Abstract
The adolescent brain, with ongoing prefrontal maturation, may be more vulnerable to drug use-related neurotoxic changes as compared to the adult brain. We investigated whether the use of methamphetamine (MA), a highly addictive psychostimulant, during adolescence affect metabolic and cognitive functions of the anterior cingulate cortex (ACC). In adolescent MA users (n = 44) and healthy adolescents (n = 53), the levels of N-acetyl aspartate (NAA), a neuronal marker, were examined in the ACC using proton magnetic resonance spectroscopy. The Stroop color-word task was used to assess Stroop interference, which may reflect cognitive functions of behavior monitoring and response selection that are mediated by the ACC. Adolescent MA users had lower NAA levels in the ACC (t = −2.88, P = 0.005) and relatively higher interference scores (t = 2.03, P = 0.045) than healthy adolescents. Moreover, there were significant relationships between lower NAA levels in the ACC and worse interference scores in adolescent MA users (r = −0.61, P < 0.001). Interestingly, early onset of MA use, as compared to late onset, was related to both lower NAA levels in the ACC (t = −2.24, P = 0.03) as well as lower performance on interference measure of the Stroop color-word task (t = 2.25, P = 0.03). The current findings suggest that metabolic dysfunction in the ACC and its related cognitive impairment, may play an important role in adolescent-onset addiction, particularly during early adolescence.
Keywords: Adolescents, anterior cingulate cortex, magnetic resonance spectroscopy, methamphetamine, N-acetyl aspartate, Stroop interference
INTRODUCTION
Adolescents are particularly vulnerable to drug use-related neuronal and cognitive changes, as their brains undergo dynamic changes during this stage of development (Smith, 2003). For instance, the use of methamphetamine (MA), a highly neurotoxic stimulant, during adolescence may be associated with cognitive (King et al. 2010) and brain structural deficits (Lyoo et al. 2015). Given the region-specific developmental trajectories and continued prefrontal maturation of the adolescent brain (Giedd et al. 1999), repeated exposure to neurotoxic substances during this critical period may interfere with neurodevelopment of the prefrontal cortex (Cass et al. 2013).
The medial prefrontal regions including the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) are thought to be critically involved in the pathophysiology of addiction (Goldstein & Volkow, 2011). Given the role of the ACC in behavioral monitoring and response selection (Goldstein & Volkow, 2011) and the prematurity of the ACC during adolescence (Velanova et al. 2008), exposure to psychostimulants during this period may lead to dysfunction in the ACC and may render adolescent recreational drug users more vulnerable to chronic addiction.
Based on previous animal studies showing that the selected brain regions including the frontal cortex undergo highly plastic changes in response to drug dependence (Smith, 2003; Crews & Boettiger 2009), it is thus possible that MA exposure during adolescence may have a greater influence on the developing structures, rather than already matured subcortical structures (Crews & Hodge 2007). In alignment with this assumption, adolescent rats, as compared with adult rats, were more vulnerable to amphetamine-induced cognitive impairment, however, less sensitive to psychomotor effects (Sherrill et al. 2013). A recent animal study has also suggested that the exposure to MA during adolescence may lead to a generalized and long-lasting learning impairment (Ye et al. 2014). These cognitive-behavioral deficits may be directly attributed to the neurotoxic effects on the dopaminergic neurotransmitter system (Moratalla et al. 2016; Kalechstein et al. 2008), along with the neuroadaptive changes in the frontocortico-striatal-limbic circuitry (Li & Sinha, 2008). Drug use-related frontocortical dysfunction and resultant inefficient inhibitory control by the prefrontal cortex may eventually lead to repetitive stimulant use (Feil et al. 2010). Interestingly, repeated exposure to psychostimulant during adolescence may be related to the prefrontal disinhibition by impairing the local prefrontal gamma-aminobutyric acid network (Cass et al. 2013). Likewise, using unbiased whole-brain analysis of high-resolution MR images, a recent animal study has reported adolescent-specific vulnerability of the PFC and pronounced sensitization in response to repeated exposures to the psychostimulant (Wheeler et al. 2013). However, surprisingly few human studies have examined the effects of neurotoxic stimulants on the adolescent brain, particularly on the ACC (Lyoo et al. 2015; Sung et al. 2013a), although the use of psychostimulants including MA is often initiated during adolescence (King et al. 2010).
In the current study, we examined whether MA use during adolescence was associated with neurometabolic dysfunction in the ACC as measured by proton magnetic resonance spectroscopy (1H-MRS). Given that the levels of N-acetyl aspartate (NAA) are associated with neuronal viability and integrity and may often be reduced in MA users (Yoon et al. 2013), NAA levels were examined in adolescent MA users and healthy adolescents as a metabolic measure of the ACC function. In addition, since the Stroop task has been shown to be a useful tool in evaluating cognitive conflict monitoring and inhibitory control and requires the ACC engagement and activation during the task completion (Bush et al. 2000), the Stroop color-word task was selected as a cognitive measure of the ACC function in this study. Specifically, the Stroop interference scores were acquired in adolescent MA users and healthy adolescents and the relationships between metabolic and cognitive measures of the ACC were assessed for each group. We also explored whether adolescent MA use might be associated with metabolic dysfunction in the ACC and its related cognitive deficits in a dose-dependent manner.
Converging evidence from preclinical studies indicates that the age of exposure to psychostimulants including MA is critical for their neurotoxic effects (Teixeira-Gomes et al. 2015). Therefore, we also explored whether an earlier onset of MA use was associated with greater deficits in metabolic and cognitive functions of the ACC.
MATERIALS AND METHODS
Participants
The participants in the current study are a sample from the previously published study (Lyoo et al. 2015). Among 111 late adolescents in the original study, 44 MA users and 53 healthy individuals who had valid 1H-MRS data were included in the current study.
We enrolled late adolescents who were diagnosed with MA dependence and gave written informed consent to participate in the study. For study participants who were younger than 19 years old, their parents or authorized legal representatives also provided written informed consent for study participation. Individuals who had current Axis I diagnoses other than MA or nicotine dependence, concurrent major neurological or medical diseases, head trauma history with loss of consciousness for more than 30 minutes, or any contraindications to magnetic resonance imaging (MRI) were excluded from the study. All participants were seronegative for human immunodeficiency virus infection.
This study was approved by the Institutional Review Boards of the Catholic University of Korea College of Medicine, Ewha W. University, Seoul National University Hospital, and the University of Utah.
Clinical and cognitive assessment
A diagnostic evaluation of drug use-related problems was performed and a detailed lifetime medical and substance use history was obtained by a board-certified psychiatrist. The Structured Clinical Interview for the DSM-IV was used for the diagnostic evaluation of major psychiatric disorders. Medical history taking and physical and neurological examinations were also performed to assess concurrent major medical or neurological diseases. Socioeconomic status was categorized into three levels based on the household income, parents’ educational level, and parents’ occupational status: high, middle, and low. Using the Stroop color-word task (Stroop, 1935), Stroop interference score, which may reflect inhibitory control (Goldstein & Volkow, 2011), focused attention (Eidels et al. 2010), and cognitive flexibility (Uttl & Graf 1997), was assessed as a cognitive measure of the ACC function. Stroop interference score was calculated by subtracting time per item of the color task from time per item on the color-word task (Lansbergen et al. 2007). A lower Stroop interference score reflects less susceptibility to interference and therefore higher task performance. All clinical, cognitive, and neuroimaging assessments were performed on the same day.
Neuroimaging assessment
Image acquisition
High-resolution T1-weighted and magnetic resonance spectroscopic images were obtained using a 1.5-Tesla whole-body imaging system (Signa HDx, GE Healthcare, Milwaukee, WI). A three-dimensional spoiled gradient echo sequence was used for the acquisition of T1-weighted anatomical images with the following parameters: repetition time (TR) = 24 ms, echo time (TE) = 5 ms, matrix = 256 × 256, field of view (FOV) = 240 mm, flip angle = 45°, number of excitation = 2, slice thickness = 1.2 mm, no skip. Single voxel proton magnetic resonance spectra were obtained from the midfrontal anterior cingulate area using the following parameters: water-suppressed, localized point resolved spectroscopy pulse sequence, TR = 2,000 ms, TE = 35 ms, voxel of interest (VOI) = 2 × 2 × 2 cm3.
1H-MRS data processing
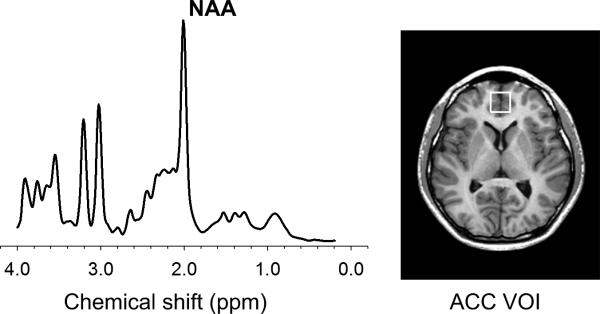
The cubic VOI, which corresponds to Brodmann area 32, was located anterior to the genu of the corpus callosum, centered on the interhemispheric fissure in the axial and coronal planes, and oriented along the line connecting the anterior commissure and posterior commissure in the sagittal plane of the T1-weighted image (Fig. 1). The pregenual ACC region was selected as the VOI based on a recent meta-analysis of structural abnormalities related to stimulant drug dependence (Ersche et al. 2013). For spectral fitting, the Linear Combination of Model Spectra (LCModel) method and the standard basis set were used (Provencher, 2001). An example of a representative fitted spectra for one healthy adolescent is presented in Figure 1. NAA levels in the ACC VOI were quantified as a metabolic measure of the ACC function using the unsuppressed water signal as an internal concentration reference (Lyoo et al.2009). Moreover, metabolite concentrations for creatine/phosphocreatine (Cr), glycerophosphocholine + phosphocholine (choline), and myo-inositol were explored. The lipid and macromolecules basis spectra were also included and simulated with the LCModel (Behar et al. 1994).
Figure 1.
Representative fitted spectra from one healthy adolescent and an axial image showing voxel size and location of the ACC
Abbreviations: ACC, anterior cingulate cortex; VOI, voxel of interest; NAA, N-acetyl aspartate.
The criteria of a full width at half maximum (FWHM) value < 0.16 ppm and a signal-to-noise ratio (SNR) > 3 were used to ensure that the spectral quality was adequate for reliable peak fitting for each metabolite. The values of FWHM and SNR in each group were adequate for spectral fitting and did not differ between groups (mean FWHM ± standard deviation [SD], the MA group = 0.070 ± 0.022, the control group = 0.067 ± 0.018, t = −0.76, P = 0.45; mean SNR ± SD, the MA group = 13.5 ± 4.0, the control group = 14.5 ± 3.4, t = 1.44, P = 0.15). Reliability of the fit for all metabolites was also measured using a Cramer-Rao lower bound (CRLB) value (Provencher, 2001). Individual metabolite spectra with CRLB values of less than 20 were selected for further analysis. Among all 105 late adolescents whose 1H-MRS data were available, 97 (44 MA users vs. 53 healthy individuals) who had all four metabolites including NAA, Cr, choline, and myo-inositol satisfying the criteria for adequate spectral quality were included in the final analysis.
Using FAST (FMRIB's Automated Segmentation Tool) implemented in FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl), tissue segmentation of the VOI was performed on T1-weighted images. Gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes of the VOI were measured and used to adjust for the effects of tissue composition on metabolite levels. There were no differences in the proportions of GM (t = 0.98, P = 0.33), WM (t = −0.91, P = 0.37), and CSF (t = −0.36, P = 0.72) volumes of the VOI between MA users and healthy individuals. GM proportion of the VOI was included as a covariate in all analyses.
Statistical analyses
Demographic and clinical characteristics were compared between the groups using two-paired t-tests, chi-square tests, or Fisher exact tests.
For the group comparison of NAA levels as a metabolic measure of the ACC function, multiple linear regression analysis was performed with NAA levels as a dependent variable and diagnosis as the main factor. Age, sex, and gray matter proportion were included as covariates. Stroop interference scores as a cognitive measure of the ACC function were also compared between the groups using multiple linear regression analysis. Age, sex, and educational level were included in the model as covariates. Cook's distance, which is often used to assess the influence of individual observations on the regression analysis, was estimated in each observation. For the multiple linear regression analysis for NAA levels and Stroop interference scores, Cook's distance was less than 1 (Cook, 1982). Cohen's d was estimated for the measurement of the effect size.
Partial correlation analysis was performed to examine the relationships between NAA levels and Stroop interference scores in each group after adjusting for age, sex, and educational level. Associations of metabolic and cognitive measures with lifetime cumulative dose of MA were also explored using partial correlation analysis.
Two-tailed P < 0.05 was considered to be statistically significant. Data were analyzed using Stata SE, v11.0 (Stata Corp, College Station, TX).
RESULTS
Participant characteristics
There were no differences in demographic characteristics including age, sex composition, socioeconomic status, and handedness between adolescent MA users and healthy adolescents (Table 1). Healthy adolescents had a slightly higher educational level (t = 1.99, P < 0.05) and a higher intelligence quotient (IQ)(t = 4.23, P < 0.001) than adolescent MA users. The proportion of current tobacco smokers was higher among adolescent MA users than in healthy adolescents (χ2 = 41.5, P < 0.001). There was also a significant difference in the scores of Fagerstrom Test for Nicotine Dependence (FTND) between the groups (mean ± SD, MA group, 1.86 ± 1.29, control group, 0.47 ± 1.20, t = −5.50, P < 0.001). Adolescent MA users had a greater pack-year history of smoking than healthy adolescents (mean ± SD, MA group, 1.17 ± 1.16, control group, 0.34 ± 0.80, t = −4.14, P < 0.001).
Table 1.
Demographic characteristics of study participants.
| Characteristics | Adolescent MA users (n = 44) | Healthy adolescents (n = 53) | P Value |
|---|---|---|---|
| Age — yr | 18.1±1.3 | 17.9±1.2 | 0.59 |
| Male sex — no. (%) | 35 (79.6) | 41 (77.4) | 0.80 |
| Education — yr | 10.8±1.7 | 11.4±1.1 | < 0.05 |
| IQ | 101.4 (8.5) | 110.0 (10.4) | < 0.001 |
| Right handedness — no. (%) | 38 (86.4) | 44 (83.0) | 0.65 |
| Race/ethnicity — no. (%) | |||
| East Asian | 44 (100) | 53 (100) | NA |
| SES — no. (%) | |||
| Low | 6 (13.6) | 7 (7.6) | 0.61 |
| Middle | 32 (72.7) | 42 (79.3) | |
| High | 6 (13.6) | 4 (13.2) | |
| Smoking — no. (%) | |||
| Current smoker | 34 (77.3) | 10 (18.9) | < 0.001 |
| Former smoker | 3 (6.8) | 0 (0.0) | |
| Never smoked | 7 (15.9) | 43 (81.1) | |
| FTND* | 1.86 (1.29) | 0.47 (1.20) | < 0.001 |
| Pack year history of smoking* | 1.17 (1.16) | 0.34 (0.80) | < 0.001 |
| Onset age of MA use — yr | 14.9 (1.1) | NA | NA |
Means and standard deviation values are denoted as mean ± standard deviation.
Continuous and categorical variables were compared between the groups using unpaired t-test and chi square tests, respectively.
There were no differences in FTND (mean ± SD, MA group, 2.38 ± 0.95, control group, 2.50 ± 1.65; t = −0.29, P = 0.78) and pack year history of smoking (mean ± SD, MA group, 1.46 ± 1.15, control group, 1.81 ± 0.87; t = 0.87, P = 0.39) of current smokers (n = 44) between adolescent MA users and healthy adolescents.
Abbreviations: yr, year; NA, not applicable; MA, methamphetamine; IQ, intelligence quotient; SES, socioeconomic status; FTND, Fagerstrom Test for Nicotine Dependence.
Mean onset age of MA use was 14.9 years (SD 1.1). Duration of regular MA use was calculated as the sum of months when MA was used more frequently than weekly. Mean duration of regular MA use was 25.5 months (SD 14.6; range 5 to 56) and lifetime cumulative dose of MA of adolescent MA users was 125.2 grams (SD 96.9; range 11.2 to 358.4). The route of MA administration included intravenous injection (n = 28, 63.6%), smoking (n = 15, 34.1%), and oral administration (n = 1, 2.3%). Among 44 adolescent MA users, 13 (29.5 %) reported ever having used cannabis. One participant in the MA group has a past history of alcohol abuse.
Effects of MA use during adolescence on metabolic and cognitive functions of the ACC
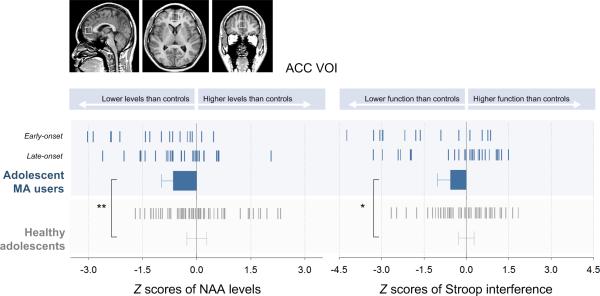
Adolescents MA users demonstrated significantly lower NAA levels in the ACC than healthy adolescents (mean ± SD, MA group, 6.68 ± 0.85 mmol/L, control group, 7.20 ± 0.80 mmol/L; effect size = 0.61; t = −2.88, P = 0.005)(Fig. 2). Stroop interference scores were higher in adolescent MA users than in healthy adolescents (mean ± SD; MA group, 0.52 ± 0.29, control group, 0.42 ± 0.18; effect size = 0.43; t = 2.03, P = 0.045).
Figure 2.
NAA levels in the ACC and Stroop interference scores measured in adolescent MA users and healthy adolescents. Adolescent MA users exhibited lower NAA levels than healthy adolescents after adjusting for age, sex, and gray matter proportion of the VOI (left bar graph, t = −2.88, P = 0.005). In addition, poorer performance in the Stroop color-word task was observed in adolescent MA users as compared with healthy adolescents after adjusting age, sex, and educational level (right bar graph, t = 2.03, P = 0.045). Z scores of NAA levels and Stroop interference scores were calculated using the mean and standard deviation of healthy adolescents and are presented in the graphs. Tick markers in the bar graphs indicate individual z scores of study participants. Error bars indicate 95% confidence interval.
Abbreviations: ACC, anterior cingulate cortex; VOI, voxel of interest; MA, methamphetamine; NAA, N-acetyl aspartate.
In an effort to determine the independent effects of MA use on the adolescent brain and cognitive function, repeated analyses including the current smoking status or IQ as a relevant covariate were performed. In addition, a subsample analysis excluding adolescents with history of cannabis use or a past history of alcohol abuse were performed. Results for NAA levels from these repeated analyses accounting for smoking history (t = −2.85, P = 0.005), IQ (t = −2.43, P = 0.02), or other substance use history (t = −2.98, P = 0.004) remained similar, although the statistical significance levels changed slightly. Likewise, the result for Stroop interference including smoking history as an additional covariate was similar (t = 2.21, P = 0.03). However, marginal statistical significance was observed in the comparisons of Stroop interference scores between the groups when accounting for IQ (t = 1.54, P = 0.13) or other substance use history (t = 1.74, P = 0.09). Detailed information on the statistical values of the models is presented in Supplementary Table.
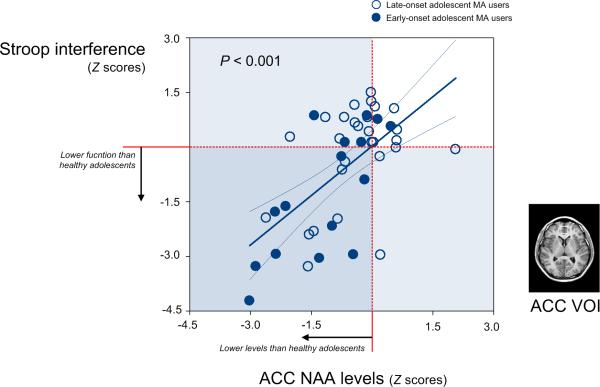
There was a significant relationship between NAA levels in the ACC and Stroop interference scores in adolescent MA users (r = −0.61, P < 0.001)(Fig. 3), but not in healthy adolescents (r = 0.12, P = 0.41). These results did not change after additionally covarying for current smoking status (r = −0.63, P < 0.001 in the MA group; r = 0.18, P = 0.21 in the control group). NAA levels in the ACC (r = −0.09, P = 0.57) and Stroop interference scores (r = 0.15, P = 0.33), respectively, were not associated with lifetime cumulative dose of MA. After additionally adjusting for current smoking status, there was no significant relationship between metabolic and cognitive function of the ACC (NAA levels, r = −0.12, P = 0.46; Stroop interference scores, r = 0.14, P = 0.40) and lifetime cumulative dose of MA.
Figure 3.
Scatter plot and regression line showing the relationship between the NAA levels in the ACC and the performance in the Stroop color-work task in adolescent MA users. Z scores of NAA levels and Stroop interference scores were calculated using the mean and standard deviation of healthy adolescents. Lower NAA levels in the ACC were significantly associated with poorer performance in the Stroop interference after adjusting for age, sex, and educational level (r = −0.61, P < 0.001).
Abbreviations: ACC, anterior cingulate cortex; VOI, voxel of interest; MA, methamphetamine; NAA, N-acetyl aspartate.
We also explored whether other metabolite levels in the ACC were associated with MA use during adolescence. There were no differences in Cr (mean ± SD; MA group, 5.05 ± 0.80 mmol/L, control group, 5.02 ± 0.73 mmol/L; t = 0.46, P = 0.65), choline (mean ± SD; MA group, 1.31 ± 0.24 mmol/L, control group, 1.35 ± 0.28 mmol/L; t = −0.65, P = 0.51), and myo-inositol (mean ± SD; MA group, 4.89 ± 1.34 mmol/L, control group, 4.61 ± 0.84 mmol/L; t = 1.40, P = 0.17) levels in the ACC between the adolescent MA users and healthy adolescents.
Onset age-specific effects of MA use on metabolic and cognitive functions of the ACC
To determine whether MA-associated metabolic and cognitive alterations in the ACC may differ by the age onset of MA use, partial correlation analysis between metabolic and cognitive functions of the ACC and the age onset of MA use was firstly performed. NAA levels in the ACC were positively associated with the onset age of MA use after adjusting for age, sex, gray matter proportion, and current smoking status (r = 0.34, P = 0.03). Stroop interference scores were negatively associated with the onset age after adjusting for age, sex, educational level, and current smoking status (r = −0.35, P = 0.03). Although there has been some debate over its sub-stage, adolescence may be categorized into early (10-14 years) and late (15-19 years) stages (Unicef, 2015; Feldman & Elliott, 1990; Irwin et al. 2002). Therefore, the current adolescent MA users were divided into two subgroups based on their age of onset for MA use (early adolescence-onset group: < 15 years old of onset age, n = 16 vs. late adolescence-onset group: ≥ 15 years old of onset age, n = 28). There were no significant differences in age (mean ± SD; early adolescence-onset group, 18.1 ± 1.4 years; late adolescence-onset group, 18.1 ± 1.3 years; t = 0.03, P = 0.97) and sex composition (early adolescence-onset group, 13 boys and 3 girls; late adolescence-onset group, 22 boys and 6 girls; Fisher's exact P = 1.00) between the subgroups. At a marginal level of statistical significance (t = −1.43, P = 0.16), the cumulative dose of MA was larger in the early-onset group (mean ± SD, 152.5 ± 98.0 grams) than in the late-onset group (mean ± SD, 109.7 ± 94.5 grams). Multiple regression analysis was used compare NAA levels in the ACC and Stroop interference scores between adolescent MA user subgroups. To compare NAA levels between two subgroups, age, sex, and gray matter fraction were included as covariates. Age, sex, and educational level were included in the model for the group comparison of Stroop interference scores.
The early adolescence-onset MA group had lower NAA (t = −2.24, P = 0.03) levels in the ACC and higher Stroop interference scores (t = 2.25, P = 0.03) than the late adolescence-onset MA group. These results remained unchanged after the additional covariation of the cumulative dose of MA (NAA levels, t = −2.17, P = 0.04; Stroop interference, t = 2.06, P = 0.047).
Interestingly, Cr levels in the ACC were also lower in early-onset adolescent MA users than in the late-onset counterpart (t = −2.79, P = 0.008). However, there were no differences in choline (t = −1.91, P = 0.06) and myo-inositol (t = −0.99, P = 0.33) levels between the MA user subgroups. Lower NAA and Cr levels in the ACC were associated with poor task performance in Stroop interference scores in early-onset adolescent MA users (NAA, r = −0.71, P = 0.007; Cr, r = −0.63, P = 0.02).
DISCUSSION
In the current study with a relatively large sample size of adolescent MA users, we found that MA use during adolescence was associated with lower NAA levels in the ACC. As compared with healthy adolescents, adolescent MA users were also likely to require more time to inhibit prepotent responses and resolve conflicts in the face of conflicting data, which are processes mediated by the ACC. Further analysis demonstrated a relationship between MA use-related metabolic dysfunction in the ACC and cognitive measures of inhibitory control. However, these MA effects on the adolescent brain, specifically the ACC, did not appear to be dose-dependent.
Although MA use-related neurochemical alterations including reduced NAA levels in the prefrontal cortex (PFC) have been previously studied in adult populations (Yoon et al. 2013), there have been surprisingly few attempts to investigate the effects of MA use on neurochemical metabolites in the adolescent brain. Considering age-dependent changes in metabolites levels from childhood/adolescence to adulthood (Horska et al. 2002), it is important to examine whether the adolescent brains may be either vulnerable or resistant to MA use-related neurochemical alterations. To date, only one study with a small sample size has reported that combined MA and cannabis use during adolescence may be related to lower NAA levels in the ACC (Sung et al. 2013a).
In addition to the well-known role of mesolimbic dopaminergic reward circuitry in addiction, disrupted function of the PFC including the ACC, OFC, and dorsolateral prefrontal cortex may predispose individuals to addiction by decreasing inhibitory control and increasing impulsivity (Goldstein & Volkow, 2011). Therefore, adolescents, who undergo substantial changes and reorganization of the PFC which has not reached its full maturity, tend to engage in risky behaviors such as drug use. Furthermore, converging evidence from preclinical studies suggests that exposure to psychostimulants during the critical period of prefrontal development may induce long-lasting changes in the brain that may contribute to the progression of addictive behaviors into adulthood (Cass et al. 2013).
In this study, we found that the MA-use was associated with neurochemical metabolic alterations in the ACC and cognitive deficits in the adolescent population, although the usual lifetime cumulative dose of MA in the adolescent MA users may be far less than the usual lifetime dose in adult MA users. Therefore, it is speculated that adolescence may be a period of greater vulnerability to effects of psychostimulants. This is supported by the lack of correlation between the amount of MA use and the observed metabolic or cognitive deficits in the ACC. Taking this into consideration, MA use-related effects may be of greater consequences in actively maturing brain regions such as the PFC. These findings are also consistent with the clinical observations that earlier drug exposure in teenagers is likely to increase the risk of lifetime drug abuse rates and further development of serious physical and mental complications, compared to those with drug exposure limited to adulthood (Anthony & Petronis 1995).
The current findings may seem discordant with results from rodent studies that reported adolescent rats to be less susceptible and more resistant to MA use-related hyperthermia and subsequent neurotoxicity than adult rats (Teixeira-Gomes et al. 2015). However, the striatal dopamine system, rather than higher cortical structures as observed in this study, has been the target brain region that is examined in most published rodent studies of repeated MA exposure (Teixeira-Gomes et al. 2015). In addition, consistent with the current finding, it has been recently reported that adolescent rodents, as compared with adult rodents, showed prefrontal vulnerability to the repeated use of psychostimulants (Cass et al. 2013; Wheeler et al. 2013).
Interestingly, the age of exposure is important for the MA use-related metabolic alterations in the ACC. Adolescents who started using MA during early adolescence, compared with those who were exposed during later adolescence, were likely to have lower NAA levels in the ACC and greater deficits in inhibitory control. Likewise, the greater vulnerability of early adolescent period to psychostimulant exposure, rather than late adolescent period, has consistently been reported in animal studies (Teixeira-Gomes et al. 2015; Wheeler et al. 2013). Furthermore, Cr levels, which are known to reflect cerebral energy metabolism as well as mitochondrial function, were also lower in the ACC in earlier onset MA users than in their later counterparts (Sung et al. 2013b; Yoon et al. 2013). Overactivation of mitochondria and induction of oxidative stress are regarded as major mechanisms underlying MA-related alterations in the brain (Shiba et al. 2011). A few previous studies, but not all, have reported MA use-related altered Cr levels in the adult brain reflecting the potential effects of MA on mitochondrial dysfunction (Ernst et al. 2000; Chang et al. 2005; Sung et al. 2013b). NAA is synthesized by mitochondria and may also reflect the level of cerebral energy metabolism. Therefore, our findings on NAA and Cr reductions in early-onset adolescent MA users suggest that an earlier exposure to MA may be related to a more profound dysfunction in mitochondria. This is consistent with the preclinical findings indicating that the specific effects of methylphenidate on oxidative damage were greater in the adolescent brain but not in the adult brain (Martins et al. 2006). However, given that the Cr spectral resonance which was measured using 1H-MRS includes contributions from both creatine and phosphocreatine, future 31-phosphorous MRS studies will be necessary to determine adolescent MA use-specific changes in brain high-energy phosphate metabolism.
We found a positive correlation between NAA levels in the ACC and Stroop interference scores in the MA group, with lower levels of NAA being linked to poorer performance, but not in the control group. This may imply a specific MA use-related alteration in the ACC. Likewise, a previous study did not find a relationship between NAA levels and task performance on Stroop color-word task in healthy individuals, despite a significant relationship in patients with schizophrenia (Delamillieure et al. 2004). However, although performed in middle-aged healthy subjects, another study has reported that lower NAA levels in the ACC were associated with lower performance on the Stroop color-word task (Grachev et al. 2001). In this regard, the analysis in the current control group was not able to detect a tentative link between the spectroscopic finding in the ACC and its cognitive function during normal developmental stage potentially due to its small effect size.
It should be noted that metabolic and cognitive alterations observed in adolescent MA users cannot be entirely accounted for by the plastic changes within the developing brain in response to MA exposure. Recently, the endophenotypic role of the prefronto-striatal connectivity has been reported in the context of developing stimulant dependence (Ersche et al. 2012). Genetically-determined underdevelopment of the prefrontal cortex could pave the way for stimulant use during earlier adolescence. Interestingly, a recent meta-analysis has suggested that gray matter deficits in the ACC may underpin some of psychiatric disorders including addiction, schizophrenia, bipolar disorder, depression, obsessive-compulsive disorders, and anxiety (Goodkind et al. 2015). Because of the cross-sectional nature of the study design, the causality of this issue could not be determined in this study. Future longitudinal studies are warranted to determine whether metabolic and functional deficits in the ACC may predispose to or be caused by MA use during adolescence.
Since additional analyses considering the history of other substances measures produced similar results, the current finding of adolescent MA use-related ACC deficits does not appear to be caused by the combined use of tobacco, alcohol, or cannabis. Participants in this study did not have current diagnoses of other substance dependence other than tobacco smoking. However, considering the high co-occurrence for use of illegal substances or alcohol in the population of drug abusers, it may be difficult to determine the exact effects of MA on the developing brain that are independent of those caused by other substances. Future studies with an adequate sample size to detect shared and independent effects of MA and other substances use will be needed in the adolescent population.
Tobacco smoking has been reported to be associated with structural and functional brain alterations not only in adults (Morales et al. 2014; Brody et al. 2004) but also in adolescents (Yip et al. 2016; Gogliettino et al. 2016; Peters et al. 2011). Although the results from repeated analyses including the scores of current smoking status as an additional covariate produced similar results, it is noted that early exposure to tobacco smoking as well as MA may have the potential to exert interactive effects on the developing brain.
Despite having similar results from the sensitivity analyses, our results should be interpreted cautiously in the context of the potential effects of confounding factors because we did not find the dose-response effects of MA use on metabolic and cognitive alterations in the ACC. A tentative explanation for the negative findings on the relationship between the cumulative dose of MA and ACC dysfunction is that the developmental stage exposed to MA rather than its dosage may play a more prominent role in determining the effects of MA on the developing brain (Lyoo et al. 2015). Although the causality could not be determined in this cross-sectional design, the results from the mediation analysis suggest the relationship between the age onset of MA use and performance on Stroop color-word task may be partly mediated by the NAA concentrations in the ACC (see the supplementary result).
It is also noted that the effect size for the group difference in Stroop interference was moderate (effect size = 0.43) and this result was not statistically significant after covarying for IQ difference between the groups. IQ stands for innate intellectual capacity and is generally regarded as being stable over time (McCall et al. 1977). However, recent studies may suggest that IQ may change during teenage years by several environmental factors including education or disease conditions (Ramsden et al. 2011). In this regard, the potential for co-linearity between MA effects on IQ and those on specific cognitive functions such as Stroop interference may also be taken into account when interpreting the data. Considering the moderate effect size, the influence of MA use on cognitive functions mediated by the ACC during adolescence should therefore be replicated in a larger sample.
Considering the differential roles of the ACC subregions in cognitive and emotional processing (Bush et al. 2000; Ridderinkhof et al. 2004), voxel-wise approach incorporating the dorsal, pregenual, and subgenual ACC will be necessary for a more comprehensive understanding of the ACC role in addictive behavior particularly in adolescence (Goldstein & Volkow, 2011).
In summary, this is the largest study using 1H-MRS to examine the MA use-related neurochemical alterations in the ACC and related cognitive dysfunction in adolescents, which may exacerbate addictive behaviors by impairing behavior monitoring and cognitive control. This finding may provide neurobiological clues as to why adolescent-onset drug use, as compared to the adult-onset drug use, is more likely to progress to life-long addiction.
Supplementary Material
ACKNOWLEDEGMENTS
This study was supported by grant 2015M3C7A1028373 and 2016R1A2B2012575 from the National Research Foundation of Korea, Institute for Information & communications Technology Promotion grant B0132-15-1001 from the MSIP, and NIDA grants 2R01DA024070-06.
Footnotes
AUTHORS CONTRIBUTION
JEK, SY and IKL were responsible for the concept and design of the study. All authors contributed equally to the data preparation and analysis. GHK, JEK, SY, and IKL wrote the paper. All authors critically reviewed the manuscript and approved the final version for publication.
REFERENCES
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals and Influence in Regression. Chapman & Hall; New York: 1982. [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamillieure P, Constans JM, Fernandez J, Brazo P, Dollfus S. Relationship between performance on the Stroop test and N-acetylaspartate in the medial prefrontal cortex in deficit and nondeficit schizophrenia: preliminary results. Psychiatry Res. 2004;132:87–89. doi: 10.1016/j.pscychresns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Eidels A, Townsend JT, Algom D. Comparing perception of Stroop stimuli in focused versus divided attention paradigms: evidence for dramatic processing differences. Cognition. 2010;114:129–150. doi: 10.1016/j.cognition.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Feldman SS, Elliott GR. At the threshold: The developing adolescent. Harvard University Press; Cambridge: 1990. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogliettino AR, Potenza MN, Yip SW. White matter development and tobacco smoking in young adults: A systematic review with recommendations for future research. Drug Alcohol Depend. 2016;162:26–33. doi: 10.1016/j.drugalcdep.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, Kumar R, Ramachandran TS, Szeverenyi NM. Cognitive interference is associated with neuronal marker N-acetyl aspartate in the anterior cingulate cortex: an in vivo (1)H-MRS study of the Stroop Color-Word task. Mol Psychiatry. 2001;496:529–539. doi: 10.1038/sj.mp.4000940. [DOI] [PubMed] [Google Scholar]
- Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15:137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Irwin CE, Jr, Burg SJ, Uhler Cart C. America's adolescents: where have we been, where are we going? J Adolesc Health. 2002;31:91–121. doi: 10.1016/s1054-139x(02)00489-5. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Jentsch JD, Kantak KM. Stimulant-associated cognitive abnormalities: mechanisms and impact on reward-related behavior and addiction. Drug Alcohol Depend. 2008;97:276–280. doi: 10.1016/j.drugalcdep.2008.05.003. [DOI] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology. 2010;212:243–249. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21:251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Yoon S, Kim TS, Lim SM, Choi Y, Kim JE, Hwang J, Jeong HS, Cho HB, Chung YA, Renshaw PF. Predisposition to and effects of methamphetamine use on the adolescent brain. Mol psychiatry. 2015;20:1516–1524. doi: 10.1038/mp.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, Ryan CM, Kim JE, Renshaw PF, Jacobson AM. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch Gen Psychiatry. 2009;66:878–887. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- Martins MR, Reinke A, Petronilho FC, Gomes KM, Dal-Pizzol F, Quevedo J. Methylphenidate treatment induces oxidative stress in young rat brain. Brain Res. 2006;1078:189–197. doi: 10.1016/j.brainres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- McCall RB. Childhood IQ's as Predictors of Adult Educational and Occupational Status. Science. 1977;197:482–483. doi: 10.1126/science.197.4302.482. [DOI] [PubMed] [Google Scholar]
- Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39:1816–1822. doi: 10.1038/npp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Khairnar A, Simola N, Granado N, Garcia-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog Neurobiol Epub ahead of print. 2016 doi: 10.1016/j.pneurobio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Richardson FM, Josse G, Thomas MS, Ellis C, Shakeshaft C, Seghier ML, Price CJ. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479:113–116. doi: 10.1038/nature10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Yamato M, Kudo W, Watanabe T, Utsumi H, Yamada K. In vivo imaging of mitochondrial function in methamphetamine-treated rats. Neuroimage. 2011;57:866–872. doi: 10.1016/j.neuroimage.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935. 1935;18:643–662. Stroop. [Google Scholar]
- Sung YH, Carey PD, Stein DJ, Ferrett HL, Spottiswoode BS, Renshaw PF, Yurgelun-Todd DA. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav Brain Res. 2013a;246:154–161. doi: 10.1016/j.bbr.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Hellem TL, Huber RS, Fiedler KK, Harrell RE, Nickerson BR, Kim SE, Jeong EK, Renshaw PF. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 2013b;129:102–109. doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Gomes A, Costa VM, Feio-Azevedo R, Bastos Mde L, Carvalho F, Capela JP. The neurotoxicity of amphetamines during the adolescent period. Int J Dev Neurosci. 2015;41:44–62. doi: 10.1016/j.ijdevneu.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Unicef [13 September 2016];Adolescence, An Age of Opportunity. 2011 http://www.unicef.org/adolescence/files/SOWC_2011_Main_Report_EN_02092011.pdf.
- Uttl B, Graf P. Color-Word Stroop test performance across the adult life span. J Clin Exp Neuropsychol. 1997;19:405–420. doi: 10.1080/01688639708403869. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Lerch JP, Chakravarty MM, Friedel M, Sled JG, Fletcher PJ, Josselyn SA, Frankland PW. Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci. 2013;33:1797–1803a. doi: 10.1523/JNEUROSCI.3830-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Pozos H, Phillips TJ, Izquierdo A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014;138:17–23. doi: 10.1016/j.drugalcdep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Balodis IM, Carroll KM, Krishnan-Sarin S, Potenza MN. Intra-individual changes in Stroop-related activations linked to cigarette abstinence in adolescent tobacco smokers: Preliminary findings. Drug Alcohol Depend Epub ahead of print. 2016 doi: 10.1016/j.drugalcdep.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Lyoo K, Renshaw PF. Application of magnetic resonance spectroscopic imaging to addiction research. In: MacKillop J, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. Willey-Blackwell; Oxford: 2013. pp. 707–750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.