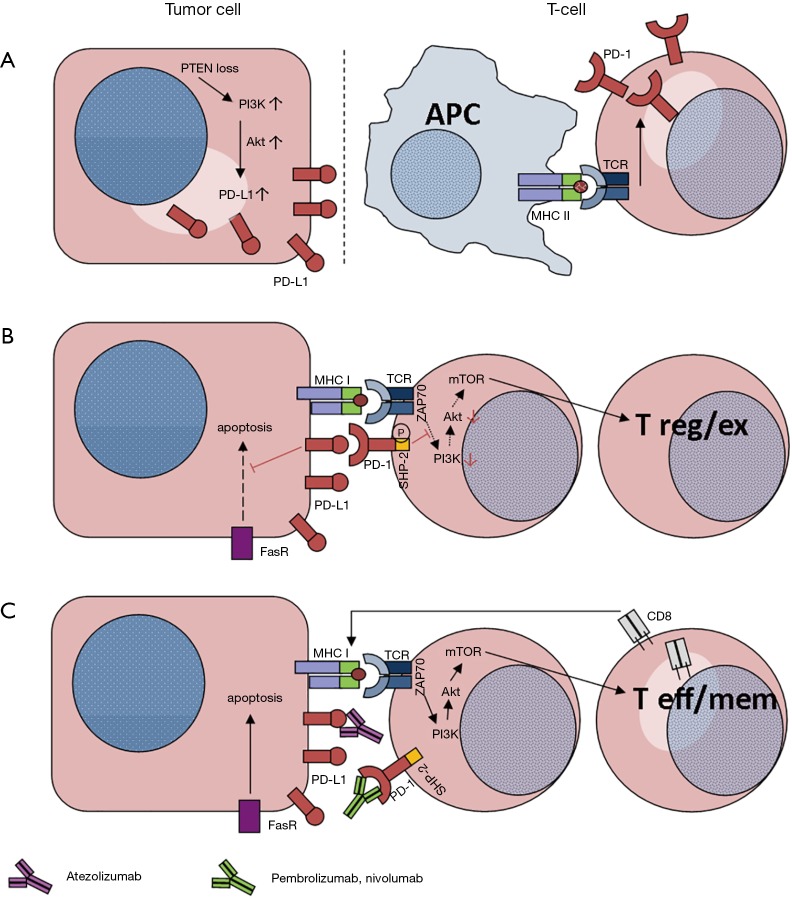
The better understanding of interactions between tumor and immune system (e.g., tumor-associated upregulation of PD-L1 to induce checkpoint for cytotoxic lymphocytes; Figure 1A) gave rise to the development of immune modulating therapies. To overcome T-cell tolerance and to boost cellular immune response, the application of monoclonal antibodies targeting CTLA-4 (4) or the PD-1/PD-L1 axis enlarged our therapeutic options for advanced cancer patients. With regard to this type of immunotherapy, non-small cell lung cancer (NSCLC) proved to be a suitable entity. The approval of immune checkpoint inhibitors [i.e., Nivolumab (5,6), Pembrolizumab (7-9) and Atezolizumab (10)] enriched our therapeutic armamentarium for advanced NSCLC patients (11).
Figure 1.
PD-1:PD-L1 mechanisms between T-cells and tumor cells. (A) Loss of phosphatase and tensin homolog (PTEN) in tumor cells induces the Phosphoinositol-3-kinase (PI3K)—Akt pathway with consecutive overexpression of PD-L1 (1); (B) once PD-L1 binds to PD-1, the resulting inhibition influences tumor surveillance such as Fas-mediated apoptosis (2). By co-activation of PD-1 concomitant to TCR in T-cells, the phosphorylation of the SHP-2 domain results in a down-regulation of the PI3K-Akt pathway. This step alters the mTOR complex, which regulates T cellular differentiation (3); (C) upon immune checkpoint inhibition, either with PD-L1-mAb (Atezolizumab) or PD-1-mAbs (Pembrolizumab and Nivolumab), T-cell differentiation is switched to CD8+ T effector (T eff) or T memory (T mem) cells, inducing apoptosis by completing the major histocompatibility complex (MHC) of the tumor cells (3).
Basically, there are two molecular pathways with impact on tumor cell survival. On the one hand, concomitant engagement of both the T-cell receptor (TCR) and the PD-1 receptor regulate T cellular differentiation (Figure 1B) (3). On the other hand the binding of PD-1 to PD-L1 impairs i.a. Fas-mediated apoptotic mechanisms (Figure 1C) (2). However, PD-L1 activity is not stable. Besides tumor heterogeneity it depends on other factors such as EGFR-mutational status (12), JAK2 gene amplification (13) or following tyrosine kinase inhibitor (TKI) therapies (14).
At present, there are two PD-1 inhibiting antibodies approved for NSCLC therapy: Nivolumab and Pembrolizumab. The design of the underlying studies pivotal for approval differs in terms of methodology. Whereas Nivolumab was tested as a 2nd line treatment in squamous (5) and non-squamous (6) NSCLC patients (against prior standard of care: docetaxel chemotherapy), the KEYNOTE-001 trial focused on the response rates of Pembrolizumab in correlation with PD-L1 expression in NSCLC tumor tissues (7). Similar to the pivotal studies for Nivolumab, Pembrolizumab was compared to docetaxel chemotherapy as second line treatment in a phase II/III-study (8). Only patients with a PD-L1 expression level of at least 50% in the investigated tumor cells were included in this study (8).
Against the background of the reported favorable therapeutic results (8), Reck et al. investigated Pembrolizumab as 1st line treatment for untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells. With focus on progression-free survival (as primary end point), overall survival, response rate, and safety (as secondary end points), Pembrolizumab (tested in n=154 patients with a PD-L1 expression) was compared to standard chemotherapy (n=151 patients) (9).
In the comparative analysis, Pembrolizumab was associated with increased progression free survival (PFS) [hazard ratio (HR) for disease progression or death =0.50; P<0.001], overall survival (OS) (HR for death =0.6; P=0.005) and improved response rates (44.8% vs. 27.8%). Moreover, less adverse events were observed for the Pembrolizumab treatment. With regard to the immune-mediated specific side effects, hypothyroidism (9.1%), hyperthyroidism (7.8%) and pneumonitis (5.8%) were observed in the Pembrolizumab cohort. Other side effects, e.g., nausea, vomiting, fatigue, and constipation were seen more often following platinum based doublet chemotherapies (9).
Due to the confirmed favorable benefit-to-risk profile of PD-1 inhibition, this therapeutic option now becomes relevance for 1st line NSCLC treatment. With regard to future therapeutic implications, the combined application of immunotherapy and chemotherapy have already been investigated in two phase I/II-trials (15,16). Whether there are beneficial effects for both neoadjuvant and adjuvant immune checkpoint inhibition, requires further investigation.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by the Section Editor Tianxiang Chen (Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13:84-8. 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 2.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008;111:3635-43. 10.1182/blood-2007-11-123141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol 2016;7:550. 10.3389/fimmu.2016.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan WL, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol 2016;17:e347-62. 10.1016/S1470-2045(16)30123-1 [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. 10.1097/JTO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Okamoto T, Okano S, et al. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:62-71. 10.1016/j.jtho.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 14.Han JJ, Kim DW, Koh J, et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:263-70.e2. 10.1016/j.cllc.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Kanda S, Goto K, Shiraishi H, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol 2016;27:2242-50. 10.1093/annonc/mdw416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. 10.1200/JCO.2016.66.9861 [DOI] [PMC free article] [PubMed] [Google Scholar]