Abstract
What is the role of normally patterned sensory signaling in development of vestibular circuits? For technical reasons, including the difficulty in depriving animals of vestibular inputs, this has been a challenging question to address. Here we take advantage of a vestibular-deficient zebrafish mutant, rock solo AN66, in order to examine whether normal sensory input is required for formation of vestibular-driven postural circuitry. We show that the rock solo AN66 mutant is a splice site mutation in the secreted glycoprotein otogelin (otog), which we confirm through both whole genome sequencing and complementation with an otog early termination mutant. Using confocal microscopy, we find that elements of postural circuits are anatomically normal in rock solo AN66 mutants, including hair cells, vestibular ganglion neurons, and vestibulospinal neurons. Surprisingly, the balance and postural deficits that are readily apparent in younger larvae disappear around 2 weeks of age. We demonstrate that this behavioral recovery follows the delayed development of the anterior (utricular) otolith, which appears around 14 days post-fertilization (dpf), compared to 1 dpf in WT. These findings indicate that utricular signaling is not required for normal structural development of the inner ear and vestibular nucleus neurons. Furthermore, despite the otolith’s developmental delay until well after postural behaviors normally appear, downstream circuits can drive righting reflexes within ∼1–2 days of its arrival, indicating that vestibular circuit wiring is not impaired by a delay in patterned activity. The functional recovery of postural behaviors may shed light on why humans with mutations in otog exhibit only subclinical vestibular deficits.
Electronic supplementary material
The online version of this article (doi:10.1007/s10162-017-0617-9) contains supplementary material, which is available to authorized users.
Keywords: zebrafish, posture, otolith, critical period, development, vestibulospinal
Introduction
In many sensory systems, both spontaneous and sensory-elicited activities are required for normal circuit development. For example, prior to eye opening, retinal activity in the form of spontaneous waves is crucial for eye-specific segregation of retinal ganglion inputs to the lateral geniculate nucleus (Torborg and Feller, 2005); after eye opening, visually elicited activity is required for normal formation of ocular dominance columns in the cortex (Hubel and Wiesel, 1970). Loss of either type of activity during a “critical period” of development can lead to permanent impairments in sensory processing (Hensch, 2004).
Whether or not normal vestibular circuit development is characterized by a critical period for sensory signaling is poorly understood. There are two types of vestibular end organs: the otoliths report linear acceleration, including gravity, whereas the semicircular canals signal rotational velocity, such as head turns. Here our focus is on the gravitoinertial otolith system, which develops functionally first (Branoner and Straka, 2015). For technical reasons, it is difficult to eliminate gravitational signaling: either animals must be reared in low or zero gravity conditions; or the vestibular end organs must be eliminated early in development, at in utero stages for mammals. Furthermore, the profound disruption associated with loss of gravitational signals typically impairs locomotion and feeding, leading to non-specific problems that complicate interpretation of results from vestibular-deficient animals. Therefore, few studies have addressed whether gravitoinertial signals are required for normal development of vestibular circuits.
The existing data on the effects of vestibular deprivation on circuit development have been conflicting. In frogs, entirely removing an ear leaves the major vestibular nucleus anatomically intact, but causes loss or dimished dendritic branching of the ipsilateral Mauthner cell, a reticulospinal neuron with significant vestibular and auditory inputs (Fritzsch, 1990; Elliott et al., 2015). These results suggest a mixture of sensory-dependent and independent processes in vestibular circuit development. For a more targeted approach, animals can be raised without the normal sensation of gravity. Exposing fish to microgravity prior to development of the vestibulo-ocular reflex (VOR) has produced contradictory results, with some authors finding reduced and others augmented VOR gains after return to 1 g (Sebastian et al., 2001; Moorman et al., 2002). These conflicting findings may be related to the fact that microgravity does not eliminate inertial cues, which also drive otolithic signaling. Therefore, animals engaging in different amounts or speeds of self-movement in microgravity will experience different linear acceleration signals through the otolithic system, potentially confounding results.
In the otolithic system, end organs signal head-tilt/translation in diverse directions (Fernandez and Goldberg, 1976), making it difficult to follow the functional circuit by anatomical tracing alone, in contrast with the unidirectionally tuned semicircular canals (Straka, 2010; Straka et al., 2014). Optimally, any manipulation depriving a developing organism of sensory input would be reversible, so that the function of the system can be properly assayed following deprivation. This is achieved in visual, auditory, and somatosensory systems via eye closing, ear plugging, or whisker trimming, respectively, but not readily accomplished in vestibular systems.
Here we characterize the genetics, anatomy, and behavioral development of a vestibular-deficient mutant zebrafish line, rock solo AN66 (Mo et al., 2010). The anterior (utricular) otolith, which serves as the sole gravitoinertial sensor in zebrafish (Riley and Moorman, 2000), is absent in larval rock solo AN66 mutants, with attendant impairments in vestibulo-ocular and vestibulospinal reflexes (Mo et al., 2010; Bagnall and McLean, 2014). We find that basic elements of the postural circuit, including hair cells, vestibular ganglion neurons, and vestibulospinal neurons, are anatomically normal even in the absence of patterned sensory signaling. We further show that the absent utricular otolith eventually develops on a grossly delayed time scale, but postural behaviors ensue normally after the otolith has arrived. These results argue that, over this early time window, vestibular-evoked postural circuits develop independently of typically patterned sensory input.
Methods
Zebrafish Lines and Husbandry
The rock solo AN66 mutant was obtained from T. Nicolson and maintained as heterozygous or homozygous adults. All larvae were obtained from heterozygous or heterozygous × homozygous crosses. Rock solo AN66 homozygous recessive mutants, which were identified by the missing anterior (utricular) otolith at 2–5 days post-fertilization (dpf), were always compared with heterozygous or wild-type (WT) siblings; heterozygotes exhibit no apparent anatomical or behavioral deficits. The otog early termination mutant was obtained from the Sanger Zebrafish Mutation Project (Kettleborough et al., 2013) as heterozygous adults and embryos. The vestibular (stato-acoustic) ganglion was visualized in the Tg (islet2b:GFP) transgenic line (Pittman et al., 2008). All experiments were approved by the Animal Studies Committee at Washington University, USA.
Zebrafish were maintained in the Washington University Zebrafish Facility at 27–29 °C under standard conditions, with a 14 h:10 h light:dark cycle. Typically, vestibular mutant animals are unable to locate the surface to fill their swim bladders and feed, causing them to die by ∼10 dpf (Riley and Moorman, 2000). To promote survival, rock solo AN66 mutant larvae were segregated from WT/heterozygous siblings by 5 days post-fertilization (dpf); both genotypes were then raised in static water tanks with a rotifer diet for the next ∼2 weeks. After this time, rock solo AN66 mutants swam normally and could be raised to adulthood with no further problems.
Sequencing
Genomic DNA was obtained from 21 5 dpf larvae from each line. Whole-genome sequencing was carried out on an Illumina HISeq 3000 with paired end reads. Reads were aligned using BWA-MEM (Li, 2014). Variants were called using SAMtools (mpileup and bcftools) (Li et al., 2009).
Fluorescent Imaging
Hair cells were visualized with the HCS-1 antibody to otoferlin (Goodyear et al., 2010). 4-5 dpf larvae were anesthetized with tricaine (MS-222; 0.02 %) then fixed with 4 % paraformaldehyde overnight at 4 °C. After application of blocking buffer (5 % normal horse serum, 1 % DMSO, 1 % Triton X-100 in PBS), HCS-1 was applied 1:200 overnight at 4 °C. Antibody staining was revealed with Cy3-conjugated anti-mouse secondary antibody (Jackson Labs; 1:200). Fish were whole mounted onto slides and coverslipped (Vectashield Hard Set; Vector Labs) for examination with confocal microscopy.
Vestibular ganglion neurons were imaged in 5 dpf larvae from the Tg (isl2b:GFP) fish line (Pittman et al., 2008). Larvae were fixed as above then treated with 0.75 μM DAPI in PBS with 1 % Triton-X overnight before rinsing and mounting as above. In some cases slide spacers (0.25 mm thick, Electron Microscopy Sciences) were used to prevent flattening of the larvae.
Retrograde labeling of vestibulospinal neurons was carried out via dye injection into the mid-body spinal cord. Tetramethylrhodamine (2–10 % w/v; 3000 MW; Molecular Probes, Thermofisher) was injected via fine glass micropipette into the ventral aspect of the spinal cord around segments 14–20 in 4 dpf larvae anesthetized with MS-222 (0.02 % in system water). Animals were allowed to recover in normal fish saline for 1 to 2 days. Following screening, they were anesthetized again and embedded in 1.2 % low melting point agarose for examination with confocal microscopy. Only animals with a significant backlabel of vestibulospinal neurons, as identified during screening, were imaged further. No differences were noted in the relative frequency of successful retrograde labeling in rock solo AN66 versus WT/heterozygous animals.
Fluorescence and transmitted light imaging was carried out predominantly with an Olympus FV1200 scanning confocal microscope, using laser lines of 488 and 559 nm with a ×20 water immersion objective (numerical aperture 0.95) for live tissue or a ×60 oil immersion objective (N.A. 1.4) for fixed tissue. In some cases, a Nikon A1Rsi scanning confocal was used instead, with a ×40 oil immersion objective (N.A. 1.35).
Behavior
High-speed imaging of freely swimming fish was accomplished with a HiSpec-1 2G monochrome camera mounted on a Leica MZ16f stereomicroscope. Infrared illumination was delivered via a 96-bulb infrared array (IR100 Illuminator; YYtrade) (Wolman et al., 2011). Fish were introduced into a 15 × 15 mm glass arena filled with fish saline. Two 45 ° prism mirrors (ThorLabs) placed adjacent to the arena provided side views of the animal. Fish were imaged at 500 frames/s during both spontaneous and stimulus-evoked swimming. To evoke swimming in quiescent animals, a fine plastic pipette tip was used to gently prod the animal. There was no difference in postural data obtained from spontaneous and evoked swimming (data not shown). Videos were acquired and scored post-hoc by an observer blind to otolith development for animal orientation during the swim. Swimming was scored as abnormal if the animal spent any significant amount of time swimming sideways (>45 ° roll; one eye occluding the other), upside-down, or vertically (perpendicular to the ground). Trials were excluded if animals ran into walls or hugged the perimeter of the arena, to minimize contributions from somatosensory/startle pathways.
Four to six distinct swim bouts were analyzed each day for each fish. In all, 29 rock solo AN66 animals were tracked over development. A smaller number of WT/heterozygous animals were analyzed during a comparable time period; none of them exhibited any posturally abnormal swimming. Following swim analysis, animals were anesthetized and examined under light microscopy for the number and location of otoliths present on each side. In all, 15 animals were excluded from the analysis of utricular development for the following reasons: (1) if the saccular otolith had shifted or expanded anteriorly to the locus where the utricular otolith was normally found (n = 4); (2) if the spine developed with a persistent visible large kink (n = 1); (3) or if the anterior otolith failed to develop or was greatly displaced from the normal position of the anterior macula (n = 11). Some members of this last group were analyzed for the effects of lagenar development.
Statistics
All statistical analyses were carried out in Igor Pro 6 (WaveMetrics) and are reported as mean ± standard deviation. Significance testing between rock solo AN66 and heterozygous/WT siblings was carried out with the Mann-Whitney rank test (also known as the Wilcoxon-Mann-Whitney). Significance of behavioral transition pre/post-otolith arrival was measured with Cochran’s Q test.
Results
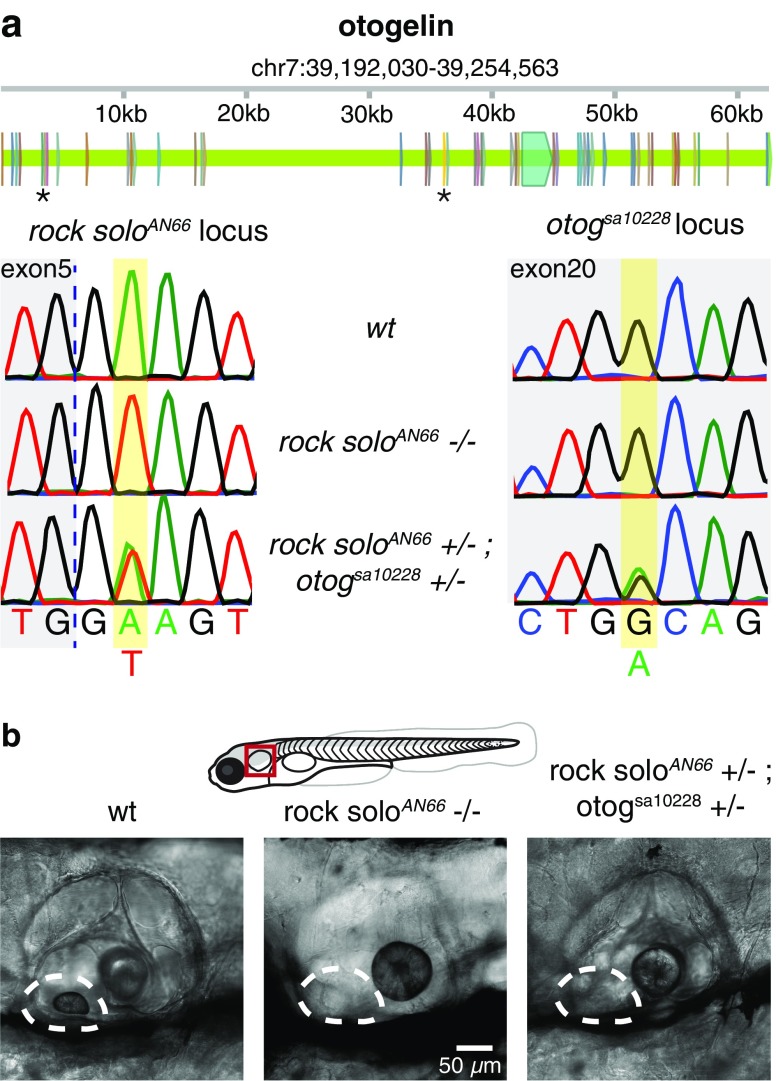
The vestibular-deficient mutant rock solo AN66 was first identified and described by Mo et al. (2010). This autosomal recessive mutation prevents formation of the anterior (utricular) otolith in larval zebrafish, and associated absence of the tilt-elicited vestibulo-ocular reflex (Mo et al., 2010). To identify the genotypic basis of the rock solo AN66 mutation, we compared the results of whole-genome sequencing of homozygous versus WT/heterozygous offspring. Alignment of reads to the zebrafish genome (GRCz10, Illumina iGenomes) identified a single long region of homozygosity on chromosome 7 (28,960,796–50,053,383) unique to the homozygous offspring. We used SnpEff (Cingolani et al., 2012) to find six genes within this region that contained small nucleotide polymorphisms (SNPs) with a high likelihood of altering protein synthesis or function, including a mutation at position 39,251,244 resulting in T to A transversion within the splice donor site of the fifth exon of otogelin (otog) (c.1522 + 2T > A) (Fig. 1a). An independently induced ENU mutation, einstein (eis te296f) also results in the loss of the utriclular otolith and was recently mapped to the splice donor site following exon 24 of otog (Stooke-Vaughan et al., 2015).To verify that the splice site mutation in otogelin was responsible for the observed phenotype, we carried out a complementation cross between heterozygous rock solo AN66/+ and otog sa10228/+ mutants, carrying an early termination mutation, obtained from the Sanger Zebrafish Mutation Project (Kettleborough et al., 2013) (Fig. 1b). Approximately one quarter of offspring (26.2 %: 146/558, from two crosses) exhibited the recessive phenotype, missing utricular otoliths, consistent with the conclusion that the disrupted gene in rock solo AN66 is otog. Here the mutant line will be referred to as otog c.1522+2T>A.
FIG.1.
A mutation in a splice site of otogelin underlies rock solo AN66 phenotype. a Top: exon structure of otog and location of the splice site mutation in rock solo AN66 (first asterisk) and the early termination mutation in the otog sa10228 line (second asterisk). Bottom: electropherograms of otog sequencing in the rock solo AN66 (left) and otog sa10228 (right) lines at the loci identified. Data are from pooled larvae. b Transmitted light images of the ear, viewed from the lateral aspect, in 5–7 dpf larvae. The anterior (utricular) otolith (dashed outline) is absent in both the rock solo AN66 homozygotes as well as in offspring from the complementation cross between rock solo AN66 and otog sa10228. Rostral is to the left and dorsal is up.
The complete absence of the sole vestibular end-organ in the otog c.1522+2T>A larval zebrafish provides an opportunity to examine the importance of normally patterned sensory signaling in vestibular development. Because the utricle (but not the saccule) is crucial for normal self-righting postural behaviors (Riley and Moorman, 2000), we examined whether the anatomical substrates of postural reflexes—hair cells in the utricular macula, the vestibular ganglion, and vestibulospinal neurons—developed normally in otog c.1522+2T>A animals despite the loss of patterned sensory input.
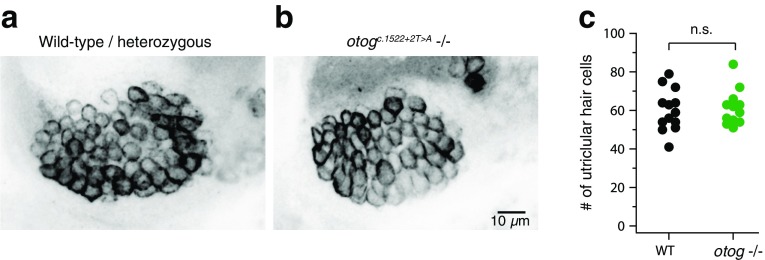
The first sensory hair cells appear at 24 h post-fertilization, shortly after the otoliths which are first seen at 18–20 hpf (Haddon and Lewis, 1996). Hair cells were visualized under confocal microscopy with immunofluorescence, using the HCS-1 antibody to otoferlin (Goodyear et al., 2010). Animals were examined at 5–7 dpf, well past the stage when the utricular otolith normally develops. The utricular macula appeared grossly normal in size and position in otog c.1522+2T>A mutants as compared with their WT/heterozygous siblings (Fig. 2a, b). The number of hair cells in the utricular macula was no different between WT/heterozygous (mean ± SD, 60.2 ± 10.8; n = 13) and otog c.1522+2T>A siblings (61.7 ± 9.0; n = 13; U = 78, p = 0.74, two-tailed Mann-Whitney rank test) (Fig. 2c), indicating that the otolith is not required for normal proliferation and stabilization of hair cells, consistent with results from analysis of the einstein mutant (Whitfield et al., 1996).
FIG. 2.
No change in the number of hair cells in the utricular macula in otog c.1522+2T>A animals. a Confocal image of the anterior/utricular macula in WT/heterozygous siblings. Hair cells are stained with the HCS-1 antibody to otoferlin. Image is the average of 15 z-planes totaling 11 μm thick, encompassing the whole macula. Anterior is to the left, dorsal to the top of this and all subsequent images. b As in a, for a otog c.1522+2T>A−/− animal. Image is the average of seven z-planes totaling 5 μm. The small cluster of hair cells in upper right of the image is from a lateral line neuromast. Cells appear slightly more oval than in WT because of a modest difference in mounting angle. c Counts of the number of utricular hair cells in WT/heterozygous and otog c.1522+2T>A animals. Each dot represents data from a different animal.
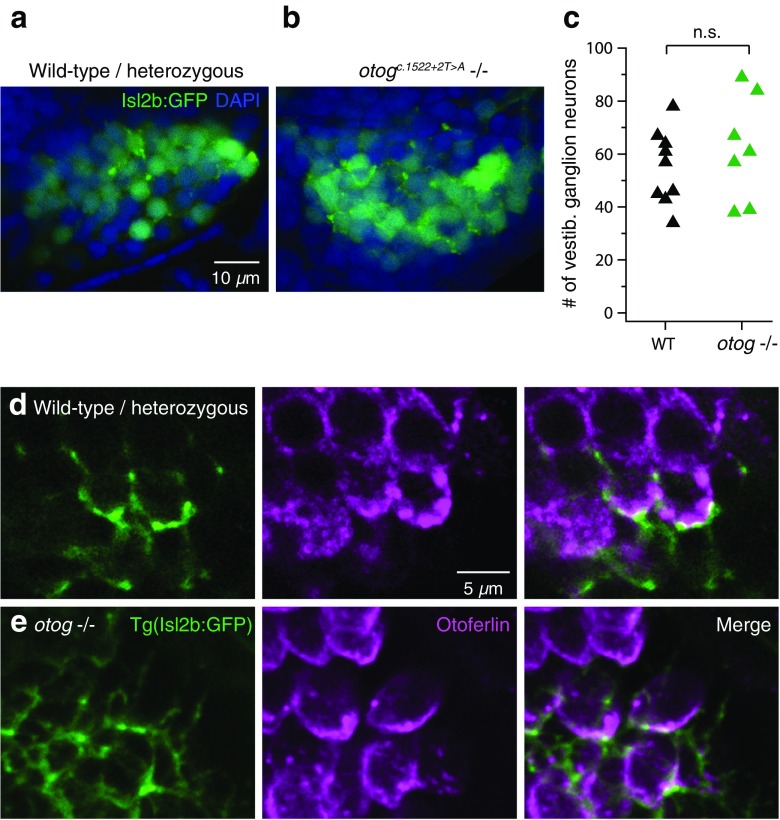
Next we asked whether the second stage in vestibular processing, the vestibular (stato-acoustic) ganglion, develops normally in the absence of the utricular otolith. To visualize the ganglion, we crossed a transgenic reporter line, Tg (isl2b:GFP), in which sensory afferent ganglia express GFP, to the otog c.1522+2T>A mutant over two generations to produce Tg (isl2b:GFP); otog c.1522+2T>A homozygotes and WT/heterozygotes. Fish were fixed and counter-stained with DAPI to aid in distinguishing the closely packed ganglion cells from one another. The utricular ganglion was identified by its position sandwiched between the anterior lateral line and saccular ganglia, as well as by the processes extending to the utricular macula. Using confocal microscopy to visualize the ganglion, we saw no difference in the number of GFP+ neurons between otog c.1522+2T>A homozygotes (62.1 ± 19.9; n = 7) and WT/heterozygous siblings (Fig. 3a–c) (55.0 ± 14.0; n = 9; U = 25.5, p = 0.54). Not only did the ganglion cells occur in normal numbers, they also extended processes toward hair cells in similar fashion in otog c.1522+2T>A and WT/heterozygous siblings, as visualized with HCS-1 immunofluorescence (Fig. 3d, e). Therefore, the general number and structure of vestibular ganglion neurons does not appear affected by the absence of sensory-evoked activity of hair cells.
FIG. 3.
No change in vestibular ganglion neurons in otog c.1522+2T>A animals. a Confocal image of the vestibular ganglion as visualized in the Tg (isl2b:GFP) line in WT/heterozygous siblings. Blue, DAPI counter-stain. Image is the average of 11 z-planes totaling 8 μm thick. b As in (a), for a otog c.1522+2T>A−/− animal. Image is the average of ten z-planes totaling 7 μm. c Counts of the number of vestibular ganglion neurons in WT/heterozygous and otog c.1522+2T>A animals. Each dot represents data from a different animal. d–e Example images of putative synaptic contacts from vestibular ganglion neurons onto utricular hair cells in Tg (isl2b:GFP) WT/heterozygous (d) and otog c.1522+2T>A (e) animals. Hair cells (magenta) are labeled with the HCS-1 otoferlin antibody as in Fig. 2. Close punctate apposition between ganglion processes and hair cells can be seen in the merged pictures (right), consistent with the known Type II (bouton-like) anatomy (Eatock and Songer, 2011).
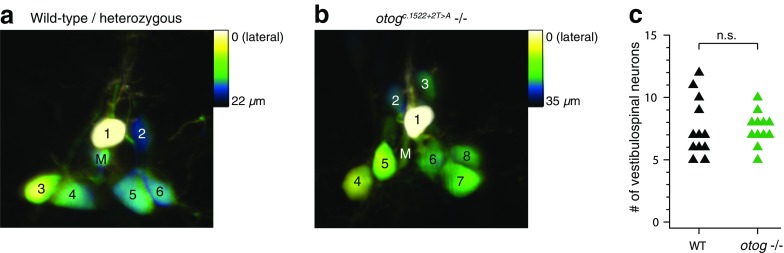
Finally, we examined the third relay in vestibular control of posture, the vestibulospinal neurons. These neurons lie underneath the ear at the lateral edge of the Mauthner cell (Kimmel et al., 1982). The vestibulospinal cluster was retrogradely labeled by dye injection into the spinal cord at mid-body levels. While the number of neurons labeled by this method differs significantly from fish to fish due to variability in the efficacy of injection, the number and arrangement of the vestibulospinal cluster was similar between otog c.1522+2T>A and WT/heterozygous siblings. Viewed from the lateral aspect, the group consists of one laterally displaced neuron (yellow in the depth-coded images) and up to 11 more neurons arranged in a roughly pyramidal shape around the Mauthner lateral dendrite in both WT/heterozygous and otog c.1522+2T>A siblings (Fig. 4a, b). Across imaged fish, there was no difference in the number of retrogradely labeled vestibulospinal neurons between WT/heterozygous (7.6 ± 2.4; n = 12) and otog c.1522+2T>A animals (7.5 ± 1.3; n = 12; U = 63.5, p = 0.62) (Fig. 4c).
FIG. 4.
No change in the number of vestibulospinal neurons in otog c.1522+2T>A animals. a Confocal image of retrogradely labeled vestibulospinal neurons in the hindbrain of a WT/heterozygous animal, viewed from the lateral aspect. Image is depth-coded with color reflecting the distance from the lateral-most image. M, Mauthner lateral dendrite, which is seen in cross-section. 1–6, vestibulospinal neurons. b As in (a), for a otog c.1522+2T>A animal. c Counts of the number of vestibulospinal neurons labeled in WT/heterozygous and otog c.1522+2T>A animals. Each dot represents data from a different animal.
Together, these findings demonstrate that the basic structural elements of the postural system—hair cells, vestibular ganglion, and vestibulospinal neurons—appear unaffected by the absence of gravitational signaling. However, this anatomical analysis cannot reveal whether circuit connectivity can form normally as well. In several sensory systems, reversible sensory deprivation is used to examine whether a deprived circuit can function normally when sensory input is restored. This approach is used to test for the presence of a critical period; a circuit that cannot recover its function even after sensory input is restored is considered to have a critical period during which sensory input is required for normal circuit development (Hensch, 2004).
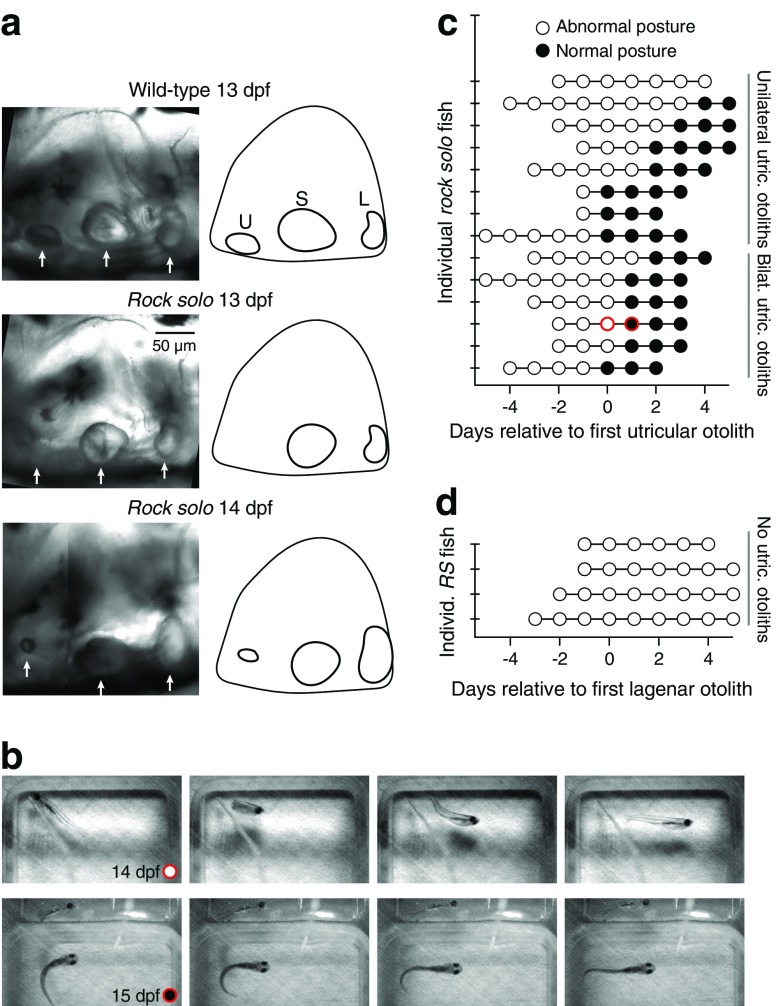
We observed that, unexpectedly, about half of the otog c.1522+2T>A animals developed normally positioned anterior otoliths either unilaterally or bilaterally at ∼14 dpf, almost 2 weeks after normal otolith development (Fig. 5a). In some animals, small otoliths sometimes formed initially in displaced positions, usually migrating to the correct general location 1–2 days later. Late developing utricular otoliths were typically small initially (e.g., Fig. 5a, bottom), and grew over subsequent days to near WT size.
FIG. 5.
Delayed otolith development triggers behavioral recovery. a Transmitted light images of the otoliths of the inner ear in three animals. In a WT animal (top), the utricular (U), saccular (S), and lagenar (L) otoliths are well-formed by 13 dpf. A typical otog c.1522+2T>A animal exhibits only the S and L otoliths at 13 dpf (middle). Around 14 dpf, many otog c.1522+2T>A animals spontaneously develop a utricular otolith, which is initially smaller than that of WT (bottom). The bottom image is stitched from two separate sets of z-planes due to the small size of the nascent utricular otolith. b Examples of abnormal and normal swimming posture in one otog c.1522+2T>A larva. This animal first developed utricular otoliths at 14 dpf, but on that day still swam abnormally, with dorsal-up posture on 0/5 swim bouts (top); here it is seen from above lying left side up and then reversing to orient right flank up during a swim. The plastic pipette used to deliver a touch stimulus can be seen contacting the head at the beginning of swim. At 15 dpf (bottom) this same animal began and maintained its swim with dorsal-up posture in 5/5 swim bouts. A 45 ° mirror can be seen at the top of these images, confirming that this animal is dorsal up. Images represent subsets of frames from high-speed videography under infrared illumination and are 2 ms exposures, 14 ms apart. c Summary of postural behaviors before and after the delayed arrival of 1 or 2 utricular otoliths in 14 otog c.1522+2T>A animals. Day 0 indicates the first day on which at least one utricular otolith was observed in approximately normal position, regardless of size (actual age at day 0, 13–17 dpf). Animals that maintained dorsal-up orientation throughout swim bouts in the dark were considered to have normal posture; animals that rolled sideways, upside-down, or pitched vertically during 50 % or more of swim bouts were considered to have abnormal posture. Each row represents observations from a different animal. Examples of swimming behavior are shown in b for the animal highlighted with red circles. d Postural behaviors before and after the development of lagenar otoliths in four animals. Day 0 indicates the first day on which at least one normally positioned lagenar otolith develops (actual age, 13–15 dpf). In these animals, which did not develop utricular otoliths over the time course represented here, posture remained abnormal.
To examine whether these late developing otoliths were capable of driving postural behaviors, freely swimming fish were imaged daily with high-speed videography under infrared illumination for 4–10 days, beginning at 11–12 dpf. The number and position of otoliths was evaluated with light microscopy each day. WT/heterozygous animals typically maintained normal, dorsal-up posture by 3–4 dpf, and no change was seen in their postural control at later ages (data not shown). In contrast, otog c.1522+2T>A animals usually swam with side-lying or upside-down postures prior to development of the anterior otoliths, but exhibited normal orientation relative to gravity after anterior otolith development (example swims, Fig. 5b) (comparing swim 1 day prior to utricular otolith development versus 2 days after: Cochran’s Q statistic =11; critical value =3.84; p = 0.0009). Figure 5c presents these behavioral outcomes aligned relative to the day on which an anterior otolith was first observed in an approximately normal position (actual age range, 13–17 dpf), although it was not possible to be certain whether the newly developed otolith was in fact in a position to contact hair cells. After otog c.1522+2T>A animals developed normal swimming, their posture and swimming were indistinguishable from those of WT/heterozygous siblings for as many days as they were observed. In cases where the utricular otoliths formed bilaterally, behavioral recovery occurred within 2 days; in cases with unilateral otolith development, behavioral recovery could take up to 4 days (in one animal, no behavioral recovery occurred through 4 days post-utricular development).
Zebrafish develop a third otolith, the lagena, which is thought to serve some vestibular, auditory, and possibly magnetic functions, in the second to third week post-fertilization (MacNaughtan and McNally, 1946; Bever and Fekete, 2002; Lu et al., 2003; Khorevin, 2008; Wu and Dickman, 2012). In otog c.1522+2T>A animals, the lagenar otolith developed at the same time as in WT/heterozygous siblings, around 12 dpf, and always prior to the utricular otoliths. Notably, in otog c.1522+2T>A animals in which the lagenar otoliths formed normally but the utricular otoliths did not form, posture did not recover in the following 5 days (n = 4; Fig. 5d). Thus, the lagenar otoliths are insufficient for normal postural control.
Discussion
Summary
The role of patterned activity in shaping vestibular circuits has been unclear, in part due to the difficulty in providing reversible manipulations to sensory circuits. Here, using otog c.1522+2T>A mutant zebrafish in which anterior otolith development is delayed by almost 2 weeks, we find that vestibular postural circuits form normally at the level of gross anatomy. Furthermore, postural behaviors recover to WT levels following the late development of the anterior otoliths, indicating that the first 2 weeks do not constitute a critical period for patterned sensory activity in vestibulospinal development. Behavioral recovery trails otolith development by approximately 1–2 days, however, suggesting that either the process of functionally tethering the otolith lags its development, or that postural behaviors may still entail some circuit refinement after the onset of sensory-elicited activity.
Otog Mutations in Humans, Mice, and Zebrafish
Otogelin is a secreted glycoprotein, specific to the inner ear, with a crucial role in anchoring the otoconial and tectorial membranes during development (Cohen-Salmon et al., 1997; El-Amraoui et al., 2001). Loss of otogelin leads to hearing and vestibular impairments in mice (Simmler et al., 2000a; Simmler et al., 2000b; El Hakam Kamareddin et al., 2015), though in humans only the hearing loss is clinically significant (Schraders et al., 2012). The reason for this discrepancy is unknown. In humans with mutations in otog there are some reports of delayed onset motor functions in infancy (e.g., delayed walking) (Schraders et al., 2012). We suggest that the anatomically delayed otolith development seen in otog c.1522+2T>A zebrafish might occur in humans with otog mutations as well, producing similar delays in gross motor coordination that eventually disappear as the otoliths develop to proper size and location.
In both this study and an independent recent analysis (Stooke-Vaughan et al., 2015), otog mutation selectively affects the utricular otolith, with typically normal saccular and lagenar otolithic development (suggesting normal hearing, though this was not explicitly tested). It is unclear why the utricular otolith eventually develops normally, albeit delayed, in otog c.1522+2T>A animals. Possibly the related protein otogelin-like (otogl) (Yariz et al., 2012; Oonk et al., 2014) functionally substitutes for otog at later stages; alternatively some gene expression patterns that are engaged during later development may trigger sufficient expression of other acellular membrane components, such as tectorins (Stooke-Vaughan et al., 2015), that eventually permit appropriate tethering of the utricular otolith.
Critical Periods in Vestibular Development
Classic experiments defining critical periods in other systems have taken advantage of reversible deprivation of sensory input. For example, the effects of monocular deprivation can be tested by suturing shut one eyelid during development, then opening it to evaluate the efficacy of the deprived eye in driving visual responses (Hubel and Wiesel, 1970). This approach has powered a large body of literature demonstrating that connectivity can develop abnormally in pathways deprived of typical sensory input (Hensch, 2004). The equivalent experiments in vestibular systems are technically prohibitive, requiring space flight or microgravity reactors (Boyle et al., 2001; Sebastian et al., 2001; Moorman et al., 2002). Furthermore, these approaches only disrupt gravitational forces; otoliths are equally sensitive to translational forces, and therefore are still predicted to drive signaling during head translation in space. Therefore, microgravity will produce a different constellation of effects than pure sensory deprivation. In addition, depriving an organism of typical gravitational forces has many non-specific effects, including changes in fluid distribution and bone and muscle metabolism. Notably, studies subjecting developing animals to microgravity have yielded a variety of effects, including both increased and decreased VOR responsivity (Sebastian et al., 2001; Moorman et al., 2002).
Because the otolithic end organs are the sole gravity sensors, a related approach is to examine vestibular circuits in animals with absent otoliths or hair cells. Mice with early hair cell degeneration exhibit some vestibular phenotypes in adulthood that exceed those of deafferented animals, suggesting a role for early signaling in circuit maturation (Eugene et al., 2009). Anatomical analysis of vestibular projections to spinal cord in the otolith-deficient head-tilt (Nox3−/−) mouse (Paffenholz et al., 2004) revealed that lateral vestibulospinal neurons are still present and exhibit preferential bias toward limb extensor motor neurons, but do not prune their contacts from flexor motor neurons, again suggesting a mixture of genetic and sensory-dependent rules governing connectivity (Basaldella et al., 2015). However, this result does not appear to have been compared with littermate controls, leaving it provisional (Holmdahl and Malissen, 2012). Even careful anatomical analysis may not be able to distinguish normal from abnormal synaptic connectivity, because otolithic pathways encode head movements in diverse directions, beginning with the hair cells. As a consequence, sensory tuning differs among ganglion neurons and vestibulospinal neurons, making simple anatomical tracing inadequate for examining how information is passed through the circuit.
Here we have found first, that the otog c.1522+2T>A mutant serves as a model of reversible sensory deprivation, with the utricular otolith grossly delayed (∼2 weeks) in development; and second, that after the late development of utricular otoliths, postural effects recover within ∼48 h. These observations suggest that the vestibulospinal circuit for posture remains plastic enough at ∼14 dpf that it can wire up appropriately once the utricular otolith is in place. That is, if there is a critical period in vestibular development, it extends beyond 2 weeks post-fertilization. In support of this hypothesis is the observation that animals with a single utricular otolith usually develop normal posture (Fig. 5c). This may indicate that the circuit can make up for under-powered sensory stimulation either through central compensation or possibly supplemental signals from the lagenar otoliths. Alternatively, the delay between initial otolith arrival and functional behavior may be attributable to subtle changes in position or tethering of the utricular otolith, which would not be apparent in these experiments. If so, then it is possible that vestibular circuit development proceeds in a largely genetically specified fashion, without a requirement for patterned activity in circuit formation at all. To test these hypotheses it will be useful to examine functional connectivity in otog c.1522+2T>A animals before and after otolith development.
The absence of the utricular otolith does not appear to have had any effect on the position or number of hair cells, vestibular ganglion neurons, or vestibulospinal neurons (Figs. 2, 3, 4) (Whitfield et al., 1996). Because the saccular otolith does not contribute to gravity sensation in zebrafish (Riley and Moorman, 2000), and the semicircular canals cannot provide functional information until ∼35 dpf (Beck et al., 2004; Lambert et al., 2008), it is reasonable to conclude that the gross anatomy of these structures forms independent of sensory-driven activity.
It remains possible that spontaneous activity is important for normal anatomical development. In the neighboring auditory system, total deafening results in both an overall reduction in size of the cochlear nuclei as well as a broadening of tonotopic projection patterns (Leake et al., 2006). In contrast, conductive hearing loss, which eliminates patterned but not spontaneous auditory nerve activity, seems to have minimal effects on cochlear nucleus organization (Rubel and Fritzsch, 2002). Our observations suggest a similar arrangement in the vestibular system, with spontaneous but not sensory activity being important for accurate formation of vestibular circuits (Levi-Montalcini, 1949). Further experiments on the development of sensory responses in vestibular circuits in WT and otog c.1522+2T>A animals may be able to address this issue.
Although some vestibular function has been attributed to the lagena (Caston et al., 1977; Khorevin, 2008), in the experiments presented here, animals with intact saccular and lagenar otoliths, but not utricular, were still completely deficient in self-righting behaviors (Fig. 5d). Therefore we postulate that any lagenar contributions to the vestibular circuit through 21 dpf are either not directed toward posture, or are insufficient to drive postural movements in the absence of additional utricular input.
Electronic supplementary material
(Rocksolo_14dpf_abnormalswim.avi) presents a high-speed video (500 frames/s) of a otog c.1522+2T>A animal swimming abnormally on the first day of utricular otolith appearance. 45 ° mirrors to reflect a side view can be seen in the upper and lower left of the image. The animal is initially side-lying; after being touched gently with a plastic probe, it turns and swims entirely upside-down (note the ventral swim bladder can be seen on the upper surface in the mirror views) before eventually rolling back to side-lying. The entire video is 480 ms long. (M4V 1531 kb)
(Rocksolo_15dpf_normalswim.avi) presents the same animal as in Video 1, 1 day later. Three swim episodes are shown with brief periods of quiescence in between removed. The animal swims normally (dorsal up) for both spontaneous and light touch-evoked swims. The swim bladder can be seen in side mirror view clearly on the underside of the animal. The video is 746 ms long. (M4V 2568 kb)
Acknowledgements
The authors are grateful to Dr. Teresa Nicolson for sharing the rock solo line, Dr. Mark Warchol for the otoferlin antibody and protocol, and to Dr. Rebecca Callahan for critical feedback on the manuscript. Funding for this research was provided by the National Institute for Deafness and Other Communication Disorders (R00DC012536); by the Pew Scholars Program; and by an Alfred P. Sloan Fellowship to M.W.B.
Compliance with Ethical Standards
Conflict of Interests
The authors declare that they have no conflict of interest.
Footnotes
Richard Roberts and Jeffrey Elsner contributed equally to the manuscript.
Contributor Information
Richard Roberts, Email: rroberts23@wustl.edu.
Jeffrey Elsner, Email: elsnerj@wustl.edu.
Martha W. Bagnall, Phone: (314) 362-9695, Email: bagnall@wustl.edu
References
- Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaldella E, Takeoka A, Sigrist M, Arber S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell. 2015;163:301–312. doi: 10.1016/j.cell.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. J Neurophysiol. 2004;92:3546–3561. doi: 10.1152/jn.00311.2004. [DOI] [PubMed] [Google Scholar]
- Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;223:536–543. doi: 10.1002/dvdy.10062. [DOI] [PubMed] [Google Scholar]
- Boyle R, Mensinger AF, Yoshida K, Usui S, Intravaia A, Tricas T, Highstein SM. Neural readaptation to Earth’s gravity following return from space. J Neurophysiol. 2001;86:2118–2122. doi: 10.1152/jn.2001.86.4.2118. [DOI] [PubMed] [Google Scholar]
- Branoner F, Straka H. Semicircular canal-dependent developmental tuning of translational vestibulo-ocular reflexes in Xenopus laevis. Developmental neurobiology. 2015;75:1051–1067. doi: 10.1002/dneu.22234. [DOI] [PubMed] [Google Scholar]
- Caston J, Precht W, Blanks RHI. Response characteristics of frog’s lagena afferents to natural stimulation. J Comp Physiol. 1977;118:273–289. doi: 10.1007/BF00614351. [DOI] [Google Scholar]
- Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, El-Amraoui A, Leibovici M, Petit C. Otogelin: a glycoprotein specific to the acellular membranes of the inner ear. Proc Natl Acad Sci U S A. 1997;94:14450–14455. doi: 10.1073/pnas.94.26.14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci. 2011;34:501–534. doi: 10.1146/annurev-neuro-061010-113710. [DOI] [PubMed] [Google Scholar]
- El Hakam Kamareddin C, Magnol L, Blanquet V. A new Otogelin ENU mouse model for autosomal-recessive nonsyndromic moderate hearing impairment. Springerplus. 2015;4:730. doi: 10.1186/s40064-015-1537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Cohen-Salmon M, Petit C, Simmler MC. Spatiotemporal expression of otogelin in the developing and adult mouse inner ear. Hear Res. 2001;158:151–159. doi: 10.1016/S0378-5955(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, DeCook R, Fritzsch B. Ear manipulations reveal a critical period for survival and dendritic development at the single-cell level in Mauthner neurons. Developmental neurobiology. 2015;75:1339–1351. doi: 10.1002/dneu.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugene D, Deforges S, Vibert N, Vidal PP. Vestibular critical period, maturation of central vestibular neurons, and locomotor control. Ann N Y Acad Sci. 2009;1164:180–187. doi: 10.1111/j.1749-6632.2008.03727.x. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Experimental reorganization in the alar plate of the clawed toad, Xenopus laevis. I. Quantitative and qualitative effects of embryonic otocyst extirpation. Brain Res Dev Brain Res. 1990;51:113–122. doi: 10.1016/0165-3806(90)90263-X. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Christiansen JR, Xia B, Korchagina J, Gale JE, Warchol ME, Corwin JT, Richardson GP. Identification of the hair cell soma-1 antigen, HCS-1, as otoferlin. J Assoc Res Otolaryngol. 2010;11:573–586. doi: 10.1007/s10162-010-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Malissen B. The need for littermate controls. Eur J Immunol. 2012;42:45–47. doi: 10.1002/eji.201142048. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fenyes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorevin VI. The lagena (the third otolith endorgan in vertebrates) Neurophysiology. 2008;40:142–159. doi: 10.1007/s11062-008-9021-8. [DOI] [Google Scholar]
- Kimmel CB, Powell SL, Metcalfe WK. Brain neurons which project to the spinal cord in young larvae of the zebrafish. J Comp Neurol. 1982;205:112–127. doi: 10.1002/cne.902050203. [DOI] [PubMed] [Google Scholar]
- Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci. 2008;28:8086–8095. doi: 10.1523/JNEUROSCI.1288-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The development of the acoustico-vestibular centers in the chick embryo in the absence of the afferent root fibers and of descending fiber tracts. J Comp Neurol. 1949;91:209–241. doi: 10.1002/cne.900910204. [DOI] [PubMed] [Google Scholar]
- Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014;30:2843–2851. doi: 10.1093/bioinformatics/btu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Acoustic response properties of lagenar nerve fibers in the sleeper goby, Dormitator latifrons. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2003;189:889–905. doi: 10.1007/s00359-003-0462-7. [DOI] [PubMed] [Google Scholar]
- MacNaughtan IPJ, McNally WJ. Some experiments which indicate that the frog’s lagena has an equilibrial function. J Laryngol Otol. 1946;61:204–214. doi: 10.1017/S0022215100007842. [DOI] [PubMed] [Google Scholar]
- Mo W, Chen F, Nechiporuk A, Nicolson T. Quantification of vestibular-induced eye movements in zebrafish larvae. BMC Neurosci. 2010;11:110. doi: 10.1186/1471-2202-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman SJ, Cordova R, Davies SA. A critical period for functional vestibular development in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;223:285–291. doi: 10.1002/dvdy.10052. [DOI] [PubMed] [Google Scholar]
- Oonk AM, Leijendeckers JM, Huygen PL, Schraders M, del Campo M, del Castillo I, Tekin M, Feenstra I, Beynon AJ, Kunst HP, Snik AF, Kremer H, Admiraal RJ, Pennings RJ. Similar phenotypes caused by mutations in OTOG and OTOGL. Ear Hear. 2014;35:e84–e91. doi: 10.1097/AUD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–2871. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BB, Moorman SJ. Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. J Neurobiol. 2000;43:329–337. doi: 10.1002/1097-4695(20000615)43:4<329::AID-NEU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Schraders M, et al. Mutations of the gene encoding otogelin are a cause of autosomal-recessive nonsyndromic moderate hearing impairment. Am J Hum Genet. 2012;91:883–889. doi: 10.1016/j.ajhg.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Esseling K, Horn E. Altered gravitational forces affect the development of the static vestibuloocular reflex in fish (Oreochromis mossambicus) J Neurobiol. 2001;46:59–72. doi: 10.1002/1097-4695(200101)46:1<59::AID-NEU6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Simmler MC, Cohen-Salmon M, El-Amraoui A, Guillaud L, Benichou JC, Petit C, Panthier JJ. Targeted disruption of otog results in deafness and severe imbalance. Nat Genet. 2000;24:139–143. doi: 10.1038/72793. [DOI] [PubMed] [Google Scholar]
- Simmler MC, Zwaenepoel II, Verpy E, Guillaud L, Elbaz C, Petit C, Panthier JJ. Twister mutant mice are defective for otogelin, a component specific to inner ear acellular membranes. Mamm Genome. 2000;11:961–966. doi: 10.1007/s003350010197. [DOI] [PubMed] [Google Scholar]
- Stooke-Vaughan GA, Obholzer ND, Baxendale S, Megason SG, Whitfield TT. Otolith tethering in the zebrafish otic vesicle requires Otogelin and alpha-Tectorin. Development. 2015;142:1137–1145. doi: 10.1242/dev.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H. Ontogenetic rules and constraints of vestibulo-ocular reflex development. Curr Opin Neurobiol. 2010;20:689–695. doi: 10.1016/j.conb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Straka H, Fritzsch B, Glover JC. Connecting ears to eye muscles: evolution of a ‘simple’ reflex arc. Brain Behav Evol. 2014;83:162–175. doi: 10.1159/000357833. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Granato M, van Eeden FJ, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Liss L, Granato M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci U S A. 2011;108:15468–15473. doi: 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LQ, Dickman JD. Neural correlates of a magnetic sense. Science. 2012;336:1054–1057. doi: 10.1126/science.1216567. [DOI] [PubMed] [Google Scholar]
- Yariz KO, et al. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am J Hum Genet. 2012;91:872–882. doi: 10.1016/j.ajhg.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Rocksolo_14dpf_abnormalswim.avi) presents a high-speed video (500 frames/s) of a otog c.1522+2T>A animal swimming abnormally on the first day of utricular otolith appearance. 45 ° mirrors to reflect a side view can be seen in the upper and lower left of the image. The animal is initially side-lying; after being touched gently with a plastic probe, it turns and swims entirely upside-down (note the ventral swim bladder can be seen on the upper surface in the mirror views) before eventually rolling back to side-lying. The entire video is 480 ms long. (M4V 1531 kb)
(Rocksolo_15dpf_normalswim.avi) presents the same animal as in Video 1, 1 day later. Three swim episodes are shown with brief periods of quiescence in between removed. The animal swims normally (dorsal up) for both spontaneous and light touch-evoked swims. The swim bladder can be seen in side mirror view clearly on the underside of the animal. The video is 746 ms long. (M4V 2568 kb)