Abstract
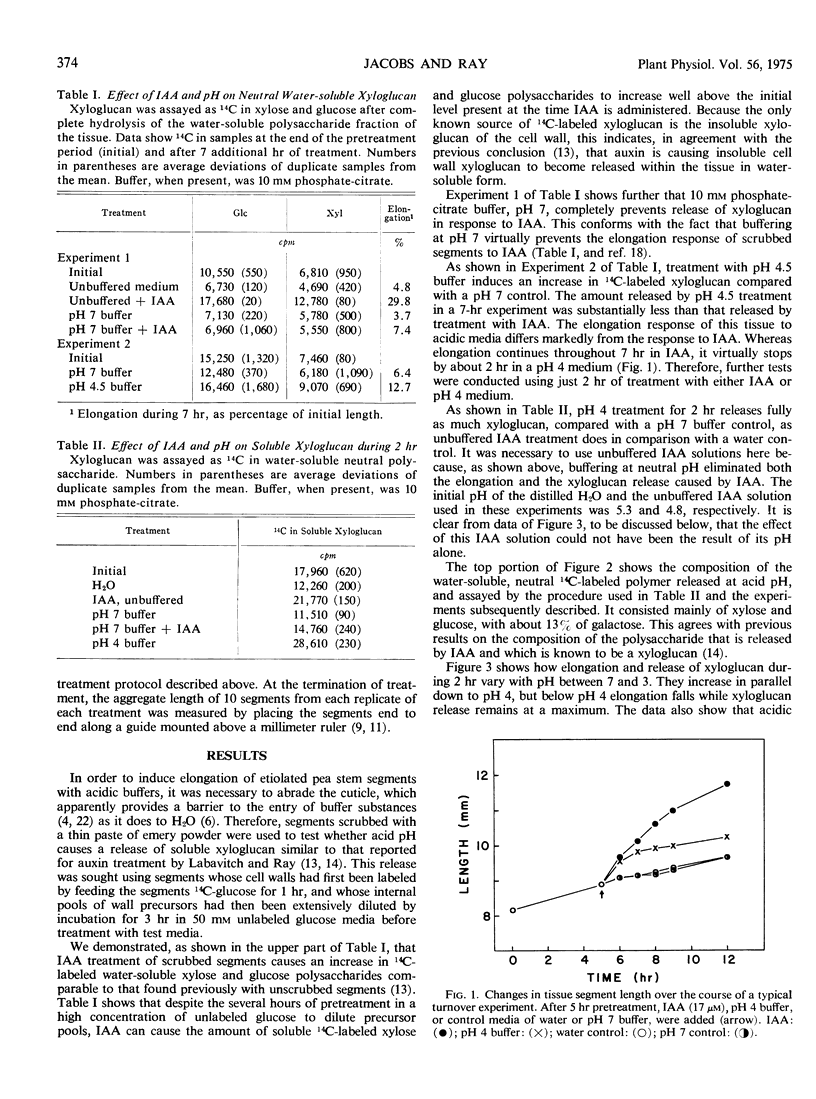
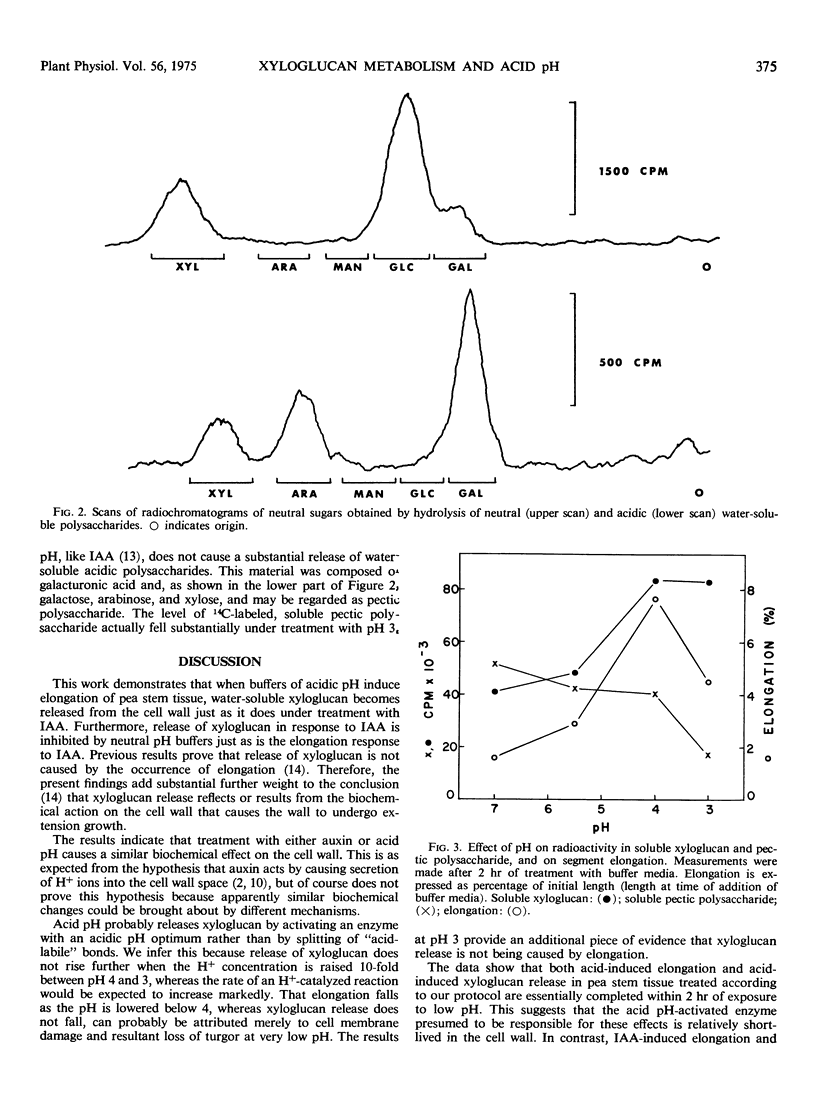
Like indoleacetic acid, buffers of acidic pH, which stimulate elongation of pea (Pisum sativum var. Alaska) stem tissue, induce the appearance within the tissue of a watersoluble xyloglucan polymer that probably arises from previously deposited wall material. Neutral pH buffers, which inhibit the elongation response to indoleacetic acid in this tissue, inhibit indoleacetic acid-induced increase in soluble xyloglucan. The findings provide further evidence that release of soluble xyloglucan from the cell walls of pea results from the biochemical action on the cell wall that is responsible for wall extension. The data also indicate that treatment of tissue with either auxin or acidic pH has a similar biochemical effect on the cell wall. This is consistent with the H+ secretion theory of auxin action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Hydrogen Ion Entry as a Controlling Factor in the Acid-growth Response of Green Pea Stem Sections. Plant Physiol. 1975 Mar;55(3):547–549. doi: 10.1104/pp.55.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H. E., Thimann K. V. Studies on the Growth Hormone of Plants: I. Proc Natl Acad Sci U S A. 1932 Jan;18(1):30–46. doi: 10.1073/pnas.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler M. J., Rayle D. L. Auxin Does Not Alter the Permeability of Pea Segments to Tritium-labeled Water. Plant Physiol. 1974 Feb;53(2):229–232. doi: 10.1104/pp.53.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L. Evidence Against the Involvement of Galactosidase or Glucosidase in Auxin- or Acid-stimulated Growth. Plant Physiol. 1974 Aug;54(2):213–215. doi: 10.1104/pp.54.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Inactivity of 3-methyleneoxindole as mediator of auxin action on cell elongation. Plant Physiol. 1973 Aug;52(2):186–189. doi: 10.1104/pp.52.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Studies on the Growth of Coleoptile and First Internode Sections. A New, Sensitive, Straight-Growth Test for Auxins. Plant Physiol. 1956 Mar;31(2):94–111. doi: 10.1104/pp.31.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Haughton P. M., Cleland R. An in vitro system that simulates plant cell extension growth. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1814–1817. doi: 10.1073/pnas.67.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]


