Abstract
Asthma and chronic obstructive pulmonary disease (COPD) are common chronic respiratory diseases. Both diseases have incompletely distinct pathophysiology, clinical manifestation, and treatment responsiveness. Pulmonary and systemic inflammations are the hallmarks of COPD. Most asthma responds to inhaled corticosteroid (ICS) treatment. In contrast, COPD is a corticosteroid-resistant disease. Bronchodilators are a preferred treatment method of COPD, with the aim of improving symptoms and preventing exacerbation. In addition, corticosteroid insensitivity is an underlying mechanism in severe asthma. An overlap of features between asthma and COPD, which was described as asthma-COPD overlap syndrome (ACOS) is not uncommon in practice. Novel nonsteroidal therapies focusing on inflammation in asthma and COPD have been developed. Selective phosphodiesterase 4 (PDE4) inhibitor is a promising class of drugs that has been studied for the treatment of COPD. Selective PDE4 inhibitor is different from xanthine in terms of mechanisms and pharmacokinetic profiles. This review focuses on clinical data on PDE4 inhibitors and its future roles in asthma, COPD, bronchiectasis, ACOS and other chronic non-pulmonary diseases.
Keywords: Roflumilast, selective phosphodiesterase 4 (PDE4) inhibitor, airway diseases
Cyclic adenosine monophosphate (cAMP)
cAMP is a second messenger which influences homeostasis and cellular functions, for instance, structural and inflammatory cells. cAMP exerts its cellular functions via protein kinase A (PKA) activation, through a non-PKA pathway, or by guanosine triphosphate (GTP) exchange protein activated by cyclic AMP (EPAC) (1,2). cAMP is converted from adenosine trisphosphate (ATP) by adenylyl cyclase (AC). However, it is unstable and rapidly hydrolyzed by phosphodiesterase enzymes (PDE). Thus, either AC activation or PDE inhibition resulting in increased cellular cAMP and physiological changes such as enhanced smooth muscle relaxation and suppressed inflammation (3,4). Mammalian PDE was classified into PDE1–11 which is based on kinetics, substrate selectivity, and cellular or tissue distribution. The most important PDE isoforms in respiratory diseases are phosphodiesterase 4 (PDE4) and PDE5. PDE4 is further divided into PDE4A, PDE4B, PDE4C and PDE4D which depend on gene encoding. PDE4B inhibition is associated with bronchodilator and anti-inflammatory effects, while PDE4D inhibition is associated with emesis due to its prominence in the brain cells (5,6).
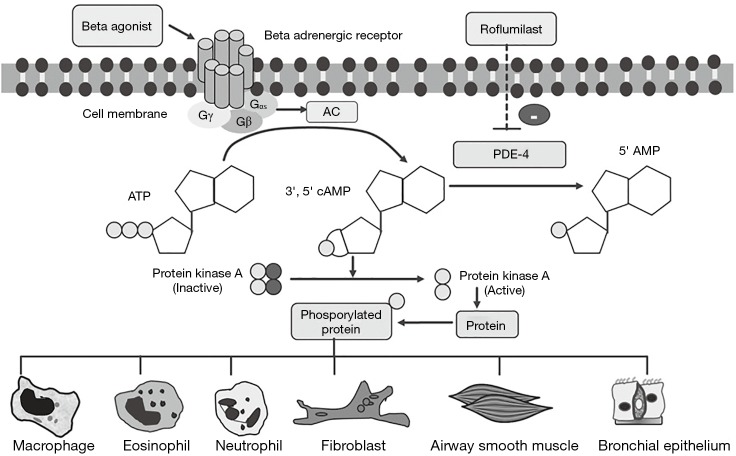
Mechanisms of roflumilast in airway disease treatment
Rolipram, the first PDE4 inhibitor, was developed for the treatment of psychiatric diseases. However, rolipram has severe side effects due to its predominant PDE4D inhibition. Hence, the more selective PDE4 inhibitors, for instance, cilomilast (GlaxoSmithKline), roflumilast (Altana and Nycomed) and piclamilast (Novartis) were developed. Meanwhile, only roflumilast is an agent that has been extensively studied and was approved for treating chronic obstructive pulmonary disease (COPD) (7). The binding affinity (Km) of various selective PDE4 inhibitors to PDE4B and PDE4D contributes to the clinical efficacy as well as unwanted side effects. According to in vitro studies, roflumilast has a significantly higher PDE4B affinity (low Km) than the prototypic drugs rolipram and cilomilast. Thus, the effective dose inhibitory concentration (IC50) of roflumilast is lower than that of other PDE4 inhibitors (7,8) (Figure 1).
Figure 1.
Molecular mechanism of roflumilast for treating obstructive airway diseases. AC, adenylyl cyclase; ATP, adenosine trisphosphate; cAMP, cyclic adenosine monophosphate; PDE4, phosphodiesterase 4.
Effects of roflumilast and other PDE4 inhibitors on inflammatory cells
PDE4 inhibitors were demonstrated to inhibit inflammatory cytokine and mediator release from inflammatory cells. In addition, it inhibits neutrophil chemotaxis or migratory activity. Lastly, PDE4 inhibitor promotes apoptosis of these cells (8,9).
Macrophages
Macrophages are key switch of airway inflammation in COPD. Roflumilast and roflumilast N-oxide reduced LPS-induced release of chemokines (CCL2, CCL3, CCL4 and CXCL10) and tumor necrosis factor alpha (TNF-α) from human lung macrophages in dose-dependent fashion (10). The decrease in chemokines and pro-inflammatory cytokine production from macrophages and monocytes plays role in pathogenesis of COPD (11).
Eosinophils and mast cells
Theophylline and PDE4 inhibitor (rolipram) suppressed platelet activating factor (PAF) and C5a-stimulated eosinophils decreasing leukotriene C4 (LTC4) synthesis and chemotaxis (12). In addition, rolipram caused concentration-dependent inhibition of zymosan-stimulated superoxide anion generation by human eosinophils (13). In addition to the inhibition of LTC4 production from eosinophils, rolipram inhibited the IgE-dependent generation of IL-4, IL-13 and histamine from basophils (14). Roflumilast inhibited IgE-mediated mast cell degranulation has been shown from in vitro study. Rolipram inhibited pulmonary eosinophilia in ovalbumin (OVA)-challenged mice (15). Roflumilast also inhibited early and late allergic response and airway hyper-responsiveness in fungal sensitized mice (16).
Neutrophils
Since neutrophilic inflammation is key pathogenesis of corticosteroid resistance asthma and COPD. In vitro study has shown that roflumilast and roflumilast N-oxide inhibit neutrophil release of interleukin 8 (CXCL8), leukotriene B4 (LTB4), matrix metalloproteinase-9 (MMP-9) and neutrophil elastase (NE) in dose dependent fashion (8). They inhibit neutrophil degranulation by selective PDE4 effects not shared by PDE3 inhibitors or theophylline (17). In addition, roflumilast N-oxide concentration-dependently suppressed neutrophil adhesion to endothelial cells and CD11b expression on bacterial peptide stimulated neutrophils. Hence, it potentially inhibits neutrophil migratory activity (18).
Effects roflumilast and other PDE4 inhibitors on airway structural cells
Structural cells of the lungs such as airway smooth muscle (ASM) cells, fibroblast and epithelial cells play roles in pathogenesis of asthma and COPD. PDE4 inhibitors inhibiting morphological and functional changes of these cells will be discussed.
ASM cells
ASM cells exert their contractile function resulting in airway obstruction in asthma and COPD. In vitro study has shown that roflumilast N-oxide in combination with formoterol significantly enhanced the effect of dexamethasone in ASM (19). It potentiated formoterol-induced expression of mitogen-activated protein kinase phosphatase 1 (MKP-1). In addition, other selective PDE4 inhibitors (cilomilast, piclamilast, rolipram) have been shown to increase cAMP and MKP-1 mRNA expression in respond to formoterol (20).
Fibroblasts
Since fibroblast proliferation contributes to small airway fibrosis in COPD (11). Roflumilast antagonizing profibrotic activity of fibroblasts stimulated by tumor growth factor β (TGF-β) has been shown from in vitro study (21). Rolipram and cilomilast attenuating fibroblast chemotaxis has been shown in in experimental study (22). Roflumilast mitigates bleomycin-induced lung fibrotic remodeling in rodents (23). Cilomilast lowered pulmonary fibrosis which are pathologically quantified examination of bleomycin instilled mice (24). In vitro and animal experimental results suggest that PDE4 inhibitors may be able to inhibit fibroblast activity and, thus, have the potential to prevent of progressive fibrosis of airways and lung parenchyma (22).
Bronchial epithelial cells
PDE4 inhibitors suppress TNF-α release from airway epithelial cells hence they exert anti-inflammatory effect (25). PDE4 inhibitor also suppresses TNF-α release from airway epithelial cells. Bronchial biopsy study has shown neutrophil infiltration in the bronchial submucosa and glands of chronic bronchitis compared to normal subjects (26). Furthermore, co-localization of immunoreactivity of MUC5AC and EGFR has been detected in human goblet cells (27). PDE4 inhibitors may reduce mucus hyper-secretion in COPD. Rolipram, cilomilast and roflumilast decreased MUC5AC expression induced by EGF in human airway epithelial cells (28). Roflumilast has been shown to improve ciliary function in the bronchial epithelium (29). The activation of cystic fibrosis transmembrane receptors (CFTR) via elevating cAMP level in airway epithelial cells could promote epithelial rehydration thereby facilitating mucous clearance (30). These effects may contribute to clinical efficacy PDE4 inhibitor in chronic bronchitis.
Synergistic effect of roflumilast and other anti-inflammatory agents
Despite the observed anti-inflammatory properties of roflumilast, the additive effect of other agents such as corticosteroids or long-acting β2-agonists is open to question. A synergistic effect between roflumilast and fluticasone propionate has been demonstrated in peripheral blood mononuclear cells (PBMCs). Roflumilast and roflumilast N-oxide were found to increase glucocorticoid receptor activity and glucocorticoid-dependent gene transcription in PBMCs compared with a control (31). Addition of formoterol to roflumilast provided superior in vitro anti-inflammatory activity. Formoterol significantly increased the inhibitory effect of roflumilast on the LPS induced release of the cytokines from human lung tissue (32). Adding roflumilast to indacaterol has shown an enhanced anti-fibrotic effect by inhibiting mediator release from fibroblasts and myofibroblasts. Activation of PKA is the mechanism underlying the additive effect between PDE4 inhibitor and long-acting beta-adrenoceptor agonists (LABAs) (33,34). However, the translation from a scientific basis to a clinical meaning is yet to be proven.
Clinical evidence of roflumilast in asthma
Inhaled corticosteroid (ICS) is an effective treatment for persistent asthma and is reserved as a first-line therapy according to most guidelines. However, clinical studies of roflumilast on asthma have been limited (35). Roflumilast inhibiting allergen-induced airway inflammation was shown by the attenuating allergen-induced sputum eosinophilia in mild asthma (36). Roflumilast was found to attenuate allergen-induced early- and late-phase asthmatic reactions in early clinical study (37). A comparison of the efficacy of roflumilast versus a placebo was conducted in a 12-week clinical study (Table 1). Improved morning peak expiratory flow rate (PEFR) in a dose-dependent fashion was noted. Higher doses (250 and 500 µg) were superior to a lower dose (100 µg) (41). Moreover, other studies have shown comparable clinical efficacy of 500 µg roflumilast and 400 µg inhaled beclomethasone dipropionate on forced expiratory volume in 1 second (FEV1) (40,43). Proof-of-concept studies were conducted in asthma patients by comparing roflumilast to placebo or ICS. Improved lung function was noted in roflumilast treated patients. Adding roflumilast to ICS in asthma provided additional FEV1 improvement from baseline to 24 weeks (44). Despite clinical studies of roflumilast and other PDE4 inhibitors in asthma have not been undertaken due to potentially severe adverse effects and the availability of effective alternative therapies, particularly ICSs. Recently, crossover study was conducted by addition roflumilast and montelukast versus montelukast alone in moderate-to-severe asthma whose symptoms are uncontrolled by ICS/LABA. Clinical meaningful change of FEV1 and patient report outcome were noted in roflumilast arm versus placebo (42). Roflumilast has been investigated in asthma, and has extensively been shown to reduce COPD exacerbation; for this reason, the role of this agent in treating inadequately controlled asthma with frequent exacerbation appears promising and needs further clinical evaluation.
Table 1. Major clinical trials of roflumilast in asthma.
| Investigators | Year | Duration (week) | Primary objective | Treatment arms |
|---|---|---|---|---|
| van Schalkwyk E et al. (37) | 2005 | 1 | Improved LAR (dose related) | RF 250 µg OD & RF 500 µg OD vs. placebo in mild-moderate asthma |
| Timmer W et al. (38) | 2002 | 4 | Prevention of exercise induced fall of FEV1 | RF 500 µg OD vs. placebo |
| Aubier M et al. (39) | 2004 | 12 | ↑ PEFR (speed of action) | RF 500 µg OD vs. placebo |
| Bousquet J et al. (40) | 2006 | 12 | ↑ FEV1, ↑ FEEFR and improve asthma control | RF 500 µg OD vs. BDP 200 µg BID |
| Leichtl S et al. (41) | 2002 | 12 | ↑ FEV1, alg OD vs. BDP 200 µg BIDfall of FEV mild-moderate control in mild-moderate asthma | RF 100 µg OD, RF 250 µg OD, RF 500 µg OD |
| Bateman ED et al. (42) | 2016 | 4 | ↑ FEV1, improve ACQ-7 in uncontrolled asthma by ICS/LABA | ML 10 mg plus RF 500 µg OD vs. ML 10 mg plus placebo (crossover) |
| Albrecht A et al. (43) | 2002 | 12 | ↑ FEV1, ↑ FEVEFR & ↓ rescued SABA | RF 500 µg OD vs. BDP 200 µg BID |
↑, increase; ↓, decrease. RF, roflumilast; BDP, beclomethasone dipropionate; ML, montelukast; OD, once-daily dose; BID, twice-daily; LAR, late asthmatic response; FEV1, forced expiratory volume at 1 second; PEFR, peak expiratory flow rate; ACQ-7, asthma control questionnaire-7; SABA, short-acting beta agonist; ICS-LABA, combined inhaled corticosteroid and long-acting beta-agonist.
Clinical evidence of the efficacy of roflumilast in COPD
The selective PDE4 inhibitors rolipram, cilomilast and roflumilast have been investigated for their inhibitory effects on neutrophilic airway inflammation. An experimental study found that PDE4 inhibitors inhibited MMP-9 and NE release from neutrophils in a concentration-dependent manner (17). However, cilomilast failed to decrease sputum neutrophilia as a marker of pulmonary inflammation in COPD patients, compared with a placebo-controlled clinical study (45). Another placebo-controlled study showed roflumilast decreased sputum cell counts, both eosinophils and neutrophils. In addition to its effect on inflammatory cells, roflumilast reduced neutrophilic and eosinophilic inflammatory mediators in sputum of COPD patients (46). The difference between the efficacy of cilomilast and roflumilast is associated with the different IC50; roflumilast is more potent than cilomilast for PDE4B inhibition in inflammatory cells (35).
The aims of COPD treatments comprise symptom improvement and future risk reduction. COPD exacerbation reduction was noted in clinical studies of long-acting bronchodilators [LABA and/or long-acting muscarinic antagonist (LAMA)] or combined inhaled corticosteroid and long-acting beta-agonist (ICS-LABA) (47). However, ICS-LABA was found to be significantly associated with pneumonia in COPD (48). PDE4 inhibitors, as a nonsteroidal treatment, were tested for their efficacy in clinical studies of COPD patients. Different doses of cilomilast (5, 10 and 15 mg) were investigated. There was significant improvement in lung function, i.e., FEV1 and PEFR, in a concentration-dependent manner. However, the effect on quality of life, as measured by St. George’s Respiratory Disease Questionnaire (SGRQ), was demonstrated at a high dose (49). Effects on PEFR were also observed in short-term studies on COPD treated with roflumilast (250 and 500 µg) compared with a placebo (50). A clinical study of roflumilast in COPD patients was conducted over a 24-week period. There was significant improvement of both pre- and post-bronchodilator FEV1 and quality of life, as measured by SGRQ score, in roflumilast-treated COPD patients compared with a placebo (51).
The long-term effects of oral 500 µg roflumilast on COPD were investigated in 52-week placebo-controlled trials (M2-124 and M2-125 trials). The studies aimed to evaluate the effects on lung function and exacerbation (52). Significant improvement of pre- and post-bronchodilator FEV1 in comparison with a placebo was noted. In addition, maximizing the benefit of a bronchodilator was noted when roflumilast was added to the bronchodilator—either an inhaled long-acting muscarinic antagonist (tiotropium) or an inhaled long-acting β2-agonist (salmeterol) (53). However, the effect on increasing FEV1 by adding roflumilast to a long-acting bronchodilator did not reach a minimal clinically important difference (MCID) in these studies. Moderate to severe COPD exacerbation and exacerbation requiring corticosteroid were significantly reduced in roflumilast-treated COPD patients. The maximal benefit of roflumilast for reducing exacerbation was noted in ICS-treated COPD patients and in those with chronic bronchitis, compared with those without ICS treatment and those without chronic bronchitis (54). Findings from subgroup analysis confirm the benefit of roflumilast in personalized medicine for specific COPD phenotypes. For these reasons, roflumilast was approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for treatment of severe COPD and those suffering from frequent exacerbation, in particular patients with chronic bronchitis phenotype (55,56).
Despite maximizing COPD therapy by using either ICS-LABA with a long-acting muscarinic antagonist (LAMA) or triple therapies, substantial numbers of COPD patients suffer from exacerbations. That is why roflumilast was investigated as an add-on treatment in COPD patents with inadequately controlled disease (57). Data from a series of 52-week-placebo trials of combined treatment with roflumilast and ICS-LABA or roflumilast and ICS-LABA-LAMA in severe COPD patients who experienced chronic bronchitis and frequent exacerbation were recently published. Adding roflumilast to ICS-LABA or ICS-LABA-LAMA resulted in a significant reduction of moderate to severe COPD exacerbation (58,59). In addition, the effect of roflumilast was more pronounced with increasing severity of exacerbation. Adding roflumilast to ICS-LABA or ICS-LABA-LAMA has also shown a reduction of severe COPD exacerbation in patients who experienced frequent exacerbation (>3 times/year) or hospitalized exacerbation in the past year (59) (Table 2).
Table 2. Major clinical trials of roflumilast in COPD.
| Clinical trials and investigators | Year | Duration (weeks) | Primary objective | Treatment arms |
|---|---|---|---|---|
| RECORD (M2-127): Rabe et al. (51) | 2005 | 24 | Post-BD FEV1, SGRQ | RF 500 µg OD, RF 250 µg OD vs. placebo |
| RATIO (M2-112): Calverley et al. (53) | 2007 | 52 | Post-BD FEV1, AECOPD | RF 500 µg OD vs. placebo |
| OPUS (M2-111): Calverley et al. (53) | 2008 | 52 | Post-BD FEV1, AECOPD | RF 500 µg OD vs. placebo |
| AURA (M2-124), HERMES (M2-125): Calverley et al. (52) | 2009 | 52 | Pre-BD FEV1, AECOPD | RF 500 µg OD vs. placebo |
| EOS (M2-127): Fabbri et al. (60) | 2009 | 24 | Pre-BD FEV1 | RF 500 µg OD vs. placebo |
| Helios (M2-128): Fabbri et al. (60) | 2009 | 24 | Pre-BD FEV1 | RF 500 µg OD vs. placebo |
| ACROSS: Zheng et al. (61) | 2014 | 24 | Pre-BD and post-BD FEV1 | RF 500 µg OD vs. placebo (ICS-LABA allowed) |
| REACT: Calverley et al. (57), Martinez et al. (58) |
2012 2015 |
52 | Rate of moderate to severe AECOPD | RF 500 µg OD vs. placebo (COPD were pre-treated with ICS-LABA with or without LAMA for 12 months) |
| RE [2] SPOND: Martinez et al. (59) | 2016 | 52 | Rate of moderate to severe AECOPD | RF 500 µg OD vs. placebo (COPD were pre-treated with ICS-LABA with or without LAMA for 3 months) |
RF, roflumilast; OD, once-daily dose; FEV1, forced expiratory volume at 1 second; BD, bronchodilator; AECOPD, rate of COPD exacerbation; SGRQ, St. George’s Respiratory Questionnaire; ICS-LABA, combined inhaled corticosteroid and long-acting beta-agonist; LAMA, long acting antimuscarinic antagonist.
Clinical evidence of the efficacy of roflumilast in other airway diseases
Bronchiectasis is a chronic respiratory disease characterized by abnormal dilatation of the bronchi, chronic productive cough and recurrent infective exacerbations. The effective treatments of disease are in most urgent need of evidence-based support (62). Both pharmacological and non-pharmacological interventions have been investigated and shown promising results (63). The PDE4 inhibitor, roflumilast, was evaluated in short term clinical trial of non-cystic fibrosis bronchiectasis. It has been shown symptomatic improvement from baseline (64). Since the bronchiectasis prevalence varies from 4% to 58% in COPD (65,66). In addition, bronchiectasis and COPD overlap syndrome (BCOS), is associated with poor outcomes (67). Despite that chronic cough and sputum production are characteristics of bronchiectasis, they have been suggested as a risk factor for exacerbations of COPD (68,69). Roflumilast has been shown benefit for inhibiting neutrophilic airway inflammation in COPD and preventing exacerbation in COPD with chronic bronchitis (46,54). In addition, neutrophils are prominent cell type involved in bronchiectasis and neutrophilic inflammation is key pathogenesis of COPD (70-72). The potential benefit of roflumilast for treating COPD with bronchiectasis has to be further investigated.
Asthma-COPD overlap syndrome (ACOS) is generally observed in 15–20% of patients in airway disease clinics, and they experience more frequent exacerbation and account for greater health care cost utilization than those with pure COPD (73,74). Treatment of ACOS currently consists of ICS/LABA with and without LAMA. Prospective studies to investigate the benefits of roflumilast and other PDE4 inhibitors for treating severe asthma and ACOS patients need to be conducted (75).
Comparing selective PDE4 inhibitors with xanthine derivatives
Roflumilast and other selective PDE4 inhibitors are different from xanthine derivatives, e.g., nonselective PDE inhibitors such as theophylline and doxofylline, in terms of chemical structure and pharmacological effects (6,7). Roflumilast and sustained release theophylline have been positioned for being the COPD pharmacological treatments (47,76). Roflumilast is recommended for being added on therapy to ICS/LABA or LAMA in COPD with frequent exacerbations. However, the roles of theophylline for CODP are limited due to relatively narrow therapeutic window and the availability of the effective inhaled bronchodilators (76-78). Despite that theophylline enhancing histone deacetylase (HDAC) activity and improving corticosteroid sensitivity were recognized (79). Oral low dose theophylline on top of ICS/LABA failed to reduce COPD exacerbation (80). Co-administration of roflumilast and xanthine derivatives is not recommended, as xanthine increases the level of roflumilast in the blood (77,81). In addition, the clinical study showing the benefit of adding roflumilast to xanthine derivative in COPD has never been conducted. Apart from its efficacy, roflumilast was generally well tolerated, according to pooled data from clinical studies (Table 2). The most commonly observed adverse side effects were diarrhea, weight loss and nausea, in approximately 9.5%, 7.5% and 4.7% of patients, respectively, which were significantly higher compared with a placebo (82,83). The potential mechanisms of gastrointestinal side effects involve both central and peripheral actions. One way is by inhibiting the PDE4D isoform binding site in the brain, causing nausea. Another way is that roflumilast stimulates cystic fibrosis transmembrane conductance regulator (CFRT)-dependent chloride secretion, causing diarrhea (30). However, these side effects typically resolve within 4 weeks after initiating treatment (3). Weight loss is a major concern in COPD, and lower body mass index (BMI) is associated with poor prognosis. Nevertheless, the average weight loss in subjects treated with roflumilast was found to be approximately 2.09 kg. Greater weight loss was more common in obese patients than in those underweight; reduced fat mass (lipolysis) contributed to the weight loss (3,52).
Future direction of PDE4 inhibitors
COPD is a corticosteroid-insensitive disease due to decreased HDAC activity as a result of tobacco smoking or oxidative stress (84). Low-dose theophylline increases corticosteroid sensitivity, as its molecular mechanism enhances HDAC activity in PBMCs in cases of severe asthma and COPD (79,85). However, the effects of roflumilast on HDAC activation and reversal corticosteroid resistance in COPD require further investigation (78,86). Despite that roflumilast was approved for COPD, its potential beyond inflammatory lung diseases such as bronchiectasis, pulmonary fibrosis and ACOS is promising. In addition, the benefits of anti-inflammatory effect of roflumilast in the preclinical studies and the adverse effects of long-term use of inhaled corticosteroid in COPD were addressed. Hence, corticosteroid sparing effects of roflumilast in the treatment of COPD need to be investigated. The effect of roflumilast on metabolic derangement in newly diagnosed diabetes mellitus type 2 was examined by the reduction in glycosylated hemoglobin (HbA1C) from baseline and the reduced fat mass in obese COPD patients (52,87). Since metabolic syndrome is commonly observed in COPD, likely due to inflammatory cytokine spillover, this drug may have the benefits on COPD and comorbidities since it is an anti-inflammatory agent rather than a bronchodilator (88). Roflumilast has also been shown to improve FEV1 in severe COPD but the magnitude does not reach MCID (52,60). In addition, roflumilast does not affect pulmonary hyperinflation and exercise tolerance in COPD (89). Roflumilast treated COPD patients experiencing small improvements in dyspnea with accompanying improvements in lung function were shown in a pooled analysis (90,91). Furthermore, the benefits of oral agents for targeting small airways in COPD are interesting when comparing roflumilast with ICS-LABA or LAMA. However, the gastrointestinal side effects of roflumilast limit its use in elderly patients with COPD. An inhaled selective PDE4 inhibitor was recently developed; however, this inhaled formulation (UK-500) failed to improve FEV1 or to decrease the breathlessness score in COPD patients (92). The development of the inhaled formulation of PDE4 inhibitors aim of minimizing side effects is critical for improving patient acceptability and tolerability.
Apart from airway diseases, apremilast a novel selective PDE4 inhibitor shows promising activity in chronic rheumatologic and dermatologic diseases. The preclinical and clinical studies conducted in psoriasis and psoriatic arthritis. They have shown equivalent clinical efficacy of apremilast to methotrexate but less than that of tumor necrosis factor inhibitor (93,94). PDE inhibitors enhance cAMP and cGMP signaling by preventing the degradation of these nucleotides. In addition, cAMP and cGMP are essential in neuroplasticity and neuroprotection (95). Since cAMP response element-binding (CREB) may be modulator of processes required for memory formation (96). Rolipram was the first compound found to effectively restore cognitive defect in Alzheimer’s disease animal models (97). Furthermore, roflumilast and rolipram ameliorate hypertension-induced impairment of learning and memory functions in rats (98). Meanwhile, the therapeutic potential of these inhibitors will be evaluated in the near future. Nevertheless, the applications of PDE inhibitors for memory dysfunction and neurological diseases have to be clinically proven and intensively investigated (99).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci 1999;36:275-328. 10.1080/10408369991239213 [DOI] [PubMed] [Google Scholar]
- 2.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 2010;159:265-84. 10.1111/j.1476-5381.2009.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011;163:53-67. 10.1111/j.1476-5381.2011.01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EN, Druey KM. Heterotrimeric G protein signaling: role in asthma and allergic inflammation. J Allergy Clin Immunol 2002;109:592-602. 10.1067/mai.2002.122636 [DOI] [PubMed] [Google Scholar]
- 5.Halpin DM. ABCD of the phosphodiesterase family: interaction and differential activity in COPD. Int J Chron Obstruct Pulmon Dis 2008;3:543-61. 10.2147/COPD.S1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page CP, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol 2012;12:275-86. 10.1016/j.coph.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 7.Boswell-Smith V, Cazzola M, Page CP. Are phosphodiesterase 4 inhibitors just more theophylline? J Allergy Clin Immunol 2006;117:1237-43. 10.1016/j.jaci.2006.02.045 [DOI] [PubMed] [Google Scholar]
- 8.Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2010;23:235-56. 10.1016/j.pupt.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 9.Sousa LP, Lopes F, Silva DM, et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-κB-independent manner. J Leukoc Biol 2010;87:895-904. 10.1189/jlb.0809540 [DOI] [PubMed] [Google Scholar]
- 10.Buenestado A, Grassin-Delyle S, Guitard F, et al. Roflumilast inhibits the release of chemokines and TNF-α from human lung macrophages stimulated with lipopolysaccharide. Br J Pharmacol 2012;165:1877-90. 10.1111/j.1476-5381.2011.01667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672-88. 10.1183/09031936.03.00040703 [DOI] [PubMed] [Google Scholar]
- 12.Tenor H, Hatzelmann A, Church MK, et al. Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis and chemotaxis of human eosinophils from normal and atopic subjects. Br J Pharmacol 1996;118:1727-35. 10.1111/j.1476-5381.1996.tb15598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dent G, Giembycz MA, Evans PM, et al. Suppression of human eosinophil respiratory burst and cyclic AMP hydrolysis by inhibitors of type IV phosphodiesterase: interaction with the beta adrenoceptor agonist albuterol. J Pharmacol Exp Ther 1994;271:1167-74. [PubMed] [Google Scholar]
- 14.Eskandari N, Wickramasinghe T, Peachell PT. Effects of phosphodiesterase inhibitors on interleukin-4 and interleukin-13 generation from human basophils. Br J Pharmacol 2004;142:1265-72. 10.1038/sj.bjp.0705892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung TT, Crawley Y, Luo B, et al. Inhibition of pulmonary eosinophilia and airway hyperresponsiveness in allergic mice by rolipram: involvement of endogenously released corticosterone and catecholamines. Br J Pharmacol 2000;130:457-63. 10.1038/sj.bjp.0703308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoymann HG, Wollin L, Müller M, et al. Effects of the Phosphodiesterase Type 4 Inhibitor Roflumilast on Early and Late Allergic Response and Airway Hyperresponsiveness in Aspergillus-fumigatus Sensitized Mice. Pharmacology 2009;83:188-95. 10.1159/000196814 [DOI] [PubMed] [Google Scholar]
- 17.Jones NA, Boswell-Smith V, Lever R, et al. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther 2005;18:93-101. 10.1016/j.pupt.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Sanz MJ, Cortijo J, Taha MA, et al. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br J Pharmacol 2007;152:481-92. 10.1038/sj.bjp.0707428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel BS, Rahman MM, Baehring G, et al. Roflumilast N-oxide in Combination with Formoterol Enhances the Anti-inflammatory Effect of Dexamethasone in ASM Cells. Am J Respir Cell Mol Biol 2017;56:532-8. 10.1165/rcmb.2016-0191OC [DOI] [PubMed] [Google Scholar]
- 20.Patel BS, Prabhala P, Oliver BG, et al. Inhibitors of Phosphodiesterase 4, but Not Phosphodiesterase 3, Increase β2-Agonist–Induced Expression of Antiinflammatory Mitogen-Activated Protein Kinase Phosphatase 1 in Airway Smooth Muscle Cells. Am J Respir Cell Mol Biol 2015; 52:634-40. 10.1165/rcmb.2014-0344OC [DOI] [PubMed] [Google Scholar]
- 21.Togo S, Liu X, Wang X, et al. PDE4 inhibitors roflumilast and rolipram augment PGE2 inhibition of TGF-β1-stimulated fibroblasts. Am J Physiol Lung Cell Mol Physiol 2009;296:L959-69. 10.1152/ajplung.00508.2007 [DOI] [PubMed] [Google Scholar]
- 22.Kohyama T, Liu X, Wen F-Q, et al. PDE4 Inhibitors Attenuate Fibroblast Chemotaxis and Contraction of Native Collagen Gels. Am J Respir Cell Mol Biol 2002;26:694-701. 10.1165/ajrcmb.26.6.4743 [DOI] [PubMed] [Google Scholar]
- 23.Cortijo J, Iranzo A, Milara X, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol 2009;156:534-44. 10.1111/j.1476-5381.2008.00041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udalov S, Dumitrascu R, Pullamsetti SS, et al. Effects of phosphodiesterase 4 inhibition on bleomycin-induced pulmonary fibrosis in mice. BMC Pulm Med 2010;10:26. 10.1186/1471-2466-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther 2001;297:267-79. [PubMed] [Google Scholar]
- 26.Saetta M, Turato G, Facchini FM, et al. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med 1997;156:1633-9. 10.1164/ajrccm.156.5.9701081 [DOI] [PubMed] [Google Scholar]
- 27.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 2001;163:511-6. 10.1164/ajrccm.163.2.2001038 [DOI] [PubMed] [Google Scholar]
- 28.Mata M, Sarria B, Buenestado A, et al. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax 2005;60:144-52. 10.1136/thx.2004.025692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milara J, Armengot M, Banuls P, et al. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br J Pharmacol 2012;166:2243-62. 10.1111/j.1476-5381.2012.01929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Veilleux A, Zhang L, et al. Dynamic Activation of Cystic Fibrosis Transmembrane Conductance Regulator by Type 3 and Type 4D Phosphodiesterase Inhibitors. J Pharmacol Exp Ther 2005;314:846-54. 10.1124/jpet.105.083519 [DOI] [PubMed] [Google Scholar]
- 31.Tannheimer SL, Sorensen EA, Haran AC, et al. Additive anti-inflammatory effects of beta 2 adrenoceptor agonists or glucocorticosteroid with roflumilast in human peripheral blood mononuclear cells. Pulm Pharmacol Ther 2012;25:178-84. 10.1016/j.pupt.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 32.Buenestado A, Chaumais M-C, Grassin-Delyle S, et al. Roflumilast Inhibits Lipopolysaccharide-Induced Tumor Necrosis Factor-α and Chemokine Production by Human Lung Parenchyma. PLoS ONE 2013;8:e74640. 10.1371/journal.pone.0074640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannheimer SL, Wright CD, Salmon M. Combination of roflumilast with a beta-2 adrenergic receptor agonist inhibits proinflammatory and profibrotic mediator release from human lung fibroblasts. Respir Res 2012;13:28. 10.1186/1465-9921-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moodley T, Wilson SM, Joshi T, et al. Phosphodiesterase 4 Inhibitors Augment the Ability of Formoterol to Enhance Glucocorticoid-Dependent Gene Transcription in Human Airway Epithelial Cells: A Novel Mechanism for the Clinical Efficacy of Roflumilast in Severe COPD. Mol Pharmacol 2013;83:894-906. 10.1124/mol.112.083493 [DOI] [PubMed] [Google Scholar]
- 35.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 2005;365:167-75. 10.1016/S0140-6736(05)17708-3 [DOI] [PubMed] [Google Scholar]
- 36.Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir Res 2011;12:140. 10.1186/1465-9921-12-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Schalkwyk E, Strydom K, Williams Z, et al. Roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, attenuates allergen-induced asthmatic reactions. J Allergy Clin Immunol 2005;116:292-8. 10.1016/j.jaci.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 38.Timmer W, Leclerc V, Birraux G, et al. The New Phosphodiesterase 4 Inhibitor Roflumilast Is Efficacious in Exercise-Induced Asthma and Leads to Suppression of LPS-Stimulated TNF-α Ex Vivo. J Clin Pharmacol 2002;42:297-303. 10.1177/00912700222011328 [DOI] [PubMed] [Google Scholar]
- 39.Aubier M, Sauer R, Boszormenyi G, et al. Roflumilast a novel selective PDE4 inhibitor shows early onset of efficacy in asthma. Am J Respir Crit Care Med 2004;169:A322 (Abstr). [Google Scholar]
- 40.Bousquet J, Aubier M, Sastre J, et al. Comparison of roflumilast, an oral anti-inflammatory, with beclomethasone dipropionate in the treatment of persistent asthma. Allergy 2006;61:72-8. 10.1111/j.1398-9995.2005.00931.x [DOI] [PubMed] [Google Scholar]
- 41.Leichtl S, Schmid-Wirlitsch C, Bredenbröker D, et al. Roflumilast, a new orally active, selective phosphodiesterase 4 inhibitor is effective in the treatment of asthma. Eur Respir J 2002;20:abstr 303S.
- 42.Bateman ED, Goehring U-M, Richard F, Watz H. Roflumilast combined with montelukast versus montelukast alone as add-on treatment in patients with moderate-to-severe asthma. J Allergy Clin Immunol 2016;138:142-9.e8. 10.1016/j.jaci.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 43.Albrecht A, Leichtl S, Bredenbröker D, et al. A new orally active, selective phosphodiesterase 4 inhibitor, with beclomethasone propionate in asthma control. Eur Respir J 2002;20:304S. [Google Scholar]
- 44.Meltzer EO, Chervinsky P, Busse W, et al. Roflumilast for asthma: Efficacy findings in placebo-controlled studies. Pulm Pharmacol Ther 2015;35:S20-7. 10.1016/j.pupt.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Gamble E, Grootendorst DC, Brightling CE, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:976-82. 10.1164/rccm.200212-1490OC [DOI] [PubMed] [Google Scholar]
- 46.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007;62:1081-7. 10.1136/thx.2006.075937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Global Strategy for Diagnosis, Management, and Prevention of COPD 2014.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html
- 48.Nannini LJ, Poole P, Milan SJ, et al. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;11:CD003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Compton CH, Gubb J, Nieman R, et al. Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging study. Lancet 2001;358:265-70. 10.1016/S0140-6736(01)05481-2 [DOI] [PubMed] [Google Scholar]
- 50.Bredenbröker D, Syed J, Leichtl S, et al. Roflumilast, a new orally active, selective phosphodiesterase 4 inhibitor, is effective in the treatment of chronic obstructive pulmonary disease. Eur Respir J 2002;20:abstr 374S.
- 51.Rabe KF, Bateman ED, O'Donnell D, et al. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563-71. 10.1016/S0140-6736(05)67100-0 [DOI] [PubMed] [Google Scholar]
- 52.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-94. 10.1016/S0140-6736(09)61255-1 [DOI] [PubMed] [Google Scholar]
- 53.Calverley PM, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:154-61. 10.1164/rccm.200610-1563OC [DOI] [PubMed] [Google Scholar]
- 54.Rennard SI, Calverley PM, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast--the importance of defining different subsets of patients with COPD. Respir Res 2011;12:18. 10.1186/1465-9921-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giembycz MA, Field SK. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther 2010;4:147-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wedzicha JA, Rabe KF, Martinez FJ, et al. Efficacy of roflumilast in the chronic obstructive pulmonary disease frequent exacerbator phenotype. Chest 2013;143:1302-11. 10.1378/chest.12-1489 [DOI] [PubMed] [Google Scholar]
- 57.Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis 2012;7:375-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66. 10.1016/S0140-6736(14)62410-7 [DOI] [PubMed] [Google Scholar]
- 59.Martinez FJ, Rabe KF, Sethi S, et al. Effect of Roflumilast and Inhaled Corticosteroid/Long-Acting beta2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE(2)SPOND). A Randomized Clinical Trial. Am J Respir Crit Care Med 2016;194:559-67. 10.1164/rccm.201607-1349OC [DOI] [PubMed] [Google Scholar]
- 60.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695-703. 10.1016/S0140-6736(09)61252-6 [DOI] [PubMed] [Google Scholar]
- 61.Zheng J, Yang J, Zhou X, et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest 2014;145:44-52. 10.1378/chest.13-1252 [DOI] [PubMed] [Google Scholar]
- 62.Welsh EJ, Evans DJ, Fowler SJ, et al. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2015: CD010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoo JK, Venning V, Wong C, et al. Bronchiectasis in the Last Five Years: New Developments. J Clin Med 2016;5:115. 10.3390/jcm5120115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J. Effect Of Roflumilast(Daxas®) In Patients With Symptomatic Non-Cystic Fibrosis Bronchiectasis. Am J Respir Crit Care Med 2014;189: A625. [Google Scholar]
- 65.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez-García MÁ, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors Associated With Bronchiectasis in Patients With COPD. Chest 2011;140:1130-7. 10.1378/chest.10-1758 [DOI] [PubMed] [Google Scholar]
- 67.Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic Value of Bronchiectasis in Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2013;187:823-31. 10.1164/rccm.201208-1518OC [DOI] [PubMed] [Google Scholar]
- 68.Miravitlles M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir Med 2011;105:1118-28. 10.1016/j.rmed.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 69.Burgel P-R, Nesme-Meyer P, Chanez P, et al. Cough and Sputum Production Are Associated With Frequent Exacerbations and Hospitalizations in COPD Subjects. Chest 2009;135:975-82. 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 70.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J 2008;31:396-406. 10.1183/09031936.00069007 [DOI] [PubMed] [Google Scholar]
- 71.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016;138:16-27. 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 72.Stockley RA. Neutrophilic inflammation: “Don’t you go to pieces on me!” Eur Respir J 2006;28:257-8. 10.1183/09031936.06.00053906 [DOI] [PubMed] [Google Scholar]
- 73.Zeki AA, Schivo M, Chan A, et al. The Asthma-COPD Overlap Syndrome: A Common Clinical Problem in the Elderly. J Allergy (Cairo) 2011;2011:861926. 10.1155/2011/861926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol 2013;6:197-219. 10.1586/ecp.13.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bateman ED, O’Byrne PM, Buhl R, et al. Roflumilast for asthma: Weighing the evidence. Pulm Pharmacol Ther 2015;35:S1-3. 10.1016/j.pupt.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 76.Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of Acute Exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015;147:894-942. 10.1378/chest.14-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Global Strategy for Diagnosis, Management, and Prevention of COP—2016. Available online: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 78.Spina D, Page CP. Xanthines and Phosphodiesterase Inhibitors. Berlin, Heidelberg: Springer Berlin Heidelberg, 2016:1-29. [Google Scholar]
- 79.Ito K, Lim S, Caramori G, et al. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A 2002;99:8921-6. 10.1073/pnas.132556899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cosío BG, Shafiek H, Iglesias A, et al. Oral Low-dose Theophylline on Top of Inhaled Fluticasone-Salmeterol Does Not Reduce Exacerbations in Patients With Severe COPD: A Pilot Clinical Trial. Chest 2016;150:123-30. 10.1016/j.chest.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 81.Böhmer G, Gleiter CH, Hunnemeyer A, et al. Study investigating pharmacokinetic interaction between theophylline and roflumilast in healthy adults. Int J Clin Pharmacol Ther 2011;49:451-60. 10.5414/CP201535 [DOI] [PubMed] [Google Scholar]
- 82.Oba Y, Lone NA. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther Adv Respir Dis 2013;7:13-24. 10.1177/1753465812466167 [DOI] [PubMed] [Google Scholar]
- 83.Mills EJ, Druyts E, Ghement I, et al. Pharmacotherapies for chronic obstructive pulmonary disease: a multiple treatment comparison meta-analysis. Clin Epidemiol 2011;3:107-29. 10.2147/CLEP.S16235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967-76. 10.1056/NEJMoa041892 [DOI] [PubMed] [Google Scholar]
- 85.Ito K, Caramori G, Lim S, et al. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 2002;166:392-6. 10.1164/rccm.2110060 [DOI] [PubMed] [Google Scholar]
- 86.Milara J, Lluch J, Almudever P, et al. Roflumilast N-oxide reverses corticosteroid resistance in neutrophils from patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2014;134:314-22. 10.1016/j.jaci.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 87.Wouters EF, Bredenbroker D, Teichmann P, et al. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:E1720-5. 10.1210/jc.2011-2886 [DOI] [PubMed] [Google Scholar]
- 88.Lipari M, Kale-Pradhan PB. Vulnerable COPD patients with comorbidities: the role of roflumilast. Ther Clin Risk Manag 2014;10:969-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Donnell DE, Bredenbröker D, Brose M, et al. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J 2012;39:1104-12. 10.1183/09031936.00096511 [DOI] [PubMed] [Google Scholar]
- 90.Rennard SI, Sun SX, Tourkodimitris S, et al. Roflumilast and dyspnea in patients with moderate to very severe chronic obstructive pulmonary disease: a pooled analysis of four clinical trials. Int J Chron Obstruct Pulmon Dis 2014;9:657-73. 10.2147/COPD.S55738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan L, Guo YZ, Zhang B, et al. Does roflumilast improve dyspnea in patients with chronic obstructive pulmonary disease? A meta-analysis. J Thorac Dis 2013;5:422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vestbo J, Tan L, Atkinson G, et al. A controlled trial of 6-weeks' treatment with a novel inhaled phosphodiesterase type-4 inhibitor in COPD. Eur Respir J 2009;33:1039-44. 10.1183/09031936.00068908 [DOI] [PubMed] [Google Scholar]
- 93.Wittmann M, Helliwell PS. Phosphodiesterase 4 Inhibition in the Treatment of Psoriasis, Psoriatic Arthritis and Other Chronic Inflammatory Diseases. Dermatol Ther (Heidelb) 2013;3:1-15. 10.1007/s13555-013-0023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010;159:842-55. 10.1111/j.1476-5381.2009.00559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heckman PR, Wouters C, Prickaerts J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer's disease: a translational overview. Curr Pharm Des 2015;21:317-31. 10.2174/1381612820666140826114601 [DOI] [PubMed] [Google Scholar]
- 96.García-Barroso C, Ugarte A, Martinez M, et al. Phosphodiesterase inhibition in cognitive decline. J Alzheimers Dis 2014;42 Suppl 4:S561-73. [DOI] [PubMed] [Google Scholar]
- 97.García-Osta A, Cuadrado-Tejedor M, García-Barroso C, et al. Phosphodiesterases as Therapeutic Targets for Alzheimer's Disease. ACS Chem Neurosci 2012;3:832-44. 10.1021/cn3000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jabaris SGSL, Sumathy H, Kumar RS, et al. Effects of rolipram and roflumilast, phosphodiesterase-4 inhibitors, on hypertension-induced defects in memory function in rats. Eur J Pharmacol 2015;746:138-47. 10.1016/j.ejphar.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 99.Martinez A, Gil C. cAMP-specific phosphodiesterase inhibitors: promising drugs for inflammatory and neurological diseases. Expert Opin Ther Pat 2014;24:1311-21. 10.1517/13543776.2014.968127 [DOI] [PubMed] [Google Scholar]