Abstract
The use of mesophyll protoplast extracts from various C4 species has provided an effective method for studying light-and substrate-dependent formation of oxaloacetate, malate, and asparate at rates equivalent to whole leaf C4 photosynthesis. Conditions regulating the formation of the C4 acids were studied with protoplast extracts from Digitaria sanguinalis, an NADP-malic enzyme C4 species, Eleusineindica, an NAD-malic enzyme C4 species, and Urochloa panicoides, a phosphoenolpyruvate (PEP) carboxykinase C4 species. Light-dependent induction of CO2 fixation by the mesophyll extracts of all three species was relatively low without addition of exogenous substrates. Pyruvate, alanine and α-ketoglutarate, or 3-phosphoglycerate induced high rates of CO2 fixation in the mesophyll extracts with oxaloacetate, malate, and aspartate being the primary products. In all three species, it appears that pyruvate, alanine, or 3-phosphoglycerate may serve as effective precursors to the formation of PEP for carboxylation through PEP-carboxylase in C4 mesophyll cells. Induction by pyruvate or alanine and α-ketoglutarate was light-dependent, whereas 3-phosphoglycerate-induced CO2 fixation was not.
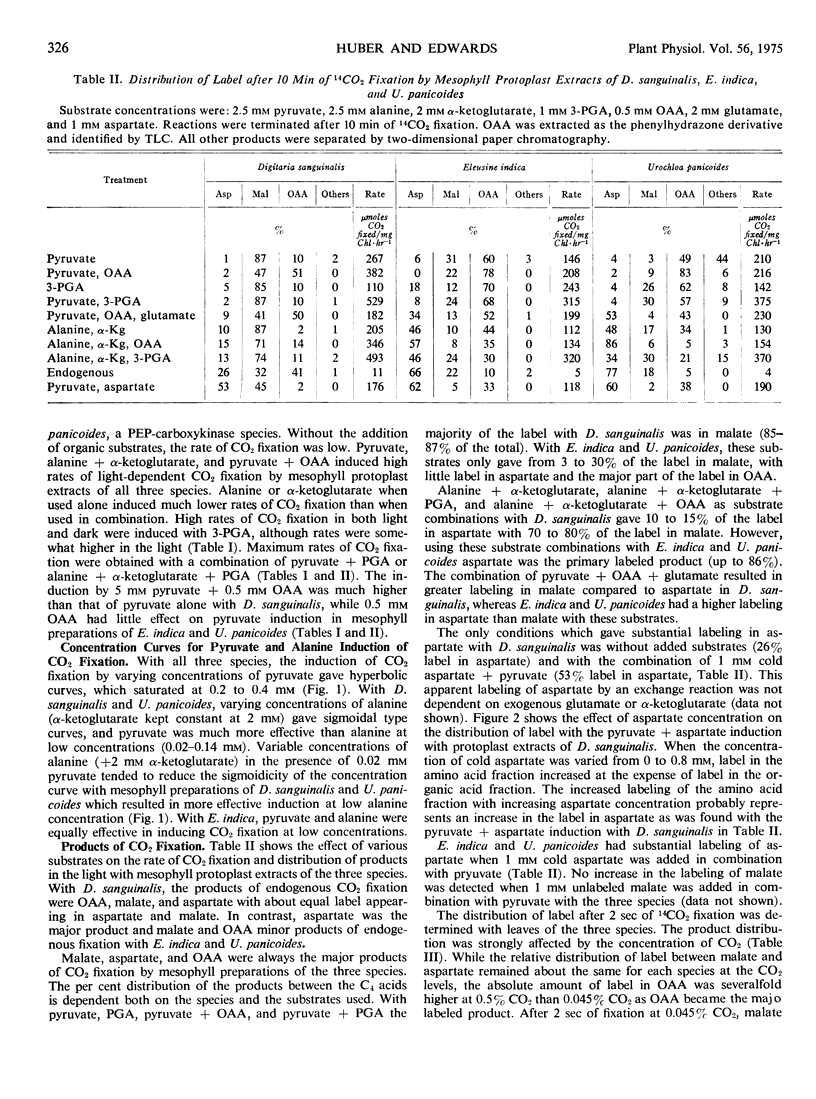
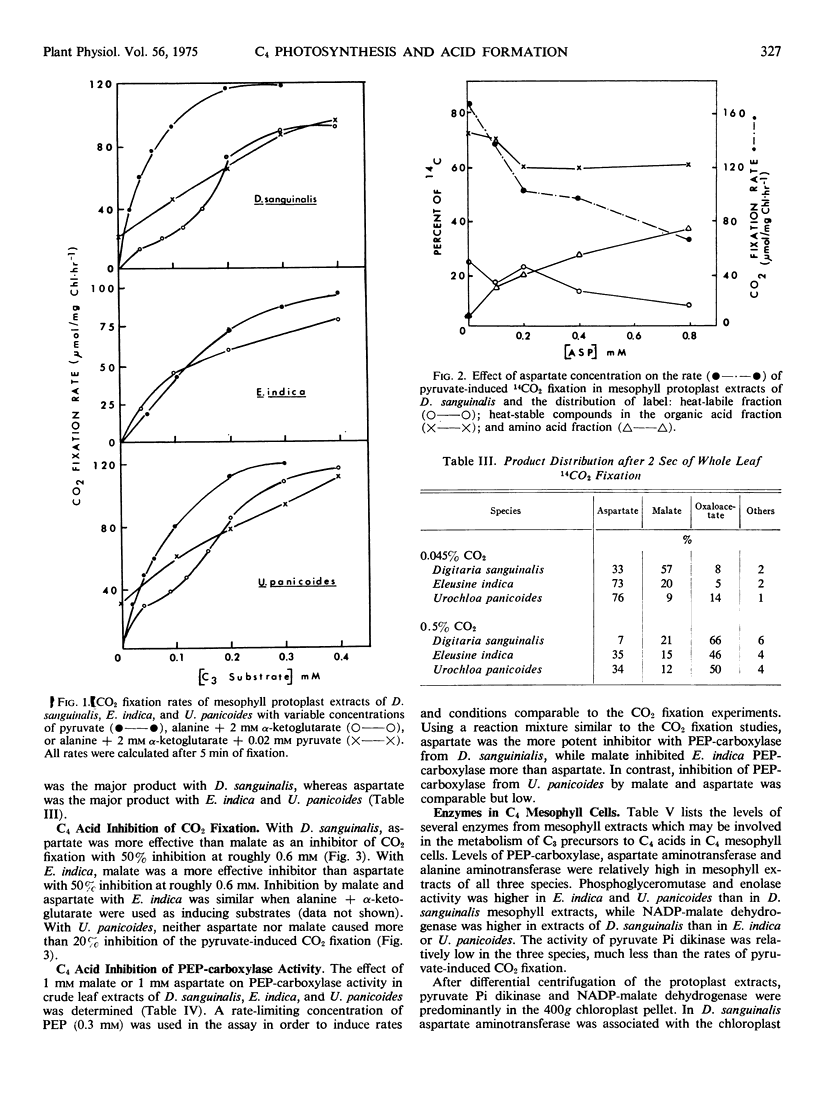
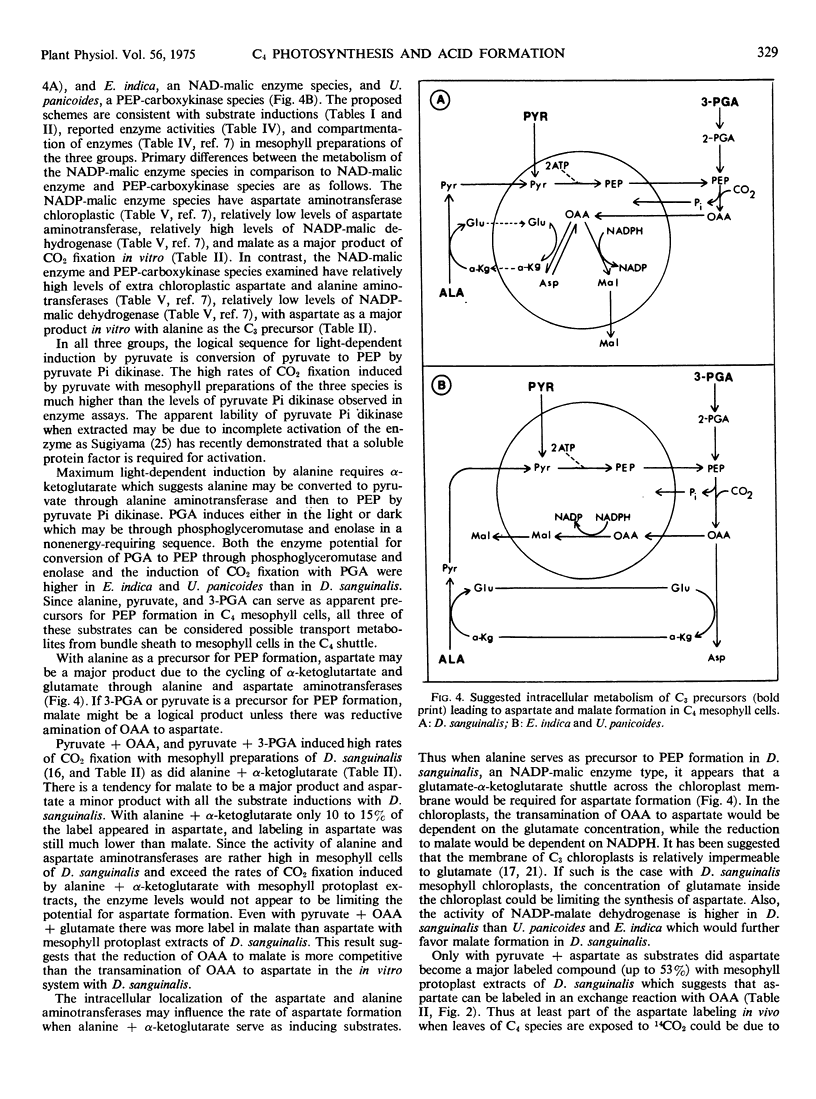
Several differences between these species representing the three C4 groups were observed. Substrate induction of CO2 fixation in mesophyll protoplast extracts of D. sanguinalis gave malate as a major product; only by an apparent exchange reaction with cold aspartate did substantial label appear in aspartate (up to 53% of labeled products). In contrast, aspartate was a major product when alanine and α-ketoglutarate served as inducing substrates with E. indica (up to 57%) and U. panicoides (up to 86%). With induction by pyruvate or 3-phosphoglycerate, mesophyll preparations of U. panicoides and E. indica were less effective in forming malate (up to 31% of products) than D. sanguinalis (up to 87% of products). After 2 seconds of whole leaf 14CO2 fixation, malate was the major labeled product (57%) with D. sanguinalis, whereas with E. indica and U. panicoides aspartate was the predominant product (73% and 76%, respectively).
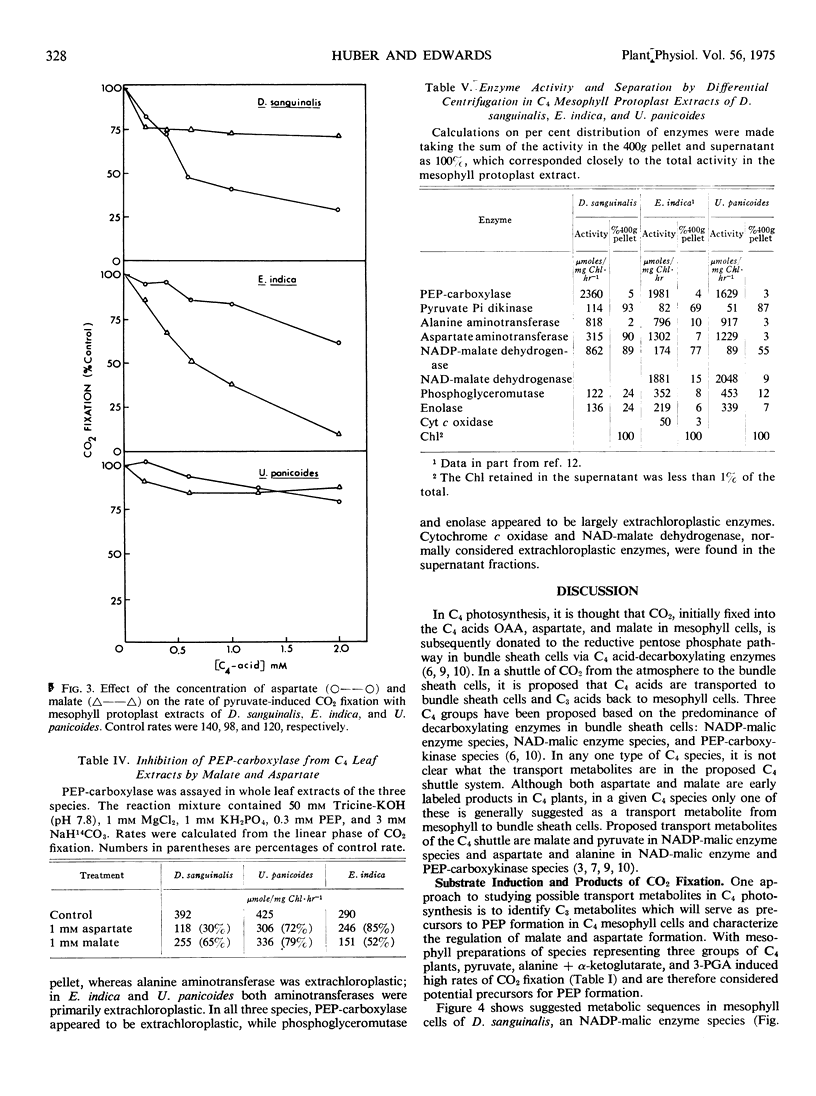
With mesophyll protoplast extracts of D. sanguinalis, aspartate inhibited CO2 fixation (about 50% at 0.6 mm), while malate was relatively uninhibitory at comparable concentrations. CO2 fixation by mesophyll protoplast extracts of E. indica was inhibited by malate (about 50% at 0.6 mm), while aspartate was relatively uninhibitory. With mesophyll preparations of U. panicoides, malate or aspartate (2 mm) caused only slight inhibition of CO2 fixation. The regulation of aspartate and malate synthesis in C4 mesophyll cells is discussed relative to initial products of photosynthesis in C4 species in vivo and species differences in the mechanisms of C4 photosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. M., Brown R. H., Black C. C. Photosynthetic CO(2) Fixation Products and Activities of Enzymes Related to Photosynthesis in Bermudagrass and Other Plants. Plant Physiol. 1971 Feb;47(2):199–203. doi: 10.1104/pp.47.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Gutierrez M. Metabolic Activities in Extracts of Mesophyll and Bundle Sheath Cells of Panicum miliaceum (L.) in Relation to the C(4) Dicarboxylic Acid Pathway of Photosynthesis. Plant Physiol. 1972 Dec;50(6):728–732. doi: 10.1104/pp.50.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLER R. G., WALKER D. A. Formation of labelled amino acids by exchange transamination. Biochem J. 1961 Jan;78:56–60. doi: 10.1042/bj0780056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. The C 4 -pathway of photosynthesis. Evidence for an intermediate pool of carbon dioxide and the identity of the donor C 4 -dicarboxylic acid. Biochem J. 1971 Nov;125(2):425–432. doi: 10.1042/bj1250425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. C(4) Photosynthesis: Light-dependent CO(2) Fixation by Mesophyll Cells, Protoplasts, and Protoplast Extracts of Digitaria sanguinalis. Plant Physiol. 1975 May;55(5):835–844. doi: 10.1104/pp.55.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Stumpf P. K. Fat metabolism in higher plants. LV. Acetate uptake and accumulation by class I and II chloroplasts from Spinacia oleracea. Arch Biochem Biophys. 1972 Dec;153(2):656–663. doi: 10.1016/0003-9861(72)90384-0. [DOI] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Slack C. R. Inhibition of maize leaf phosphopyruvate carboxylase by oxaloacetate. Biochim Biophys Acta. 1971 Apr 14;235(1):207–209. doi: 10.1016/0005-2744(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikido T., Takanashi H. Glycine activation of PEP carboxylase from monocotyledoneous C4 plants. Biochem Biophys Res Commun. 1973 Jul 2;53(1):126–133. doi: 10.1016/0006-291x(73)91410-1. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


