Abstract
Background
A considerable portion of pathological stage (p-stage) IA non-small cell lung cancer (NSCLC) patients suffered from death and recurrence after video assisted thoracoscopic surgery (VATS) lobectomy. The purpose of our study was to develop nomograms to predict which subgroup patients were more likely to suffer from recurrence or death.
Methods
Data of invasive p-stage IA NSCLC patients who underwent VATS lobectomy at Peking University People’s Hospital from September 2006 to April 2014 were analyzed. Multivariate Cox proportional hazards regression was used to develop nomograms. The performance of the nomograms was evaluated by Harrell’s concordance index (C-index), calibration plots and risk group stratification.
Results
A total of 422 patients with NSCLC of invasive p-stage IA were included in the study. The median follow-up time was 40 months. Age [hazards ratio (HR) =1.067, 95% confidential interval (CI): 1.006–1.131], lymphovascular invasion (LVI) (HR=4.062, 95% CI: 1.278–12.912) and differentiation (HR =5.747, 95% CI: 2.151–15.353) were independent prognostic factors to predict overall survival (OS). Tumor diameter (HR =3.299, 95% CI: 1.814–6.001), LVI (HR =3.260, 95% CI: 1.221–8.708) and differentiation (HR =3.607, 95% CI: 1.776–7.327) were independent predictors of recurrence free survival (RFS). The nomogram for predicting OS demonstrated stronger discriminatory power than the 7th and 8th T stage systems (C-index: 0.894 for the nomogram, 0.700 for the 7th T stage and 0.742 for the 8th T stage). Likewise, the C-index of the nomogram for predicting RFS was higher than that of the 7th and 8th T stage systems (0.838 for the nomogram, 0.670 for the 7th T stage and 0.723 for the 8th T stage).
Conclusions
We developed nomograms that could predict individual accurate prognosis for invasive p-stage IA NSCLC patients after VATS lobectomy. Risk stratification by the nomograms might guide further adjuvant chemotherapy and follow-up.
Keywords: Nomogram, non-small cell lung cancer (NSCLC), video assisted thoracoscopic surgery (VATS), prognosis
Introduction
Lung cancer still causes the most cases of cancer-related death in the world (1). Computed tomography has increased the detection of early lung cancer. Surgical resection is the foundation of treatment in pathological stage (p-stage) IA non-small cell lung cancer (NSCLC). Despite the relative early stage, some patients still suffered from recurrence and some patients died. According to the statistics from lung cancer staging project of the International Association for the Study of Lung Cancer (IASLC), the 5-year overall survival (OS) rate of p-stage IA NSCLC was 73% (2). Nearly 10% patients suffered from recurrence (3). So, it is important to identify which subgroup patients are more likely to recur and which subgroup patients are more susceptible to suffer from death after curative surgery. Conventional tumor, node, metastasis (TNM) staging system is the most commonly used prognosis predicting tool for NSCLC, but it doesn’t incorporate some other clinical and pathological predictors, thus is powerless to deal with this problem. Nomograms have been demonstrated to be more accurate than the conventional TNM staging system since they are biologically and clinically integrated (4). As a result, they have been recommended as alternative or even surrogate prognostic devices.
Besides video assisted thoracoscopic surgery (VATS) lobectomy could reduce intra-operative blood loss, shorten chest drainage time and lower complication incidence compared to open thoracotomy (5), VATS lobectomy has been advocated as an appropriate procedure for early-stage NSCLC as it can even lower systemic recurrence rate and improve 5-year survival (6). It gets more popular than ever before and becomes the routine surgical approach for early-stage NSCLC in many institutes.
However, to our knowledge, nomograms predicting survival of p-stage IA NSCLC patients who underwent VATS lobectomy has not been established. The aim of our single-institute retrospective study was to develop nomograms to predict recurrence and death of p-stage IA NSCLC patients treated by VATS lobectomy.
Methods
Patients and data collection
A prospectively maintained database of consecutive patients who underwent VATS lobectomy for lung tumors at Peking University People’s Hospital between September 2006 and April 2014 was retrospectively analyzed. Patients with invasive p-stage IA NSCLC were included. And patients who received preoperational chemotherapy, radiotherapy or TKI treatment were excluded from the study. All patients were recommended to be followed up every 6 months after surgery for 2 years and every 12 months thereafter. Chest computed tomography, brain magnetic resonance imaging, abdominal ultrasound and bone scan were recommended at every follow-up to detect recurrence. Follow-up information was obtained through telephone calls every 6 months after surgery. As the study was a retrospective study and did not involve human subjects, approval for the study was exempted by the Institutional Review Board of Peking University People’s Hospital. The study was waivered the requirement of obtaining patient informed consent because the patients remained anonymous in the study. All aspects of the study conformed to the Declaration of Helsinki.
Data analysis and statistical methods
All statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc., NC, USA) and R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). The length of OS was measured from the date of surgery to the date of death from any cause or the time point of last follow-up. Recurrence free survival (RFS) was measured as the number of months from surgery to recurrence, death from any cause or the time point of last follow-up. OS and RFS were estimated using the Kaplan-Meier method, and differences in variables were determined with the log-rank test. Multivariate Cox proportional hazards regression was used to build models predicting OS and RFS. Cox models were refined using backward selection with the minimal Akaike information criterion (AIC) value to obtain a good model fit but avoid overfitting. Nomograms of 3-year and 5-year OS and RFS probabilities were developed based on the Cox regression models by using the “survival” and “rms” packages in R 3.2.4. The nomograms were subjected to 1,000 bootstrap resamples for internal validation. The performance of the two models was evaluated with discriminatory power by calculating Harrell’s concordance index (C-index). The value of C-index varies from 0.5–1.0. A higher C-index indicated a better ability to assign patients to an accurate risk stratification. Calibration assessed how close the nomogram estimated risk is to the observed risk. Calibration of the nomograms for 3-year OS and RFS was depicted by calibration plots. All statistical tests were two tailed with the alpha threshold of significance set at 0.05.
Results
A total of 422 patients with invasive p-stage IA NSCLC were analyzed including 224 female and 198 male patients. Patients’ demographics and characteristics of the cohort were presented in the Table 1. The median age of the patients was 62 years with an interquartile range (IQR) of 52–68 years. Median tumor size was 1.7 cm (IQR: 1.0–2.1 cm). Four hundred and seven patients received total video assessed thoracic surgery while 15 patients were converted to open thoracotomy surgery. An overwhelming majority (415, 98.3%) of the patients underwent lobectomy. Adenocarcinoma was the most common type of histologically diagnosis (367, 87.0%). The median follow-up time was 40 months.
Table 1. Demographics and characteristics of patient cohort.
| Patients demographics and characteristics (n=422) | Value |
|---|---|
| Gender, n (%) | |
| Male | 198 (46.9) |
| Female | 224 (53.1) |
| Age (years), median (IQR) | 62 [53–68] |
| Tumor diameter (cm), median (IQR) | 1.7 (1.0–2.1) |
| Charlson comorbidity index, n (%) | |
| 0 | 317 (75.1) |
| 1 | 66 (15.6) |
| ≥2 | 39 (9.2) |
| FEV1 (L), median (IQR) | 2.46 (1.97–2.89) |
| DLCO (%), median (IQR) | 86.0 (77.0–95.3) |
| Surgical approach, n (%) | |
| VATS | 407 (96.4) |
| VATS to open surgery | 15 (3.6) |
| Resection extent, n (%) | |
| Lobectomy | 415 (98.3) |
| Sleeve lobectomy | 3 (0.7) |
| Bilobectomy | 4 (0.9) |
| Histological diagnosis, n (%) | |
| Adenocarcinoma | 367 (87.0) |
| Squamous cell carcinoma | 44 (10.4) |
| Other | 16 (2.6) |
| Histological differentiation, n (%) | |
| Poor | 65 (15.4) |
| Moderate/well | 357 (84.6) |
| Lymphovascular invasion (LVI), n (%) | |
| Present | 13 (3.1) |
| Absent | 409 (96.9) |
| Surgical margin positive, n (%) | 1 (0.2) |
| No. of dissected lymph nodes stations, median (IQR) | 7 (6.0–7.0) |
| No. of harvested lymph nodes, median (IQR) | 15 (11.0–20.0) |
| 30-day mortality, n (%) | 1 (0.2) |
FEV1, forced expiratory volume in 1 second; IQR, interquartile range; DLCO, diffusing capacity of carbon monoxide; VATS, video assisted thoracoscopic surgery.
Independent prognostic factors
Cox regression models were built based on the minimal AIC value indicating a good model fit. Multivariate analysis was shown in the Table 2. Age, tumor diameter, lymphovascular invasion (LVI) and histological differentiation were all incorporated in both OS model and RFS model. All the factors except tumor diameter were independent prognostic factors to predict OS. Also, except age, tumor diameter, LVI and differentiation were independent predictors of RFS.
Table 2. Multivariate analysis of the predictors of OS and RFS in patients with p-stage IA NSCLC after VATS lobectomy.
| Variable | OS | RFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | 1.067 (1.006–1.131) | 0.030 | 1.034 (0.995–1.075) | 0.085 | |
| Tumor diameter (cm) | 2.083 (0.891–4.870) | 0.090 | 3.299 (1.814–6.001) | <0.001 | |
| LVI (present vs. absent) | 4.062 (1.278–12.912) | 0.018 | 3.260 (1.221–8.708) | 0.018 | |
| Histological differentiation (poor vs. moderate/well) | 5.747 (2.151–15.353) | <0.001 | 3.607 (1.776–7.327) | <0.001 | |
OS, overall survival; RFS, overall survival; p-stage, pathological stage; NSCLC, non-small cell lung cancer; VATS, video assisted thoracoscopic surgery; HR, hazards ratio; CI, confidential interval; LVI, lymphovascular invasion.
Nomograms
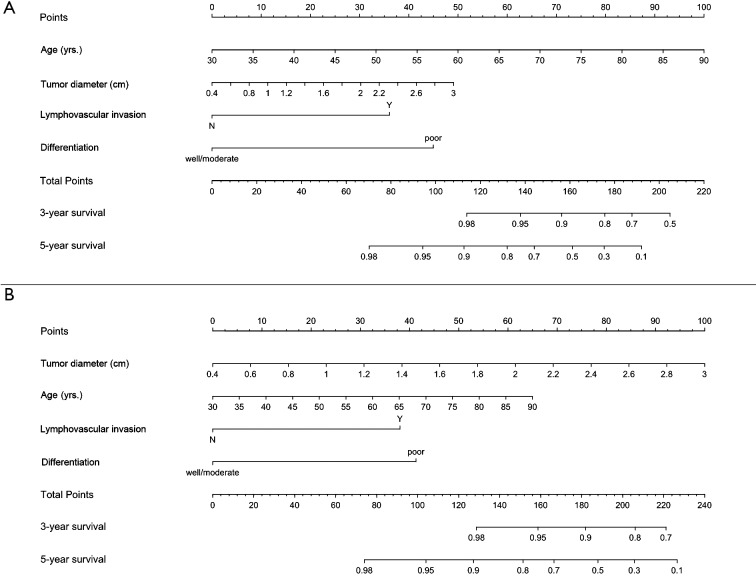
Based on the multivariate Cox regression models, nomograms that integrated all above factors were developed to calculate the 3-year and 5-year OS (Figure 1A) and RFS (Figure 1B) probability.
Figure 1.
Prognostic nomograms predicting overall survival (OS) (A) and recurrence free survival (RFS) (B) for patients with invasive pathological stage (p-stage) IA non-small cell lung cancer (NSCLC) after video assisted thoracoscopic surgery (VATS) lobectomy. Y, lymphovascular invasion (LVI) present; N, LVI absent.
Validation and calibration of the nomograms
In the bootstrap resampling cohort, the C-indexes for the nomograms to predict OS and RFS were 0.894 [95% confidential interval (CI): 0.745–1.042] and 0.838 (95% CI: 0.734–0.943) respectively. The discriminatory power of the nomograms and conventional T stage both by the AJCC 7th and 8th editions of TNM staging system was compared. The C-index for OS prediction by the 7th (0.700, 95% CI: 0.587–0.813) or the 8th (0.742, 95% CI: 0.605–0.879) T stage alone was lower than that of the OS nomogram. Similarly, the C-index for RFS prediction by the 7th (0.670, 95% CI: 0.591–0.749) or the 8th (0.723, 95% CI: 0.627–0.819) T stage alone was also lower than that of the developed RFS nomogram. It indicated that the two nomograms were more effective for predicting both OS and RFS of this patient population than conventional T stage.
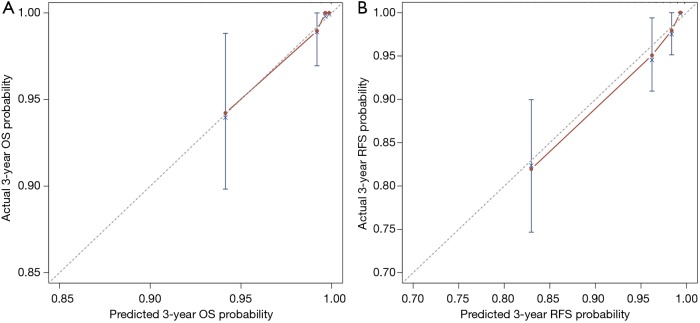
The calibration plot for the probability of 3-year OS indicated an excellent agreement between the predicted probability and the actual observation (Figure 2A). Likewise, the calibration plot for the probability of 3-year RFS also demonstrated a good consistence between the prediction and the observation (Figure 2B).
Figure 2.
The calibration curves for predicting overall survival (OS) (A) and recurrence free survival (RFS) (B) at the time point of 3 years in the patient cohort. Nomogram-predicted survival probability is plotted on the x-axis; actual survival probability is plotted on the y-axis. A curve along the 45-degree line indicates perfect calibration models.
Risk stratifications based on the nomograms
Also, to evaluate the performance of the two nomograms in stratifying risk of patients, the total points predicting OS and RFS were calculated based on the two Cox regression models by the two following formulas:
| (I) OS: total points = [0.06480 × (age, yrs.) +0.73388 × (tumor diameter, cm) + 1.40173 × LVI + 1.74869 × differentiation] × 25.72016461−57.55020576; |
| (II) RFS: total points = [0.03362 × (age, yrs.) + 1.19374 × (tumor diameter, cm) + 1.18179 × LVI + 1.28294 × differentiation] × 32.21935971−47.88106159. |
LVI was calculated as 1 (present) or 0 (absent). Differentiation was also calculated as 1 (poor) or 0 (well/moderate).
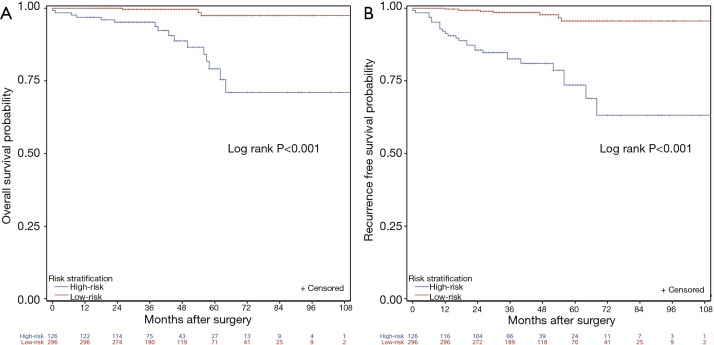
We stratified the cohort patients into high-risk subgroup and low-risk subgroup by determining the cutoff value at the highest 30% total point among the patients. For the OS model, the cutoff value was 96.3. And for the RFS model, it was 108.69. Survival curves of the high-risk subgroup and low-risk subgroup were illustrated in the Figure 3. Significant difference between Kaplan-Meier curves for both OS and RFS was demonstrated (both P<0.001). It also indicated that the nomograms functioned well in assigning patients into correct risk stratification.
Figure 3.
Risk stratifications according to the nomograms. Kaplan-Meier curves of overall survival (OS) (A) and recurrence free survival (RFS) (B) are plotted.
Discussion
Although patients with NSCLC of p-stage IA are always considered with good prognosis after radical resection surgery, cancer recurrence and death also occur to a considerable portion of the patients. Because of the heterogeneity of p-stage IA NSCLC, it is of great significance to predict which patient subgroup is relatively more likely to relapse and die. Several studies had developed prediction models or nomograms for early-stage NSCLC (7-10). Yang’s study developed nomogram to predict recurrence in stage I lung adenocarcinoma patients (7) and Zhang’s study established nomogram to predict recurrence in stage IA NSCLC patients (9). Our study had some unique features. First, only the patients underwent VATS lobectomy with p-stage IA were included in the study, which could diminish the bias caused by the choices of surgical approaches and adjuvant therapies. Second, despite its wide use, nomogram had not been used for predicting OS for the cohort of p-stage IA NSCLC patients to our knowledge. We provided both OS and RFS nomograms to predict accurate probability of death and recurrence in this cohort. Third, in the TNM staging system, tumor size was a vitally important predictor for survival and thus the most important factor in the T stage descriptors (2,11,12). In the forthcoming 8th IASLC TNM staging system, stage T1 was proposed to be divided into more categories according to tumor size (13). However, the data of tumor size was all categorized. In our study, the tumor size was integrated into the models as a continuous variable which may predict more precise prognosis than both 7th and 8th T staging systems and it might result a relative higher C-index and stronger discriminatory power than previous studies. Finally, Cox models of our study were developed based on minimal AIC value which assured good model fits and avoiding overfitting. As a whole, we developed visualization and practical tools, which could predict the probabilities of death and recurrence in individual p-stage IA NSCLC patient after VATS lobectomy. They would be helpful for the individual treatment or follow-up strategy. Patients with high recurrence probability were more likely to need further treatment and more frequent follow-up.
Previous studies had demonstrated that tumor size lager than 2 cm, LVI and poor differentiation was associated with a poor prognosis in patients with p-stage IA NSCLC after complete resection which was consistent with our study (14-16). Even in some studies, patients with LVI were proposed to be upstaged (17,18). But it is still controversial whether chemotherapy should be administered to patients of p-stage IA NSCLC with tumor size larger than 2 cm, poor differentiation or LVI after radical resection.
A pooled analysis by the LACE collaborative group indicated that postoperative cisplatin-based chemotherapy did not demonstrate a survival benefit in patients of p-stage IA (19). Base on the evidence, the guidelines of National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) did not recommend adjuvant chemotherapy in p-stage IA NSCLC regardless of other clinical and pathological features (20,21). But in a meta-analysis of six randomized controlled trials in Japan, postoperative Tegafur-Uracil (UFT) chemotherapy significant improved survival in p-stage IA patients with tumor size more than 2 cm compared to surgery alone (22). Since all the trials were conducted in Japan, postoperative UFT chemotherapy was generally recommended to T1b subgroup of p-stage IA patients in Japan but not in other parts of the world. There was no prospective randomized controlled trial dealing with adjuvant chemotherapy in patients of p-stage IA with poor differentiation or LVI as far as we were concerned. A retrospective study found that adjuvant chemotherapy was effective in patients with invasive component larger than 2 cm or LVI (23). Other two retrospective studies indicated that adjuvant chemotherapy might be beneficial to p-stage IA NSCLC patients with vessel invasion (24) and poor differentiation (25) separately. As stated above, it did not come to an agreement on which patient subgroup harbored a high risk of recurrence and might benefit from adjuvant therapy. Selecting candidates for chemotherapy based on conventional single risk factor might be inefficient since it did not take into account all the factors and the varying weight of each factor. But when applying the developed RFS nomogram by the study, we could calculate an accurate recurrence probability for every individual patient. The patients with a high recurrence risk and a relatively younger age might benefit from adjuvant therapy.
The study has limitations and bias that should be noticed. As a retrospective single institutional study, selection bias and recall bias might be evitable. Besides, since not only adenocarcinoma was involved, the IASLC/ATS/ERS classification of lung adenocarcinoma and radiologic solid components were not included in the analyses. Also, as the developed nomograms were not externally validated among databases of other institutes, its generally application might be questionable. It called for further studies to validate the nomograms in databases of other institutes.
Conclusions
The nomograms developed in this study predicted more precise prognosis of patients with invasive p-stage IA NSCLC after VATS lobectomy than conventional T stage. Patients with high recurrence or death risk stratified by the nomograms displayed significant poorer prognosis than the low recurrence or death risk subgroups. Risk stratification by the nomograms might guide further adjuvant chemotherapy and follow-up. Additional studies are required to externally validate the nomograms.
Acknowledgements
Funding: The study was funded by Beijing Municipal Science & Technology Commission (D141100000214004).
Ethical Statement: As the study was a retrospective study and did not involve human subjects, approval for the study was exempted by the Institutional Review Board of Peking University People’s Hospital. The study was waivered the requirement of obtaining patient informed consent because the patients remained anonymous in the study.
Disclaimer: The sponsor did not involve in the study design, data acquisition, statistical analysis, and manuscript writing.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 3.Maeda R, Yoshida J, Ishii G, et al. Long-term outcome and late recurrence in patients with completely resected stage IA non-small cell lung cancer. J Thorac Oncol 2010;5:1246-50. 10.1097/JTO.0b013e3181e2f247 [DOI] [PubMed] [Google Scholar]
- 4.Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. 10.1016/j.ejso.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 6.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. 10.1200/JCO.2008.18.2733 [DOI] [PubMed] [Google Scholar]
- 7.Yang HC, Kim HR, Jheon S, et al. Recurrence Risk-Scoring Model for Stage I Adenocarcinoma of the Lung. Ann Surg Oncol 2015;22:4089-97. 10.1245/s10434-015-4411-9 [DOI] [PubMed] [Google Scholar]
- 8.Melloni G, Gajate AMS, Sestini S, et al. New positron emission tomography derived parameters as predictive factors for recurrence in resected stage I non-small cell lung cancer. Eur J Surg Oncol 2013;39:1254-61. 10.1016/j.ejso.2013.07.092 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Sun Y, Xiang J, et al. A clinicopathologic prediction model for postoperative recurrence in stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1193-9. 10.1016/j.jtcvs.2014.02.064 [DOI] [PubMed] [Google Scholar]
- 10.Ou S-HI, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007;110:1532-41. 10.1002/cncr.22938 [DOI] [PubMed] [Google Scholar]
- 11.Gajra A, Newman N, Gamble GP, et al. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer 2003;42:51-7. 10.1016/S0169-5002(03)00285-X [DOI] [PubMed] [Google Scholar]
- 12.Birim O, Kappetein AP, Takkenberg JJ, et al. Survival after pathological stage IA nonsmall cell lung cancer: tumor size matters. Ann Thorac Surg 2005;79:1137-41. 10.1016/j.athoracsur.2004.09.051 [DOI] [PubMed] [Google Scholar]
- 13.Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003. [DOI] [PubMed] [Google Scholar]
- 14.Port JL, Kent MS, Korst RJ, et al. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest 2003;124:1828-33. 10.1378/chest.124.5.1828 [DOI] [PubMed] [Google Scholar]
- 15.Shimada Y, Saji H, Yoshida K, et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 2012;7:1263-70. 10.1097/JTO.0b013e31825cca6e [DOI] [PubMed] [Google Scholar]
- 16.Funai K, Sugimura H, Morita T, et al. Lymphatic vessel invasion is a significant prognostic indicator in stage IA lung adenocarcinoma. Ann Surg Oncol 2011;18:2968-72. 10.1245/s10434-011-1729-9 [DOI] [PubMed] [Google Scholar]
- 17.Kudo Y, Saji H, Shimada Y, et al. Proposal on incorporating blood vessel invasion into the T classification parts as a practical staging system for stage I non-small cell lung cancer. Lung Cancer 2013;81:187-93. 10.1016/j.lungcan.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya T, Hashizume S, Akamine S, et al. Upstaging by Vessel Invasion Improves the Pathology Staging System of Non-Small Cell Lung Cancer. Chest 2007;132:170-7. 10.1378/chest.06-1950 [DOI] [PubMed] [Google Scholar]
- 19.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer (Version 5, 2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [DOI] [PubMed]
- 21.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. 10.1093/annonc/mdt241 [DOI] [PubMed] [Google Scholar]
- 22.Hamada C, Tsuboi M, Ohta M, et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 2009;4:1511-6. 10.1097/JTO.0b013e3181bbf1f2 [DOI] [PubMed] [Google Scholar]
- 23.Tsutani Y, Miyata Y, Kushitani K, et al. Propensity score-matched analysis of adjuvant chemotherapy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1179-85. 10.1016/j.jtcvs.2014.05.084 [DOI] [PubMed] [Google Scholar]
- 24.Tsuchiya T, Akamine S, Muraoka M, et al. Stage IA non-small cell lung cancer: vessel invasion is a poor prognostic factor and a new target of adjuvant chemotherapy. Lung Cancer 2007;56:341-8. 10.1016/j.lungcan.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 25.Liu CH, Peng YJ, Wang HH, et al. Heterogeneous prognosis and adjuvant chemotherapy in pathological stage I non-small cell lung cancer patients. Thorac Cancer 2015;6:620-8. 10.1111/1759-7714.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]