Abstract
The expression of Basigin gene products and monocarboxylate transporter-1 (MCT1) has been investigated within the mammalian neural retina and suggests a role for these proteins in cellular metabolism within that tissue. The purpose of the present study was to investigate the expression of these same proteins in the pineal gland of the mouse brain. Mouse pineal gland and neural retina RNA and protein were subjected to quantitative reverse transcription-polymerase chain reaction (q-RT-PCR) and immunoblotting analyses. In addition, paraffin-embedded sections of each tissue were analyzed for expression of Basigin gene products and MCT1 via immunohistochemistry. The results indicate that MCT1 and Basigin variant-2, but not Basigin variant-1, are expressed within the mouse pineal gland. The expression of Basigin variant-2 and MCT1 was localized to the capsule surrounding the gland. The position and relative amounts of the gene products suggest that they play a much less prominent role within the pineal gland than in the neural retina.
Keywords: Basigin gene products, monocarboxylate transporter-1, pineal gland, neural retina
Introduction
This research group has previously studied the expression and function of Basigin gene products in the mammalian neural retina. It was determined that the Basigin gene expresses two products (Ochrietor et al. 2003). One product, a transcript of 1.5 kb, translates to a glycoprotein of 45 kDa and is ubiquitously expressed on epithelial cells (Ochrietor et al. 2003; Muramatsu 2015). In the mouse eye, this Basigin transcript, named Basigin variant-2, is expressed on the surface of Müller cells and the retinal pigmented epithelium (Ochrietor et al. 2003). The second product, named Basigin variant-1, is a transcript of 1.8 kb that translates to a glycoprotein of 55 kDa and is expressed only by photoreceptors (Ochrietor et al. 2003). It is thought that the two gene products bind each other to connect photoreceptors to Müller cells and align monocarboxylate transporters (MCTs) for delivery of metabolites to the photoreceptors for oxidative phosphorylation (reviewed in Ochrietor and Linser 2004). The expression of MCT1 at the plasma membrane within the neural retina is dependent on the expression of Basigin gene products. Mice in which the gene for Basigin was deleted have no MCT1 on the surface of photoreceptors, Müller cells, or the retinal pigmented epithelium, as is observed in control littermates (Philp et al. 2003). These animals are blind from the time of eye opening and have a reduced number of mitochondria within their neural retinas (Ochrietor et al. 2002; Pablo and Ochrietor 2013).
The pineal gland is located within the mammalian brain and has traditionally been associated with light entrainment and circadian rhythms. More recent studies indicate that mammals do not possess non-retinal photoreceptive cells, like non-mammal vertebrates. Rather, light entrainment occurs via a subset of ganglion cells within the neural retina that express melanopsin (reviewed in Doyle and Menaker 2007). In mice, the pineal gland is a tiny structure located in the median plane between the two hemispheres of the brain (Matsunaga et al. 2011). The gland is composed of pinealocytes, which express melanopsin, and astrocytes, and is surrounded by an epithelium, called the capsule, that derives from the pia mater (Matsunaga et al. 2011).
In previous studies, the expression of Basigin gene products was evaluated within the mouse brain, but not the pineal gland itself (Ochrietor et al. 2003). Therefore, the purpose of the present study was to characterize the expression of Basigin gene products, as well as MCT1, within the mouse pineal gland.
Materials and Methods
Isolation of RNA and protein samples from mouse pineal gland
Five mice (C57Bl/J-129 hybrid) were euthanized following an accepted protocol and the eyes and pineal glands were immediately removed and washed in phosphate buffered saline (PBS). The retinas were dissected from the eyes and pooled. The pineal glands were also pooled. RNA and proteins from each tissue type were isolated using the TRI reagent protocol (MRC, Inc., Cincinnati, OH) following the instructions of the manufacturer. The RNA was quantified via spectrophotometric analyses and the protein was quantified using the Coomassie-Bradford protocol (Pierce Thermo Scientific, Rockland, IL).
Quantitative polymerase chain reaction (q-PCR)
Mouse pineal gland and neural retina RNA (0.1 µg) was subjected to one-step RT-PCR using the iScript protocol (Bio-Rad, Hercules, CA), with the addition of random hexamer primer (2.5 µM). Primer sets used included those for mouse 18s rRNA, Basigin variant-1, and Basigin variant-2 (Ochrietor et al., 2003). Runs were performed in triplicate on a MiniOpticon Real-Time PCR system using the default cycling program of 50°C for 10 minutes, 95°C for 5 minutes, followed by 49 cycles of 95°C for 10 seconds, 55°C for 30 seconds, then 95°C for 1 minute, 55°C for 1 minute. A melt curve analysis was performed. All runs were performed in triplicate and the values obtained for the Basigin gene products were normalized to those of 18s rRNA within each tissue.
Immunoblotting analyses
Mouse pineal and retina proteins (~10 µg) were subjected to immunoblotting analyses, as previously described (Ochrietor et al. 2003). Briefly, proteins were separated on a NuPAGE 4–12% gradient Bis-Tris gel in NuPAGE buffer (Invitrogen Corporation, Carlsbad, CA). The proteins were electroblotted onto a nitrocellulose membrane (GE Osmonics, Pittsburgh, PA), stained with Fast Green, and destained. The blot was incubated in blocking solution (tris buffered saline [TBS] containing 2.5% dry milk and 0.2% Tween-20) for 1 hour at room temperature. An antibody specific for Basigin gene products (Ochrietor et al., 2003) was diluted in blocking buffer to a final concentration of 5 µg/mL, and incubated with the blot for 1 hour at 37°C. After washing with several changes of TBS, the blot was incubated in blocking buffer containing alkaline phosphatase (AP)-conjugated goat-anti-rabbit secondary antibody (Pierce/Thermo Scientific) for 30 minutes at 37°C. After washing with several changes of TBS, the blot was developed using AP conjugate substrate (Bio-Rad). The blot was documented using a scanner (ScanJet 6100C, Hewlitt Packard, Palo Alto, CA) and the figures were assembled using Microsoft PowerPoint software.
Immunohistochemistry
Mouse pineal glands and neural retinas were fixed with 4% paraformaldehyde in 0.1 M cacodylate (pH 7.4) and embedded in paraffin wax as previously described (Ochrietor et al., 2001). Tissues were sectioned at 6 µm, applied to gelatin-coated slides, deparaffinized, and incubated in blocking solution (TBS containing 0.1% Tween 20 and 2% normal goat serum [Pierce/Thermo Scientific]) overnight at 4°C. The tissues were incubated with antibodies specific for Basigin gene products (Ochrietor et al., 2003) or MCT1 (Millipore Corporation, Billerica, MA), diluted to 1 µg/mL in blocking solution, for 1 hour at 37°C and then at 4°C overnight. After washing with several changes of TBS, the tissues were incubated with Alexa 594-conjugated goat-anti-rabbit secondary antibody (Invitrogen Corporation), diluted 1:1000 in blocking solution, for 1 hour at 37°C. Coverslips were mounted with 30% glycerol containing p-phenylenediamine (Sigma Chemical Company, St. Louis, MO) and the tissues were viewed with an Olympus Fluoview F1000 confocal microscope (Pittsburgh, PA). Images were gathered digitally using Olympus FV10-ASW 4.0 software and assembled for publication using Microsoft PowerPoint software.
Results
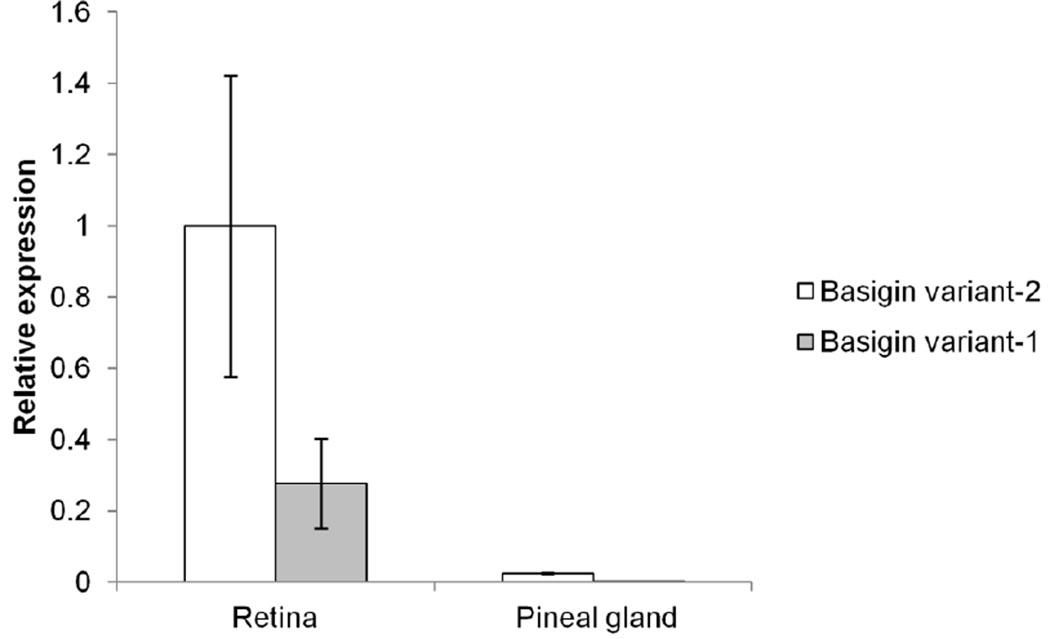
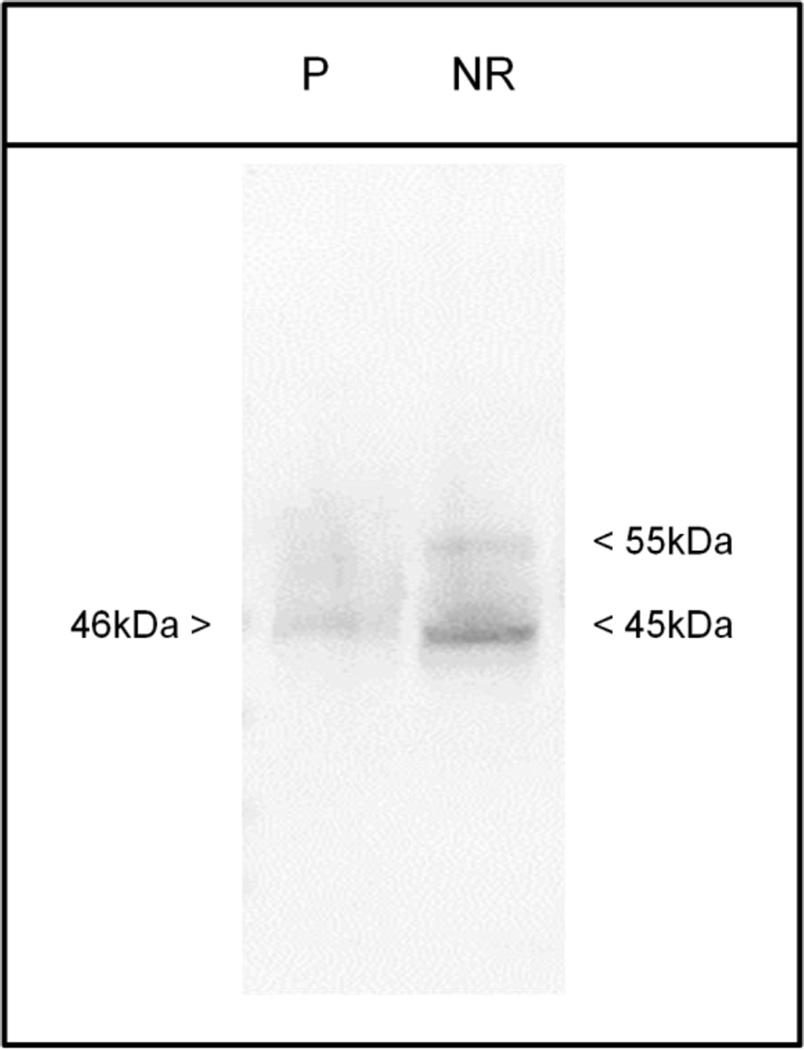
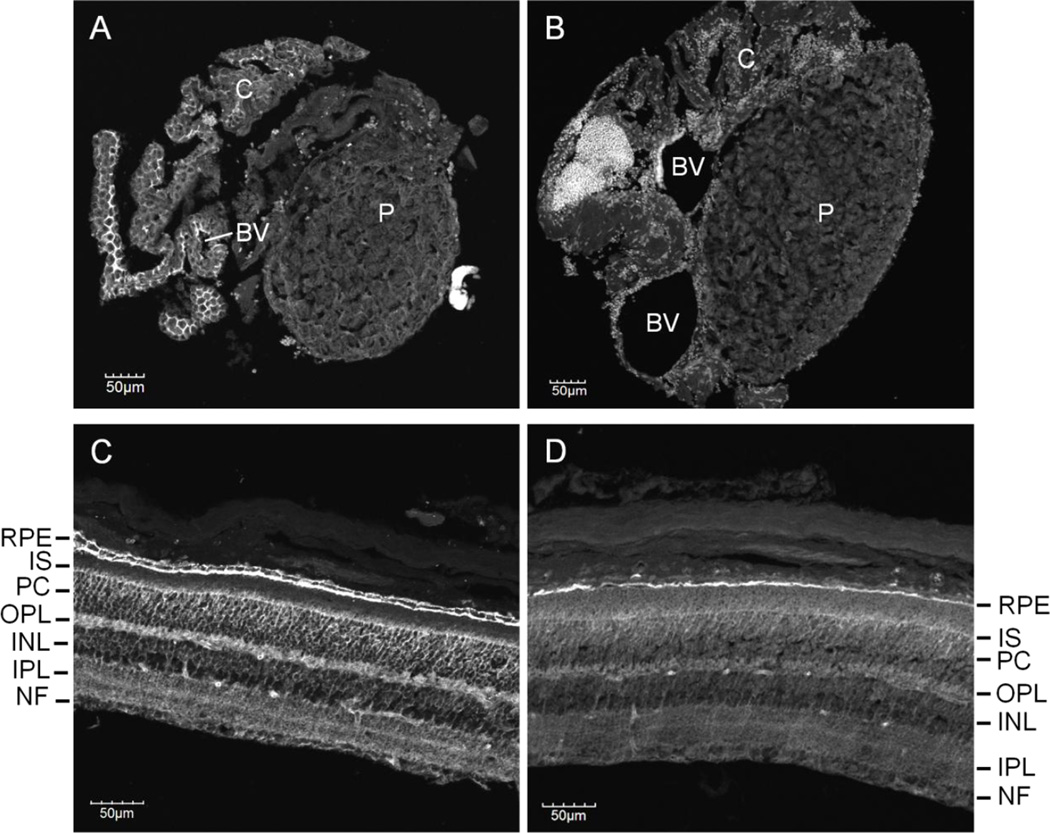
Expression of Basigin gene products within the mouse pineal gland was assessed via several biochemical methods. Quantitative reverse transcription polymerase chain reaction (q-RT-PCR) of mouse pineal gland RNA indicated that the Basigin variant-2 transcript is expressed within the tissue, whereas only trace amounts of the Basigin variant-1 transcript are present (Figure 1). This is in contrast with the neural retina, in which both transcripts are detected (Figure 1; Ochrietor et al., 2003). It was also noted that the expression of Basigin variant-2 was significantly lower (~40-fold) within the pineal gland than the neural retina. Immunoblotting analyses indicated that only the Basigin variant-2 protein, but not the Basigin variant-1 protein, is expressed within the pineal gland (Figure 2). To date, the neural retina is the only tissue in which both Basigin gene products are expressed (Figure 2; Ochrietor et al. 2003). Immunohistochemical analyses of mouse pineal tissue indicate that expression of Basigin variant-2 is not present in the pineal parenchyma, but is found on the capsule that surrounds the tissue (Figure 3). Basigin variant-2 is also expressed by the endothelial cells that line the blood vessels of the tissue (Figure 3). In the neural retina, Basigin gene products are found on the apical and basal membranes of the RPE, the Müller glial cells, which extend from the inner segments of the photoreceptor cells to the nerve fiber layer, and the photoreceptor cell bodies and inner segments (Figure 3, Ochrietor et al. 2001). The MCT1 protein is also present in the pineal gland and neural retina of mice and has an expression pattern identical to that of Basigin gene products in both tissues, with the exception of the basal membrane of the RPE (Figure 3; Philp et al. 2003).
Figure 1. Basigin variant-2 transcript is present in the mouse pineal gland.
Quantitative reverse transcription polymerase chain reaction (q-RT-PCR) was performed on mouse pineal and neural retina RNA using primers specific for Basigin variant-1 and Basigin variant-2. The data were normalized via analyses of 18s rRNA expression. All runs were performed in triplicate. The average relative expression of each transcript, in each tissue, is represented within the bar graph. The error bars represent the coefficients of variation.
Figure 2. Basigin variant-2 protein is present in the mouse pineal gland.
Immunoblotting analyses were performed on mouse pineal (P) and neural retina (NR) protein lysates using an antibody that detects both Basigin gene products. A representative blot is shown. The pineal gland tissue expresses only the Basigin variant-2 protein (~46 kDa), whereas the neural retina expresses both Basigin variant-1 (55 kDa) and Basigin variant-2 (45 kDa) proteins.
Figure 3. Basigin variant-2 and MCT1 are expressed on the capsule surrounding the pineal gland.
Immunohistochemical analyses were performed on paraffin-embedded sections of mouse pineal (A and B) and neural retina (C and D) using antibodies specific for Basigin gene products (A and C) or MCT1 (B and D). Expression of Basigin gene products and MCT1 is localized to the capsule of the pineal gland, as well as the RPE, Müller cells and photoreceptors of the neural retina. Abbreviations in A and B: C, capsule; BV, blood vessel; P, parenchyma. Abbreviations in C and D: RPE, retinal pigmented epithelium; IS, inner segments; PC, photoreceptor cell bodies; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; NF, nerve fiber layer. The magnification bars represent 50 µm.
Discussion
Basigin gene products are intriguing molecules, as they have been implicated in a variety of biological functions (reviewed in Muramatsu 2015). Previous studies have investigated the function of Basigin gene products within the mouse neural retina, and their patterns of expression within various mouse tissues. It was determined that MCT1 expression is linked to that of Basigin gene products in the neural retina (Philp et al., 2003). When Basigin is not expressed, MCT1 is not expressed at the plasma membrane of Müller cells and photoreceptor cells (Philp et al., 2003). This results in blindness from the time of eye opening (Ochrietor et al., 2002) and a significant decrease in the number of mitochondria in the neural retina (Pablo and Ochrietor, 2013). Biochemical analyses indicate that the transmembrane domain of Basigin gene products interacts via hydrophobic associations with MCT1 (Finch et al., 2009). While a significant number of studies have been conducted on Basigin gene products and MCT1 in the neural retina, this is the first study to characterize the expression of Basigin gene products and their relationship with MCT1 within the pineal gland.
In the present study, it was noted that only Basigin variant-2 is expressed within the mouse pineal gland (Figures 1 and 2), and that expression was not present within the gland itself, but rather on the epithelial cells that surround the gland (Figure 3). The levels of Basigin gene transcripts within the pineal tissue were much lower than that of the neural retina (Figure 1), which suggests that Basigin variant-2 plays a less-prominent role within that tissue.
It was also noted that the transporter protein MCT1 has an expression pattern that mimics Basigin within the pineal gland (Figure 3). Again, the expression of MCT1, like Basigin variant-2, is restricted to the capsule surrounding the tissue, and is not found within the parenchyma. It is likely that MCT1 contributes to basal monocarboxylate transport within the pineal gland.
Previous studies of Basigin gene products and MCT1 in the neural retina suggested that these two protein types are necessary for the rapid and efficient transport of metabolic substrates from Müller glial cells to the photoreceptor neurons. Basigin gene products and MCT1 are expressed throughout the neural retina, and in the absence of their expression, the neurons do not function and eventually die (Ochrietor et al., 2001). In the pineal gland, however, Basigin variant-2 and MCT1 do not appear to be as critical for the overall function of the tissue, since expression was restricted to the capsule surrounding the tissue and not within the parenchyma of the gland itself (Figure 3). The two proteins likely work to supply the tissue with monocarboxylates from the blood stream but are not required for rapid and efficient transport of metabolites to the pinealocytes.
Acknowledgments
This work was supported by NIH F32EY13918, the University of North Florida Offices of Academic Affairs and Undergraduate Studies (to JDO), and by NSF IBN-0113697 (to PJL).
Footnotes
Derek Tokar performed the q-RT-PCR, immunoblotting, and immunohistochemical analyses; Leslie van Ekeris and Paul Linser generated the paraffin-embedded samples for immunohistochemistry; Judith Ochrietor generated the confocal micrographs.
Ethical approval: All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Florida and the University of North Florida. All applicable guidelines for the care and use of animals were followed.
References
- Doyle S, Menaker M. Circadian photoreception in vertebrates. Cold Spring Harb Symp Quant Biol. 2007;72:499–508. doi: 10.1101/sqb.2007.72.003. [DOI] [PubMed] [Google Scholar]
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan Q-W, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T. A null mutation in Basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–165. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- Matsunaga MM, Crunfli F, Fernandez GJM, Rossi WC, Jr, Esteves A. Morphologic analysis of mice’s pineal gland. J Morphol Sci. 2011;28:157–160. [Google Scholar]
- Muramatsu T. Basigin (CD 147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2015 doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochrietor JD, Linser PJ. 5A11/Basigin gene products are necessary for proper maturation and function of the retina. Dev Neurosci. 2004;26:380–387. doi: 10.1159/000082280. [DOI] [PubMed] [Google Scholar]
- Ochrietor JD, Moroz TM, Kadamatsu K, Muramatsu T, Linser PJ. Retinal degeneration following failed photoreceptor maturation in 5A11/Basigin null mice. Exp Eye Res. 2001;72:467–477. doi: 10.1006/exer.2000.0974. [DOI] [PubMed] [Google Scholar]
- Ochrietor JD, Moroz TP, Clamp MF, Timmers AM, Muramatsu T, Linser PJ. Inactivation of the Basigin gene impairs normal retinal development and maturation. Vis Res. 2002;42:447–453. doi: 10.1016/s0042-6989(01)00236-x. [DOI] [PubMed] [Google Scholar]
- Ochrietor JD, Moroz TP, van Ekeris L, Clamp MF, Jefferson SC, deCarvalho AC, Fadool JM, Wistow G, Muramatsu T, Linser PJ. Retina-specific expression of 5A11/Basigin-2, a member of the immunoglobulin gene superfamily. Invest Ophthalmol Vis Sci. 2003;44:4086–4096. doi: 10.1167/iovs.02-0995. [DOI] [PubMed] [Google Scholar]
- Pablo KAV, Ochrietor JD. Deletion of the Basigin gene results in reduced mitochondria in the neural retina. Biochem Biophys Res Comm. 2013;438:546–550. doi: 10.1016/j.bbrc.2013.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/Basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]