Abstract
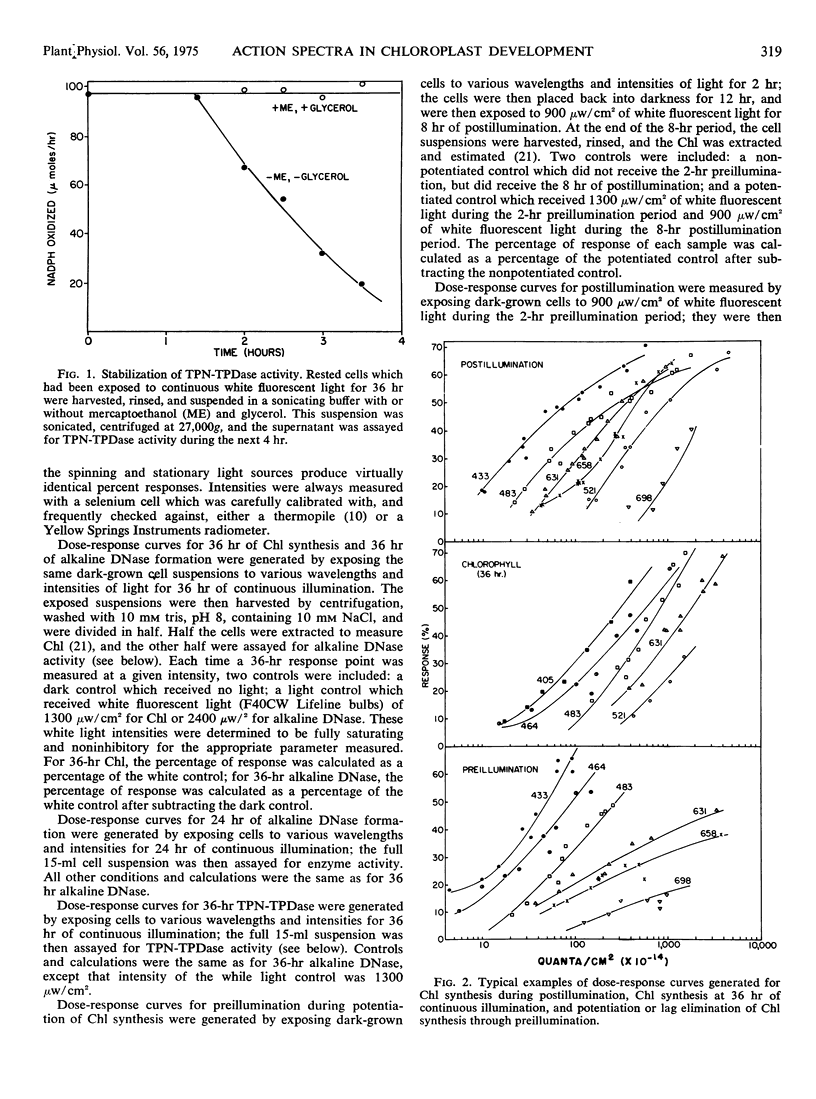
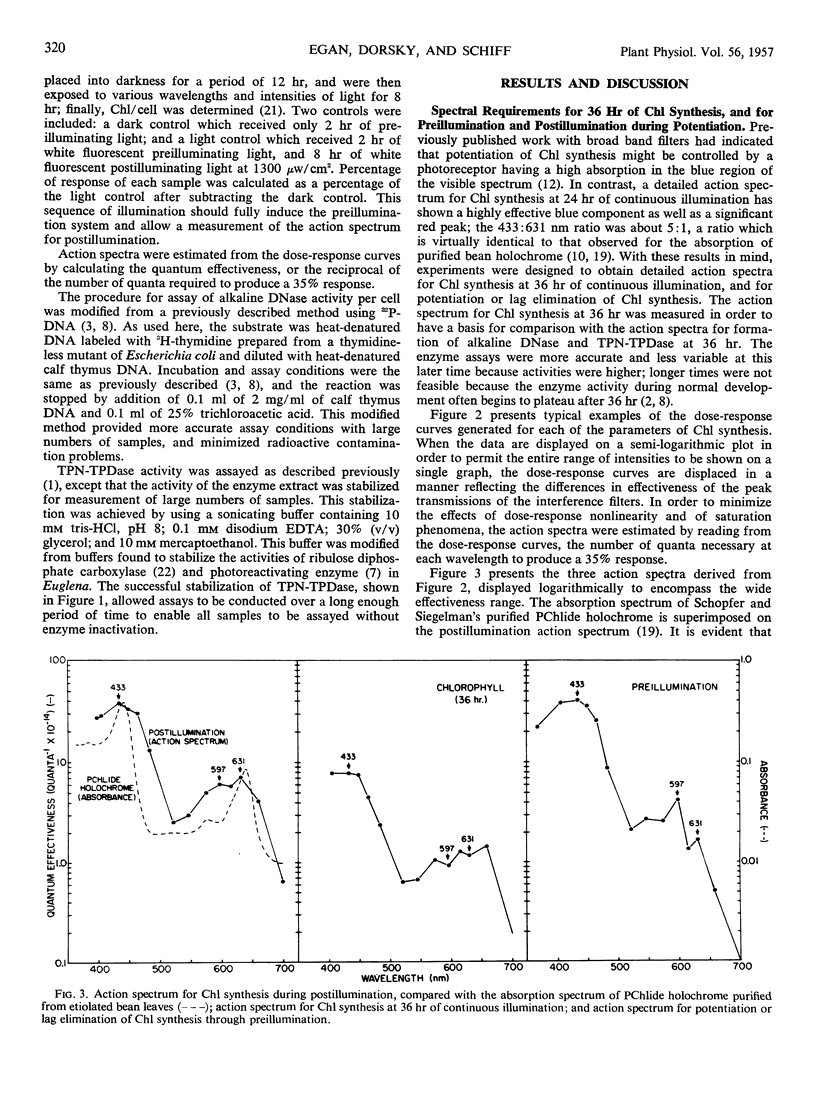
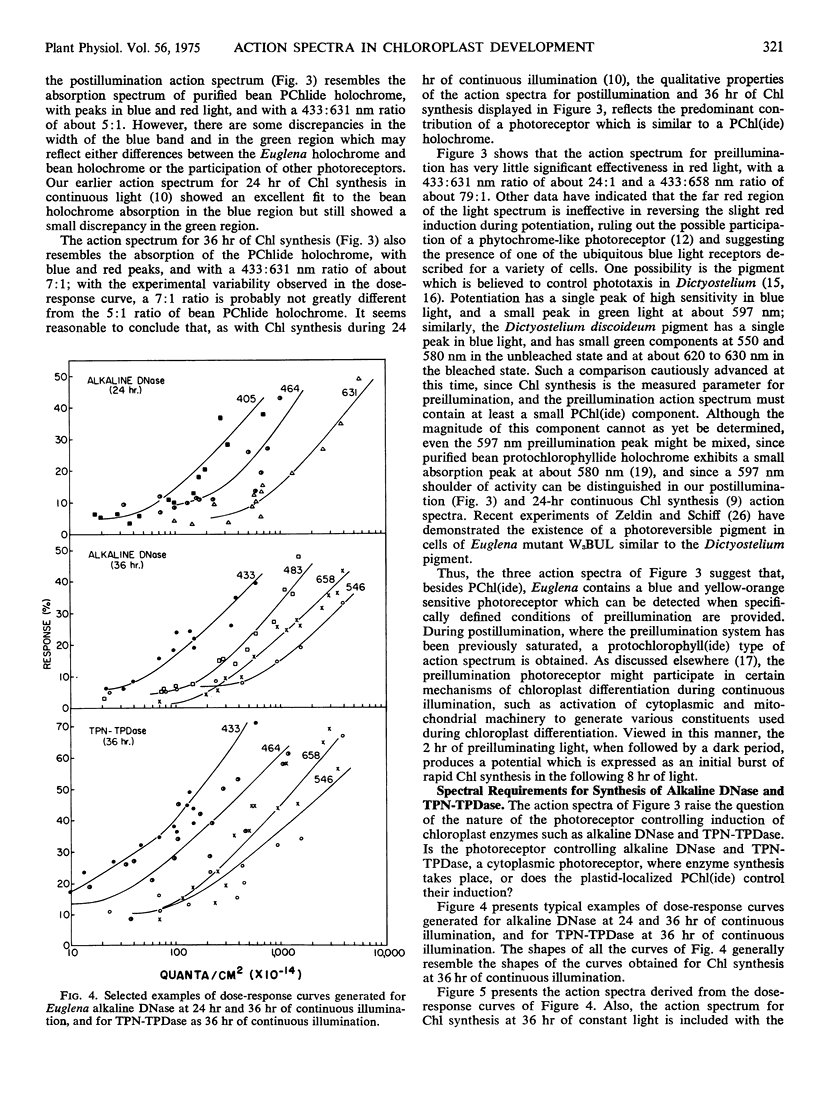
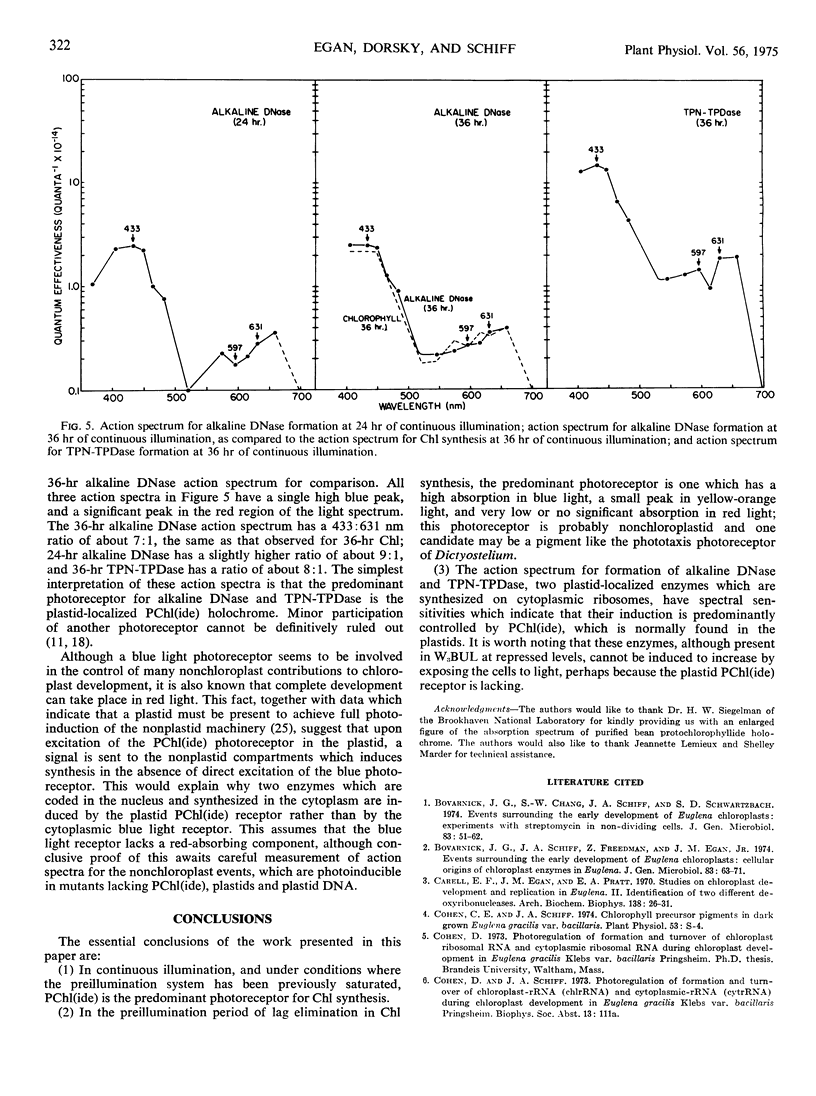
Action spectra derived from dose-response curves measured for various processes associated with chloroplast development in Euglena gracilis var. bacillaris are presented. The action spectrum for chlorophyll synthesis during the first 36 hours of continuous illumination of dark-grown resting cells resembles the absorption spectrum of protochlorophyll(ide). The action spectrum for the preillumination phase of potentiation, during which preillumination followed by a dark period brings about lag elimination in chlorophyll synthesis when the cells are subsequently exposed to postilluminating light, shows a high peak in the blue region (at about 433 nm) with a small peak in the yellow-orange region (at about 597 nm); the postillumination phase yields an action spectrum very similar to that obtained for chlorophyll synthesis in continuous light in normal, unpotentiated cells, with peaks at 433 and 631 nm. Alkaline DNase and TPN-linked triose phosphate dehydrogenase, two plastid enzymes which are synthesized outside the chloroplast, yield action spectra which are consistent with protochlorophyll(ide) being the major light receptor. The action spectra which implicate pigments resembling protochlorophyll(ide) holochrome have blue to red peak ratios in the vicinity of 5:1 as does the absorption spectrum of the protochlorophyllide holochrome from beans; the action spectrum is not identical with the holochrome spectrum indicating that the Euglena holochrome may differ from the bean pigment in details of its absorption spectrum. The action spectrum for preillumination, shows a ratio of the blue peak to the red effectiveness of about 24:1. This suggests that preillumination is controlled by a photoreceptor different from the protochlorophyll(ide) holochrome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Shaul Y., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. VII. Fine Structure of the Developing Plastid. Plant Physiol. 1964 Mar;39(2):231–240. doi: 10.1104/pp.39.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Carell E. F., Egan J. M., Pratt E. A. Studies on chloroplast development and replication in Euglena. II. Identification of two different deoxyribonucleases. Arch Biochem Biophys. 1970 May;138(1):26–31. doi: 10.1016/0003-9861(70)90279-1. [DOI] [PubMed] [Google Scholar]
- Ecklund P. R., Moore T. C. Correlations of Growth Rate and De-etiolation with Rate of Ent-Kaurene Biosynthesis in Pea (Pisum sativum L.). Plant Physiol. 1974 Jan;53(1):5–10. doi: 10.1104/pp.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecklund P. R., Moore T. C. Correlations of Growth Rate and De-etiolation with Rate of Ent-Kaurene Biosynthesis in Pea (Pisum sativum L.). Plant Physiol. 1974 Jan;53(1):5–10. doi: 10.1104/pp.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. M., Carell E. F. Studies on Chloroplast Development and Replication in Euglena: III. A Study of the Site of Synthesis of Alkaline Deoxyribonuclease Induced during Chloroplast Development in Euglena gracilis. Plant Physiol. 1972 Sep;50(3):391–395. doi: 10.1104/pp.50.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Poff K. L., Butler W. L. Spectral characteristics of the photoreceptor pigment of phototaxis in Dictyostelium discoideum. Photochem Photobiol. 1974 Sep;20(3):241–244. doi: 10.1111/j.1751-1097.1974.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Schopfer P., Siegelman H. W. Purification of protochlorophyllide holochrome. Plant Physiol. 1968 Jun;43(6):990–996. doi: 10.1104/pp.43.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLKEN J. J., MELLON A. D. The relationship between chlorophyll and the carotenoids in the algal flagellate, Euglena. J Gen Physiol. 1956 May 20;39(5):675–685. doi: 10.1085/jgp.39.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


