Abstract
In 2003, select families with familial hypercholesterolemia were first identified to have gain-of-function mutations for proprotein convertase subtilisin kexin type 9 (PCSK9) followed, in 2006, by the identification of those with lifelong low levels of LDL-C and lowered atherosclerotic cardiovascular disease (ASCVD) risk who had loss-of-function PCSK9 mutations. These discoveries led to the rapid development of PSCK9-targeted monoclonal antibody (PCSK9 mAb) therapies and, in 2015, 2 ‘fully-humanized’ PCSK9 mAbs (alirocumab and evolocumab) were marketed in the United States, Europe, and other countries. In a wide range of high risk patients, with and without ASCVD, these PCSK9 mAbs, as once or twice monthly subcutaneous injections, potently reduce LDL-C 50–65% beyond levels achieved by maximally tolerated statin therapy; approximately one-third of patients achieve LDL-C levels <25 mg/dL. In the US, PCSK9 mAb therapy has current limited indications for persons with ASCVD or familial hypercholesterolemia requiring additional LDL-C reduction beyond maximally tolerated statin therapy. The first of the ASCVD outcomes-driven trials, the FOURIER trial has very recently shown in over 27,000 subjects randomized to evolocumab or placebo on top of moderate or high intensity statin therapy, a 15% risk reduction in the primary and 20% reduction in the secondary outcome over 2.2 years of treatment. Also of interest in patients with coronary artery disease on statin therapies, once-monthly evolocumab treatment, for only 76 weeks, resulted in significant plaque atheroma volume regression, as assessed by serial intravascular ultrasonography imaging, in approximately two-thirds of treated patients. Finally, in development is a highly durable RNA interference therapeutic inhibitor of PCSK9 synthesis which from a single dosage has been shown to maintain, for 6 months, a 75% reduction in PCSK9 levels and 50% reductions in LDL-C levels. The potential role of this vaccination-like product, as well as currently available PCSK9 mAb therapies, represents significant therapeutic advances to address ASCVD residual risk.
Keywords: Proprotein convertase subtilisin kexin type 9 (PCSK9), dyslipidemia, LDL-cholesterol, atherosclerosis, cardiovascular disease
The most significant advance in clinical cardiology and lipidology this decade is the emergence, and availability, of proprotein convertase subtilisin kexin type 9 (PCSK9) monoclonal antibody (mAb) therapies. Not since statin therapies became available nearly three decades ago has there been as much excitement and hope for the efficacious management of dyslipidemia and reduction of atherosclerotic cardiovascular disease (ASCVD) residual risk. Two fully-human PCSK9 mAbs, alirocumab (Praluent™) and evolocumab (Repatha™), were approved by the US Food and Drug Administration (FDA) in the summer of 2015. A third humanized PCSK9 mAb, LY3015014 is currently under development. In late 2016, a humanized PCSK9 mAb, bococizumab, was discontinued from further clinical development due to increased immunogenicity and attenuated LDL-C response.
The primary pathway for the clearance of circulating low-density lipoprotein particles (LDL-P) is LDL receptor (LDLR)-mediated hepatic internalization or endocytosis of the LDL apolipoprotein B (apo B)-LDLR complex. Under normal circumstances, LDLRs can potentially recycle, up to 150 times, returning to the cell surface, after each delivery of their LDL-C particles intracellularly. PCSK9 is a 692-amino acid mature protein mainly expressed as a secreted protease in the liver, intestines, and kidneys that forms a complex with the LDLR-LDL-P extracellular domain, which after subsequent clathrin-mediated endocytosis then acts as a “chaperone” protein resulting in lysosomal degradation of the entire complex, including the LDLR. This step results in decreased LDLR recycling, and fewer hepatocyte cell surface LDLRs able to process LDL, and consequently increased circulating plasma LDL particles (1-3). PCSK9 mAbs act by binding to PCSK9, preventing the association between PCSK9 and the LDLR, thus blocking the effects of PCSK9-mediated LDLR degradation. This allows the LDLRs to recycle to their higher potential, resulting in dramatic lowering of LDL-C. While statin therapy is first-line, lowering hepatic cholesterol content upregulates not only LDLRs, but also the synthesis and secretion of PCSK9 (4), which may account for the limited increase in efficacy with each doubling of statin doses (further LDL-C lowering of 6%).
PCSK9 mAb safety and efficacy
A wealth of phase II and III trials, involving a variety of patient populations, examined the efficacy and safety of alirocumab, evolocumab and the now discontinued bococizumab. These trials showed PCSK9 mAb-induced LDL-C reductions of 50 to 60 percent or greater in statin-treated, or statin-intolerant patients, taking maximally-tolerated statins or no statins at all, with, or without, known ASCVD, and in those with, and without, heterozygous familial hypercholesterolemia (HeFH). Furthermore, approximate 50 percent reductions in non-HDL-C and apolipoprotein B, and 25 percent reductions in lipoprotein(a) typically are achieved by these therapies (5).
Moreover, when treating patients with the much more devastating and rare homozygous familial hypercholesterolemia (HoFH), average approximately 30% reductions in LDL-C are typically seen, consistent with the presence of variable genetic defects and LDL receptor activity ranging from defective to negative; i.e., some more responsive, as in patients with two LDL receptor-defective mutations than others, as in those with even a single LDL receptor-negative mutation (6).
Two key studies pooled data from open label studies provided further insight into safety and efficacy as well as preliminary outcomes from these products. The Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG-TERM) placebo-controlled trial evaluated 2,341 patients with hyperlipidemia on maximally tolerated statins who were at high risk for coronary heart disease (CHD) (69 percent with prior CHD and 35 percent with diabetes) and showed alirocumab (150 mg Q2W) to lower LDL-C by 62 percent at 24 weeks, compared to placebo. A mean LDL-C of 48 mg/dL was achieved in the alirocumab group compared to 119 mg/dL in those on placebo, and a persistent LDL-C reduction of 52 percent was observed out to 78 weeks (7). Among the alirocumab group, 79 percent of subjects achieved an LDL-C <70 mg/dL at week 24, compared to only 8 percent in the placebo group. The most common adverse events that were higher in the alirocumab group compared to placebo included injection site reactions (5.9% vs. 4.2%), myalgia (5.4% vs. 2.9%), neurocognitive events (1.2% vs. 0.5%), and ophthalmologic events (2.9% vs. 1.9%). Of interest, adverse events in the subgroup of 575 patients who achieved an LDL-C <25 percent were similar to the overall alirocumab group. Finally, in a post-hoc analysis of the composite of cardiovascular events over the relatively short exposure of 78 weeks—including CHD death, myocardial infarction, ischemic stroke and unstable angina requiring hospitalization—those treated with alirocumab (n=27 events/1,550 patients) compared to placebo (n=26 events/788 patients) had a 48 percent reduced risk of such events [1.7% vs. 3.3%, hazard ratio (HR) =0.52, 95% confidence interval (CI): 0.31–0.60]. The Open-Label Study of Long-Term Evaluation Against LDL-cholesterol (OSLER) (8) studied 4,465 patients who completed one of 12 phase 2 or 3 studies of evolocumab who were randomized either to evolocumab 420 mg every 4 weeks plus standard of care versus standard of care alone in an open-label extension study averaging 11.1 months. Those assigned to evolocumab showed a 61 percent reduction in LDL-C, from 120 to 48 mg/dL at 12 weeks. There was no difference in the rate of serious adverse events (7.5% in each group). Neurocognitive events, while uncommon, were more frequent (0.9% vs. 0.3%) in the evolocumab group. Importantly, there appeared to be no excess of adverse events in groups ranging down to treated LDL-C levels of <25 mg/dL. Importantly, there was a 53% reduction in the incidence of the pre-specified composite endpoint of death, myocardial infarction, hospitalization for unstable angina, coronary revascularization, stroke, transient ischemic attack and hospitalization for heart failure was seen in those randomized to evolocumab (29 events/2,976 patients) versus placebo (31 events/1,489 patients) (0.95% vs. 2.18%, HR=0.47, 95% CI: 0.28–0.78) (8).
A large pooled analysis of 24 randomized phase 2 and 3 trials comprising 10,159 patients showed that compared to placebo, those randomized to PCSK9 mAb had a 49% LDL-C reduction (P=0.001), accompanied by relative risk reductions (RRR) of 55% (P=0.015), 50% (P=0.08), and 51% (P=0.03) in all-cause mortality cardiovascular mortality, and myocardial infarction, respectively (9). More recently among patients within 10 double-blind pooled alirocumab trials of 24–104 weeks duration (10) mean baseline LDL-C levels ranged from 123.2 to 126.8 mg/dL, non-HDL-C between 154.2 to 156.9 mg/dL, and apoB between 101.8 to 104.3 mg/dL. The mean LDL-C levels achieved during treatment were: in placebo-controlled studies, 56.9 and 126.5 mg/dL among those treated with alirocumab and placebo, and in ezetimibe-controlled studies, 64.0 and 100.9 mg/dL with alirocumab and ezetimibe, respectively. Percent reductions in LDL-C from baseline were inversely correlated with MACE rates [HR 0.71 (0.57 to 0.89) per additional 50% reduction from baseline; P=0.003]. As with achieved average LDL-C, lower (average) achieved non-HDL-C and apoB levels were associated with a lower risk of MACE. A 39 mg/dL difference in LDL-C corresponded to a 42 mg/dL difference in non-HDL-C and 27 mg/dL difference in apoB in the present pooled datasets. For each 42 mg/dL lower non-HDL-C, as a continuous relationship, at least down to a non-HDL-C level of 50 mg/dL, there was a 23% lower MACE rate; HR was 0.77 (95% CI: 0.65–0.93; P=0.0056). For each 27 mg/dL lower apoB, as a continuous relationship, at least down to an apo B level of 40 mg/dL, there was a 28% lower MACE rate; HR was 0.72 (95% CI: 0.60–0.86; P=0.0002). Furthermore, the low LDL-C levels achieved were not associated with excess treatment emergent adverse events. These findings are consistent with that of the Cholesterol Treatment Trialists Collaboration, where an absolute reduction in LDL-C levels of 1 mmol/L (39 mg/dL) was associated with a 23 percent risk reduction in major coronary events that is consistent across baseline levels of LDL-C (11) as well as the IMPROVE-IT trial (12).
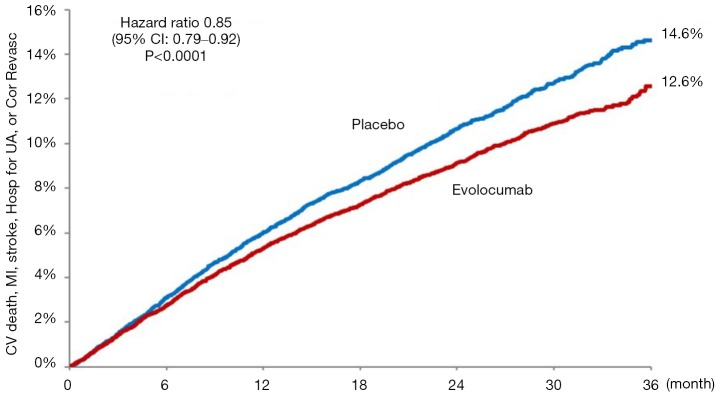
Possibly what was the most widely anticipated lipid-lowering trial in a decade, just reported and published is the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) (13) the first published outcomes-driven trial of a proprotein convertase subtilisin-kexin type 9 (PCSK9) monoclonal antibody therapy. FOURIER randomized 27,564 patients with ASCVD and LDL-C levels of ≥70 mg/dL (1.8 mmol/L) on statin therapy to either evolocumab or matching placebo. The primary composite endpoint included cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization. Overall, 81% of patients had a prior myocardial infarction and the median baseline LDL-C was 92 mg/dL (2.4 mmol/L). Evolocumab treatment resulted in a 59% reduction in LDL-C down to 30 mg/dL (0.78 mmol/L) with 42% of patients achieving an LDL-C of <25 mg/dL (0.65 mmol/L). After 2.2 years of follow-up, the trial met its primary endpoint with a 15% RRR (HR=0.85, 95% CI: 0.79–0.92, P<0.001) (Figure 1). In addition, the key secondary endpoint of cardiovascular death, myocardial infarction, or stroke was reduced by 20% (HR=0.80, 95% CI: 0.73–0.88, P<0.001). Landmark analyses showed the treatment-control group difference to widen with time with a 2% absolute risk reduction seen by 3 years [number needed to treat (NNT) of 50]. Finally, event rates appear to be successively lower with achieved LDL-C levels down to below 25 mg/dL (0.65 mmol/L). Importantly, there were no differences in treatment emergent adverse events, except for expected increased injection site reactions seen in the evolocumab group. A more definitive and objective assessment of evolocumab’s effects on cognitive function was recently completed in the EBBINGHAUS trial (a substudy of FOURIER—see below). The primary endpoint of noninferiority on spatial working memory strategy index was met after 20 months of follow-up among the 1,974 patients enrolled, demonstrating no difference between groups in cognitive function outcome (14). These results show the further benefit of evolocumab provided to statin-treated high risk patients. Further outcomes data are expected with the release of the ODESSEY outcomes trial involving alirocumab due out in 2018.
Figure 1.
Cumulative primary cardiovascular composite endpoint rates for evolocumab and control groups in the FOURIER trial (13) (reproduced with permission).
Additive impact of combined PCSK9 mAb and statin therapies on regression of atherosclerosis
ASCVD events are attributed to disruption of a “vulnerable” atherosclerotic plaque defined as a lipid-rich plaque with a thin fibrous cap lacking proper collagen and smooth muscle cell support (15-17). Aggressive statin therapy has demonstrated reduced progression and actual regression of atherosclerosis in some patients especially when LDL-C levels <70 mg/dL are achieved, with an increase in IVUS hypoechogenicity and an increase in hyperechogenicity, most likely due to a change in composition from lipid-rich to lipid poor plaque (18). Clinical ASCVD outcomes have been correlated to atherosclerotic burden utilizing intravascular ultrasound (IVUS) imaging technology (19,20). In a meta-analysis of 8 IVUS trials (21) a continuous relation between changes in percent atheroma volume (PAV) versus achieved LDL-C, down to levels well below 35 mg/dL, underscores the rationale of greater anti-atherosclerotic benefit in the setting of lower achieved LDL-C levels.
To assess the impact of PCSK9 inhibitors on ASCVD burden, the GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound) multicenter, double-blind, placebo-controlled randomized clinical trial, Nicholls et al. (22) studied 968 patients with angiographic coronary disease, 98% of whom were already on statin therapies, randomized to a monthly injection of evolocumab (420 mg) or placebo and examined as the primary endpoint, PAV, over a 84 week treatment period. They showed that compared with placebo, the evolocumab group achieved lower mean, time-weighted LDL-C levels [93.0 vs. 36.6 mg/dL; difference, −56.5 mg/dL (95% CI: −59.7 to −53.4); P<0.001]. Furthermore, despite history of prior statin therapy [duration of use not reported), PAV increased 0.05% with placebo, compared to a decrease of 0.95% with evolocumab; between-group difference of −1.0% (95% CI: −1.8% to −0.64%); P<0.0001] (Figure 2). In addition, evolocumab induced plaque regression in 64.3% of patients, compared to 47.3% in those on placebo, in addition to statin (P<0.001); the percent of patients remaining either unchanged or progressing was 35.7% and 52.7%, respectively. Estimates of plaque compositional changes were not reported. Of interest, exploratory pre-specified post hoc analyses generated several important observations: (I) the relationship between achieved LDL-C level and change in PAV showed a continuous linear relation between LDL-C achieved and change in PAV down to LDL-C levels as low as 20 mg/dL, without any evidence of a threshold effect; (II) the first MACE occurred in 74 patients (15.3%) on placebo and 59 patients (12.2%) on evolocumab; while not powered to evaluate clinical outcomes, the 20.3% relative and 3.1% absolute risk reduction trends and NNT of 32 in the relatively short 18-month duration trial, is in the right direction and promising; and (III) in the subgroup of patients with a baseline LDL-C level <70 mg/dL (n=144), the percentage of patients with regression of PAV for evolocumab cohort compared with placebo was 81.2% vs. 48.0% [between-group difference, 33.2% (95% CI: 18.6% to 47.7%); P<0.001] and these patients had a more markedly favorable effect on the change in PAV [−1.97% vs. −0.35%; between-group difference −1.62% (95% CI: −2.50% to −0.74%); P<0.001]. While the baseline characteristics of this ‘LDL-C <70 mg/dL’ cohort was not reported, one could speculate that those with the higher atherogenic particle concentration are likely to receive the greatest absolute LDL-P clearance benefit from the PSCK9 mAb-induced upregulated LDL receptor recycling.
Figure 2.
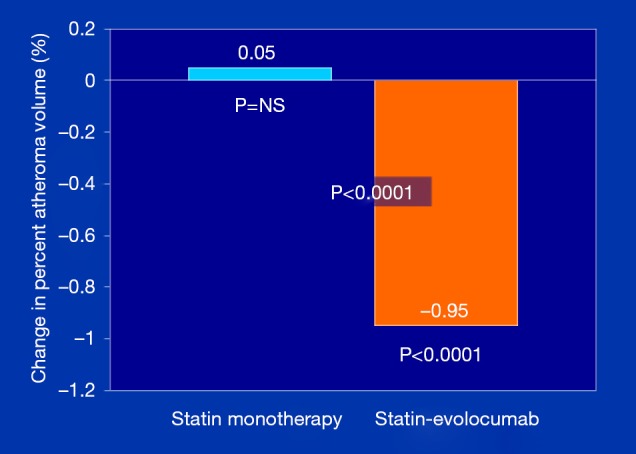
GLAGOV primary endpoint: change in percent atheroma volume (PAV). Data adapted from Nicholls et al. (22).
Who is appropriate for PCSK9 mAb treatment?
The current indications for both alirocumab and evolocumab, as approved by the FDA, call for their usage as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with HeFH or clinical ASCVD, who require additional lowering of LDL-C (23,24); additionally, evolocumab is indicated for such persons with HoFH who require further LDL-C lowering beyond what other lipid-lowering therapies provide (24). In Europe, the European Medicines Association (25) approved a broader indication for evolocumab in which usage can be considered for adults with primary hypercholesterolaemia (HeFH and non-familial) or mixed dyslipidemia. It can be an adjunct to diet in combination with a statin or with a statin with other lipid-lowering therapies in patients unable to reach LDL C goals via the maximum tolerated dose of a statin or alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contra-indicated. There also is an indication for use by adults and adolescents ages 12 years and older with HoFH in combination with other lipid-lowering therapies. However, both products clearly state in their labeling that the effects on cardiovascular outcomes have not been determined.
Roughly 1 in 300 to 500, and possibly as many as 1 in 250 adults have HeFH (26) and, given their marked CHD risk, associated with a relatively high lifetime cholesterol burden, most experts would classify them as a CHD-risk equivalent, warranting a need for additional LDL-C lowering, if the achieved LDL-C on a high-intensity (or maximum tolerated) statin is still remains ≥70 mg/dL or greater. An approximate 50 percent further lowering of LDL-C would result in LDL-C levels well below 50 mg/dL in many such persons from the addition of a PCSK9 MAb. A much smaller proportion of persons (1 in 500,000 to 1 million) have HoFH; however, these persons have substantially higher LDL-C levels than do those with HeFH and they normally cannot achieve reasonable “goal” levels, despite being on a high-intensity statin. LDL-C targets in HoFH are <2.5 mmol/L (<100 mg/dL) or <1.8 mmol/L (<70 mg/dL) if accompanied with ASCVD. Even at the highest doses of the most efficacious statins, however, only modest reductions in LDL-C plasma levels, of 10–25%, are observed in most patients. Autosomal recessive hypercholesterolemia seems relatively more responsive to treatment. Combinations of statins with other lipid lowering agents, including ezetimibe, bile acid sequestrants, niacin, fibrates, and probucol have been successful in HoFH further lowering LDL-C levels, although their use may be limited by tolerability and availability (26,27).
Aside for LDL apheresis and statin drugs, mipomersen (an antisense oligonucleotide therapy), and lomitapide (a microsomal triglyceride transfer protein inhibitor), evolocumab also has an indication (in Europe and the U.S.) for HoFH, based on the Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) Part B, a randomized, double-blind, placebo-controlled phase 3 trial, undertaken at 17 sites in ten countries in North America, Europe, Middle East, and South Africa. Compared with placebo, evolocumab significantly reduced ultracentrifugation LDL cholesterol at 12 weeks by 30.9% (95% CI: −43.9% to −18.0%; P<0.0001) (6). The LDL-C reduction response to PCSK9mAb in HoFH does require at least some LDL receptor activity (which is present in many despite HoFH).
A recent report by Wong and colleagues (28) from statin-treated U.S. adults in the National Health and Nutrition Examination Survey (NHANES) 2011–2012 showed that only 64 percent of those with ASCVD were on statins, and of those on statins, 80% were not at a target of LDL-C <70 mg/dL. And of those with an actual or estimated pre-treatment LDL-C ≥190 mg/dL, only 61% were on statins, and of these, 98% were not at LDL-C target. While it is unclear how many such persons were on recommended moderate or high-intensity therapy, these data suggest a significant opportunity for the consideration of newer therapies, such as PCSK9 monoclonal antibodies, when reasonable targets cannot be reached. While the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (29) focus on use of high-intensity statin therapy in such individuals, consideration for evidence-based non-statin therapy also is an option, especially when there is inadequate response or tolerability from statin therapy alone.
Recent guidance has been given for the use of PCSK9 mAb therapy by both the National Lipid Association (NLA) (30) and by the American College of Cardiology (ACC) (31). The NLA first noted in part 2 of their recently published dyslipidemia recommendations that until the results of cardiovascular outcomes trials are available, a conservative approach would be to use PCSK9 inhibitors primarily in those with ASCVD who have LDL-C ≥100 mg/dL (or non-HDL-C ≥130 mg/dL) while on maximally tolerated statin (± ezetimibe) therapy and in those with heterozygous FH without ASCVD who have an LDL-C ≥130 mg/dL (or non-HDL-C ≥160 mg/dL) while on maximally tolerated statin (± ezetimibe). They also note such therapy may be considered for selected high risk patients with ASCVD (e.g., with recurrent events) with LDL-C ≥70 mg/dL (or non-HDL ≥100 mg/dL) despite the use of other lipid-lowering therapies (30). Following this, the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk (31) that PCSK9 mAb therapy may be considered (after considering ezetimibe) when a patient still has not achieved at least a 50% LDL-C reduction (or LDL-C <100 mg/dL in the case of stable ASCVD without comorbidities, or <70 mg/dL for ASCVD with comorbidities) on maximally tolerated statin therapy. For those without ASCVD but with a baseline LDL-C ≥190 mg/dL a PCSK9 mAb could be considered first before ezetimibe because of its greater LDL-C lowering efficacy in those not having at least a 50% LDL-C reduction or LDL-C <100 mg/dL without comorbidities or <70 mg/dL with comorbidities despite maximally tolerated statin therapy. A PCSK9 inhibitor was not recommended at this time by the ACC for those without ASCVD who have diabetes or 10-year ASCVD risk >7.5%.
Further considerations for use of PCSK9 mAb therapy
Besides patients with ASCVD or FH in whom PCSK9 mAb therapy is currently indicated, many persons with multiple risk factors have substantial residual risk for ASCVD events despite maximally tolerated therapy, indicating a much larger segment of the population could potentially benefit from such therapy This includes older persons, those with unfavorable discordance between LDL-C and other atherogenic markers (i.e., non-HDL-C, apo B and LDL-P), including those with metabolic syndrome or diabetes of long duration, especially in the presence of multiple or single severe risk factors, and those with substantial subclinical atherosclerosis. Such factors would bring many up to a “true CHD risk equivalent” (or higher) status that could be seen by some clinicians as reasonable for such therapy if response to statin therapy alone, or combination therapies with non-statins, is inadequate. For instance, among those with metabolic syndrome or diabetes who have coronary calcium scores ≥100, annual CHD event rates are >2 percent, placing them at such increased risk (32). Moreover, current ACC/AHA guidelines indicate a 10-year ASCVD risk >7.5 percent identifies a higher-risk person with diabetes, warranting high-intensity statin therapy (29). In addition, those with diabetes who have additional risk factors have a ≥50 percent lifetime risk of CHD (33), demonstrating the limitation of focusing on 10-year risk estimates. Finally, those who are completely statin intolerant—estimates range from 10–15 percent of statin users (34)—may be an important patient population for these agents; however, prescribers will need to document the details concluding the patient is statin intolerant (e.g., using an objective comparison of pre- vs. post-statin complaints) and ideally has failed ≥2 statin drugs, as was the requirement for most patients in PCSK9 trials studying statin intolerance (35,36). Additionally, it is important to remember that before labeling a patient “statin intolerant”, a careful search for reversible causes should be made. These include high intake of dietary grapefruit juice that contains CYP3A4 enzyme blockers, drug-drug interactions, sub-clinical hypothyroidism, slow metabolizers (SLOCO-1B1 gene), vitamin D deficiency, overly vigorous exercise programs, etc. Also, occasionally there are patients who have an excellent response to lower doses of statins and may reach their LDL goals without the use of high intensity therapy.
Finally, not widely appreciated is the extent of statin non-responders, which can be substantial, even among those on high-intensity statins. In these cases, true non-responders should be differentiated from “pseudo non-responders”, as non-adherence and poor compliance should be excluded which unfortunately has been shown to be yet another cause for lack of “goal attainment”. A major problem contributing to residual risk is the considerable variability of LDL-C response with statins and that achieving a goal of LDL-C <70 mg/dL is more difficult than achieving a goal of >50 percent reduction in LDL-C. For instance, the VOYAGER high-risk patient database (n=20,539) of subjects with mean baseline LDL-C 168 mg/dL showed that, even on atorvastatin 80 mg/dL, while 60 percent achieved >50 percent reduction in LDL-C, only 30 percent achieved <70 mg/dL. On rosuvastatin 40 mg/day, 78 percent achieved >50 percent reduction in LDL-C, while only 42 percent achieved <70 mg/dL (37). These patient groups suggest a need for the potency associated with PCSK9 inhibitors for addressing what can be substantial residual lipid risk but do not explicitly meet the current FDA indications of HeFH or clinical ASCVD.
The wider inclusion of these therapies in future clinical practice guidelines as well as optimal cost negotiations with payers (given current list pricing of approximately $1,200 per month) will be important for accessibility of these agents by patients. It has been recently estimated that annual drug costs per patient would need to be reduced to $4,536 to be cost-effective at the accepted <$100,000 per quality adjusted life year (QALY) (38). Broader indications for PCSK9 mAb therapy may be possible once further data become available in certain patient subgroups, cost-effectiveness is better documented, and most importantly, an improvement in clinical outcomes has been demonstrated. Presently, the use of these PCSK-9 inhibitors requires a detailed prior authorization. It is the responsibility of the health care provider to show that the approved FDA indications have been carefully documented in order to limit denials by insurance companies for these expensive newer therapies.
RNA interference therapy for inhibition of PCSK9 synthesis
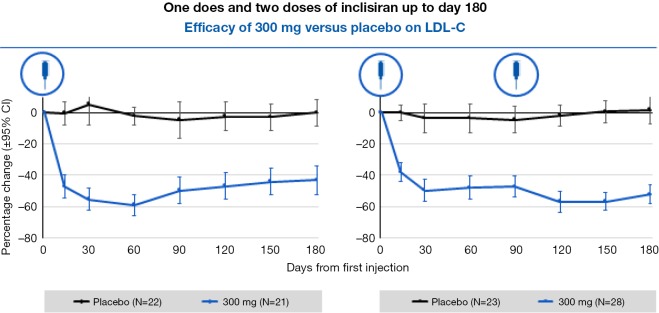
Administration of ‘small interfering RNA’ (siRNA) molecules is a recently discovered means to decrease PCSK9 levels, thereby reducing LDL-C levels. These siRNA molecules bind intracellularly to the RNA-induced silencing complex (RISC) enabling it to cleave messenger RNA (mRNA) molecules that encode PCSK9. This cleaved mRNA is degraded and thus not available for protein translation, resulting in decreased levels of PCSK9. Inclisiran (ALN-PCSsc) is a long-acting, subcutaneously delivered, synthetic siRNA directed against PCSK9 that is taken up specifically by hepatocytes. Fitzgerald and colleagues (39) recently demonstrated in a phase 1 trial involving randomization of healthy volunteers to inclisiran or placebo, that a single 300 mg dose of inclisiran was able to reduce the PCSK9 level by 75% and LDL-C levels of 51% for 6 months or longer (Figure 3), with two dosages reducing LDL-C by 57% at 6 months. This product appeared to be very well-tolerated and offers the potential for bi- or tri-annual dosing. Given the positive results, phase 3 outcomes trials are now being planned. ORION-1’s primary endpoint was the percentage change in LDL cholesterol and circulating PCSK9 levels at 180 days. Secondary end points included LDL cholesterol levels at 90 days and PCSK9 levels over time plus safety and tolerability (40). A total of 501 patients were randomly assigned to 1 of 6 treatment arms (average age: 63 years; 48.1% with ASCVD; 14.2% with diabetes). More than 80% were taking statins at the study entry and 28.1% were on ezetimibe with a baseline LDL cholesterol of 128 mg/dL. A mean 51% reduction in LDL cholesterol was seen in the group assigned to the 300-mg dose; a 57% reduction was seen in the group assigned to 2 doses of 300 mg. Additionally, side effects including elevated liver and muscle enzymes, myalgia, and injection site skin reactions were mild.
Figure 3.
Change in LDL-C level in single-dose cohorts treated with inclisiran. Adapted from Fitzgerald et al. (39).
Conclusions
Despite potent high-intensity statins reducing ASCVD events and favorably altering the atherosclerotic vulnerable plaque, considerable residual risk and plaque burden persists. Possibly the most significant development this decade in cardiology is the emergence and availability of PCSK9 mAb therapies as a new and highly efficacious LDL-C lowering class of therapies now providing clinicians with the opportunity to address the significant residual atherogenic lipid risk present in many high-risk patients because of inadequate response or unacceptable drug intolerance of existing therapies. Recently reported results of atherogenic cholesterol lowering, efficacy, safety, reduction in MACE, and coronary plaque burden are promising. Clinical practice guidelines have already adopted PCSK9 mAbs, but they are currently reserved for those at highest risk, consistent with current FDA restrictive labeling, until the results of large clinical event-driven trials are taken into account and long-term estimates of cost-effectiveness have been demonstrated. Moreover, newer therapies in development, such as mRNA interference therapies targeting PCSK9 may hold further promise in providing more sustained reductions in LDL-C and addressing ASCVD residual risk.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dr. Wong has received research support thorough his institution from Amgen and Regeneron, speaking fees from Sanofi and Regeneron, consulting fees from Pfizer, and has served on advisory boards with Merck and Amgen; Dr. Greenfield has received speaking fees from Amgen. Dr. Rosenblit has no conflicts of interest to declare.
References
- 1.He G, Gupta S, Yi M, et al. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem 2002;277:44044-9. 10.1074/jbc.M208539200 [DOI] [PubMed] [Google Scholar]
- 2.Wong ND, Bloch MJ. The PCSK9 Revolution: Hope or Hype? Latest Clinical Trial Results and Implications. Lipid Spin 2014;3:7-11. [Google Scholar]
- 3.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009;50:S172-7. 10.1194/jlr.R800091-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubuc G, Chamberland A, Wassef H, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis - regulated convertase - 1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2004;24:1454-9. 10.1161/01.ATV.0000134621.14315.43 [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015;65:2638-51. 10.1016/j.jacc.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Raal FJ, Honarpour N, Blom DJ, et al. TESLA Investigators Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial Lancet 2015;385:341-50. 10.1016/S0140-6736(14)61374-X [DOI] [PubMed] [Google Scholar]
- 7.Robinson JG, Farnier M, Krempf M, et al. For theODYSSEY LONG TERM investigators Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489-99. 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]
- 8.Sabatine MS, Giugliano RP, Wiviott SD, et al. For the Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Instigators. Efficacy and safety of evolucumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500-9. 10.1056/NEJMoa1500858 [DOI] [PubMed] [Google Scholar]
- 9.Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:40-51. 10.7326/M14-2957 [DOI] [PubMed] [Google Scholar]
- 10.Ray KK, Ginsberg HN, Davidson MH, et al. Reductions in Atherogenic Lipids and Major Cardiovascular Events: A Pooled Analysis of 10 ODYSSEY Trials Comparing Alirocumab to Control. Circulation 2016;134:1931-43. 10.1161/CIRCULATIONAHA.116.024604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Blazing MA, Giugliano RP, et al. IMPROVE-IT Investigators Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372:2387-97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017. [Epub ahead of print]. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 14.No Evidence of Cognitive Issues When Evolocumab Added to Statin Therapy. Available online: http://www.acc.org/about-acc/press-releases/2017/03/17/11/11/sat-8am-no-evidence-of-cognitive-issues-when-evolocumab-added-to-statin-therapy
- 15.Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 16.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. 10.1161/01.CIR.0000087480.94275.97 [DOI] [PubMed] [Google Scholar]
- 17.Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol 2013;61:1041-51. 10.1016/j.jacc.2012.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation 2001;104:387-92. 10.1161/hc2901.093188 [DOI] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55: 2399-2407. 10.1016/j.jacc.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 20.Puri R, Nissen SE, Shao M, et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J 2013;34:3182-90. 10.1093/eurheartj/eht260 [DOI] [PubMed] [Google Scholar]
- 21.Puri R, Nissen SE, Shao M, et al. Impact of Baseline Lipoprotein and C-Reactive Protein Levels on Coronary Atheroma Regression Following High-Intensity Statin Therapy. Am J Cardiol 2014;114:1465-72. 10.1016/j.amjcard.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016;316:2373-84. 10.1001/jama.2016.16951 [DOI] [PubMed] [Google Scholar]
- 23.Package insert for Praluent. Available online: www.regeneron.com/Praluent/Praluent-fpi.pdf. Accessed August 28, 2015.
- 24.Package insert for Repatha. Available online: www.repathahcp.com/. Accessed August 28, 2015.
- 25.European Medicines Association. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003766/smops/Positive/human_smop_000828.jsp&mid=WC0b01ac058001d127. Accessed August 21, 2015.
- 26.Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:133-40. 10.1016/j.jacl.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Cuchel M, Bruckert E, Ginsberg HN, et al. European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146-57. 10.1093/eurheartj/ehu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ND, Young D, Zhao Y, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011-2012. J Clin Lipidol 2016;10:1109-18. 10.1016/j.jacl.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 29.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889-934. 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 30.Jacobson TA, Maki KC, Orringer CE, et al. NLA Expert Panel. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J Clin Lipidol 2015;9:S1-122.e1. 10.1016/j.jacl.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2016;68:92-125. 10.1016/j.jacc.2016.03.519 [DOI] [PubMed] [Google Scholar]
- 32.Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in persons with metabolic syndrome and diabetes: the Multiethnic Study of Atherosclerosis. Diabetes Care 2011;34:2285-90. 10.2337/dc11-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of Lifetime Risk for Cardiovascular Disease by Risk Factor Burden at 50 Years of Age. Circulation 2006;113:791-8. 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 34.Banach M, Rizzo M, Toth PP, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Expert Opin Drug Saf 2015;14:935-55. 10.1517/14740338.2015.1039980 [DOI] [PubMed] [Google Scholar]
- 35.Stroes E, Colquhoun D, Sullivan D, et al. For theGAUSS-2 Investigators Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541-8. 10.1016/j.jacc.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 36.Moriarty PM, Thompson PD, Cannon CP, et al. ODYSSEY ALTERNATIVE: Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody, alirocumab, versus exetimibe, in patients with statin intolerance as defined by a placebo run-in and statin rechallenge arm (abstr). Circulation 2014;130:2108-9. [Google Scholar]
- 37.Karlson BW, Nicholls SJ, Lundman P, et al. Achievement of 2011 European low-density lipoprotein cholesterol (LDL-C) goals of either <70 mg/dL or 50 percent reduction in high-risk patients: Results from VOYAGER. Atherosclerosis 2013;228:265-9. 10.1016/j.atherosclerosis.2013.02.027 [DOI] [PubMed] [Google Scholar]
- 38.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 Inhibitor Therapy in Patients With Heterozygous Familial Hypercholesterolemia or Atherosclerotic Cardiovascular Disease. JAMA 2016;316:743-53. 10.1001/jama.2016.11004 [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald K, White S, Borodovsky A, et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med 2017;376:41-51. 10.1056/NEJMoa1609243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray KK, Landmesser U, Leiter LA, et al. LBCT.03: Insights from new therapeutic trials for lipids. ORION-1. Inclisiran inhibits PCSK9 synthesis by RNA interference. Planned interim analysis of a multi-center randomized controlled dose-finding trial. American Heart Association's Scientific Sessions 2016. November 12-16, 2016, New Orleans, LA. [Google Scholar]