Abstract
Electrical stimulation of auditory nerve fibers using cochlear implants (CI) shows psychophysical forward masking (pFM) up to several hundreds of milliseconds. By contrast, recovery of electrically evoked compound action potentials (eCAPs) from forward masking (eFM) was shown to be more rapid, with time constants no greater than a few milliseconds. These discrepancies suggested two main contributors to pFM: a rapid-recovery process due to refractory properties of the auditory nerve and a slow-recovery process arising from more central structures. In the present study, we investigate whether the use of different maskers between eCAP and psychophysical measures, specifically single-pulse versus pulse train maskers, may have been a source of confound.
In experiment 1, we measured eFM using the following: a single-pulse masker, a 300-ms low-rate pulse train masker (LTM, 250 pps), and a 300-ms high-rate pulse train masker (HTM, 5000 pps). The maskers were presented either at same physical current (Φ) or at same perceptual (Ψ) level corresponding to comfortable loudness. Responses to a single-pulse probe were measured for masker-probe intervals ranging from 1 to 512 ms. Recovery from masking was much slower for pulse trains than for the single-pulse masker. When presented at Φ level, HTM produced more and longer-lasting masking than LTM. However, results were inconsistent when LTM and HTM were compared at Ψ level. In experiment 2, masked detection thresholds of single-pulse probes were measured using the same pulse train masker conditions. In line with our eFM findings, masked thresholds for HTM were higher than those for LTM at Φ level. However, the opposite result was found when the pulse trains were presented at Ψ level.
Our results confirm the presence of slow-recovery phenomena at the level of the auditory nerve in CI users, as previously shown in animal studies. Inconsistencies between eFM and pFM results, despite using the same masking conditions, further underline the importance of comparing electrophysiological and psychophysical measures with identical stimulation paradigms.
Keywords: electrical stimulation, electrically evoked compound action potential, recovery from forward masking, detection threshold, stimulation rate, neural adaptation
INTRODUCTION
Psychophysical forward masking (pFM) refers to the increase in detection threshold of a probe when presented after a masker, compared with the probe’s unmasked threshold. This effect can persist up to several hundreds of milliseconds in both normal-hearing (NH) and cochlear implant (CI) listeners and depends on the duration and level of the masker (Plomp 1964; Shannon 1990). The amplitude-normalized temporal course of masking was found to be similar for these two subject groups, and it has been thus hypothesized that pFM likely involves processes at or more central than the auditory nerve (Shannon 1990).
Contemporary CIs are able to record the composite auditory nerve response to electrical stimulation, a measure known as the electrically evoked compound action potential (eCAP). Using different recording paradigms, Brown et al. (1990) and Morsnowski et al. (2006) used this functionality to measure the eCAP response to a probe pulse presented after a single-pulse masker for several masker-probe intervals. Both studies used masker and probe levels at or just below the loudest acceptable levels. They showed that eCAP recovery from forward masking (eFM) was more rapid than what is typically found in pFM and that the masker had no effect on the probe response when the interval was larger than a few milliseconds. Morsnowski et al. (2006) reported absolute refractory periods and exponential recovery from masking with time constants in the range of a few hundreds of microseconds for human CI users. These findings agree well with single-fiber recordings in cats using a similar paradigm (Miller et al. 2001). Also, using single-pulse maskers, Nelson and Donaldson (2001) reported that psychophysical recovery from forward masking was dominated by a rapid-recovery process similar to that observed in physiological recovery functions and, therefore, suggested that this process reflects refractory properties of the auditory nerve.
Apart from Nelson and Donaldson (2001), most pFM studies in CI users employed maskers with long durations, i.e., pulse train maskers. For example, using 320-ms maskers, Nelson and Donaldson (2002) found slow recovery functions with a mean time constant greater than 50 ms, which is consistent with data from previous studies in both NH and CI listeners. They thus theorized that the slow-recovery pFM is mediated by more central processes in both subject groups. Lee et al. (2012) reached a similar conclusion when comparing younger with older CI users. Although eCAP recovery from single-pulse maskers showed no difference between these groups, psychophysical detection thresholds showed slower recovery in older CI users. Due to this difference in performance, the authors proposed that changes in the central auditory system may be the main contributors to slow recovery from pFM, rather than peripheral mechanisms.
However, the use of different maskers between eCAP and psychophysical measures, specifically single-pulse versus pulse train maskers, may have been a source of confound. Two animal studies showed that the amount of masking generated at the level of the auditory nerve depends on various properties of the masker, including its duration, pulse rate, and current level: Using 100-ms sinusoidal electrical maskers in guinea pigs, Killian et al. (1994) found eCAP recovery functions that were sometimes incomplete, even after 500 ms. More recently, Miller et al. (2011) measured single-fiber responses in cats following 300-ms pulse train maskers and found a decrease in the response probability to a probe stimulus for several hundreds of milliseconds. These observations are in contrast with the fast recovery obtained with single-pulse maskers. The first aim of the present study was to test whether such long eFM recovery could be observed at the level of the auditory nerve in human CI users when using pulse train maskers.
Miller et al. (2011) also found that the masker pulse rate had a significant effect on eFM recovery. Specifically, for a fixed current level, single units recovered faster after a 250-pps than after a 5000-pps pulse train masker. This observation has relevance for contemporary CI speech coding strategies, which continuously stimulate the auditory nerve at high stimulation rates up to 2500 pps per channel depending on the speech processing strategy (reviews in Loizou 1998; Zeng et al. 2008), yielding aggregate rates with possibly an order of magnitude higher. Such high stimulation rates are likely to induce longer adaptation than lower rates (Miller et al. 2008; Miller et al. 2011). The second aim of this study was to test whether recovery from eFM slowed down as a function of masker rate using the same low and high rates investigated by Miller et al. (2011). In human CI listeners, a direct comparison between low and high rates is further complicated by the fact that both stimuli do not elicit the same loudness percept when presented at the same current level. When increasing the stimulation rate in CI users, the current level needs to be decreased in order to maintain the same loudness (Kreft et al. 2004). This decrease in current level may in turn decrease the amount of adaptation (Miller et al. 2011). Therefore, changing the electrical stimulation rate in CI strategies may have two opposite effects on the amount of adaptation.
These study aims were addressed in experiment 1 where we compared eFM functions for low-rate and high-rate pulse train maskers in two cases: when presented at the same current level and when presented at loudness-balanced levels. A single-pulse masker served as reference. In experiment 2, we attempted to relate our eFM findings with perception in order to understand which part of the percept may be explained by the auditory nerve response. To that end, pFM data using the same stimuli as used for eFM are also reported.
EXPERIMENT 1: ELECTROPHYSIOLOGICAL FORWARD MASKING
Methods
Subjects
Experiment 1 included nine adult CI users (U01–U09). They were implanted with Cochlear CI24RE implants (Cochlear Limited, Sydney, Australia), except for U05 who had the Cochlear CI512 implant. All had Contour Advance electrode arrays with 22 intracochlear contacts. Table 1 provides more information on the subjects. They received financial compensation and reimbursement of their traveling costs. A local ethics committee approved this study (Eudract 2012-A00438-35).
TABLE 1.
Subject information
| CI user | Gender | Age | Ear | Etiology | Duration of CI use | Measurement electrodesa |
|---|---|---|---|---|---|---|
| U01 | F | 57 | R | Genetic | 5 years | S11-R09; S20-R22 |
| U02 | F | 55 | R | Genetic | 3 years | S11-R13; S20-R22 |
| U03 | F | 27 | R | Meningitis | 9 months | S11-R13; S20-R22 |
| U04 | F | 50 | R | Genetic | 6 years | S11-R13; S20-R22 |
| U05 | F | 48 | L | Unknown | 2 years | S11-R13; S20-R22 |
| U06 | F | 36 | L | Congenital progressive | 1 year | S11-R13; S20-R22 |
| U07 | F | 77 | R | Unknown | 7 years | S20-R22 |
| U08 | F | 56 | L | Pendred syndrome | 8 years | S04-R06 |
| U09 | M | 61 | L | Trauma | 5 years | S15-R17 |
CI cochlear implant, F female, M male, L left, R right
aS## indicates the stimulation electrode, and R## indicates the recording electrode
Stimuli
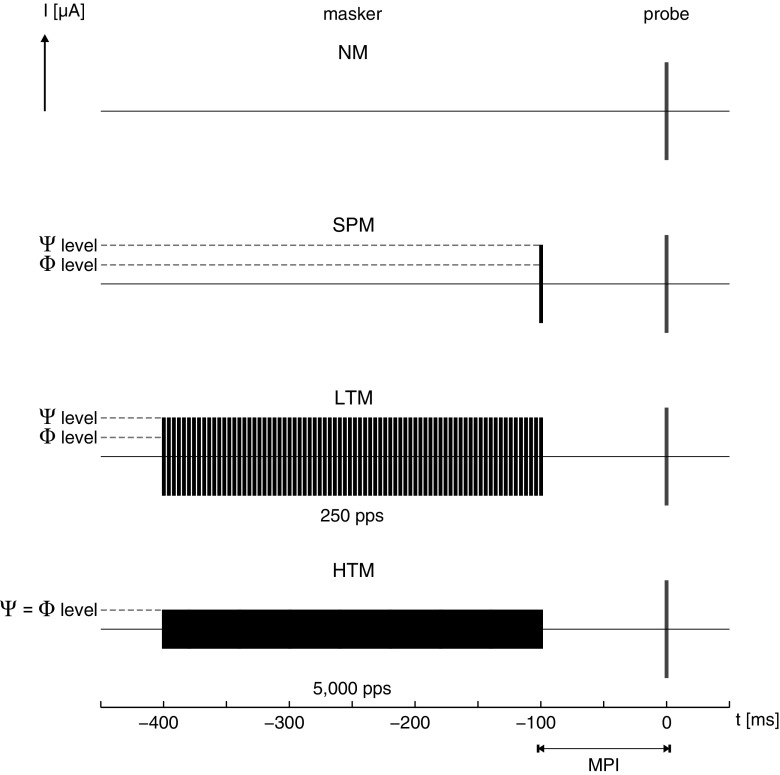
The eCAPs were measured in response to a probe stimulus, a single pulse set at a current level eliciting an N1-P2 amplitude response of approximately 50 μV when no masker (NM) was present. In addition, the probe response was measured in five masking conditions, where masker duration, pulse rate, and current level were varied. Maskers were either (1) a single-pulse masker (SPM), (2) a 300-ms low-rate pulse train masker (LTM) at 250 pps, or (3) a 300-ms high-rate pulse train masker (HTM) at 5000 pps. HTM was always presented at a current level evoking comfortable loudness (C level). LTM was presented either at the same physical current level (Φ) or at a level eliciting the same loudness as HTM, i.e., psychophysical level (Ѱ). SPM was also presented at two different current levels, equal to those of LTM. Besides masker levels, the masker-probe interval (MPI) was varied as an experimental condition. This interval was defined by the time between the offset of the masker and the onset of the probe and was 2n ms, where n is an integer in the range 0–9. Figure 1 provides an overview of the masking conditions.
FIG. 1.
Overview of the masking conditions: no masker (NM), single-pulse masker (SPM), low-rate pulse train masker (LTM) at 250 pps, and high-rate pulse train masker (HTM) at 5000 pps. Pulse train maskers were presented at the same physical current level (Φ) or at the same psychophysical loudness (Ѱ). SPM was presented at current levels equal to those of LTM. The masker-probe interval (MPI; here, 100 ms) was varied as an experimental condition.
All electrical pulses were biphasic, symmetric, and rectangular. They were presented in monopolar mode. The two phases had durations of 25 μs each and were separated by an 8-μs inter-phase gap. All masker stimuli (SPM, LTM, and HTM) had pulses with leading cathodic phases, while the leading polarity of the probe was alternated.
Procedures
Prior to data collection, we determined whether eCAP responses could be detected. If such was the case, the eCAP growth function was measured and the masker levels were established. Finally, forward masking was determined electrophysiologically by measuring eCAP recovery functions. These procedures are explained in the following subsections.
eCAP Detection
The eCAP detection procedure checked whether a response could be detected and determined the current level eliciting an N1-P2 amplitude response of approximately 50 μV. It started by choosing a stimulating electrode in the apical or middle range, with the external electrode (MP1) as reference. Initially, the recording electrode was two positions apical to the stimulation electrode, with the CI stimulator housing (MP2) as reference. The probe was presented at a rate of 80 pps. Its current level was increased in small steps, ranging from 0.16 to 0.78 dB, while monitoring loudness acceptability on a 10-point rating scale. The alternating polarity method (e.g., Miller et al. 1998) was used for artifact suppression. The procedure terminated once the probe evoked an N1-P2 amplitude response of approximately 50 μV. If no eCAP response was found or if no sufficient amplitude could be obtained before the CI user rated the loudness above C level, i.e., rating 7 on the 10-point scale, then recording gain, recording electrode position, or both stimulating and recording electrodes were varied and the detection procedure was started again. If the detection of an eCAP response was successful, the amplitude growth as a function of stimulation level was measured with a low probe rate of 14 pps to limit the effect of neural adaptation (Clay and Brown 2007). Again, subjective loudness acceptability was monitored during the procedure. Table 1 shows the electrodes that were used for each subject.
Establishing Masker Levels
To establish masker levels, LTM or HTM was presented at increasing current level while monitoring loudness perception on the 10-point rating scale to determine comfortable loudness levels. For each masker, the procedure terminated when a loudness rating of 8 (labeled as “loud”) was reached. Subsequently, the loudness of LTM was balanced to that of HTM using the following adjustment paradigm: Each loudness-balancing trial consisted of two pulse trains presented consecutively with a 500-ms inter-stimulus gap. The first pulse train was the reference and its level was fixed across the adjustment. The current level of the second stimulus pulse train was adjustable in step sizes 0.16, 0.31, or 0.47 dB with a graphical user interface provided to the subjects. They were asked to balance its loudness to the first pulse train and were encouraged to make over- and undershoots before deciding on the final level. First, HTM was the reference and was set to the current level invoking a rating of 6 (labeled as “most comfortable”), while LTM was initially set to the current levels invoking a rating of 3 or 7 (labeled as “soft” and “loud but comfortable,” respectively). This procedure was carried out once for each of the previously mentioned initial levels of LTM. Then, a new procedure was carried out with LTM as the reference. Its level was fixed at the average of the two final LTM levels adjusted to the loudness of HTM. The subject next adjusted the level of HTM, which was also initially set to the current levels invoking a rating of 3 or 7. The loudness-balanced level of LTM was calculated by
| 1 |
where L B is the loudness-balanced level, L r6 is the level invoking a loudness rating of 6, and is the average of the two final levels set by the subject during the adjustment paradigm. L B(LTM) was thus defined as having same loudness (i.e., psychophysical level Ѱ) as HTM at its fixed current level L r6(HTM). Presenting LTM at L r6(HTM) was defined as having the same physical current level (Φ) as HTM.
Forward Masking: eCAP Recovery Functions
Recovery functions were obtained with measurement sequences, each of which started with a 200-ms pulse train at a rate of 10,000 pps and at current level zero to power up the implant. A measurement sequence consisted of a masker stimulus, a defined MPI, and a probe pulse with a given leading polarity. After a 98-μs measurement delay, 32 points were recorded at a 20-kHz sampling rate. The gap between a probe and the onset of a subsequent masker was 400 ms. Artifact suppression was accomplished using a modified alternating polarity method, which compensated for the pulse train masker artifact. Ordinarily, the alternating polarity method measures responses for cathodic-leading (A 1) and anodic-leading (A 2) biphasic pulses. Inverting the polarity of the stimulus results in inverting the polarity of the artifact, whereas the polarity of the neural response remains the same. Averaging these two measurements cancels out most of the stimulus artifact. In this study, each measurement sequence had a preceding pulse train masker, which itself could generate an artifact. Thus, a third measurement (B) with the probe at current level zero was carried out. The neural response as a function of time (t) was finally calculated by
| 2 |
The telemetry system allows one to average neural responses on the internal memory (so-called sweeps) before transferring the averaged data back to the computer. Each measurement sequence consisted of 8 sweeps and was performed 8 times, effectively resulting in 64 neural responses per experimental condition.
Measurement sequences were grouped in blocks with a defined order and set of experimental conditions. Each block had a fixed MPI of 2n ms, where n is an integer in the range 0–9. A block started with an NM condition as control, followed by five combinations of different masker types (SPM, LTM, and HTM) and stimulation levels (Φ and Ѱ) in a randomized order. After the last block within a session, another measurement was conducted in the NM condition.
Sessions
Given a particular position of the testing electrode, apical or middle, two sessions were conducted. In the first session, we determined whether neural responses could be measured using the eCAP detection procedure. After defining measurement electrodes, the eCAP growth function was measured. Masker loudness growth was determined and loudness balancing was conducted, thereby establishing masker levels. In the second session, we first confirmed the eCAP growth function and subject’s tolerance of the masking levels. Then, eCAP recovery functions were measured for different experimental conditions. Each session had a duration of approximately 3 h, whereas the second session was interrupted in case no forward masking was found for MPIs of 4 ms or less. At the start and end of each session, electrode impedances were checked.
Material
A Cochlear Pod served as a USB interface to the speech processors. The eCAP detection was conducted using the clinical software Cochlear Custom Sound EP 3.2 and the Cochlear SP12 speech processor. Masker loudness growth and loudness balancing were conducted using the APEX research platform (Laneau et al. 2005) and the Cochlear L34 speech processor. The eCAP forward masking measurement sequences were programmed in Python using the Nucleus Implant Communicator (NIC2) software interface and again the Cochlear SP12 speech processor.
Analysis
Analysis of eCAP responses was done in MATLAB (MathWorks, Inc., Natick, MA, USA). After artifact suppression using the modified alternating polarity method, each eCAP trace consisted of 32 points, sampled at 20 kHz. These traces were interpolated at the 10-fold sampling frequency using shape-preserving piecewise cubic interpolation (interp1 function in MATLAB). Then the minimum and maximum amplitude points corresponding to N1 and P2, respectively, were calculated using zero crossings of the first derivative. Their difference was defined as the eCAP amplitude. Estimates for each experimental condition were calculated from the mean of eight eCAP amplitudes, each consisting of eight sweeps (internal memory averages).
To validate eCAP responses and establish the noise floor, all traces were checked by visual inspection. Three judges blinded to the experimental condition of a given trace classified the response as “eCAP present” or “eCAP absent.” Each judge checked all traces three times, with the majority defined as their respective judgment, and the majority judgment defined as the final judgment. Due to the stochastic nature of eCAP generation, the noise floor was defined when 25 % or less traces were classified as eCAP present in the final judgment.
Finally, to quantify eFM over time, eCAP recovery functions were fitted to an exponential model adopted from Morsnowski et al. (2006)
| 3 |
where V(t) is the eCAP amplitude for a given time interval t between the offset of the masker and the onset of the probe (i.e., MPI), V ∞ is the average of all measurements in the NM condition, τ is the time constant of the exponential decay, and c is a constant representing the absolute refractory period. The Nelder-Mead simplex algorithm (fminsearch function in MATLAB) was used to fit the parameters τ and c using unconstrained nonlinear optimization.
Additional statistical analysis was done using SPSS Statistics (IBM Corporation, Armonk, NY, USA) and MLwiN (Centre for Multilevel Modelling, University of Bristol, England).
RESULTS
Probe and Masker Levels
Probe levels determined in the eCAP detection procedure and established masker levels are shown in Table 2 for all subjects. When examining eFM data, one needs to consider the masker to probe level differences, which inherently affect the amount of forward masking. The eCAP detection procedure yielded a mean probe level of 53.4 dB re 1 μA. Psychophysically established HTM levels had a mean of 49.9 dB re 1 μA, which in 13/15 cases were lower than the respective probe level. LTM at Φ level was per definition at the same respective current level as HTM. LTM at its loudness-balanced Ѱ levels had a mean level of 54.3 dB re 1 μA, and in 9/15 cases was greater than the respective probe level. Loudness-balanced LTM always had current levels greater than HTM with a mean difference of +4.4 dB. This level difference as a function of stimulation rate is in accordance with previous findings (Kreft et al. 2004), showing that higher current levels are needed to achieve the same loudness percept with a low-rate pulse train (LTM at 250 pps) compared with a high-rate pulse train (HTM at 5000 pps).
TABLE 2.
Probe and masker levels
| CI user | Electrode position | Probe level [dB re 1 μA] | SPM = LTM | HTM | |
|---|---|---|---|---|---|
| Φ level [dB re 1 μA] | Ѱ level [dB re 1 μA] | Φ = Ѱ level [dB re 1 μA] | |||
| U01 | Middle | 54.66 | 50.90 | 56.23 | 50.90 |
| Apical | 53.57 | 54.82 | 57.65 | 54.82 | |
| U02 | Middle | 55.92 | 51.53 | 56.23 | 51.53 |
| Apical | 54.35 | 51.53 | 54.82 | 51.53 | |
| U03 | Middle | 53.41 | 52.31 | 56.23 | 52.31 |
| Apical | 52.78 | 51.21 | 56.23 | 51.21 | |
| U04 | Middle | 54.04 | 49.96 | 53.41 | 49.96 |
| Apical | 51.06 | 50.27 | 53.10 | 50.27 | |
| U05 | Middle | 53.10 | 53.88 | 57.17 | 53.88 |
| Apical | 52.63 | 52.00 | 54.66 | 52.00 | |
| U06 | Middle | 53.25 | 47.14 | 52.00 | 47.14 |
| Apical | 50.90 | 44.78 | 49.65 | 44.78 | |
| U07 | Apical | 52.47 | 43.84 | 51.06 | 43.84 |
| U08 | Basala | 54.98 | 47.61 | 52.31 | 47.61 |
| U09 | Middle | 53.57 | 46.51 | 53.25 | 46.51 |
| Mean | 53.38 | 49.89 | 54.27 | 49.89 | |
| SD | 1.35 | 3.23 | 2.38 | 3.23 | |
CI cochlear implant, Φ same physical current level, Ѱ same psychophysical loudness level, SPM single-pulse masker, LTM low-rate pulse train masker, HTM high-rate pulse train masker, SD standard deviation
aA basal electrode was used here due to difficulty detecting an eCAP response otherwise
At these masker and probe levels, only subjects U01–U05 showed eFM at both electrode positions. Their results demonstrate the general trend of lower HTM (8/10 cases) and higher loudness-balanced LTM (9/10 cases) current levels than corresponding probe levels. For the remaining subjects U06–U09, no masking was found for MPI > 4 ms or no masking was evident at all. They are thus excluded from further analysis of eFM. For this subject group, it appears that the differences between established masker levels (for LTM and HTM) and respective probe levels were generally larger than those for U01–U05, i.e., established masker current levels were relatively lower (c.f. Table 2). To assess the contribution of the masker-probe current level difference to the probability of observing forward masking, we fitted the following binary logistic regression model to the data:
| 4 |
where X 1 is the difference between masker and probe current levels (scalar predictor), X 2 is the pulse train masker (categorical predictor with baseline LTM = 0 and HTM = 1), β 0 , u is a random intercept across CI users u ∈ {1, … , 9}, β 1 is the main effect coefficient of X 1, β 2 is the main effect coefficient of X 2, β 3 is the interaction effect coefficient of X 1 X 2, and ε is the residual term. The categorical outcome measure Y was defined as “masking” = 1 when forward masking was found for MPIs greater than 4 ms; otherwise, it was defined as “no masking” = 0. Table 3 shows the fitted coefficients and their respective Wald statistic and odds ratio. The model was able to correctly predict 84.4 % of the observed outcomes. The Wald statistic confirms that both predictors X 1 and X 2 have significant contributions to the model (p = .016 and p = .044, respectively), which is not the case for their interaction nor for the random intercept. The odds ratio of β 1 at 4.179 shows that a larger positive difference between masker and probe current levels increases the odds that the outcome measure “masking” occurs. This suggests that the negative differences between established masker levels and respective probe levels for subjects U06–U09 did contribute to the absence of forward masking.
TABLE 3.
Fitting parameters of the binary logistic regression model
| Coefficient | Fitted value | Standard error | Odds ratio | Wald statistic (p) |
|---|---|---|---|---|
| β 0 , u a | −1.247 | 0.842 | 0.287 | 0.187 (.665) |
| β 1 | 1.430 | 0.595 | 4.179 | 5.785 (.016) |
| β 2 | 7.990 | 3.983 | 2.951 × 103b | 4.024 (.044) |
| β 3 | −0.132 | 0.942 | 0.876 | 0.020 (.887) |
aWhere β 0,u = β 0 + p 0,u with p 0,u ~N (0,0.886 ± 2.048)
bVery large odds ratio indicates that the model saturates in the step from LTM = 0 (baseline) to HTM = 1
The very large odds ratio of β 2 indicates that the model saturates in the step from LTM = 0 (baseline) to HTM = 1, which can be traced to the fact that masking occurred in 9/30 cases with LTM (Φ and Ѱ) as opposed to 10/15 cases with HTM (Φ = Ѱ). To alleviate this problem, one could remove the corresponding variable from the model and collapse the data or just consider the baseline data, i.e., only LTM at Φ and Ѱ levels. Such a simplified model includes only the random intercept and the main effect of X 1. Still, fitting this model showed a significant contribution (p = .019, data not shown here) of the predictor X 1 and not the random intercept, which further underlines the importance of the masker-probe current level difference to the probability of forward masking in this CI subject group.
eCAP Amplitude Estimates
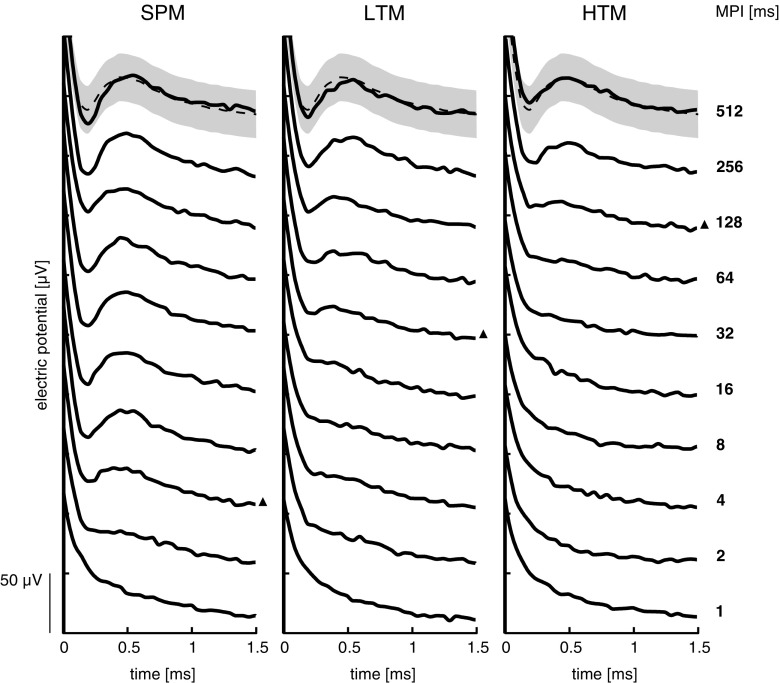
An illustrative example of eCAPs in response to the masked probe for subject U05, middle electrode, is shown in Figure 2. All maskers were presented at Ѱ level for MPI in the range of 1–512 ms. Note that the eCAP response starts to recover at MPI = 4 ms for SPM, which is indicated by the black triangle. This is in contrast to pulse train maskers where valid responses were first detected at MPI = 32 ms for LTM and at MPI = 128 ms for HTM. Furthermore, eCAP responses to SPM appear to rapidly reach full recovery, which was not the case for either pulse train masker.
FIG. 2.
Illustrative example (subject U05, middle electrode) of eCAP traces in response to the masked probe as a function of the masker-probe interval (MPI), for the masking conditions: single-pulse masker (SPM), low-rate pulse train masker (LTM), and high-rate pulse train masker (HTM). Pulse train maskers were presented at the same psychophysical loudness level (Ѱ). SPM was presented at current levels equal to those of LTM. For each masking condition, the first response judged as “eCAP present” is marked by a black triangle on its right. Mean eCAP and ±1 standard deviation of all responses in the no masker (NM) condition are shown as a dashed line and shaded area, respectively. A 50-µV scale bar is shown in the bottom left corner.
The eCAP N1-P2 amplitudes were used as a measure of eFM and are shown as a function of MPI in Figures 3 and 4 for each subject and electrode and for each masker presented at Φ and Ψ level, respectively: white circles for SPM, gray squares for LTM, and black triangles for HTM. The average of all amplitude estimates in the NM condition is shown as a solid horizontal line and two times the standard deviation (σ) below that (i.e., 95.45 % confidence interval) as a dashed horizontal line. Masking was considered when amplitude estimates were lower than NM − 2σ. The NM condition range was 30.5–86.7 μV with a mean of 50.4 μV. For all subjects, eCAP measurements in NM condition over the course of the experimental sessions showed some variability but did not demonstrate any sign of neural fatigue (data not shown here).
FIG. 3.
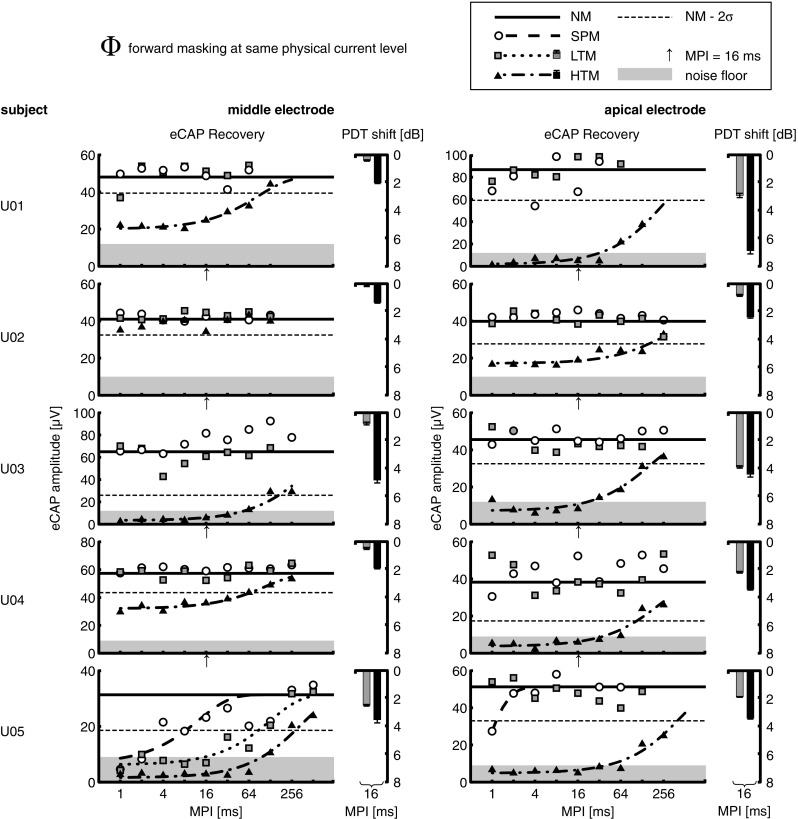
Individual results for the masking conditions: no masker (NM), single-pulse masker (SPM), low-rate pulse train masker (LTM), and high-rate pulse train masker (HTM), for each electrode position (apical and middle). The average of all amplitude estimates in the NM condition is shown as a solid horizontal line, and two times the standard deviation (σ) below that as a dashed horizontal line. The noise floor, which was defined when 25% or less traces were valid eCAP responses, is shown as a shaded area. Pulse train maskers were presented at the same physical current level (Φ), corresponding to a current level evoking comfortable loudness for HTM. SPM was always presented at current levels equal to those of LTM. Electrophysiological forward masking (eFM) was measured using eCAP amplitude estimates as a function of the masker-probe interval (MPI). Fitted exponential models of the eCAP recovery functions are only shown when eFM occurred. Psychophysical forward masking (pFM) results are shown as shifts in the probe detection threshold (PDT) at an MPI of 16 ms. Error bars indicate the standard error of the mean. Note that the ordinate is inverted.
FIG. 4.
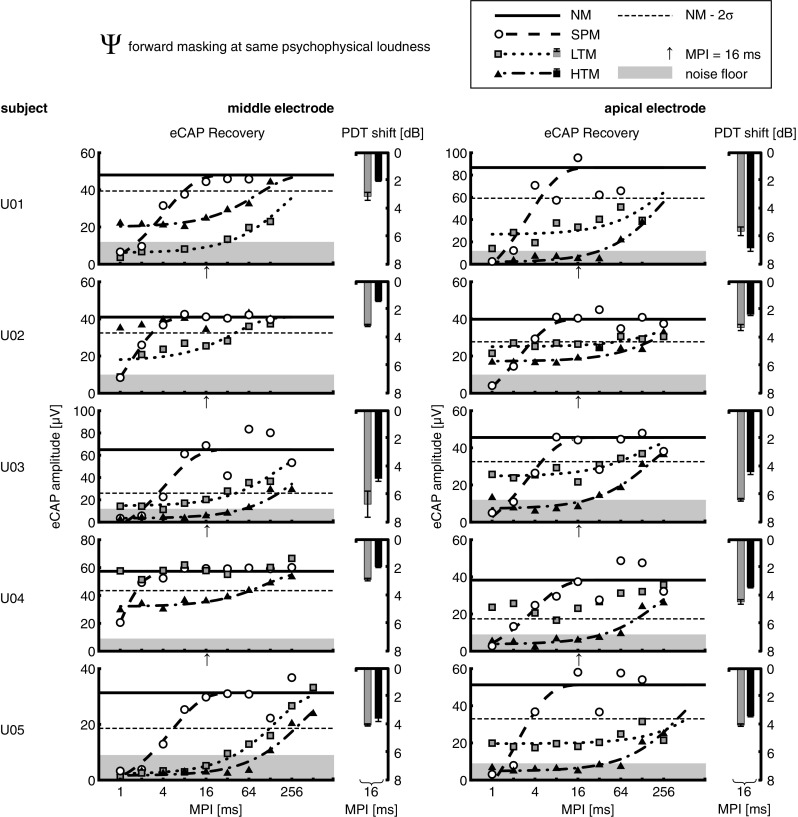
Same as Figure 3, but with pulse train maskers presented at the same psychophysical loudness level (Ѱ). SPM was always presented at current levels equal to those of LTM.
All eCAP responses were also validated by visual inspection to establish the noise floor (see above). Krippendorff’s alpha coefficient (Hayes and Krippendorff 2007), a measure of inter-judge reliability, was α = 0.87. The probability of failure to achieve α min = 0.90 was 0.73, which indicates very high judgment reliability. The noise floor, which was defined when 25 % or less traces were valid eCAP responses, was in the range of 9–12 μV. This range is consistent with noise floor values previously reported for the CI24RE device (McKay et al. 2013a). The noise floor is shown as a shaded area in each eCAP recovery plot. The mean dynamic range between NM level (average of all amplitude estimates in the NM condition) and respective noise floor was 13.4 ± 2.2 dB re 1 μV.
eCAP Recovery Functions
In cases where forward masking occurred, i.e., when eCAP amplitude estimates were lower than NM − 2σ, eCAP recovery functions were fitted to the exponential model described in Eq. 3. These are shown in Figures 3 and 4 as a function of MPI for each masker: dashed lines for SPM, dotted lines for LTM, and dash-dotted lines for HTM. Tables 4 and 5, respectively, show values for V ∞, which is the average of all measurements in the NM condition, and V ∞ − 2σ (two times the standard deviation). Fitted parameters τ and c are shown for each masking condition, in addition to intersections with the time axis T 0 for SPM and intersections with the amplitude axis V 0 for LTM and HTM.
TABLE 4.
Fitting parameters of the eCAP recovery model, pulse train maskers at same physical current level (Φ)
| CI user | Electrode position | V ∞ [μV] | V ∞ − 2σ [μV] | SPM at Φ level | LTM at Φ level | HTM at Φ = Ѱ level | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ [ms] | c | T 0 [ms] | τ [ms] | c | V 0 [μV] | τ [ms] | c | V 0 [μV] | ||||
| U05 | Middle | 31.33 | 18.58 | 12.67 | −0.24 | <0.00a | 128.32 | −0.22 | 6.16 | 339.70 | −0.05 | 1.57 |
| Apical | 51.27 | 32.98 | 0.54 | 1.08 | 0.59 | 428.56 | −0.10 | 4.82 | ||||
Fitting parameters are only shown for subjects where forward masking occurred in at least one experimental condition
CI cochlear implant, Φ same physical current level, Ѱ same psychophysical loudness level, SPM single-pulse masker, LTM low-rate pulse train masker, HTM high-rate pulse train masker
a V 0 = 6.69 µV
TABLE 5.
Fitting parameters of the eCAP recovery model, pulse train maskers at same psychophysical loudness level (Ѱ)
| CI user | Electrode position | V ∞ [μV] | V ∞ − 2σ [μV] | SPM at Ѱ level | LTM at Ѱ level | HTM at Φ = Ѱ level | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ [ms] | c | T 0 [ms] | τ [ms] | c | V 0 [μV] | τ [ms] | c | V 0 [μV] | ||||
| U01 | Middle | 48.02 | 39.37 | 4.28 | 0.12 | 0.52 | 208.63 | −0.14 | 6.10 | 85.89 | −0.54 | 20.03 |
| Apical | 86.74 | 59.19 | 3.25 | 0.31 | 1.00 | 260.14 | −0.37 | 26.64 | 252.22 | −0.02 | 1.72 | |
| U02 | Middle | 40.98 | 32.44 | 1.34 | 0.51 | 0.69 | 40.49 | −0.56 | 17.50 | |||
| Apical | 39.85 | 27.68 | 2.37 | 0.34 | 0.80 | 286.58 | −0.99 | 25.02 | 256.65 | −0.56 | 17.18 | |
| U03 | Middle | 65.04 | 25.96 | 4.41 | 0.27 | 1.18 | 162.72 | −0.25 | 14.44 | 367.77 | −0.05 | 3.34 |
| Apical | 45.56 | 32.54 | 3.32 | 0.24 | 0.80 | 128.50 | −0.78 | 24.69 | 162.14 | −0.17 | 7.17 | |
| U04 | Middle | 57.34 | 43.49 | 0.71 | 0.97 | 0.68 | 111.39 | −0.81 | 31.95 | |||
| Apical | 38.26 | 17.35 | 3.83 | 0.15 | 0.56 | 213.09 | −0.10 | 3.70 | ||||
| U05 | Middle | 31.33 | 18.58 | 5.33 | 0.15 | 0.81 | 150.92 | −0.07 | 2.09 | 339.70 | −0.05 | 1.57 |
| Apical | 51.27 | 32.98 | 2.30 | 0.47 | 1.08 | a | −0.48 | 19.58 | 428.56 | −0.10 | 4.82 | |
Fitting parameters are only shown for subjects where forward masking occurred in at least one experimental condition
CI cochlear implant, Φ same physical current level, Ѱ same psychophysical loudness level, SPM single-pulse masker, LTM low-rate pulse train masker, HTM high-rate pulse train masker
aNo recovery even after 256 ms
At Φ level (Fig. 3 and Table 4), HTM showed masking in 9/10 cases (exception was U02, middle electrode) with long recovery time constants τ in the range 85.9–428.6 ms. Except for one case (U05, middle electrode), none of the subjects tested showed masking for SPM or LTM at this current level. For the middle electrode of subject U05, LTM had τ = 128.3 ms, which was shorter than that for HTM with τ = 339.7 ms. Interestingly, this case showed relatively long forward masking for SPM with τ = 12.7 ms and the fitted recovery function had a V 0 = 6.69 μV. This is contrary to what is otherwise observed for single-pulse maskers. Note, however, that this case had a high noise floor at 28.7 % re V ∞ and a high standard deviation for the NM condition at 20.35 % re V ∞.
At Ѱ level (Fig. 4 and Table 5), LTM showed masking in 8/10 cases (exception was U04, both electrodes), with recovery time constants τ in the range 128.5–286.6 ms, while two cases apparently did not recover from masking after 128 ms (U01, both electrodes) and one case even after 256 ms (U05, apical electrode). LTM generally showed less forward masking than HTM except in two cases (U01 and U02, middle electrodes), with mean τ = 176.85 ± 83.46 ms for LTM versus mean τ = 246.38 ± 116.70 ms for HTM. In one case (U01, middle electrode), LTM showed longer masking with τ = 208.6 ms compared with τ = 85.9 ms for HTM. Interestingly, in the one case where HTM showed no masking (U02, middle electrode), LTM achieved forward masking with τ = 40.5 ms. Both of these exceptions could not be traced back to differences in current level (c.f. Table 2). In all cases, SPM showed shorter forward masking than both pulse train maskers, with τ in the range 0.7–5.33 ms and mean τ = 3.11 ± 1.44 ms. Note that while having the same current level as LTM, SPM achieved more masking at MPI = 1 ms in six cases (U02, both electrodes; U03, apical electrode; U04, both electrodes; and U05, apical electrode), which is discussed below.
DISCUSSION
In experiment 1, eFM in CI users was found to be much longer with both pulse train maskers than with single-pulse maskers, with time constants over 100 ms in contrast to a few milliseconds, respectively. Single-pulse maskers were found to produce at least as much masking as pulse train maskers (low-rate, LTM; and high-rate, HTM) at the shortest masker-probe intervals. Data at the same physical current level (Φ) showed that high-rate pulse trains can mask more and longer than low-rate pulse trains or single pulses. Data at the same psychophysical loudness level (Ѱ) had variable results; high-rate pulse trains did not always produce more masking than low-rate ones. This underlines the importance of comparing these maskers at the same perceptual level. In the next experiment, we attempt to relate our eFM findings to perception in order to investigate whether psychophysical masking may be explained by neural masking at the level of the auditory nerve.
In the following, we discuss why long eFM was not observed in a subset of subjects for the given experimental conditions. Then, we examine potential limitations of the method used to extract the neural response from telemetry recordings. Finally, we discuss a possible explanation of how single pulses could mask more than pulse trains at the shortest masker-probe intervals.
Current Level Differences
In our subject group and for the given conditions, long eCAP recovery from forward masking was measurable in 5/9 subjects, i.e., 10/15 electrodes. Since the absence of forward masking in one electrode was an exclusion criterion, not all subjects were tested in two electrode positions. When considering all tested electrodes, the binary logistic regression model showed a significant contribution of the difference between masker and probe current levels to the outcome of forward masking at MPIs greater than 4 ms, where a larger positive difference increased the odds that forward masking occurred. The model also showed that the pulse train masker rate (LTM or HTM) had a significant contribution to that outcome.
Inspection of the loudness-balancing results (c.f. Table 2) revealed that the current level difference between LTM and HTM at equal loudness was larger on average for subjects who did not show masking (5.7 dB) than for subjects who showed masking (3.7 dB), with a significant group difference, two-tailed Student’s t test, p = .0048. It can be thus hypothesized that this subject group either had less tolerance to high current levels when determining the loudness of pulse train maskers or had more temporal integration of loudness for pulse trains. However, the additional variability of probe current levels between subjects makes it difficult to draw such conclusions from the present data.
Our study design determined the current level eliciting an N1-P2 amplitude response of approximately 50 μV and did not fix the probe current level across subjects (typically just below the loudest acceptable level), which was motivated by several reasons; in order to maximize the chances of measuring forward masking of the probe response, the current level had to be low enough to avoid recruiting too many nerve fibers. However, the eCAP amplitude range needed to be sufficiently above the noise floor to allow statistical analysis. Still, the large variance of eCAP amplitude growth functions between subjects and the low resolution of stimulation levels in CI made this difficult to achieve (results showed mean NM level of 50.4 μV, in the range 30.5–86.7 μV). More importantly, while eCAP studies typically use single-pulse probes at high current levels to obtain clear responses, these levels may not always have clinical relevance. When presented as a pulse train, such high current levels would most likely exceed acceptable loudness. Here, for the subject group that showed forward masking, probe current levels were generally higher than HTM (5000 pps) and lower than LTM at Ψ level (250 pps; c.f. Table 2). Consequently, these probe current levels can be assumed to be at or below comfortable loudness when presented at clinical stimulation rates. Our eCAP recovery functions for pulse train maskers thus confirm the presence of slow-recovery phenomena at the level of the auditory nerve in CI users, most probably at clinical stimulation rates and levels.
Probe Stimulus Polarity
Artifact suppression of eCAP recordings could have also been achieved using the forward-masking paradigm (Brown and Abbas 1990; Brown et al. 1990). This method adds a preceding masker stimulus to evoke a refractory response to the probe stimulus, which is in turn used to reduce the artifact from the probe response. The paradigm can be adjusted to use pulse train instead of single-pulse maskers (Abbas and Brown 2015). With increasing MPI, the forward-masking paradigm would record an eCAP equal to the difference between the unmasked and the masked probe responses (Miller et al. 2000), which can be used to derive the masked probe response. In the current experiment, we used a modified alternating polarity method which has at least two limitations. First, it has been shown that the averaged waveform (from both polarities) produces significantly smaller amplitudes and higher thresholds than that obtained with the forward-masking method for short MPIs (Baudhuin et al. 2016; Eisen and Franck 2004; Frijns et al. 2002). This can be traced back to previously observed differences in eCAP amplitude and latency for cathodic- versus anodic-leading biphasic stimulation (Macherey et al. 2008). In this regard, the second limitation of our method is the inability to separate differences in masking between polarities. In a guinea pig model, Matsuoka et al. (2000) found that the amount of adaptation measured with eCAPs was greater for anodic than for cathodic stimulation. It is, therefore, possible that the long recovery time constants observed were mainly due to one polarity.
To evaluate this possibility, we mathematically derived eCAP responses using the forward-masking paradigm from our existing data set, isolating the responses using either anodic- or cathodic-leading probe pulses. The results had overall more noise (data not shown here), which may be due to the calculation requiring more averages and/or the maskers being at suboptimal current levels with regard to the probe levels. Nevertheless, when the data could be analyzed, there was no evidence of one leading polarity showing more masking than the other in the derived forward-masking paradigm.
Neural Response Alternation
Another observation was that the single-pulse masker could sometimes produce more masking than pulse train maskers at MPI = 1 ms in six cases (see Fig. 4). A possible explanation for this unexpected result is the alternating response pattern observed in eCAP recordings to each pulse in a train (Hughes et al. 2012; Rubinstein et al. 1999). At specific pulse rates, individual eCAP responses sometimes alternate between higher and lower amplitudes for odd and even pulse train counts or vice versa. This pattern is thought to be the result of variance in absolute and relative refractory periods for different auditory nerve fibers. Since the pulse train maskers LTM and HTM each had an even number of pulses, it could be argued that a direct comparison with masking from SPM (single pulse, i.e., odd number of pulses) oversees the possible alternating response pattern.
In a control experiment, we collected additional eCAP data for all subjects (U01–U05, both electrode positions) and for all maskers (SPM, LTM, and HTM) at Ѱ level and at MPIs 1, 8, and 64 ms. The following experimental conditions were added for each pulse train masker: −1 pulse, ±0 pulse, and +1 pulse, i.e., removing a pulse, no change, and adding a pulse at the beginning of the pulse train, respectively. The results were similar to those measured in experiment 1 (data not shown here) and confirmed that the long time constants of eCAP recovery from LTM and HTM were independent from the parity of the pulse counts. As previously observed, SPM masked more than LTM at MPI = 1 ms in 3/10 cases and more than HTM in 4/10 cases but this did not depend on whether the maskers had an even or odd number of pulses. The absence of an effect for HTM can be explained by the so-called “stochastic independence” state for high-rate stimulation, where alternating response patterns are no longer observed when reaching a sufficiently high rate (Hughes et al. 2012). Low-rate stimulation below 200 pps has been shown to yield steady eCAP amplitudes across individual pulses (Wilson et al. 1997), where nerve fibers have the time to recover from depolarization. It is thus possible that LTM at 250 pps had a rate too low to show the alternating response pattern in these subjects.
EXPERIMENT 2: PSYCHOPHYSICAL FORWARD MASKING
Methods
Subjects
This experiment included the five adult CI users (U01–U05) who showed eFM at both electrode positions (apical and middle) in experiment 1. They received financial compensation and reimbursement of their traveling costs. The existing approval from the local ethics committee also covered the current experiment.
Stimuli
In accordance with experiment 1, the probe stimulus was a single pulse. Its current level was varied to measure the psychophysical detection threshold when no masker (NM) was present. In addition, the probe detection threshold (PDT) was measured in three masking conditions, where masker pulse rate and current level were varied. Pulse train maskers were as follows: (1) HTM presented at a current level evoking comfortable loudness (C level), (2) LTM presented at the same current level as HTM (Φ level), and (3) LTM presented at the same loudness as HTM (Ѱ level). The single-pulse masker (SPM) was excluded from this experiment, since it showed short eFM with τ in the range 0.7–5.33 ms. The masker-probe interval (MPI) was fixed at 16 ms, and the presentation levels of LTM and HTM were kept the same as those used in the previous experiment (c.f. Table 2).
All electrical pulses were biphasic, symmetric, and rectangular. They were presented in monopolar mode. The two phases had durations of 25 μs each and were separated by an 8 μs inter-phase gap. All stimuli (probe, LTM, and HTM) had pulses with leading cathodic phases.
Procedures
Forward Masking: Probe Detection Thresholds
Forward masking was determined psychophysically by comparing masked with unmasked probe detection thresholds (PDTs). Thresholds were measured using a two-interval, two-alternative forced choice task with a two-down-one-up adaptive procedure. In the masked condition, a pulse-train masker (LTM or HTM) was presented in both intervals, while the single-pulse probe was only presented in one randomly selected interval after an MPI of 16 ms. In the unmasked condition, only the single-pulse probe was presented in one randomly selected interval. Subject’s task was to detect the interval which contained the probe. The initial probe level was at comfortable loudness (C level) and was adapted by 0.63 dB for the first two reversals and 0.16 dB for the remaining eight reversals. Thresholds were estimated from the mean of the last six reversal points. The procedure started with an NM condition and then three combinations of different masker types (LTM and HTM) and stimulation levels (Φ and Ѱ) in a randomized order, for each stimulating electrode (apical and middle). Each condition was measured there times, and the mean was defined as the final PDT for a given condition.
Sessions
The data for the middle and apical electrodes were collected in two different sessions. Prior to data collection, we verified that the masker levels established in experiment 1 still evoked a comfortable percept. Then, PDTs were measured for the different experimental conditions. At the start and end of each session, electrode impedances were checked.
Material
A Cochlear Pod served as a USB interface to the Cochlear L34 speech processor. PDT measurements were conducted using the APEX research platform (Laneau et al. 2005).
Analysis
Statistical analysis was done using SPSS Statistics (IBM Corporation, Armonk, NY, USA).
RESULTS
Probe Detection Threshold Shifts
As a measure of psychophysical forward masking (pFM) for different experimental conditions, PDT shifts were calculated as the difference between a masked PDT and the PDT in the no masker (NM) condition. Figures 3 and 4 show mean PDT shifts at MPI = 16 ms for each pulse train masker: gray bars for LTM and black bars for HTM. Error bars indicate the standard error of the mean. Note that the ordinate is inverted such that “more masking” corresponds to visually lower eCAP recovery functions (longer time constants) in eFM and visually lower PDT shifts (greater threshold differences) in pFM.
At Φ level (Fig. 3), PDT shifts for LTM were in the range 0.1–3.8 dB, and for HTM, they were in the range 1.3–6.8 dB. HTM always had larger PDT shifts than LTM, with positive shift differences in the range 0.5–4.0 dB (mean of 1.8 dB). This was consistent with our eFM findings, where HTM showed more masking and longer time constants than LTM when compared at the same current level.
At Ѱ level (Fig. 4), PDT shifts for LTM were in the range 2.8–6.7 dB. In contrast to the results at Φ level, HTM had lower PDT shifts than LTM in all but one case (U01, apical electrode), with negative shift differences in the range 0.5–2.0 dB. The positive shift in the one exception was 1.2 dB. These results were opposite to the eFM results in 7/10 cases, where HTM showed more eCAP masking than LTM. In the remaining three cases, either pFM or eFM results coincided (U01, apical and middle electrodes) or partly coincided since HTM sometimes showed no eFM at all (U02, middle electrode). To explore the effect of pulse train maskers across each subject’s electrodes, we calculated the difference between apical and middle electrodes for mean eCAP amplitude at MPI = 16 ms normalized re the respective NM level (Fig. 4, upwards arrow) and mean PDT shift for each masker. While no correlation between these two measures was found for LTM, within-subject (across electrodes) results for HTM showed a strong negative correlation between mean eCAP amplitudes and mean PDT shifts, Spearman’s ρ = −0.90, p = .037. It thus appears that higher eFM (lower eCAP amplitude) is correlated with higher pFM (higher PDT shift) for HTM.
A two-way repeated-measures analysis of variance (ANOVA) was performed with the factors electrode position (apical or middle) and masker condition (LTM at Φ level, LTM at Ѱ level, or HTM at Φ = Ѱ level). Mauchly’s test indicated that the assumption of sphericity was not violated for any of the model effects. The within-subject effect of electrode position and its interaction with the masker effect were not significant. By contrast, the masker effect was significant, F (2,8) = 32.5, p = .00014. Pairwise comparisons using Tukey’s LSD post hoc test revealed a significant difference between LTM at Φ and Ѱ level, p = .004, and also between LTM at Φ level and HTM at Φ = Ѱ level, p = .002. The difference between LTM and HTM at Ѱ level was also significant, p = .045; however, it would not survive the more conservative Bonferroni correction.
Normalized Data
In order to find a general trend in the data from experiments 1 and 2, each subject’s results (eCAP amplitude estimates) were normalized re the respective average of all measurements in the no masker condition, i.e., NM level. All normalized data were then collapsed and the mean calculated for each condition. Figure 5 shows the results. At Φ level, HTM showed long eFM with τ = 327.4 ms, while LTM and SPM did not show any masking. This coincided with pFM results where HTM produced more masking than LTM (3.4 versus 1.6 dB, respectively). At Ѱ level, LTM showed eFM with τ = 169.4 ms, which is shorter than HTM. However, this contradicts pFM results where HTM masked less than LTM (3.4 versus 4.3 dB, respectively). SPM showed much shorter forward masking than both pulse train maskers, with τ = 3.0 ms. Despite having the same current level as LTM, SPM achieved more masking at MPI = 1 ms.
FIG. 5.
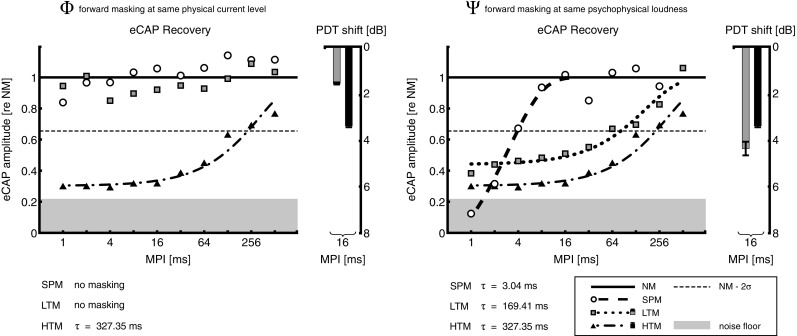
Same as Figures 3 and 4, but with data normalized re the respective no masker (NM) level and collapsed across subjects. Results are shown for pulse train maskers presented at the same physical current level (Φ, left panel) and at the same psychophysical loudness level (Ѱ, right panel). SPM was always presented at current levels equal to those of LTM. Time constants (τ) of the exponential eCAP recovery functions are shown at the bottom.
DISCUSSION
In experiment 2, pulse train maskers (low-rate, LTM; and high-rate, HTM) presented at the same physical current level (Φ) showed significantly higher pFM of a single-pulse probe at MPI = 16 ms for HTM compared with LTM. The opposite was found when the maskers were presented at the same psychophysical loudness (Ѱ), but this effect was marginally significant. Furthermore, when comparing LTM at its respective Φ and Ѱ levels, a significant effect of the masker current level could be found. These results are consistent with eFM data when considering the effect of current level at fixed stimulation rate or the effect of stimulation rate at a fixed current level. However, the results are not consistent with eFM when pulse train maskers are compared at the same Ѱ level. Finally, when within-subject results are compared across electrodes at the Ѱ level, the magnitudes of change in eFM and pFM are correlated for HTM but not for LTM.
Normalized results from experiments 1 and 2 show a general trend in eFM data which is mostly in line with the current understanding of the neural response to biphasic stimulation, as reviewed in Boulet et al. (2015): (1) pulse trains can induce much longer adaptation than single-pulse maskers at the level of the auditory nerve, (2) pulse trains at fixed current level induce more adaptation as stimulation rate is increased, and (3) pulse trains at fixed stimulation rate induce more adaptation as current level is increased. Our pFM data showed results that were coinciding with the last two effects. However, when comparing pulse trains of different rates at their loudness-balanced level, no consistent results were found; while two subjects suggest a peripheral contribution to the psychophysical results, the other three subjects showed the opposite effect.
In the following, we examine possible explanations for the inconsistencies we found between eFM and pFM data despite using identical stimulation paradigms. Then, we discuss the long neural recovery phenomenon shown by our eFM data in the human auditory nerve and relate it to previous findings in humans and animal models. Finally, we address possible implications of such long time-constant temporal interactions in CI users.
Electrophysiology Versus Psychophysics
Three possible explanations for the inconsistencies found between eFM and pFM are discussed below. First, when comparing electrophysiological and psychophysical data in CI users, it is generally assumed that the reduction of the neural response at the auditory nerve level can be correlated to an increase in the probe detection threshold measured behaviorally. However, Relkin and Turner (1988) pointed out that such direct relationship may not necessarily hold in all cases. The detectability of a given signal is dependent on both its neural response magnitude relative to the spontaneous activity and the variance of that neural response. Relkin and Pelli (1987) described a two-interval forced-choice procedure to measure such “neural detectability” in auditory nerve fibers. To this end, the neural response to a probe stimulus is compared to the spontaneous activity of the nerve fiber. This method allows measuring the so-called “neurometric function,” the counterpart of a behavioral psychometric function. However, it remains unclear whether spontaneous neural activity occurs in deaf ears, and whether gross potentials could detect such activity (Pfingst et al. 2011). Furthermore, current CI hardware would not allow recording spontaneous neural activity in CI users (if it exists) due to a high noise floor. And since eCAP amplitudes capture simultaneous firing of groups of nerve fibers, they are not a direct measure of the “number of neurons excited.” Consequently, there is an additional stochastic variable which is impacted by the synchronicity of the activity to be considered when comparing them to single nerve fiber recordings. The quintessence is that one should be cautious in making conclusions about peripheral and central neural mechanisms when interpreting psychophysical results, even if collected from CI users with direct electric stimulation of the auditory nerve.
Another possible explanation comes from the fact that the probe level at which the eFM data were collected is different than the probe level at psychophysical threshold. As our adaptive threshold procedure does not measure the whole psychometric function, there is always the possibility that the slopes of the psychometric functions of probe detection vary for different masker rates. This means that the masking difference found for a given condition may only be valid at this particular point of the psychometric function (here at P = .707). In an attempt to reconcile eFM and pFM at Ѱ level (see Fig. 4), we collected additional eCAP data for one subject (U05, data not shown here) with the probe current level set to the masked detection threshold obtained in the HTM masker condition and at an MPI of 16 ms. Incidentally, this condition was already met in another subject collected in experiment 1 (U04, apical electrode). By fixing the probe current level, we can make sure that the data collected for eFM and pFM are measured for the same point of the psychometric function of forward masking by HTM. The results were the same as before: eFM showed more masking for HTM than for LTM, while pFM showed greater PDT shifts for LTM than HTM. Therefore, the inconsistent results between eFM and pFM are unlikely to be explained by differences in the slope of the psychometric functions for these two cases.
Finally, pFM inherently involves central processes in one way or another. But it is still disputed whether psychophysical forward masking represents the effect of neural adaptation (decrease in probe response due to the masker) or rather integration, i.e., that the subject’s judgment is based on the output of an integrator of neural activity over a certain time window in which both the probe and the part of the masker are present (McKay et al. 2003; McKay et al. 2013b). The inconsistent results found in eFM and pFM could be explained by adaptation and integration phenomena: pulse train maskers adapt over their 300-ms duration, with higher adaptation expected for higher stimulation rates. Thus, the amount of neural activity produced at the end of the high-rate masker (e.g., at the level of the auditory nerve) may be smaller than at the end of the low-rate masker. Higher adaptation would thus result in “less energy” in the integration window, which in turn would allow better probe detection. Therefore, we could hypothesize two concurrent effects: high-rate pulse trains producing more masking of the probe at the periphery, yet its psychophysical detection becoming easier since the masker is strongly adapted at the end of its duration, i.e., before the probe is presented. By contrast, low-rate pulse trains produce less masking at the periphery and yet result in higher psychophysical masking of the probe stimulus since they are less adapted at the end of their duration.
Single-Pulse Versus Pulse Train Adaptation
Another finding of the current study was that long neural recovery from adaptation can be measured in the human auditory nerve. Previous studies in the animal model showed similar long-lasting phenomena that cannot be explained by refractoriness alone (review in Boulet et al. 2015). Miller et al. (2011) investigated the adaptation to and recovery from low-rate and high-rate pulse train maskers in cats’ auditory nerve fibers. They showed that recovery from adaptation to supra-threshold pulse trains could last up to 200 ms. They also showed that subthreshold pulse trains can induce so-called accumulated accommodation for several tens of milliseconds. To our knowledge, only one review of eCAP characteristics in CI users reported that recovery from masking by 1800-pps pulse trains can last up to 200 ms (Abbas and Brown 2015). In the present study, we contrasted 250- and 5000-pps pulse trains, where in some cases, no complete recovery was found even after 256 ms. By contrast, previous studies in CI users collected eCAP recovery functions using a single-pulse masker at or just below the loudest acceptable level and the probe between 1.5 and 2 dB lower than that (Brown et al. 1990; Lee et al. 2012; Morsnowski et al. 2006), showing time constants in the range of a few hundreds of microseconds. Our data showed relatively longer time constants for the single-pulse masker when the masker current level was on average 2 dB higher than the probe. This can possibly be explained by the fact that our probe levels were generally lower than in other studies utilizing eCAP measures, while it was previously shown that neural recovery rate decreases as a function of probe level when the masker level is held constant (Miller et al. 2001). The relatively low probe levels in this study were chosen to possibly fall within the dynamic range of clinically used stimulation levels. This appears to be the case in the subject group which showed eFM, since probe levels were generally higher than the C level of HTM (5000 pps) and lower than that of LTM (250 pps; c.f. Table 2). Consequently, the probe levels used here can be assumed to be at or below comfortable loudness levels at clinical stimulation rates. Our eCAP recovery functions for pulse train maskers thus confirm the presence of slow-recovery phenomena at the level of the auditory nerve in CI users, most probably at clinical stimulation rates and levels. This observation may thus have implications for sound coding strategies in CIs. And in terms of the internal representation of sound stimuli in CI listeners, increasing the pulse rate may have two concurrent effects: better sampling of sound intensity fluctuations and conversely deteriorating the neural representation by increasing the amount of adaptation. These two concurrent effects may in turn explain why increasing the pulse rate does not consistently improve speech perception or detection of amplitude modulations in CI listeners (Green et al. 2012; Vandali et al. 2000).
Temporal Interactions of the Neural Response
In general, CI users show limitations on many temporal processing tasks, including rate discrimination, gap detection, and amplitude modulation detection and discrimination (Bierer et al. 2015; Garadat et al. 2012; Macherey et al. 2011). These tasks probably involve different processes at the level of the auditory nerve, including refractoriness, facilitation, accommodation, and spike-rate adaptation, as recently reviewed by Boulet et al. (2015). Such limitations may be caused by temporal interactions between consecutive pulses delivered by several CI electrodes. Trying to separate these different sources may help us understand the reasons behind temporal processing limitations experienced by CI subjects. By using the masker-probe paradigm to record eCAPs, one may reveal short time-constant phenomena such as refractoriness, facilitation, and accommodation. However, such paradigms would fail to reveal accumulated accommodation and spike-rate adaptation, which are active in a time range of a few hundreds of milliseconds (Boulet et al. 2015). These phenomena can become relevant in contemporary CI strategies, which continuously stimulate the auditory nerve at high stimulation rates. To date, most psychophysical data were only interpreted with regard to refractoriness, which corresponds to short time-constant phenomena (Nelson and Donaldson 2001; Nelson and Donaldson 2002; Shannon 1990). Other neural response phenomena such as facilitation (short time constants), accommodation, and spike-rate adaptation (long time constants) were not considered. Our data confirm that such an approach is unsuitable for temporal interactions induced by pulse trains, which usually incorporate more than one of these phenomena. We presume to have measured the recovery from a combined effect between accumulated accommodation and spike-rate adaptation for the pulse train maskers (Boulet et al. 2015; Miller et al. 2011). Furthermore, several groups tried to correlate the time needed to recover from masking (measured by means of pFM or eFM) to speech perception scores, hypothesizing that fast recovery would better allow subjects to follow fast amplitude fluctuations and would, therefore, yield better speech scores. Results have been inconsistent across studies, sometimes showing the expected effect (Brown et al. 1990; Lee et al. 2012) and sometimes not (review in Botros and Psarros 2010; Chatterjee 1999). Others observed no correlation between pFM and eFM (Lee et al. 2012). Our data showed inconsistencies between eFM and pFM results despite using the same stimuli, further underlining the importance of comparing electrophysiological and psychophysical measures with the same stimulation paradigms.
CONCLUSION
In this study, we showed that using different maskers between electrophysiological and psychophysical measures of forward masking in CI users, specifically single-pulse versus pulse train maskers, can be a source of confound. Recovery of electrically evoked compound action potentials from forward masking was much slower for pulse trains than for a single-pulse masker. When compared at the same current level, high-rate pulse trains produced more and longer-lasting masking than low-rate ones. This confirms the presence of slow-recovery phenomena at the level of the auditory nerve in cochlear implant users. But results were variable when pulse train maskers of different rates were compared at the same perceptual level; for some combination of subjects and electrodes, low-rate maskers produced more masking than high-rate maskers, while the opposite trend was observed for other combinations. Psychophysical detection thresholds of a probe using the same masking conditions were in line with our electrophysiological findings for pulse trains presented at the same current level. However, the opposite trend was found when maskers of different rates were presented at the same perceptual level.
The electrophysiological data presented here generally conform to the current understanding of the neural response to biphasic stimulation, while underlining the importance of comparing electrophysiological and psychophysical measures with identical stimulation paradigms. Still, future studies are needed to disentangle the contributions of peripheral and central neural mechanisms to psychophysical masking in cochlear implant users.
Acknowledgements
We would like to thank all subjects for their participation. We would also like to thank the editors and two anonymous reviewers for their insightful comments and helpful suggestions. This study was funded by the Agence Nationale de Recherche (ANR-11-PDOC-0022) and by the Mission pour l’interdisciplinarité du CNRS (Project DEFI-SENS). Parts of this work were presented at the 8th International Symposium on Objective Measures in Auditory Implants October 15–18, 2014 in Toronto, Ontario, Canada and at the Conference on Implantable Auditory Prostheses July 12–17, 2015 in Granlibakken, Lake Tahoe, CA, USA.
Compliance with Ethical Standards
A local ethics committee approved this study (Eudract 2012-A00438-35).
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Youssef Adel, Phone: +49 (0)69 6301 83321, Email: youssef.adel@kgu.de.
Gaston Hilkhuysen, Email: g.hilkhuysen@ucl.ac.uk.
Arnaud Noreña, Email: arnaud.norena@univ-amu.fr.
Yves Cazals, Email: yves.cazals@univ-amu.fr.
Stéphane Roman, Email: stephane.roman@ap-hm.fr.
Olivier Macherey, Email: macherey@lma.cnrs-mrs.fr.
References
- Abbas PJ, Brown CJ. Assessment of responses to cochlear implant stimulation at different levels of the auditory pathway. Hear Res. 2015;322:67–76. doi: 10.1016/j.heares.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin JL, Hughes ML, Goehring JL. A comparison of alternating polarity and forward masking artifact-reduction methods to resolve the electrically evoked compound action. Potential Ear Hear. 2016;37:e247–e255. doi: 10.1097/AUD.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Deeks JM, Billig AJ, Carlyon RP. Comparison of signal and gap-detection thresholds for focused and broad cochlear implant electrode configurations. J Assoc Res Otolaryngol. 2015;16:273–284. doi: 10.1007/s10162-015-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros A, Psarros C. Neural response telemetry reconsidered: II. The influence of neural population on the ECAP recovery function and refractoriness. Ear Hear. 2010;31:380–391. doi: 10.1097/AUD.0b013e3181cb41aa. [DOI] [PubMed] [Google Scholar]
- Boulet J, White M, Bruce IC. Temporal considerations for stimulating spiral ganglion neurons with cochlear implants. J Assoc Res Otolaryngol. 2015 doi: 10.1007/s10162-015-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ. Electrically evoked whole-nerve action potentials: parametric data from the cat. J Acoust Soc Am. 1990;88:2205–2210. doi: 10.1121/1.400117. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz B. Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J Acoust Soc Am. 1990;88:1385–1391. doi: 10.1121/1.399716. [DOI] [PubMed] [Google Scholar]
- Chatterjee M. Temporal mechanisms underlying recovery from forward masking in multielectrode-implant listeners. J Acous Soc Am. 1999;105:1853. doi: 10.1121/1.426722. [DOI] [PubMed] [Google Scholar]
- Clay KM, Brown CJ. Adaptation of the electrically evoked compound action potential (ECAP) recorded from nucleus CI24 cochlear implant users. Ear Hear. 2007;28:850–861. doi: 10.1097/AUD.0b013e318157671f. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrically evoked compound action potential amplitude growth functions and HiResolution programming levels in pediatric CII implant subjects. Ear Hear. 2004;25:528–538. doi: 10.1097/00003446-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Briaire JJ, de Laat JA, Grote JJ. Initial evaluation of the clarion CII cochlear implant: speech perception and neural response imaging. Ear Hear. 2002;23:184–197. doi: 10.1097/00003446-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, Pfingst BE. Across-site patterns of modulation detection: relation to speech recognition. J Acoust Soc Am. 2012;131:4030–4041. doi: 10.1121/1.3701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Faulkner A, Rosen S. Variations in carrier pulse rate and the perception of amplitude modulation in cochlear implant users. Ear Hear. 2012;33:221–230. doi: 10.1097/AUD.0b013e318230fff8. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data communication. Methods and Measures. 2007;1:77–89. doi: 10.1080/19312450709336664. [DOI] [Google Scholar]
- Hughes ML, Castioni EE, Goehring JL, Baudhuin JL. Temporal response properties of the auditory nerve: data from human cochlear-implant recipients. Hear Res. 2012;285:46–57. doi: 10.1016/j.heares.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian MJ, Klis SF, Smoorenburg GF. Adaptation in the compound action potential response of the guinea pig VIIIth nerve to electric stimulation. Hear Res. 1994;81:66–82. doi: 10.1016/0378-5955(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate on threshold and dynamic range in clarion cochlear-implant users (L) J Acoust Soc Am. 2004;115:1885. doi: 10.1121/1.1701895. [DOI] [PubMed] [Google Scholar]
- Laneau J, Boets B, Moonen M, van Wieringen A, Wouters J. A flexible auditory research platform using acoustic or electric stimuli for adults and young children. J Neurosci Methods. 2005;142:131–136. doi: 10.1016/j.jneumeth.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lee ER, Friedland DR, Runge CL. Recovery from forward masking in elderly cochlear implant users. Otol Neurotol. 2012;33:355–363. doi: 10.1097/MAO.0b013e318248ede5. [DOI] [PubMed] [Google Scholar]
- Loizou PC. Mimicking the human ear. IEEE Signal Process Mag. 1998;15:101–130. doi: 10.1109/79.708543. [DOI] [Google Scholar]
- Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Deeks JM, Carlyon RP. Extending the limits of place and temporal pitch perception in cochlear implant users. J Assoc Res Otolaryngol. 2011;12:233–251. doi: 10.1007/s10162-010-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: evoked compound action potential recordings. Hear Res. 2000;149:115–128. doi: 10.1016/S0378-5955(00)00172-6. [DOI] [PubMed] [Google Scholar]
- McKay CM, Chandan K, Akhoun I, Siciliano C, Kluk K. Can ECAP measures be used for totally objective programming of cochlear implants? J Assoc Res Otolaryngol. 2013;14:879–890. doi: 10.1007/s10162-013-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Henshall KR, Farrell RJ, McDermott HJ. A practical method of predicting the loudness of complex electrical stimuli. J Acous Soc Am. 2003;113:2054. doi: 10.1121/1.1558378. [DOI] [PubMed] [Google Scholar]
- McKay CM, Lim HH, Lenarz T. Temporal processing in the auditory system: insights from cochlear and auditory midbrain implantees. J Assoc Res Otolaryngol. 2013;14:103–124. doi: 10.1007/s10162-012-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Brown CJ. An improved method of reducing stimulus artifact in the electrically evoked whole-nerve potential. Ear Hear. 2000;21:280–290. doi: 10.1097/00003446-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. Response properties of the refractory auditory nerve fiber. J Assoc Res Otolaryngol. 2001;2:216–232. doi: 10.1007/s101620010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Rubinstein JT, Robinson BK, Matsuoka AJ, Woodworth G. Electrically evoked compound action potentials of guinea pig and cat: responses to monopolar, monophasic stimulation. Hear Res. 1998;119:142–154. doi: 10.1016/S0378-5955(98)00046-X. [DOI] [PubMed] [Google Scholar]
- Miller CA, Hu N, Zhang F, Robinson BK, Abbas PJ. Changes across time in the temporal responses of auditory nerve fibers stimulated by electric pulse trains. J Assoc Res Otolaryngol. 2008;9:122–137. doi: 10.1007/s10162-007-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Woo J, Abbas PJ, Hu N, Robinson BK. Neural masking by sub-threshold electric stimuli: animal and computer model results. J Assoc Res Otolaryngol. 2011;12:219–232. doi: 10.1007/s10162-010-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsnowski A, Charasse B, Collet L, Killian M, Muller-Deile J. Measuring the refractoriness of the electrically stimulated auditory nerve. Audiol Neurootol. 2006;11:389–402. doi: 10.1159/000095966. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS. Psychophysical recovery from single-pulse forward masking in electric hearing. J Acoust Soc Am. 2001;109:2921–2933. doi: 10.1121/1.1371762. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS. Psychophysical recovery from pulse-train forward masking in electric hearing. J Acoust Soc Am. 2002;112:2932–2947. doi: 10.1121/1.1514935. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, et al. Cochlear infrastructure for electrical hearing. Hear Res. 2011;281:65–73. doi: 10.1016/j.heares.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp R. Rate of decay of auditory sensation. J Acoust Soc Am. 1964;36:277–282. doi: 10.1121/1.1918946. [DOI] [Google Scholar]
- Relkin EM, Pelli DG. Probe tone thresholds in the auditory nerve measured by two-interval forced-choice procedures. J Acoust Soc Am. 1987;82:1679–1691. doi: 10.1121/1.395159. [DOI] [PubMed] [Google Scholar]
- Relkin EM, Turner CW. A reexamination of forward masking in the auditory nerve. J Acoust Soc Am. 1988;84:584–591. doi: 10.1121/1.396836. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/S0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Forward masking in patients with cochlear implants. J Acoust Soc Am. 1990;88:741–744. doi: 10.1121/1.399777. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. Otol Neurotol. 1997;18:S30–S534. [PubMed] [Google Scholar]
- Zeng FG, Rebscher S, Harrison W, Sun X, Feng H. Cochlear implants: system design, integration, and evaluation IEEE. Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]