Abstract
Background
There is increased use of early nasal continuous positive airway pressure (NCPAP) to manage respiratory distress in preterm infants but optimal methods and factors associated with successful wean are not well defined. A systematic review was performed to define the corrected gestational age (CGA), weight to wean NCPAP and the methods associated with successful weaning of the NCPAP among preterm infants, along with factors affecting it.
Methods
Searches were made of PubMed using the keywords-NCPAP, CPAP, weaning, withdrawal, preterm, and infants from its inception to January 1st, 2014, for studies in all languages but limited to humans. Previous reviews (including cross references) were also searched. We included all randomized and quasi-randomized controlled trials where preterm neonates were randomized to different NCPAP weaning strategies. Details of CGA, weight and methods used for weaning NCPAP were extracted along with factors which affect its withdrawal.
Results
Seven studies met the search criteria. The successful wean was at 32 to 33 weeks CGA and at 1600 g. Three different methods were used for weaning were sudden, gradual pressure wean and gradual graded time off wean. Criteria for readiness, success and failure to wean were defined. Factors affecting successful weaning were intubation, anemia, infection and gastro-esophageal reflux.
Conclusions
The successful wean was at 32 to 33 weeks CGA and 1600 g. Criteria for readiness, success and failure to wean are well defined. Sudden weaning may be associated with a shorter weaning time. Future trials are needed comparing weaning methods using defined criteria for readiness and success of NCPAP wean and stratify the results by gestational age and birth weight.
Keywords: continuous positive airway pressure, infants, preterm, systematic review, weaning
Introduction
With wider acceptance of the early use of nasal continuous positive airway pressure (NCPAP) for treatment of respiratory distress syndrome in preterm infants, the use of intubation and invasive ventilation has been significantly reduced.[1,2] Early use of NCPAP has been extensively studied and has been associated with decreased incidence of significant pulmonary[3–5] and non-pulmonary morbidities associated with invasive ventilation.[6,7] NCPAP is not only safe due to its noninvasive nature,[8,9] but it is also associated with lung growth.[10] There are certain risks which are associated with NCPAP, including nasal trauma, increased incidence of pneumothorax[11] and an unproven relationship with increased intraventricular ventricular hemorrhage (IVH).[12] There is a learning curve related to the process of administrating NCPAP, but the time is easily recovered by the lower cost of the NCPAP equipment and lower incidence of morbidities associated with invasive ventilation.[13–17]
While CPAP use and benefits have been elucidated, weaning of NCPAP has not been as extensively investigated, and is associated with risks, especially when weaning is performed prematurely. These risk are due to atelectasis, apnea and bradycardia, leading to prolonged use of CPAP, or possibly intubations with mechanical ventilation and prolonged oxygen use.[17,18] There are considerable variations in the timing and methods used for weaning of NCPAP. The weaning methods included sudden removal of NCPAP with or without oxygen supplementation, gradual increase in time off NCPAP, gradual reduction of pressure followed by removal of NCPAP, or a combination of these methods.[19–25]
Surveys done among neonatologists identified a lack of consensus regarding the optimal method of weaning NCPAP.[25] Only 6% of responders followed set standards within their units, and the majority used a combination of various methods. The timing of optimal weaning method for the preterm infants was also variable, and was dependent on the subjective clinical judgment of the neonatologist, frequently implemented on an ad hoc basis, thereby identifying a need for development of evidence based practice. This review was therefore performed, to define the optimal timing, methods and factors associated with successful wean from NCPAP in preterm neonates.
The objectives of this systematic review were to determine the corrected gestational age (CGA) and weight of preterm neonate at the successful wean and compare the methods used to wean off NCPAP. Further to define the criteria for NCPAP weaning success and failure, and factors that may affect it.
Methods
Literature search
A systematic literature review was performed to identify studies conducted on NCPAP wean in preterm infants. PubMed was queried using the keywords-NCPAP, CPAP, weaning, withdrawal, preterm, and infants from its inception to January 1st, 2014, for studies in all languages but limited to humans. All reviewers evaluated the titles and abstracts yielded by our search to determine the articles that met the eligibility criteria. The authors also searched the reference section of each relevant article from the initial screening for additional relevant studies. Studies were chosen for based on the relevance of the article to our study questions. Given the heterogeneity and the relatively small number of studies that met the eligibility criteria, a decision was made not to perform meta-analysis of the eligible studies.
Eligibility criteria
The eligibility criteria used to identify the participants consisted of preterm infants who were born less than 37 weeks gestational age (GA) and were managed by use of NCPAP and its subsequent successful weaning during their hospital course. The methods for weaning such as sudden discontinuation of NCPAP, gradual weaning of NCPAP by gradual increase in time off CPAP or gradual reduction of pressure were compared. The outcomes that were studied were the weight and CGA when the CPAP was successfully weaned off and the factors affecting its success. Further we defined the criteria for readiness of CPAP wean along with its success or failure of weaning from CPAP.
Study selection
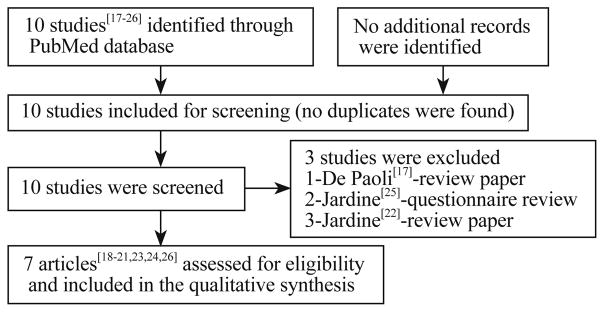
We initially identified 10 studies which comprised of randomized controlled trials (RCT), retrospective study, questionnaire survey and review that assessed the weaning of NCPAP in preterm infants. Out of these, three[17,22,25] were excluded. Of which, 2 were reviews and a questionnaire. As the studies were few, publications in abstract form and those not restricted in English, were included (Fig.).[20,21]
Fig.
Flow chart of selection process for the publication included in the review.
Outcomes studied
We abstracted the CGA and weight of preterm infants at successful weaning. Further, we defined the detailed criteria for readiness for weaning of NCPAP along with details of defining success and failure to wean. Various methods used to wean NCPAP were studied and compared with the purpose of studying if one was better and would lead to earlier successful weaning from NCPAP. Factors, both epidemiological and associated comorbidities, which affected weaning of NCPAP were also studied. We assessed the studies for bias regarding the blinding of randomization, intervention, outcome (Table 1). No meta-analysis or further analysis was performed during this review.
Table 1.
Risk of bias within studies
| Risk of bias | Abdel-Hady 1998[19] | Singh 2006[20] | Soe 2006[21] | Rastogi 2011[26] | Abdel Hady 2011[18] | Todd 2012[23] | Rastogi 2013[24] |
|---|---|---|---|---|---|---|---|
| Blinding of randomization | Yes | Unknown | Unknown | Not applicable | Yes | Yes | Yes |
| Blinding of intervention | No | No | No | Not applicable | No | No | No |
| Blinding of outcome | Unknown | Unknown | Unknown | Not applicable | No | Unknown | Unknown |
Results
Details of included search
The characteristics of 6 of the 7 studies[18–21,23,24] included were RCT or Quasi-RCT that compared various strategies of NCPAP wean (Table 2). Todd et al[23] demonstrated sudden wean off NCPAP was better than gradual wean off NCPAP, but lacked a clear criteria or methodology used to define failure and success for weaning of NCPAP. Rastogi et al[24] did not show any difference between these two methods, although they had clear criteria for initiation of weaning, and for defining success of weaning from NCPAP. Abdel-Hady et al,[19] Singh et al[20] and Soe et al[21] showed that gradual pressure wean was better than sudden wean off NCPAP. A large retrospective study[26] was included in this review that defined the various demographic and clinical factors including antenatal and postnatal morbidities that affected weaning. We excluded a review by De Paoli, which briefly mentioned the problems associated but lacks any details of information regarding weaning of CPAP.[17] Another review included only 3 studies, two of which were abstracts.[22] Lastly, Jardine et al published a questionnaire article that discussed the current practice of withdrawal of CPAP in the preterm.[25]
Table 2.
Summary of studies that met eligibility criteria
| Author/year | n | Study type | Participants
|
Interventions
|
Outcomes
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion | Exclusion | Weaning method A | Comparison method B | Results | CGA at successful wean (wk) | Weight at successful wean (g) | Weaning period in A (d) | Weaning period in B (d) | |||
| Abdel-Hady, 1998[19] | 88 | RCT | ≤33 wk, NCPAP without supplemental O2, no apnea requiring bagging and ≤6 apneas requiring stimulation during the preceding 24 h | None defined | Pressure decreased over 1 h (every 20 min) to 3 cm H2O, then CPAP removed | Control group: continued unchanged on CPAP for 6 h | Successful weaned off NCPAP in A is approx. 60% Incidence of ROP=2% Incidence of PVL=2%, equal in both A and B No BPD, IVH, pneumothorax, nasal injury, infection |
29±2 | 1264±332 | 1 | 9.5 |
| Singh, 2006[20] | 105 | RCT | <1500 g <0.3 FiO2 on NCPAP |
Gradual pressure wean group (actual method not described) | Gradual time off wean group with transition to nasal cannula low flow O2 | Successfully weaned in A=65% Successfully weaned in B=37% Duration on NCPAP in A=6 d Duration on NCPAP in B=13.2 d |
29 | 1.5 | 8.9 | ||
| Soe, 2006[21] | 98 | RCT | <32 wk who required respiratory support for surfactant deficiency or immature lung disease | Gradual pressure off weaning group with CPAP at 6 cm H2O for 2 d, then 5 cm of FiO2 for 2 d, then 4 cm H2O for 2 d | Gradual time off weaning-start with 7 h on NCPAP, 1 h off, next 5 d-the time on CPAP was decreased 1 h daily | Successfully weaned in A=92% Successfully weaned in B=78% Successfully weaned in A (23–27 wk subgroup)=82% Successfully weaned in B (23–27 wk subgroup)=55% Successfully weaned in 28–31 wk sub group=all but one Duration of NCPAP in A (23–27 wk)=10 d Duration of NCPAP in B (23–27 wk)=15 d Incidence of CLD in A=6% Incidence of CLD in B=26% |
|||||
| Rastogi 2011[26] | 454 | Retrospective chart review | Babies who were born ≤32 wk | Demographics-Younger (increased by 0.4 wk for every week decrease in GA) and smaller babies weaned late (increase by 0.2 wk for every 100 g decrease in BW). Gender and ethnicity had no effect on weaning Clinical data: intubation affected time and weight to weaning (no intubation: 32 wk/1580 g; for intubated: 35 wk/1869 g). Chorioamnionitis, anemia, GER, IVH were independent predictors for successful weaning in infants who were not intubated, time on NCPAP increased by 1.1 wk for chorioamnionitis, 1 wk for those who had anemia, 1.9 wk for those who had GER |
32.9±2.4 | 1611±432 | |||||
| Abdel- Hady, 2011[18] | 60 | RCT | ≥28 wk 0.30 FiO2, PEEP 5 Maintain O2 sat ≥87% Clinically stable for 24 h-RR <60/min No apnea requiring bagging and ≤6 apneas requiring stimulation during the preceding 24 h ABG-pH >7.25, PCO2 <60, base deficit <8 |
Life threatening congenital anomalies, cyanotic heart disease Congenital airway/chest wall abnormalities, pulmonary hypoplasia, known or suspected neuromuscular disease, congenital neurological disorder, severe IVH-grade 3 or 4, PVL, hydrocephalus |
Non NC group-on NCPAP till FiO2 =0.21 for 24 h, then weaned from press 5 cm H2O to room air | NC group Weaned from NCPAP of FiO2 ≤0.30 to NC (2 L/min) with FiO2=0.30 FiO2 then adjusted for SpO2 87%–93% After FiO2 at 0.21, NC flow decreased by 0.5 L/min every 6 h until 0.5 L/min is reached, then NC removed if stable |
Failed initial weaning trial in A is 20%, failed initial weaning trial in B is 23% (both group were successfully weaned in subsequent trials), 63% of NC group (B) were successfully weaned off O2 but continued to require flow of air via NC for few days, then weaned to room air Duration of O2 in A=5 d Duration of O2 in B=14 d Duration of any respiratory support in A=10.5 d Duration of any respiratory support in B=18 d Duration of CPAP in A=11.2±8.9 d Duration of CPAP in B=7.6±3.2 d |
||||
| Todd, 2012[23] | 154 | RCT | <30 wk gestation with CPAP from birth or following extubation | CPAP <24 h, started wean before consent, transferred or surgery >0.21 FiO2 >grade 2 IVH or congenial malformations |
Sudden removal of NCPAP (M1) | CPAP was weaned with gradually increasing time off with 6 h on CPAP Once the infant was 16 h off CPAP, attempt made to stop CPAP (M2) CPAP was weaned off similar to above but weaned to nasal cannula with O2 at 0.5 L/min for 24 h prior to removal of support (M3) |
M1: 32% came off CPAP in first attempt, 42% came off CPAP in 2–3 attempts, 28% took 4–13 attempts to come off, immature premature infants significantly take more attempts (24/25 wk=3, 26 wk=3, 27 wk=3.5, 28 wk=2, 29 wk=1) CPAP duration in M1=24.4±0.1 d CPAP duration in M2=38.6±0.1 d CPAP duration in M3=30.5±0.1 d O2 duration in M1=24.1±1.5 d O2 duration in M2=45.8±2.2 d O2 duration in M3=34.1±2.0 d BPD incidence in M1=12.5% BPD incidence in M2=42% BPD incidence in M3=19% Length of admission in M1=58.5±0.1 d Length of admission in M2=73.8±0.1 d Length of admission in M3=69.5±0.1 d |
M1-29.6±1.7 M2-30.5±2.3 M3-29.7±1.9 |
M1-1014±38 M2-1112±34 M3-1074±40 |
M1-11.3±0.8 | M2-16.8±1.0 M3-19.4±1.3 |
| Rastogi, 2013[24] | 56 | RCT | ≤32 wk who required NCPAP for at least 48 h | Outborn Chromosomal defects Severe congenital anomalies including congenital heart disease, neurological malformations, chest wall or airway abnormalities, lung hypoplasia |
Sudden weaning -NCPAP was removed and kept off completely | Gradual wean-NCPAP was cycled off for 3 h with 3 h on for 48 h, if tolerated, increased to 6 h off and 3 h on for 48 h. If tolerated then discontinued | Successfully weaned in first attempt in A=46% Successfully weaned in first attempt in B=43% Number of wean trial in A and B=2 (median) Length of hospital stay in A=61.3±19.6 d Length of hospital stay in B=66.0±27.1 d |
A:33.8±2.6; B: 33.7±2.8 |
A:1736±487 B: 1736±501 |
||
ABG: arterial blood gas: BPD: bronchopulmonary dysplasia; BW: birth weight; CGA: corrected gestational age; CLD: chronic lung disease; FiO2: fractional inspired oxygen; GER: gastro-esophageal reflux; IVH: intra-ventricular hemorrhage; NC: nasal cannula; NCPAP: nasal continuous positive airway pressure; NEC: necrotizing enterocolitis; PVL: periventricular leukomalacia; PEEP: positive end expiratory pressure; RCT: randomized controlled trial; ROP: retinopathy of prematurity; SpO2: saturation of peripheral oxygen.
Outcomes
The results for the objectives of the systematic review were as follows.
CGA at successful wean
There is a significant relationship between the time to come off NCPAP and prematurity.[26] In a retrospective chart review, Rastogi et al[26] found that babies had a successful wean at CGA of 32.9±2.5 weeks. Similarly, Todd et al[23] found that when babies were weaned by sudden and complete removal of NCPAP, after predefined weaning criteria, they weaned earlier, at CGA of 31.9±0.1 weeks, than those weaned by cycling CPAP off. Rastogi et al[24] in a RCT showed that sudden wean was successful at CGA 33.8±2.6 weeks, as compared to gradual wean, which was successful at 33.7±2.8 weeks. Based on the above data, we summarize that optimal corrected GA for the successful wean is usually achieved at 32 to 33 weeks, and was inversely related to the GA.
Weight at successful wean
Rastogi et al[26] reported that babies weaned successfully when they reached average weight of 1611±432 g, with those more preterm weaning off later, after gaining more weight. Rastogi et al[24] in a RCT showed that when babies were successfully weaned from NCPAP suddenly and by gradual time off method at 1736±487 g and 1736±501 g, respectively. Based on this limited data, we conclude that successful wean is rare before a weight of 1600 g, and is inversely related to the GA.
Methods used to wean NCPAP
Abdel-Hady et al[20] were the first to report on discontinuation of NCPAP by reducing pressure in increments over 1 hour. The gradual pressure reduction over 1 hour before weaning showed that upon discontinuation, these neonates were on shorter duration on NCPAP, as compared to sudden weaning. Singh et al[20] compared decreasing pressure with increasing the time off CPAP, using oxygen or low flow nasal cannula, but the details of reduction of pressure were not reported. Soe et al[21] described in reduction of pressure in greater detail where the pressure was reduced by 1 cm H2O every 48 hours before discontinuation of NCPAP.
In the two RCT, Todd et al[23] and Rastogi et al[24] described the comparison between sudden and gradual graded time off NCPAP. Sudden wean meant removal of NCPAP completely at once after predefined stability criteria were met. In gradual graded time off, the time on NCPAP was gradually decreased with increasing in time off NCPAP before removing the NCPAP completely. Todd et al[23] found sudden removal decreased time on NCPAP, while Rastogi et al did not find a difference between the 2 methods. Abdel-Hady et al[18] used the weaning method of sudden removal of NCPAP to room air or to oxygen by nasal cannula. Latter method was associated with increased time to completely wean off NCPAP and increased time on oxygen.
Three methods to wean NCPAP have been used, though their exact details varied with the studies. 1) Sudden wean: NCPAP is taken off completely after meeting predefined stability criteria;[24,25] 2) Gradual wean by gradual increase in time off NCPAP: After meeting the stability criteria, weaning off of NCPAP for different lengths of duration have been used. In one of the studies, NCPAP was cycled 3 hours off alternating with 3 hours on for 48 hours, if tolerated, NCPAP was kept off for 6 hours and on for 3 hours for the next 48 hours, if tolerated then, NCPAP is discontinued after the 48 hours.[24] Todd et al[23] used a total of 24 hours to wean NCPAP off with gradually increasing time off NCPAP; 3) Gradual wean by gradual reduction of pressure: There is variation in the rate and the amount of pressure reduction. It has been usually described over 1–2 days before completely weaning off NCPAP. One of the methods was to reduce pressure after the neonate was on 5 cm H2O for 8 hours, then to 4 cm for 8 hours to 3 cm for 8 hours before removing the NCPAP. Others such as Soe et al[21] completed weaning over 48 hours and Todd et al[23] over 24 hours.
Based on these studies, the optimal method of weaning for most neonates is still not known. The sudden wean method has been shown to have a successful outcome in terms of shorter weaning period, shorter duration of CPAP, decreased length of hospital stay and lower incidence of broncho pulmonary dysplasia compared to gradual graded time off method but the neonate needs to meet the pre-defined criteria for readiness to wean.[18,23] Rastogi et al[24] reported that there was no significant difference between the sudden weaning and gradually increasing time off NCPAP. This difference could explain the differences in the length of gradual graded time off NCPAP protocol used by these authors.[23,24]
Defining the timing to initiate NCPAP wean
Criteria used in various studies[23,24] to define respiratory stability before considering weaning were described and used by Todd et al[23] and Rastogi et al[24] (Table 3). Studies by Abdel-Hady et al,[19] Singh et al[20] and Soe et al,[21] where these criteria were not as well defined or comprised of very few clinical indicators. Predefined criteria to initiate NCPAP wean reduces variation and may decrease time on NCPAP. Utilization of criteria for identification for readiness for NCPAP wean is important, since premature weaning not only leads to failure, but may prolong the time on CPAP and oxygen due to undue stress caused by increased work of breathing due to premature wean.
Table 3.
Stability criteria for weaning of nasal continuous positive airway pressure
| The following should be present for 24–48 h prior to weaning: |
|---|
| Continuous positive airway pressure (CPAP) pressure of 5 cm H2O; |
| FiO2 of 0.21; |
| Normal work of breathing: no persistent tachypnea (>60 breaths for >2 h), marked retractions; |
| No apnea (cessation of respiration >20 sec) associated with bradycardia or cyanosis with >2 episodes in 12 h or >3 in 24 h with at least one requiring bag and mask ventilation; |
| Saturation >90% most of the time or PaO2/transcutaneous PaO2 >45 mmHg; |
| Not currently treated for PDA or sepsis at the time of weaning; |
| Tolerated time off CPAP during nursing cluster care up to 15 min or more. |
PDA: patent ductus arteriosus; FiO2: fractional inspired oxygen; PaO2: partial pressure of arterial oxygen.
Definition of successful CPAP wean
We found variability in the definition of successful wean off NCPAP including the time post NCPAP wean to be considered a successful wean, that were used in the studies included in this review. The average time taken to wean off from NCPAP varied from 1–7 days depending on the definition of the success used in different studies.[4–6] Rastogi et al[24] have defined a successful wean off NCPAP most stringently, with the infant being stable on room air for 7 days with absence of persistent tachypnea, marked retractions, apneic episodes, on room air without any respiratory support or supplemental oxygen for 7 days.
Definition of NCPAP weaning failure
Criteria used for defining failure of wean have been adopted from Todd et al and Rastogi et al[23,24] (Table 4). There is variability not only in the criteria but also the timing for which they should be observed before considerable successful wean, which varied from 1 to 7 days. Although data is limited, longer duration of observation before claiming success may be important as many of the infants after coming off NCPAP fail beyond 1–2 days when there is loss of their lung volumes.
Table 4.
Clinical criteria for defining failure of trial off nasal continuous positive airway pressure
| The following should be present up to 7 d after weaning: |
|---|
| Increased work of breathing: persistent tachypnea (>60 for >2 h) and marked retractions; |
| Apnea (cessation of respiration >20 sec) associated with bradycardia or cyanosis with >2 episodes in 12 h or >3 in 24 h with at least one requiring bag and mask ventilation; |
| Increased O2 requirement >0.21 to maintain the oxygen saturations >90%; |
| Abnormal blood gases (2 arterial samples >2 h apart) with low pH <7.2, PaCO2 >65 mmHg, PaO2 <50 mmHg. |
PaCO2: partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen.
Attempts needed to successful NCPAP wean
Success of NCPAP wean at the first attempt varied from 20% to 60%, usually effective in about 40% of the infants, with number of trials ranging between 1 to 7 attempts, with a median of 2 attempts before successful NCPAP wean.[24] It took 11.3±0.8 days for NCPAP weaned off using the sudden method, as compared to 16.8±1.0 days when weaned using gradual time off.[25] Though this has not been confirmed by other authors.[24]
Factors affecting NCPAP wean
Earlier studies showed that comorbidities such as IVH, retinopathy of prematurity, bronchopulmonary dysplasia (BPD) and periventricular leukomalacia did not significantly affect the success of NCPAP wean.[19] Rastogi et al[26] in a retrospective study, discussed the factors affecting NCPAP wean and classified them into modifiable and non-modifiable factors (Table 5).
Table 5.
Clinical factors affecting successful weaning of nasal continuous positive airway pressure
| Non-modifiable factors | Modifiable factors |
|---|---|
| Birth weight | Chorioamnionitis |
| Gestational age | Intubation |
| Anemia | |
| GERD | |
| Apnea | |
| IVH | |
| Sepsis/NEC | |
| Caffeine |
GERD: gastro-esophageal reflux disease; IVH: intra-ventricular hemorrhage; NEC: necrotizing enterocolitis.
Non-modifiable or demographic factors: While birth weight and GA were inversely related to the duration of NCPAP, ethnicity and gender were not related to the successful weaning of NCPAP. Modifiable factors or comorbidities: intubation, chorioamnionitis, anemia, gastro-esophageal reflux disease, apnea, IVH and sepsis are associated with duration on NCPAP while antenatal steroids, magnesium sulfate use, intra uterine growth restriction and BPD were not. Recently, Broom et al[27] observed that sudden weaning not only decreased the weaning duration but also did not affect the weight gain, time to full feeds and time to stop caffeine.
Discussion
From the present literature, we conclude that criteria for readiness for NCPAP wean is usually when neonates attain a CGA of 32 to 33 weeks and weight of 1600 g. The available methods of weaning include sudden wean, gradually increase in time off NCPAP or gradually decrease in NCPAP pressure. The strength of this review paper is the detailed assessment of the stability criteria for initiation of weaning, methods of weaning and detailed definition of success and failure of NCPAP weaning. Further, it details the various methods used to wean off NCPAP. It also highlights modifiable factors which may influence NCPAP wean, correction of which may increase the success and decrease time to wean off NCPAP. Therefore, this information may be useful for developing guidelines for clinical care of the preterm infants and for planning future studies investigating NCPAP wean among preterm infants.
One of the limitations of this review is the paucity of available studies on NCPAP wean. As a result, we included abstracts which had described and compared weaning methods. Despite their inclusion, optimal method of NCPAP weaning in preterm infants is still not well defined. Also due to small number of studies and variability in methods used for weaning further statistical analysis or meta-analysis was not possible. Other limitations include the bias of the medical provider and inability to blind to the method used for weaning. There are variations in both methods of weaning for graded time off or pressure decrease to wean off NCPAP that precludes direct comparisons between the studies. The factors affecting weaning also need to be further validated from multicenter data as nuances of single center can influence the results which might not only be related to a single intervention. The effect of caffeine on NCPAP weaning is a still largely unknown.[24,26] Schmidt et al[28] have shown decreased incidence of apnea and BPD in preterm neonates and increased use of caffeine may be related to decrease in time to wean off NCPAP. Furthermore, anemia and gastro-esophageal reflux disease significantly increase the time to wean NCPAP,[24,26] corrective interventions might decrease the time and needs to be studied further.
However, the paucity of studies on optimal timing and method of weaning highlights the need for future RCT to perform direct comparisons of these methods. In summary, future studies are needed to study and confirm the criteria for readiness for weaning, defining successful and failure to wean NCPAP. To compare and contrast the methods used for weaning and defining any modifiable factors correction of which would lead to earlier successful weaning from NCPAP.
Conclusions
The successful wean occurs at CGA of 32 to 33 weeks and weight of 1600 g. Criteria for readiness, success and failure from weaning are well defined. There is no one method is consistently associated with earlier weaning though one large multicenter weaning trial demonstrated sudden weaning may be associated with a shorter weaning time. There are factors that affect successful weaning are intubation, anemia, infection and gastro-esophageal reflux, presence of which are associated with prolonged time from weaning.
Acknowledgments
Funding: No external funding was secured for this study.
Footnotes
Ethical approval: Not required.
Competing interest: The authors have no conflict of interest.
Contributors: Amatya S contributed to acquisition of data, drafting and final approval. Rastogi D contributed to concept and design, analysis and interpretation of data, critical revision final approval. Bhutada A contributed to interpretation of data, critical revision and final approval. Rastogi S contributed to concept and design, acquisition of data, drafting, critical revision and final approval.
Contributor Information
Shaili Amatya, Division of Neonatology, Maimonides Infants & Children’s Hospital, 4802 Tenth Ave, K-113, Brooklyn NY 11219, USA.
Deepa Rastogi, Division of Neonatology, Maimonides Infants & Children’s Hospital, 4802 Tenth Ave, K-113, Brooklyn NY 11219, USA.
Alok Bhutada, Division of Neonatology, Maimonides Infants & Children’s Hospital, 4802 Tenth Ave, K-113, Brooklyn NY 11219, USA.
Shantanu Rastogi, Children’s Hospital at Montefiore, Albert Einstein College of Medicine, Bronx, NY 10467, USA.
References
- 1.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–708. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 2.Aly H. Ventilation without tracheal intubation. Pediatrics. 2009;124:786–789. doi: 10.1542/peds.2009-0256. [DOI] [PubMed] [Google Scholar]
- 3.Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med. 1971;284:1333–1340. doi: 10.1056/NEJM197106172842401. [DOI] [PubMed] [Google Scholar]
- 4.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79:26–30. [PubMed] [Google Scholar]
- 5.Bhandari V. The potential of non-invasive ventilation to decrease BPD. Semin Perinatol. 2013;37:108–114. doi: 10.1053/j.semperi.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. J Perinatol. 2003;23:195–199. doi: 10.1038/sj.jp.7210904. [DOI] [PubMed] [Google Scholar]
- 7.De Klerk AM, De Klerk RK. Nasal continuous positive airway pressure and outcomes of preterm infants. J Paediatr Child Health. 2001;37:161–167. doi: 10.1046/j.1440-1754.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- 8.Aly H, Milner JD, Patel K, El-Mohandes AA. Does the experience with the use of nasal continuous positive airway pressure improve over time in extremely low birth weight infants? Pediatrics. 2004;114:697–702. doi: 10.1542/peds.2003-0572-L. [DOI] [PubMed] [Google Scholar]
- 9.Polin RA, Sahni R. Newer experience with CPAP. Semin Neonatol. 2002;7:379–389. doi: 10.1053/siny.2002.0132. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol (1985) 1996;81:1471–1476. doi: 10.1152/jappl.1996.81.4.1471. [DOI] [PubMed] [Google Scholar]
- 11.Davis PG, Morley CJ, Owen LS. Non-invasive respiratory support of preterm neonates with respiratory distress: continuous positive airway pressure and nasal intermittent positive pressure ventilation. Semin Fetal Neonatal Med. 2009;14:14–20. doi: 10.1016/j.siny.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Levene MI, Fawer CL, Lamont RF. Risk factors in the development of intraventricular haemorrhage in the preterm neonate. Arch Dis Child. 1982;57:410–417. doi: 10.1136/adc.57.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aly H, Hammad TA, Essers J, Wung JT. Is mechanical ventilation associated with intraventricular hemorrhage in preterm infants? Brain Dev. 2012;34:201–205. doi: 10.1016/j.braindev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bowe L, Clarke P. Current use of nasal continuous positive airways pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 2005;90:F92–F93. doi: 10.1136/adc.2004.061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aly H. Ventilation without tracheal intubation. Pediatrics. 2009;124:786–789. doi: 10.1542/peds.2009-0256. [DOI] [PubMed] [Google Scholar]
- 16.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:S9–S15. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 17.De Paoli AG, Morley C, Davis PG. Nasal CPAP for neonates: what do we know in 2003? Arch Dis Child Fetal Neonatal Ed. 2003;88:F168–F172. doi: 10.1136/fn.88.3.F168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Hady H, Shouman B, Aly H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: a randomized controlled trial. Early Hum Dev. 2011;87:205–208. doi: 10.1016/j.earlhumdev.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Hady H, Mohareb S, Khashaba M, Abu-Alkhair M, Greisen G. Randomized controlled trial of discontinuation of nasal-CPAP in stable preterm infants breathing room air. Acta Paediatr. 1998;87:82–87. doi: 10.1080/08035259850157921. [DOI] [PubMed] [Google Scholar]
- 20.Singh SD, Bowe L, Clark P, Glover K, Pasquill A, Robinson MJ, et al. Is decreasing pressure or increasing time of a better strategy in weaning VLBW infants from NCPAP. Eur J Paediatr. 2006;165(Suppl 1):48. [Google Scholar]
- 21.Soe A, Hodgkinson J, Jani B, Ducker DA. Nasal continuous positive airway pressure weaning in preterm infants. Eur J Paediatr. 2006;165(Suppl 1):48–49. [Google Scholar]
- 22.Jardine LA, Inglis GD, Davies MW. Strategies for the withdrawal of nasal continuous positive airway pressure (NCPAP) in preterm infants. Cochrane Database Syst Rev. 2011;2:CD006979. doi: 10.1002/14651858.CD006979.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Todd DA, Wright A, Broom M, Chauhan M, Meskell S, Cameron C, et al. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2012;97:F236–F240. doi: 10.1136/adc.2011-300133. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi S, Wong W, Gupta A, Bhutada A, Rastogi Deepa Maimonides Neonatal Group. Gradual versus sudden weaning from nasal CPAP in preterm infants: a pilot randomized controlled trial. Respir Care. 2013;58:511–516. doi: 10.4187/respcare.01999. [DOI] [PubMed] [Google Scholar]
- 25.Jardine L, Davies MW. Withdrawal of neonatal continuous positive airway pressure: current practice in Australia. Pediatr Int. 2008;50:572–575. doi: 10.1111/j.1442-200X.2008.02617.x. [DOI] [PubMed] [Google Scholar]
- 26.Rastogi S, Rajasekhar H, Gupta A, Bhutada A, Rastogi D, Wung JT. Factors Affecting the Weaning from Nasal CPAP in Preterm Neonates. Int J Pediatr. 2012;2012:416073. doi: 10.1155/2012/416073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broom M, Ying L, Wright A, Stewart A, Abdel-Latif ME, Shadbolt B, et al. CeasIng Cpap At standarD criteriA (CICADA): impact on weight gain, time to full feeds and caffeine use. Arch Dis Child Fetal Neonatal Ed. 2014;99:F423–F425. doi: 10.1136/archdischild-2013-304581. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]