Abstract
Purpose
Meniscus tears are a common knee injury and are associated with the development of post-traumatic osteoarthritis (OA). The purpose of this study was to evaluate potential OA mediators in the synovial fluid and serum of meniscus tear subjects compared to those in the synovial fluid of radiographic non-OA control knees.
Materials and Methods
Sixteen subjects with an isolated unilateral meniscus injury and six subjects who served as reference controls (knee Kellgren-Lawrence grade 0–1) were recruited. Twenty-one biomarkers were measured in serum from meniscus tear subjects and in synovial fluid from both groups. Meniscus tear subjects were further stratified by tear type to assess differences in biomarker levels.
Results
Synovial fluid total matrix metalloproteinase (MMP) activity and prostaglandin E2 (PGE2) were increased 25-fold and 290-fold, respectively, in meniscus tear subjects as compared to reference controls (p<0.05). Synovial fluid MMP activity and PGE2 concentrations were positively correlated in meniscus tear subjects (R=0.83, p<0.0001). In meniscus tear subjects, synovial fluid levels of MMP activity, MMP-2, MMP-3, sGAG, COMP, IL-6, and PGE2 were higher than serum levels (p<0.05). Subjects with complex meniscus tears had higher synovial fluid MMP-10 (p<0.05) and reduced serum TNFα and IL-8 (p<0.05) compared to other tear types.
Conclusions
Given the degradative and pro-inflammatory roles of MMP activity and PGE2, these molecules may alter the biochemical environment of the joint. Our findings suggest that modulation of PGE2 signaling, MMP activity, or both following a meniscus injury may be targets to promote meniscus repair and prevent OA development.
Keywords: inflammatory, metabolic, collagen, proteoglycan, cartilage oligomeric matrix protein, tumor necrosis factor alpha, interleukin
Introduction
Meniscus tears are among the most common knee conditions, with roughly 850,000 meniscus surgical procedures performed annually in the United States and nearly twice as many worldwide (1, 2). Meniscus tears, which can affect all age groups (1), disrupt the ability of the meniscus to perform its primary functions of distributing loads (3–5) and stabilizing the joint during loading (6, 7). These alterations in joint function can lead to degenerative changes in the knee and the development of post-traumatic osteoarthritis (PTOA) (3, 8, 9). In order to prevent PTOA, current clinical treatments seek to preserve the meniscus tissue. While tissue preservation and repair are recommended for horizontal and longitudinal tears in the vascularized outer zone of the meniscus (10–12), tears that are complex or in the avascular inner zone frequently require partial or total meniscectomy. Meniscectomy is strongly associated with articular cartilage degradation (3, 13, 14), such that as many as two-thirds of partial or total meniscectomy patients develop PTOA within 5–15 years of injury (15).
Meniscus damage and loss results in both local biological and mechanical responses in the meniscus and joint tissues (16–19), which may also be reflected systemically. These biological changes can be quantified by measuring biomarkers that reflect the processes of extracellular matrix (ECM) synthesis and degradation and inflammation, presumably reflecting joint remodeling and disease progression (16, 20). Therefore, a further understanding of biomarker profiles and their relationship to joint injury may identify patients at risk for meniscus injury and PTOA following meniscus injury. In order to identify candidate biomarkers relevant to joint injury and potentially OA progression, synovial fluid, serum, and urine samples from healthy individuals and OA patients have been evaluated for biomarkers of both joint tissue metabolism and inflammation (21–28). While many of these biomarkers have been researched in subjects with anterior cruciate ligament (ACL) tears and intra-articular fractures (28–35), limited biomarker data are available on meniscus tear subjects (16, 18, 21).
Therefore, the goal of this study was to measure synovial fluid and serum for a panel of joint tissue metabolic and inflammatory biomarkers in subjects with a meniscus tear compared to synovial fluid of radiographic non-OA control knees and to determine the relationship between serum and synovial fluid biomarker levels in the meniscus tear subjects. We hypothesized that inflammatory and joint tissue metabolic biomarkers would be upregulated in the synovial fluid of subjects with a meniscus tear.
Materials and Methods
Sample Collection and Subject Demographics
Sixteen subjects with an isolated unilateral meniscus injury were recruited and provided informed consent for this study with approval by the Duke University Medical Center Institutional Review Board (IRB). Eleven subjects had a complex tear of the medial meniscus, while the remaining five subjects had other types of tears including horizontal, complex lateral, and bucket handle tears. Thirteen subjects could recall when the time of injury occurred, while 3 subjects could not. For these 13 subjects, the time between injury and surgery ranged from 4 to 34 weeks with a median of 7 weeks and an average of 10.8 weeks. As part of the IRB approved protocol, serum and synovial fluid were collected by small volume lavage at the time of meniscal surgery. Serum and synovial fluid samples were centrifuged for 15 minutes at 3500 rpm and 4°C. Aliquots of the samples were prepared and stored at −80°C until biomarker analyses were performed.
Synovial fluid was also analyzed from six subjects who participated in the Strategies to Predict OA Progression (POP) study (36). Synovial fluid from radiographic non-OA knees with Kellgren Lawrence (KL) grades of 0–1 (37) served as reference controls for biomarker analyses.
Biomarker Analyses
Matrix metalloproteinase (MMP) activity, MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, MMP-13, sulfated glycosaminoglycan (sGAG), cartilage oligomeric matrix protein (COMP), type II collagen cleavage (C2C) neoepitopes, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, tumor necrosis factor α (TNFα), prostaglandin E2 (PGE2), and interferon-gamma (IFN-γ) were measured in the serum of meniscus tear subjects and the synovial fluid of both meniscus tear and reference control subjects. Details about the analytes and the assays can be found in Table 1.
Table 1.
Biomarker Assay Parameters
| Analyte | Description | Lower Limit of Detection (pg/ml) | Mean Intra-assay Coefficient of Variation (within plate) (%) | Number of Undetectable Synovial Fluid Samples for: Meniscus Tear (Control) Subjects | Number of Undetectable Serum Samples for Meniscus Tear Subjects |
|---|---|---|---|---|---|
|
| |||||
| Active MMP Activity | Activity due to active MMPs | ND | - | 12 (ND) | 9 |
| Total MMP Activity | Activity due to active and latent MMPs | ND | - | 0 (0) | 0 |
| MMP-1 | Degrades fibrillar collagens | 3.29 | 2.9 | 0 (0) | 0 |
| MMP-2 | Gelatinases that degrade collagens | 109 | 2.2 | 0 (0) | 0 |
| MMP-3 | Degrades collagens, gelatins, aggrecan, and link protein | 2.71 | 1.8 | 0 (0) | 0 |
| MMP-9 | Gelatinases that degrade collagens | 30.3 | 1.4 | 0 (0) | 0 |
| MMP-10 | Degrades collagens, gelatins, aggrecan, and link protein | 4.09 | 6.1 | 0 (0) | 0 |
| MMP-13 | Degrades fibrillar collagens | 6 | 1.7 | 14 (3) | 7 |
| sGAG | Sulfated glycosaminoglycans | N/A | 3.9 | 0 (0) | 0 |
| COMP | Cartilage oligomeric matrix protein | 0.4 | 2.1 | 0 (0) | 0 |
| C2C | Type II collagen cleavage neoepitopes | N/A | 3.3 | 0 (0) | 0 |
| IL-1β | Pro-inflammatory cytokine | 0.363 | 6.9 | 16 (6) | 16 |
| IL-2 | Pro-inflammatory cytokine | 0.123 | 8.5 | 13 (3) | 15 |
| IL-4 | Anti-inflammatory cytokine | 0.022 | 13.5 | 16 (5) | 10 |
| IL-6 | Pro- and anti-inflammatory cytokine | 0.096 | 5.5 | 3 (0) | 0 |
| IL-8 | Pro-inflammatory cytokine | 0.076 | 3.5 | 0 (0) | 0 |
| IL-10 | Anti-inflammatory cytokine | 0.035 | 8.2 | 14 (4) | 0 |
| IL-12p70 | Pro-inflammatory cytokine | 0.173 | 23.7 | 16 (6) | 16 |
| IL-13 | Anti-inflammatory cytokine | 0.898 | 9.7 | 16 (6) | 16 |
| TNFα | Pro-inflammatory cytokine | 0.108 | 4.8 | 3 (2) | 0 |
| PGE2 | Pro-inflammatory mediator | 30.9 | 3.9 | 0 (0) | 0 |
| IFN-γ | Pro-inflammatory cytokine | 0.419 | 5.5 | 13 (3) | 0 |
ND: not detectable
N/A: not applicable
MMP activity was measured, as previously published, using the quenched fluorogenic substrate Dab-Gly-Pro-Leu-Gly-Met-Arg-Gly-Lys-Flu (Sigma-Aldrich; St. Louis, MO) (38, 39). In order to activate proMMPs, 10mM p-aminophenylmercuric acetate (APMA, Sigma-Aldrich) in 0.1M NaOH was diluted 1:4 in assay buffer (40), containing 200 mM NaCl, 50 mM Tris, 5 mM CaCl2, 10 μM ZnSO4, 0.01% Brij 35 and the pH was adjusted to 7.5. In duplicate, the lavaged synovial fluid and 1:2 diluted serum samples (45μl) were incubated with 5μl of 2.5mM APMA at 37°C for 5 hours. These samples (total MMP activity) and samples without prior APMA activation (active MMP activity) were then diluted two-fold in assay buffer containing 20 μM of the quenched fluorogenic substrate and 100 ng/mL of the broad spectrum MMP inhibitor GM6001 (EMD Biosciences Inc, San Diego, CA) or 100 ng/mL GM6001 negative control (EMD Biosciences). The fluorogenic substrate sequence is most specific for MMP-13 cleavage, but can also be cleaved by MMP-1, MMP-2, MMP-3, and MMP-9 (40). Samples were incubated for 2 hours in the dark at 37°C and then fluorescence was measured at 485 nm excitation and 535 nm emission. Total MMP activity was calculated for each sample by subtracting the fluorescence in the samples incubated with GM6001 from the fluorescence in the samples incubated with the GM6001 negative control. All values were corrected for dilution. For meniscus tear subjects, active MMP activity (without APMA activation) at the time of surgery was undetectable in most samples (Table 1). Due to volume constraints, active MMP activity was not measured in control subjects.
All other assays were performed according to the manufacturers’ instructions. MMP-1, MMP-3, and MMP-9 levels were measured using the MMP 3-plex assay (MSD, Rockville, MD). MMP-2 and MMP-10 levels were measured using the MMP 2-plex assay (MSD). Due to limited sample volume, only 5 of the 6 control synovial fluid samples were tested for the MMP multiplex assays. The MSD kits used for MMP profiling can detect both latent and active enzymes.
Sulfated GAG content was measured using an alcian blue assay (Kamiya Biomedical Company, Seattle, WA). Enzyme-linked immunosorbent assays (ELISAs) were used to measure the following biomarker levels in serum and synovial fluid: MMP-13 (Abcam, Cambridge, MA), cartilage oligomeric matrix protein (COMP, Biovendor, Czech Republic), C-terminal ¾ fragment of type II collagen (C2C, Ibex, Montreal Canada), and prostaglandin E2 (PGE2, R&D Systems, Minneapolis, MN). MMP-13 was undetectable in most samples, as shown in Table 1.
The pro-inflammatory panel 1 (MSD) was used to determine the levels of the following cytokines: IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, and IFN-γ. IL-1β, IL-2, IL-4, IL-10, IL-12p70, IL-13, and IFN-γ were undetectable in some samples, as indicated in Table 1. If an analyte was undetectable in at least 50% of samples, then the analyte was excluded from further analyses. In cases where less than 50% of samples were undetectable, the values were input as half the lower limit of detection (Table 1).
The final biomarker concentrations in the lavaged synovial fluid were corrected for dilution based on urea concentrations (41). Urea nitrogen reagents (Pointe Scientific, Canton, MI) and urea standards (CMA Microdialysis, North Chelmsford, MA) were used to measure urea in the matched serum and synovial fluid samples in order to determine the dilution factor (DF) for the lavaged synovial fluid based on the following calculation: DF = (Serum Urea + 0.897)/Synovial Fluid Urea.
Statistical Analyses
Descriptive statistics in the form of means and standard errors (SE) and medians and interquartile range (IQR) were calculated for each biomarker across each group. Mann-Whitney U tests were performed to detect differences between demographic data for the control and meniscus tear groups, between biomarker concentrations of meniscus tear and control subjects, and between meniscus tear types. Serum and synovial fluid concentrations were compared using Wilcoxon Matched-Pairs. In addition, Spearman correlations were performed to determine if there were correlations between serum and synovial fluid concentrations. Spearman correlations were also performed to determine the relationship between time since injury and serum and synovial fluid concentrations and to determine correlations between the synovial fluid biomarkers in meniscus tear subjects. Differences were considered statistically significant where p < 0.05.
Results
Subject Demographics
The meniscus tear group consisted of 13 males and 3 females with ages ranging from 33 to 62 years and an average age of 48 years (Table 2). Their body mass indices (BMI) ranged from 21 to 33 kg/m2 with an average BMI of 27 kg/m2.
Table 2.
Subject Demographics
| Subject | Sex | Age (years) | BMI (kg/m2) | Tear Type |
|---|---|---|---|---|
| Control - 1 | Male | 69 | 29 | N/A |
| Control - 2 | Male | 69 | 31 | N/A |
| Control - 3 | Female | 44 | 25 | N/A |
| Control - 4 | Female | 42 | 33 | N/A |
| Control - 5 | Male | 80 | 30 | N/A |
| Control - 6 | Male | 69 | 29 | N/A |
| Meniscus Tear - 1 | Male | 39 | 24 | Other |
| Meniscus Tear - 2 | Male | 51 | 24 | Complex |
| Meniscus Tear - 3 | Male | 41 | 27 | Complex |
| Meniscus Tear - 4 | Male | 52 | 30 | Other |
| Meniscus Tear - 5 | Male | 49 | 32 | Other |
| Meniscus Tear - 6 | Male | 33 | 25 | Other |
| Meniscus Tear - 7 | Female | 36 | 21 | Other |
| Meniscus Tear - 8 | Male | 59 | 22 | Complex |
| Meniscus Tear - 9 | Male | 62 | 28 | Complex |
| Meniscus Tear - 10 | Female | 51 | 24 | Complex |
| Meniscus Tear - 11 | Female | 53 | 29 | Complex |
| Meniscus Tear - 12 | Male | 55 | 28 | Complex |
| Meniscus Tear - 13 | Male | 35 | 25 | Complex |
| Meniscus Tear - 14 | Male | 47 | 33 | Complex |
| Meniscus Tear - 15 | Male | 58 | 31 | Complex |
| Meniscus Tear - 16 | Male | 48 | 25 | Complex |
N/A: not applicable
Other: Horizontal, bucket handle, or complex lateral
The reference control group, which included subjects who had at least one KL 0–1 knee, consisted of 4 males and 2 females with an age range of 42–81 years and average age of 62 years (Table 2). These subjects had BMI ranging from 25 to 33 kg/m2 with an average BMI of 30 kg/m2. No statistically significant differences were detected between the subjects in the reference control group (KL 0–1) and meniscus tear groups with regard to age (p=0.06), sex (p=0.64), or BMI (p=0.07). Additionally, after separating the meniscus tear group into subsets based on tear type, consisting of complex or other (horizontal, complex lateral, or bucket handle) tear types, there were no significant differences by age (p=0.09), sex (p=1.00), or BMI (p=0.91).
Synovial Fluid Biomarkers Are Altered in Meniscus Tear Subjects
In order to identify biomarkers that are altered in subjects with a meniscus injury, levels of synovial fluid biomarkers were compared between meniscus tear knees and reference control radiographic non-OA knees. Average and median joint tissue metabolic and inflammatory biomarkers concentrations for the reference control group (synovial fluid) and meniscus tear group (synovial fluid and serum) are reported in Table 3.
Table 3.
Synovial Fluid and Serum Biomarker Levels for Control and Meniscus Tear Subjects
|
|
||||||
|---|---|---|---|---|---|---|
| Biomarker | Control Synovial Fluid mean±SE (median, IQR) |
Meniscus Tear Synovial Fluid mean±SE (median, IQR) |
Control Synovial Fluid vs Meniscus Tear Synovial Fluid p-value |
Meniscus Tear Serum mean±SE (median, IQR) |
Meniscus Tear Synovial Fluid vs Serum p-value |
|
| Total MMP Activity (FU/ml) | 1451±616 (1124, 1724) | 35524±8147 (34140, 59258) | <0.001 | 853±165 (942, 1012) | <0.001 | |
| MMP-1 (ng/ml) | 23.55±19.60 (5.32, 5.18) | 26.33±15.62 (4.62, 20.08) | 0.93 | 13.24±3.03 (9.87, 8.12) | 1.00 | |
| MMP-2 (ng/ml) | 188±46 (220, 135) | 150±14 (164, 45) | 0.35 | 80±2 (81, 12) | 0.002 | |
| MMP-3 (ng/ml) | 1633±1412 (205, 270) | 838±586 (160, 368) | 0.45 | 16±2 (15, 10) | 0.002 | |
| MMP-9 (ng/ml) | 1.38±0.41 (1.20, 1.09) | 1.05±0.24 (0.84, 1.27) | 0.45 | 184±25 (200, 129) | 0.0004 | |
| MMP-10 (ng/ml) | 0.70±0.13 (0.72, 0.45) | 0.16±0.07 (0.02, 0.17) | <0.01 | 0.96±0.10 (0.85, 0.36) | <0.001 | |
| s-GAG (μg/ml) | 143±31 (153, 34) | 69±12 (61, 44) | <0.05 | 9±1 (10, 5) | <0.001 | |
| COMP (ng/ml) | 34745±14723 (18265, 33013) | 19636±3928 (14723, 13172) | 0.51 | 798±779 (76.90, 486.21) | 0.0004 | |
| C2C (ng/ml) | 104±23 (93, 34) | 100±13 (79, 54) | 0.66 | 172±7 (171, 41) | 0.001 | |
| IL-6 (pg/ml) | 49.42±37.64 (9.65, 31.60) | 30.22±14.83 (2.81, 17.51) | 0.59 | 0.86±0.23 (0.44, 0.40) | 0.0004 | |
| IL-8 (pg/ml) | 22.03±4.35 (25.61, 11.72) | 12.84±4.02 (6.81, 6.34) | 0.08 | 33.84±14.50 (10.99, 15.15) | <0.05 | |
| IL-10 (pg/ml) | ND | ND | - | 0.22±0.02 (0.19, 0.08) | - | |
| TNFα (pg/ml) | 0.90±0.25 (0.85, 0.68) | 0.97±0.11 (0.91, 0.50) | 0.69 | 7.59±2.65 (2.40, 7.34) | 0.0004 | |
| PGE2 (pg/ml) | 136±97 (43.62, 34.77) | 39877±16773 (24652, 21268) | <0.0001 | 792±400 (212, 162) | <0.0005 | |
| IFN-γ (pg/ml) | ND | ND | - | 6.12±0.89 (5.87, 3.66) | - | |
The p-values in bold indicate significant differences between meniscus tear synovial fluid and serum concentrations. ND = not detectable
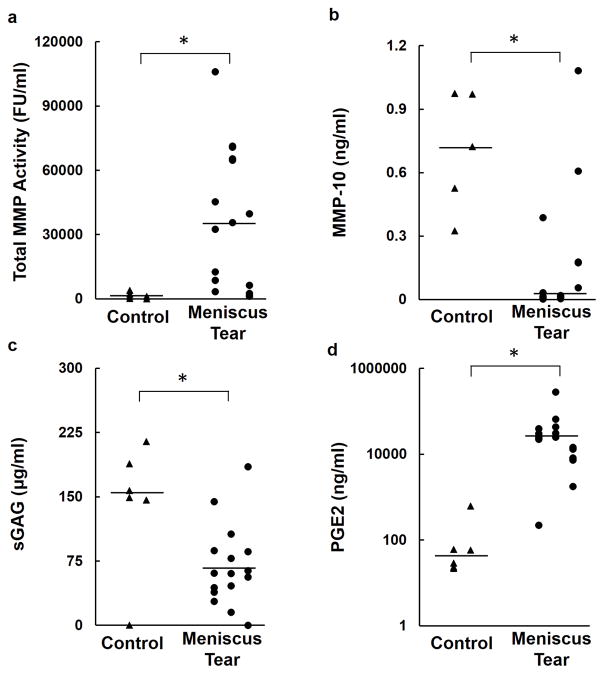
Total MMP activity was elevated nearly 25-fold in the synovial fluid of meniscus tear knees compared to control knees (p<0.001, Figure 1a). In contrast, the meniscus tear group had significantly lower synovial fluid concentrations of MMP-10 (p<0.01, Figure 1b) and sGAG (p<0.05, Figure 1c) than the control knees. Among the inflammatory markers, synovial fluid PGE2 concentrations were elevated over 290-fold in meniscus tear knees compared to the control knees (p<0.0001, Figure 1d). No significant differences were detected between groups for any of the other synovial fluid markers (Table 3).
Figure 1.
Synovial fluid levels of (a) Total MMP Activity (b) MMP-10 (c) sGAG and (d) PGE2 in control and meniscus tear subjects. The line indicates the median of the data. * p<0.05.
Synovial Fluid and Serum Biomarkers in Meniscus Tear Subjects
In order to assess the predominant source of the joint tissue metabolic and inflammatory biomarkers, we compared the concentrations in matched synovial fluid and serum (Table 3). In meniscus tear subjects, total MMP activity (p<0.001), MMP-2 (p=0.002), MMP-3 (p=0.002), sGAG (p<0.001), COMP (p=0.0004), IL-6 (p=0.0004), and PGE2 (p<0.0005) concentrations were all significantly higher in the synovial fluid compared to serum. In contrast, MMP-9 (p=0.0004), MMP-10 (p<0.001), C2C (p=0.001), TNFα (p=0.0004), and IL-8 (p<0.05) concentrations were all significantly lower in synovial fluid than serum. In the meniscus tear subjects, no significant correlations were detected between the serum and synovial fluid for any individual biomarker (data not shown).
In addition, we assessed the effect of time from injury on the meniscus tear biomarkers. Serum concentrations of MMP-10 (R=−0.76, p=0.002) and IL-6 (R=−0.59, p<0.05) decreased significantly with time from injury (data not shown). There was no detectable relationship between time from injury and any of the other measured synovial fluid or serum biomarkers.
Synovial Fluid Biomarker Correlations
Synovial fluid biomarkers that were altered in the meniscus tear knees were further evaluated to assess relationships with other synovial fluid biomarkers (Table 4). Importantly, there was a strong correlation (R=0.83, p<0.0001) between total MMP activity and PGE2, the two biomarkers most elevated after meniscal tear. On the other hand, synovial fluid total MMP activity was negatively correlated with synovial fluid MMP-3 (R=−0.56, p<0.05), MMP-9 (R=−0.63, p<0.01), and MMP-10 (R=−0.75, p<0.001). Synovial fluid sGAG was positively correlated to multiple synovial fluid biomarkers including: MMP-1 (R=0.51, p<0.05), MMP-2 (R=0.70, p=0.003), MMP-3 (R=0.70, p<0.05), MMP-9 (R=0.59, p<0.05), COMP (R=0.78, p=0.0004), IL-6 (R=0.58, p<0.05), and IL-8 (R=0.58, p<0.05). PGE2 was also negatively correlated with MMP-1 (R=−0.64, p<0.05), MMP-3 (R=−0.74, p<0.001), and MMP-9 (R=−0.78, p=0.0003).
Table 4.
Spearman Correlations of Synovial Fluid Biomarkers in Meniscus Tear Subjects
| Synovial Fluid Correlations | R | p-value |
|---|---|---|
| MMP Activity | ||
| Synovial Fluid MMP-3 | −0.56 | <0.05 |
| Synovial Fluid MMP-9 | −0.63 | <0.01 |
| Synovial Fluid MMP-10 | −0.75 | <0.001 |
| Synovial Fluid PGE2 | 0.83 | <0.0001 |
| MMP-10 | ||
| Synovial Fluid MMP-9 | 0.61 | <0.05 |
| Synovial Fluid PGE2 | −0.77 | <0.001 |
| sGAG | ||
| Synovial Fluid MMP-1 | 0.51 | <0.05 |
| Synovial Fluid MMP-2 | 0.70 | 0.003 |
| Synovial Fluid MMP-3 | 0.59 | <0.05 |
| Synovial Fluid MMP-9 | 0.59 | <0.05 |
| Synovial Fluid COMP | 0.78 | 0.0004 |
| Synovial Fluid IL-6 | 0.58 | <0.05 |
| Synovial Fluid IL-8 | 0.58 | <0.05 |
| PGE2 | ||
| Synovial Fluid MMP-1 | −0.64 | <0.05 |
| Synovial Fluid MMP-3 | −0.74 | <0.001 |
| Synovial Fluid MMP-9 | −0.78 | 0.0003 |
Biomarkers Are Altered by Meniscus Tear Type
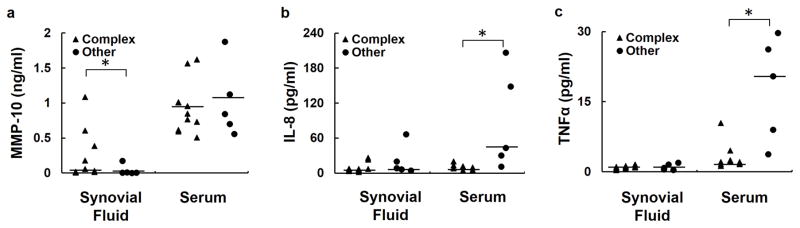
Meniscus tear biomarkers were further examined to determine if there were significant differences between subjects with complex medial meniscus tears versus other tear types, including horizontal, complex lateral, and bucket handle tears. The complex tear subjects had significantly higher synovial fluid (p<0.05) but not serum (p=0.87) MMP-10 compared to the other tear group (Figure 2a). Complex tear subjects had significantly reduced serum IL-8 (p<0.05, Figure 2b) and TNFα (p<0.05, Figure 2c) compared to the subjects with other tear types. For all other biomarkers, no differences in synovial fluid concentrations between groups (p>0.25) were detected (data not shown).
Figure 2.
Serum and synovial fluid levels of (a) MMP-10, (b) IL-8, and (c) TNFα by meniscus tear type. The line indicates the median of the data. * p<0.05.
Discussion
The present study evaluated synovial fluid and serum biomarkers in meniscus tear subjects and synovial fluid biomarkers in reference control knees, providing a snapshot into the joint tissue metabolic and inflammatory changes associated with meniscus injury. This study identified significant differences in both total MMP activity and PGE2 concentrations in the synovial fluid of subjects with meniscus tears relative to subjects with a radiographic non-OA knee. The results suggest that these catabolic and pro-inflammatory mediators may be biomarkers of meniscus injury and may play a role in subsequent joint degeneration. We did not detect a correlation between synovial fluid and serum biomarker levels, highlighting the importance of collecting synovial fluid for surveillance of post-injury inflammation and joint tissue metabolism. This small sample also provided preliminary evidence that local and systemic biological differences may exist between subjects with different types of meniscus tears.
One of the primary findings of this study is that total MMP activity in the synovial fluid of meniscus tear subjects was significantly elevated, as compared to the reference control subjects. Given that we previously observed a significant positive correlation of total synovial fluid MMP activity with increased cartilage strain following meniscal injury (18), the elevated MMP activity detected in our current study likely reflects increased cartilage strain following meniscus injury in these subjects. The collagenases, MMP-1, MMP-8, MMP-13, and membrane type 1 MMP (MT1-MMP), are the only enzymes capable of degrading intact triple helical collagen (42). The MMP activity measured in this study may be attributable to a broad range of different MMPs. However, we observed that synovial fluid total MMP activity in meniscus tear subjects was negatively correlated with synovial fluid concentrations of MMP-3, MMP-9, and MMP-10, indicating that these enzymes are likely not contributing to the increased MMP activity. MMP-10 was also decreased in the synovial fluid of meniscus tear subjects as compared to the reference controls, further suggesting that MMP-10 is likely not mediating the enhanced synovial fluid total MMP activity in the meniscus tear subjects. Although MMP-2 was elevated in synovial fluid as compared to serum, it was not increased in meniscus tear knees compared to the reference controls and there was no correlation between MMP-2 and total MMP activity in the synovial fluid. While a study in a mouse model of medial meniscus destabilization showed that MMP-13 activity was elevated for at least eight weeks following surgery (43), in this study MMP-13 was undetectable in the majority of the synovial fluid samples. Our results suggest that other MMPs or a combination of MMPs are likely contributing to the elevated activity. Recently, MMP-12 gene expression and MMP activity were shown to be elevated in a mouse model of meniscus destabilization (44). In addition, gene expression of MMP-1, MMP-9, and MMP-13 is elevated in patients following a meniscus tear (45). In a rat medial meniscal tear model of joint injury, broad spectrum MMP inhibition protects against the development of PTOA (46). Our findings are also consistent with previous work showing that broad spectrum MMP inhibition, rather than individual MMP inhibitors, is necessary to promote integrative meniscal repair in the presence of IL-1 (38). These findings suggest that a combination of MMPs may be responsible for the increased total MMP activity in the synovial fluid of meniscus tear subjects.
The total MMP activity measured in this study is a combination of the activity attributable to both secreted pro-MMPs and active MMPs. Similarly, the individual MMP protein concentrations indicate the quantity of these proteins but do not indicate the percentage of latent or active enzymes. We were not able to detect active MMPs in the majority of the synovial fluid samples from the meniscus tear subjects; therefore, most of the MMPs are currently in the latent form. However, several different factors in the joint can activate the pro-MMPs, including MMP-3, MMP-10 (47), MT-MMPs, plasmin (48, 49), and activated protein C (50). Therefore, the total MMP activity provides a surrogate measure of the potential MMP activity in the joint that could contribute to the degradation of ECM components in joint tissues and ultimately lead to PTOA development.
Synovial fluid total MMP activity and synovial fluid PGE2 concentrations in meniscus tear subjects were strongly correlated and higher than reference controls. Similar to our findings, a previous study in OA cartilage showed that PGE2 causes upregulation of MMP-13 expression and activity, downregulation of MMP-1, and decreased sGAG synthesis (51). Previous studies have also shown that PGE2 is increased in OA synovial fluid (52) and is mechanically regulated in cartilage and meniscus (53–55). PGE2 is a lipid mediator involved in inflammation, apoptosis, and potentially structural changes characteristic of OA (56, 57). Additionally, prostaglandin E synthase, an enzyme that regulates PGE2 production, is upregulated by the pro-inflammatory cytokines IL-1 and TNFα, but not IL-4, IL-6, IL-8, IL-10, or IFN-γ (58) and is increased in a rat model following ACL transection and partial medial meniscectomy (59). Therefore, immediately after meniscus injury, increased levels of IL-1, TNFα, cartilage strain (18), or a combination of these factors may drive the profoundly elevated levels of synovial fluid PGE2 detected at the time of surgery.
This study was cross-sectional in nature; therefore, we are not able to determine if the elevated total MMP activity and PGE2 levels in the meniscus tear subjects may have been present prior to meniscal injury or were a consequence of injury. In a recent study, serum C1, 2C and C2C concentrations were found to be associated with subsequent ACL injury in patients (35), suggesting that these systemic concentrations of type I and II collagen neoepitopes may reflect biochemical, genetic, or biomechanical changes in subjects that are susceptible to joint injury (35). In this study, we found that C2C levels were significantly lower in synovial fluid than serum of meniscus tear subjects. However, it is not possible to conclude from this study if the serum C2C concentrations were elevated in meniscus tear subjects prior to injury or indicative of type II collagen cleavage following injury. Future studies will be needed to evaluate subjects prior to joint injury, as well as after injury, to determine if any of these biomarker changes are predictive of PTOA development.
Nearly all measurable biomarkers were significantly different between the serum and synovial fluid of meniscus tear subjects. These findings are consistent with previous work in patients with an acute ACL injury (29). These patients also had different concentrations of synovial fluid and serum biomarkers, including COMP and MMP-3, which were significantly higher in synovial fluid than serum (29). Higher concentrations of biomarkers in the synovial fluid likely reflect the local joint origin of the biomarkers, while serum concentrations reflect the systemic response due to injury and may be confounded by other tissue sources (29, 60). This suggests that synovial fluid and serum collection are important to understanding local and systemic biological changes associated with a meniscus tear. In addition, many of the biomarkers measured in this study are not specific to either the cartilage or the meniscus. Currently, there are no known meniscus-specific biomarkers. Therefore, future work to identify markers that are specific to meniscus tissue would represent a significant advance for monitoring meniscus turnover.
Upon stratification of the meniscus tear subjects by type of meniscus tear, we detected differences in both local synovial fluid and systemic biomarkers between the tear types. It is well known that meniscus tears in the outer zone tend to heal more readily than tears in the inner zone, which has largely been attributed to the vascular supply (61). As well, horizontal and longitudinal tears in the vascularized outer region are generally recommended for repair, but repair of other types of tears is generally less favorable (61, 62). Still, there is a lack of data on the biologic changes at the joint level and systemically following different types of meniscus tears. Despite our small sample sizes and broad grouping of meniscus tears, our results suggest that the type of tear influences the biological changes associated with meniscus injury. Therefore, future work is needed to investigate biomarker profiles in different meniscus tear locations and types in order to determine the mechanistic biological changes that are occurring in these patients and their relationship to subsequent clinical outcomes.
In our study, we found that MMP-10 and sGAG levels were significantly lower in the meniscus tear group than the reference control knees. The reference control knees were classified radiographically as non-OA (KL grade 0–1), but the contralateral knee of each reference control subject was symptomatic and classified as KL grade 1–3. The reference control synovial fluid was taken from the radiographically non-OA knee but may have been confounded by OA biomarkers from the serum of these patients. On the other hand, the low sGAG in our meniscal tear subjects is consistent with a recent ACL injury study showing similar concentrations of synovial fluid sGAG at 9 weeks following injury (63). Furthermore, in the synovial fluid of meniscus tear subjects, the sGAG concentrations were positively correlated with the pro-inflammatory cytokines IL-6 and IL-8 and the degradative enzymes MMP-1, MMP-2, MMP-3, and MMP-9, which may be mediating the degradation and release of sGAG from the ECM of the cartilage and meniscus (64, 65).
Other studies investigating inflammatory biomarker levels in the synovial fluid of subjects with meniscus tears observed much higher levels of IL-6, IL-8, and TNFα (21, 66). Additionally, levels of IL-1β, IL-2, IL-4, IL-10, IL-12p70, IL-13, and IFN-γ were detected in their meniscus tear groups (21, 66), while we did not detect these synovial fluid cytokines in our meniscus tear subjects. These differences may be attributed to the varying methodological approaches. The present study evaluated subjects without clinical OA changes that were recommended for surgery after meniscus injury, while the previous studies investigated subjects that were recommended for non-operative management (21) or subjects that had concomitant OA (66). There were also varying analytical methods used between these studies. The present study corrected for synovial fluid dilution due to lavage, while a previous study also collected synovial fluid by lavage but did not correct for the inherent dilution (21), potentially explaining some differences in our findings. The time from injury (mean 10.8 weeks) to sample collection may have affected our ability to detect transient elevations of pro-inflammatory cytokines (65, 67), particularly for serum MMP-10 and serum IL-6, which showed a negative correlation with time from injury in this study. Future studies would benefit from serial biospecimen collection starting at the time of meniscus injury and at multiple and standardized time points thereafter.
In conclusion, based on analyses of inflammatory and joint tissue metabolic biomarkers, we have shown that synovial fluid total MMP activity and synovial fluid PGE2 were both significantly elevated and correlated in knees with meniscus tears. Given the pro-inflammatory and degradative roles of these molecules, our findings suggest that modulation of PGE2 signaling, total MMP activity, or both following a meniscus injury may alter the biochemical environment of the joint to promote meniscus repair and possibly prevent OA development. Future studies are needed to investigate the relationship of these biomarkers with clinical outcomes following meniscus injury to identify patients that may be at risk of developing OA and to develop therapeutic treatments that may prevent OA development.
Acknowledgments
Funding
National Institutes of Health grants AR63325, AR65527, AR48182, AR48852, AG15768, AR50245, AG46927, and AG28716 and a VA Rehabilitation Research Service Award.
The technical assistance provided by Elizabeth Pennington and Dr. William R. Wilson, Jr. and the intellectual contributions by Dr. John Martin are gratefully acknowledged.
Footnotes
Declaration of Interest
The authors of this manuscript have no conflicts of interest related to the content of this study.
References
- 1.Salata MJ, Gibbs AE, Sekiya JK. A systematic review of clinical outcomes in patients undergoing meniscectomy. Am J Sports Med. 2010;38(9):1907–1916. doi: 10.1177/0363546510370196. [DOI] [PubMed] [Google Scholar]
- 2.Baker BS, Lubowitz JH, Wolf BT. Meniscus injuries: Emedicine from WebMD. [updated Oct 3, 2012. Available from: http://www.emedicine.com/sports/TOPIC160.HTM.
- 3.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664–670. [PubMed] [Google Scholar]
- 4.Seedhom BB. Loadbearing function of the menisci. Physiotherapy. 1976;62(7):223. [PubMed] [Google Scholar]
- 5.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Walker PS, Arno S, Bell C, Salvadore G, Borukhov I, Oh C. Function of the medial meniscus in force transmission and stability. J Biomech. 2015;48(8):1383–1388. doi: 10.1016/j.jbiomech.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000;18(1):109–115. doi: 10.1002/jor.1100180116. [DOI] [PubMed] [Google Scholar]
- 8.Badlani JT, Borrero C, Golla S, Harner CD, Irrgang JJ. The effects of meniscus injury on the development of knee osteoarthritis: Data from the osteoarthritis initiative. Am J Sports Med. 2013;41(6):1238–1244. doi: 10.1177/0363546513490276. [DOI] [PubMed] [Google Scholar]
- 9.Roos H, Laurén M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: Prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis & Rheumatism. 1998;41(4):687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Cannon WD, Jr, Vittori JM. The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am J Sports Med. 1992;20(2):176–181. doi: 10.1177/036354659202000214. [DOI] [PubMed] [Google Scholar]
- 11.Venkatachalam S, Godsiff SP, Harding ML. Review of the clinical results of arthroscopic meniscal repair. Knee. 2001;8(2):129–133. doi: 10.1016/s0968-0160(01)00061-8. [DOI] [PubMed] [Google Scholar]
- 12.Mordecai SC, Al-Hadithy N, Ware HE, Gupte CM. Treatment of meniscal tears: An evidence based approach. World J Orthop. 2014;5(3):233–241. doi: 10.5312/wjo.v5.i3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 14.McAdams TR, Mithoefer K, Scopp JM, Mandelbaum BR. Articular cartilage injury in athletes. Cartilage. 2010;1(3):165–179. doi: 10.1177/1947603509360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res. 1994;12(1):21–28. doi: 10.1002/jor.1100120104. [DOI] [PubMed] [Google Scholar]
- 16.Koryem HK, Wanas MAAEQ, Rizk MMA, Kotb HT, Naguib AH, Shafei MMAHE, Naby HMAAE. Evaluation of early changes of cartilage biomarkers following arthroscopic meniscectomy in young Egyptian adults. Alexandria Journal of Medicine. 2015;51(3):191–197. [Google Scholar]
- 17.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, Nair A, Lee DM, Richmond JC, Katz JN, Crow MK, Goldring SR. Synovial inflammation in patients undergoing arthroscopic meniscectomy: Molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63(2):391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter TE, Taylor KA, Spritzer CE, Utturkar GM, Taylor DC, Moorman CT, 3rd, Garrett WE, Guilak F, McNulty AL, DeFrate LE. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech. 2015;48(8):1461–1468. doi: 10.1016/j.jbiomech.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31(7):1039–1045. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, Collette J, Cooper C, Giacovelli G, Kanis JA, Karsdal MA, Kraus V, Lems WF, Meulenbelt I, Pelletier JP, Raynauld JP, Reiter-Niesert S, Rizzoli R, Sandell LJ, Van Spil WE, Reginster JY. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann Rheum Dis. 2013;72(11):1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009;91(10):2313–2320. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- 22.Larsson S, Englund M, Struglics A, Lohmander LS. Association between synovial fluid levels of aggrecan ARGS fragments and radiographic progression in knee osteoarthritis. Arthritis Res Ther. 2010;12(6):R230. doi: 10.1186/ar3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Arman MM, El-Fayoumi G, El-Shal E, El-Boghdady I, El-Ghaweet A. Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSS J. 2010;6(2):171–176. doi: 10.1007/s11420-010-9157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozler K, Aktas E, Atay C, Yilmaz B, Arikan M, Gungor S. Serum and knee synovial fluid matrix metalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late-stage osteoarthritis. Acta Orthop Traumatol Turc. 2016;50(3):356–361. doi: 10.3944/AOTT.2015.15.0115. [DOI] [PubMed] [Google Scholar]
- 25.Sharif M, Granell R, Johansen J, Clarke S, Elson C, Kirwan JR. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology (Oxford) 2006;45(5):522–526. doi: 10.1093/rheumatology/kei216. [DOI] [PubMed] [Google Scholar]
- 26.Otterness IG, Swindell AC, Zimmerer RO, Poole AR, Ionescu M, Weiner E. An analysis of 14 molecular markers for monitoring osteoarthritis: Segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage. 2000;8(3):180–185. doi: 10.1053/joca.1999.0288. [DOI] [PubMed] [Google Scholar]
- 27.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54(8):2496–2504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]
- 28.Ahlen M, Roshani L, Liden M, Struglics A, Rostgard-Christensen L, Kartus J. Inflammatory cytokines and biomarkers of cartilage metabolism 8 years after anterior cruciate ligament reconstruction: Results from operated and contralateral knees. Am J Sports Med. 2015;43(6):1460–1466. doi: 10.1177/0363546515574059. [DOI] [PubMed] [Google Scholar]
- 29.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams SB, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler T, Kraus VB, Leimer EM, Olson SA, Nettles DL. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot Ankle Int. 2015;36(11):1264–1271. doi: 10.1177/1071100715611176. [DOI] [PubMed] [Google Scholar]
- 31.Furman BD, Kimmerling KA, Zura RD, Reilly RM, Zlowodzki MP, Huebner JL, Kraus VB, Guilak F, Olson SA. Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol. 2015;67(5):1234–1239. doi: 10.1002/art.39064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svoboda SJ, Harvey TM, Owens BD, Brechue WF, Tarwater PM, Cameron KL. Changes in serum biomarkers of cartilage turnover after anterior cruciate ligament injury. Am J Sports Med. 2013;41(9):2108–2116. doi: 10.1177/0363546513494180. [DOI] [PubMed] [Google Scholar]
- 33.Tourville TW, Poynter ME, DeSarno MJ, Struglics A, Beynnon BD. Relationship between synovial fluid ARGS-aggrecan fragments, cytokines, MMPs, and TIMPs following acute ACL injury: A cross-sectional study. J Orthop Res. 2015;33(12):1796–1803. doi: 10.1002/jor.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumahashi N, Sward P, Larsson S, Lohmander LS, Frobell R, Struglics A. Type II collagen C2C epitope in human synovial fluid and serum after knee injury--associations with molecular and structural markers of injury. Osteoarthritis Cartilage. 2015;23(9):1506–1512. doi: 10.1016/j.joca.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Svoboda SJ, Owens BD, Harvey TM, Tarwater PM, Brechue WF, Cameron KL. The association between serum biomarkers of collagen turnover and subsequent anterior cruciate ligament rupture. Am J Sports Med. 2016;44(7):1687–1693. doi: 10.1177/0363546516640515. [DOI] [PubMed] [Google Scholar]
- 36.Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, Coleman RE. Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis. 2009;68(11):1673–1679. doi: 10.1136/ard.2008.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467(6):1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res. 2008;26(4):504–512. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng SJ, Bickett DM, Mitchell JL, Lambert MH, Blackburn RK, Carter HL, 3rd, Neugebauer J, Pahel G, Weiner MP, Moss ML. Substrate specificity of human collagenase 3 assessed using a phage-displayed peptide library. Journal of Biological Chemistry. 2000;275(40):31422–31427. doi: 10.1074/jbc.M004538200. [DOI] [PubMed] [Google Scholar]
- 41.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, Guilak F. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002;46(2):420–427. doi: 10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 42.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim NH, Meinjohanns E, Meldal M, Bou-Gharios G, Nagase H. In vivo imaging of MMP-13 activity in the murine destabilised medial meniscus surgical model of osteoarthritis. Osteoarthritis Cartilage. 2014;22(6):862–868. doi: 10.1016/j.joca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Vermeij EA, Koenders MI, Blom AB, Arntz OJ, Bennink MB, van den Berg WB, van de Loo FA. In vivo molecular imaging of cathepsin and matrix metalloproteinase activity discriminates between arthritic and osteoarthritic processes in mice. Molecular Imaging. 2014;13(2) [PubMed] [Google Scholar]
- 45.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94(5):385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10(10):785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 47.Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, Lakey R, Middleton J, Cawston TE, Richards CD, Rowan AD. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: Implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54(10):3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 48.Milner JM, Elliott S-F, Cawston TE. Activation of procollagenases is a key control point in cartilage collagen degradation: Interaction of serine and metalloproteinase pathways. Arthritis and Rheumatism. 2001;44(9):2084–2096. doi: 10.1002/1529-0131(200109)44:9<2084::AID-ART359>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 49.Loffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: A critical balance. Eur Respir J. 2011;38(1):191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 50.Jackson MT, Moradi B, Smith MM, Jackson CJ, Little CB. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol. 2014;66(6):1525–1536. doi: 10.1002/art.38401. [DOI] [PubMed] [Google Scholar]
- 51.Attur M, Al-Mussawir HE, Patel J, Kitay A, Dave M, Palmer G, Pillinger MH, Abramson SB. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J Immunol. 2008;181(7):5082–5088. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura M, Segami N, Kaneyama K, Suzuki T, Miyamaru M. Relationships between pain-related mediators and both synovitis and joint pain in patients with internal derangements and osteoarthritis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(3):328–332. doi: 10.1067/moe.2002.124106. [DOI] [PubMed] [Google Scholar]
- 53.Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47(12):3185–3195. doi: 10.1016/j.jbiomech.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 54.Hennerbichler A, Fermor B, Hennerbichler D, Weinberg JB, Guilak F. Regional differences in prostaglandin E2 and nitric oxide production in the knee meniscus in response to dynamic compression. Biochem Biophys Res Commun. 2007;358(4):1047–1053. doi: 10.1016/j.bbrc.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10(10):792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 56.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33(3):155–167. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 58.Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. 2004;6(4):R355–365. doi: 10.1186/ar1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56(6):1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 60.Kushner I, Somerville JA. Permeability of human synovial membrane to plasma proteins. Relationship to molecular size and inflammation. Arthritis Rheum. 1971;14(5):560–570. doi: 10.1002/art.1780140503. [DOI] [PubMed] [Google Scholar]
- 61.DeHaven KE. Meniscus repair. Am J Sports Med. 1999;27(2):242–250. doi: 10.1177/03635465990270022301. [DOI] [PubMed] [Google Scholar]
- 62.Mordecai SC, Al-Hadithy N, Ware HE, Gupte CM. Treatment of meniscal tears: An evidence based approach. World Journal of Orthopedics. 2014;5(3):233–241. doi: 10.5312/wjo.v5.i3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amano K, Stabler T, Kraus V, Li X, Ma C. Correlating biochemical changes of synovial fluids with cartilage T1ρ after ACL injury. Osteoarthritis and Cartilage. 2016;24:S60–S61. [Google Scholar]
- 64.McDonnell S, Morgan M, Lynch C. Role of matrix metalloproteinases in normal and disease processes. Biochem Soc Trans. 1999;27(4):734–740. doi: 10.1042/bst0270734. [DOI] [PubMed] [Google Scholar]
- 65.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vangsness CT, Jr, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis--a pilot study. Bull NYU Hosp Jt Dis. 2011;69(2):122–127. [PubMed] [Google Scholar]
- 67.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA. Targeting pro-inflammatory cytokines following joint injury: Acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16(3):R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]