S, Dziewiatkowski J, Luczynska A, Kurlapska R, Morys J. Postnatal development of the basolateral complex of rabbit amygdala: a stereological and histochemical study. J Anat. 2003;203(5):513–521. doi: 10.1046/j.1469-7580.2003.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, … Thomas KM. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Dev Psychopathol. 2015;27(4 Pt 2):1577–1589. doi: 10.1017/S0954579415000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, … Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13(4):482–8. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S, Grossmann T. Neural signatures of conscious and unconscious emotional face processing in human infants. Cortex. 2015;64:260–270. doi: 10.1016/j.cortex.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Julich S. Stockholm syndrome and child sexual abuse. J Child Sex Abus. 2005;14(3):107–29. doi: 10.1300/J070v14n03_06. [DOI] [PubMed] [Google Scholar]
- Kaba H, Huang GZ. Long-term potentiation in the accessory olfactory bulb: a mechanism for olfactory learning. Chem Senses. 2005;30(Suppl 1):i150–1. doi: 10.1093/chemse/bjh158. [DOI] [PubMed] [Google Scholar]
- Karantzas GC, McCabe MP, Karantzas KM, Pizzirani B, Campbell H, Mullins ER. Attachment Style and Less Severe Forms of Sexual Coercion: A Systematic Review. Arch Sex Behav. 2016;45(5):1053–68. doi: 10.1007/s10508-015-0600-7. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5(12):e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kovach JK, Hess EH. Imprinting: effects of painful stimulation upon the following response. J Comp Physiol Psychol. 1963;56:461–4. doi: 10.1037/h0047033. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Dev Neurosci. 2012;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Leon M. The neurobiology of filial learning. Annual Review of Psychology. 1992;43:377–398. doi: 10.1146/annurev.ps.43.020192.002113. [DOI] [PubMed] [Google Scholar]
- Letcher P, Smart D, Sanson A, Toumbourou JW. Psychosocial Precursors and Correlates of Differing Internalizing Trajectories from 3 to 15 Years1. Social Development. 2009;18(3):618–646. [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behav Neurosci. 1985;99(3):399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev Psychobiol. 1999;34(1):29–35. [PubMed] [Google Scholar]
- Maity S, Rah S, Sonenberg N, Gkogkas CG, Nguyen PV. Norepinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learn Mem. 2015;22(10):499–508. doi: 10.1101/lm.039222.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16(2):237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Mason WA, Kosterman R, Hawkins JD, Herrenkohl TI, Lengua LJ, McCauley E. Predicting depression, social phobia, and violence in early adulthood from childhood behavior problems. J Am Acad Child Adolesc Psychiatry. 2004;43(3):307–315. doi: 10.1097/00004583-200403000-00012. [DOI] [PubMed] [Google Scholar]
- Mazza JJ, Fleming CB, Abbott RD, Haggerty KP, Catalano RF. Identifying trajectories of adolescents’ depressive phenomena: an examination of early risk factors. J Youth Adolesc. 2010;39(6):579–593. doi: 10.1007/s10964-009-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey LA. The intergenerational transfer of mother-daughter risk for gender-based abuse. Psychodyn Psychiatry. 2013;41(2):303–28. doi: 10.1521/pdps.2013.41.2.303. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Consolidating memories. Annu Rev Psychol. 2015;66:1–24. doi: 10.1146/annurev-psych-010814-014954. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am J Clin Nutr. 2014;99(3):704S–11S. doi: 10.3945/ajcn.113.067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. Experience with a flavor in mother’s milk modifies the infant’s acceptance of flavored cereal. Dev Psychobiol. 1999;35(3):197–203. doi: 10.1002/(sici)1098-2302(199911)35:3<197::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107(6):E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Lukasewycz LD, Castor SM, Beauchamp GK. The timing and duration of a sensitive period in human flavor learning: a randomized trial. Am J Clin Nutr. 2011;93(5):1019–24. doi: 10.3945/ajcn.110.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarresi S, Mukherjee B, McLean JH, Harley CW, Yuan Q. CaMKII mediates stimulus specificity in early odor preference learning in rats. J Neurophysiol. 2016;116(2):404–10. doi: 10.1152/jn.00176.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning: VI. Learned and unlearned responses to visual stimulation in the infant hooded rat. Dev Psychobiol. 1985;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Murray L, de Rosnay M, Pearson J, Bergeron C, Schofield E, Royal-Lawson M, Cooper PJ. Intergenerational transmission of social anxiety: the role of social referencing processes in infancy. Child Dev. 2008;79:1049–1064. doi: 10.1111/j.1467-8624.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J Neurophysiol. 1987;58(3):510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Nicholas MW. The compulsion to repeat relationships with abusive partners and how group therapy can help. Int J Group Psychother. 2013;63(3):346–65. doi: 10.1521/ijgp.2013.63.3.346. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Cameron JL. Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am J Prev Med. 2006;31(6 Suppl 1):S175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Okutani F, Yagi F, Kaba H. Gabaergic control of olfactory learning in young rats. Neuroscience. 1999;93(4):1297–300. doi: 10.1016/s0306-4522(99)00224-9. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandipati S, Schoppa NE. Age-dependent adrenergic actions in the main olfactory bulb that could underlie an olfactory-sensitive period. J Neurophysiol. 2012;108(7):1999–2007. doi: 10.1152/jn.00322.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, … Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annu Rev Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev Psychobiol. 1982;15(4):349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Perry R, Sullivan RM. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev Psychobiol. 2014;56(8):1626–1634. doi: 10.1002/dev.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Santiago AN, Sullivan RM. From trauma to safety: Rescuing adults from neurobehavioral impacts of infant abuse submitted. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Altbäcker V. Ontogeny of the responses of European rabbits (Oryctolagus cuniculus) to aerial and ground predators. Can J Zool. 2000;78:655–65. [Google Scholar]
- Poulos AM, Reger M, Mehta N, Zhuravka I, Sterlace SS, Gannam C, … Fanselow MS. Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol Psychiatry. 2014;76(4):306–314. doi: 10.1016/j.biopsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman BJ, Coss RG, Clark RW. The ontogeny of antipredator behavior: age differences in California ground squirrels (Otospermophilus beecheyi) at multiple stages of rattlesnake encounters. Behav Ecol Sociobiol. 2015;69:1447–1457. [Google Scholar]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, Menon V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat Neurosci. 2014;17(9):1263–1269. doi: 10.1038/nn.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010a;20(9):1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010b;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Pickenhagen A, Roth TL, Babstock DM, McLean JH, Harley CW, … Sullivan RM. The neurobiology of infant maternal odor learning. Braz J Med Biol Res. 2010c;43(10):914–919. doi: 10.1590/s0100-879x2010007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ, … Sullivan RM. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40(4):906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki D, Lamb M, Obmascher P. Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behav Brain Sci. 1978;3:417–464. [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Hunt PS. Ontogeny of fear conditioning. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 527–545. [Google Scholar]
- Richardson R, Paxinos G, Lee J. The ontogeny of conditioned odor potentiation of startle. Behav Neurosci. 2000;114(6):1167–73. [PubMed] [Google Scholar]
- Rincon-Cortes M, Barr GA, Mouly AM, Shionoya K, Nunez BS, Sullivan RM. Enduring good memories of infant trauma: rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proc Natl Acad Sci U S A. 2015;112(3):881–886. doi: 10.1073/pnas.1416065112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Sullivan RM. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne) 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés MS, Sullivan RM. Abuse-induced social behavior deficits linked to blunted corticolimbic activty and adult depression. doi: 10.1038/tp.2016.205. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol. 2014;56:1755–1763. doi: 10.1002/dev.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Raineki C, Salstein L, Perry R, Sullivan-Wilson TA, Sloan A, … Sullivan RM. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes Brain Behav. 2013;12(7):673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Examining the role of endogenous opioids in learned odor–stroke associations in infant rats. Dev Psychobiol. 2006;48(1):71–78. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20(20):7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107(5):887–91. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Hyson RL. Ontogenesis of learning: III. Variation in the rat’s differential reflexive and learned responses to sound frequencies. Dev Psychobiol. 1984;17:285–300. doi: 10.1002/dev.420170308. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Salzen E. Imprinting and environmental learning. In: Aronson L, Tobach E, Lehrman D, Rosenblatt J, editors. Development and evolution of behavior. San Francisco: W.H. Freeman; 1970. [Google Scholar]
- Sanchez M, Ladd C, Plotsky P. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 2015;10(5):512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal B. Prenatal and postnatal human olfactory development: influences on cognition and behavior. In: Doty RL, editor. Handbook of Olfaction. 3. John Wiley & Sons, Inc; Hoboken, New Jersey: 2015. [Google Scholar]
- Schaal B, Coureaud G, Doucet S, Delaunay-El Allam M, Moncomble AS, Montigny D, … Holley A. Mammary olfactory signalisation in females and odor processing in neonates: ways evolved by rabbits and humans. Behav Brain Res. 2009;200(2):346–58. doi: 10.1016/j.bbr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J Comp Neurol. 1984;223(2):177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, … Sullivan RM. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Dev Cogn Neurosci. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Sullivan RM, Messaoudi B, Mouly AM. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn Mem. 2008;15(9):649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci. 2008;28(42):10711–9. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm Behav. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, … Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Langencrantz H. The “stress” of being born. Sci Ame. 1986;254(4):100–7. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus) J Comp Psychol. 1987;101(2):190–196. [PubMed] [Google Scholar]
- Solomonia RO, McCabe BJ. Molecular mechanisms of memory in imprinting. Neurosci Biobehav Rev. 2015;50:56–69. doi: 10.1016/j.neubiorev.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N. Processing memories: forgetting and retention. Hillsdale, New Jersey: Erlbaum; 1978. [Google Scholar]
- Stanley W. Differential human handling as reinforcing events and as treatments influencing later social behavior in basenji puppies. Psychol Rep. 1962;10:775–788. [Google Scholar]
- Stanton ME, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiol Behav. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol. 1987;20(2):131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Stehouwer D, Campbell B. Habituation of the forelimb-withdrawal response in neonatal rats. J Exp Psychol Anim Behav Process. 1978;4(2):104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Stone EA, Bonnet KA, Hofer MA. Survival and development of maternally deprived rats: role of body temperature. Psychosom Med. 1976;38(4):242–249. doi: 10.1097/00006842-197607000-00002. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Dominick KC, Patino LR, Doyle CD, Picard LS, Phan KL. Neurobiology of Pediatric Anxiety Disorders. Curr Behav Neurosci Rep. 2014;1(3):154–160. doi: 10.1007/s40473-014-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, Phan KL. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81–88. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res Dev Brain Res. 1993;75(2):185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Dufresne MM. Mesocortical dopamine and HPA axis regulation: role of laterality and early environment. Brain Res. 2006;1076(1):49–59. doi: 10.1016/j.brainres.2005.12.100. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000a;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392(1–2):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Opendak M. Neurobiology of Infant Sensitive Period for Attachment and its Reinstatement through Maternal Social Buffering submitted. [Google Scholar]
- Sullivan RM, Perry RE. Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Soc Neurosci. 2015;10(5):500–511. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000b;114(5):957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psychobiology (Austin, Tex) 1991;19(4):308–312. doi: 10.3758/bf03332084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res Dev Brain Res. 1990;53(2):243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak D, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70(2):279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. AnnN YAcad Sci. 2003;1008(1):132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Neural networks and synaptic transmission in immature hippocampus. Adv Exp Med Biol. 1990;268:161–171. doi: 10.1007/978-1-4684-5769-8_19. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Rubin WW. Corticosteroid induction of threat-induced behavioral inhibition in preweanling rats. Behav Neurosci. 1993;107(5):860–6. doi: 10.1037//0735-7044.107.5.860. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Development of stress-induced responses in preweanling rats. Dev Psychobiol. 1991;24(5):341–60. doi: 10.1002/dev.420240504. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol. 2014;35(2):245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todrank J, Heth G, Restrepo D. Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc Biol Sci. 2011;278(1714):1949–55. doi: 10.1098/rspb.2010.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–92. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K, Moriceau S, Sullivan RM, Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol Motil. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann N Y Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden C, Uylings H. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 2004;241(3):268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varendi H, Porter RH, Winberg J. Attractiveness of amniotic fluid odor: evidence of prenatal olfactory learning? Acta Paediatr. 1996;85(10):1223–7. doi: 10.1111/j.1651-2227.1996.tb18233.x. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Rudy JW. Ontogenesis of learning: I. Variation in the rat’s reflexive and learned responses to gustatory stimulation. Dev Psychobiol. 1984;17:11–33. doi: 10.1002/dev.420170103. [DOI] [PubMed] [Google Scholar]
- Wakefield CL, Levine MS. Early postnatal development of basolateral amygdala in kitten: a Golgi morphometric analysis. Brain Res Bull. 1985;14(2):159–167. doi: 10.1016/0361-9230(85)90075-9. [DOI] [PubMed] [Google Scholar]
- Walden TA, Ogan TA. The development of social referencing. Child Dev. 1988;59(5):1230–1240. doi: 10.1111/j.1467-8624.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Walker C, Sapolsky R, Meaney M, Vale W, Rivier C. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119(4):1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- Weber EM, Olsson IAS. Maternal behaviour in Mus musculus sp.: An ethological review. Appl Anim Behav Sci. 2008;114(1–2):1–22. [Google Scholar]
- Wiedenmayer CP, Lyo D, Barr GA. Rat pups reduce ultrasonic vocalization after exposure to an adult male rat. Dev Psychobiol. 2003;42(4):386–91. doi: 10.1002/dev.10112. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987;7(11):3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Res. 1984;318(1):61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Res. 1988;470(1):69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci Biobehav Rev. 2003;27(4):307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development—II. α2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1996;76(1):261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, McLean JH. Isoproterenol increases CREB phosphorylation and olfactory nerve–evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor prference learning. Learn Mem. 2000;7(6):413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knöpfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J Neurophysiol. 2002;87(6):3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Shakhawat AM, Harley CW. Mechanisms underlying early odor preference learning in rats. Prog Brain Res. 2014;208:115–56. doi: 10.1016/B978-0-444-63350-7.00005-X. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94(8):4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]