Graphical abstract
Highlights
-
•
Iron deficiency and anemia are major threats to public health worldwide.
-
•
Biofortification is a promising and sustainable strategy to target iron deficiency.
-
•
Three randomized efficacy trials of iron-biofortification have been conducted to date.
-
•
Iron-biofortification improved iron status, with the greatest effects in iron deficient individuals.
-
•
Future trials are needed to assess the effects on functional outcomes and in other high-risk populations.
Abstract
Iron deficiency is the most common micronutrient deficiency globally and represents a major threat to public health. Biofortification, the process of enhancing micronutrient content and bioavailability in staple crops, represents an exciting sustainable food-based strategy to combat and prevent iron deficiency, particularly in resource-limited settings. In this review, we examine the evidence to date of the efficacy of iron-biofortified staple food crops on improving iron status in at-risk populations, including rice, pearl millet, and beans. Three randomized efficacy trials of iron biofortified interventions were included in this analysis, conducted in the Philippines, India, and Rwanda. Iron status (hemoglobin, serum ferritin, soluble transferrin receptor (sTfR), C-reactive protein, alpha-1 acid glycoprotein) was measured at enrollment, midline, and endline in each trial. The primary outcomes evaluated included hemoglobin, serum ferritin, sTfR, and total body iron. A meta-analysis using random effects models was conducted to examine the effects of interventions on hematological outcomes, with the DerSimonian and Laird method. In meta-analyses of data from the three trials, iron-biofortified interventions significantly increased serum ferritin concentrations and total body iron. Evidence to date from randomized trials suggest that iron-biofortified crops are an efficacious intervention to improve iron status. In particular, findings from all three trials also indicate that the effects of biofortified staple crops were highest among individuals who were iron deficient at baseline, suggesting the greatest potential to benefit. Assessment of functional outcomes and consideration of other high-risk populations such as young children, are warranted to elucidate the impact of iron-biofortified interventions on human health.
Current Opinion in Biotechnology 2017, 44:138–145
This review comes from a themed issue on Food biotechnology
Edited by Patrick Stover and Saurabh Mehta
For a complete overview see the Issue and the Editorial
Available online 25th January 2017
http://dx.doi.org/10.1016/j.copbio.2017.01.003
0958-1669/© 2017 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Iron deficiency is the most common micronutrient deficiency worldwide and disproportionately affects the poorest and most vulnerable populations in resource-limited settings [1]. A substantial body of evidence supports the relationships between iron deficiency and adverse health outcomes, and even mild iron deficiency can lead to deficits in cognitive function in children [2, 3, 4, 5], and reduced physical work capacity in adults [4, 6].
Biofortification is the process of increasing the content and bioavailablity of essential vitamins and minerals in staple crops, through plant breeding or agronomic practices, to improve nutritional status [7]. With micronutrient malnutrition, or hidden hunger, continuing to affect nearly one-third of the world’s population, biofortification is a promising and sustainable agriculture-based strategy to target iron deficiency, particularly in high-risk populations in resource-limited settings [8•, 9, 10•, 11].
In this review, we summarize the findings from the three randomized efficacy trials that have been published to date on the effects of iron-biofortified staple food crops on iron status in at-risk populations. We also present findings from a meta-analysis combining the results from these three randomized trials on the efficacy of iron-biofortified staple food crops on improving iron status, to inform public health programs incorporating biofortification as a strategy to target iron deficiency in diverse population groups.
Randomized efficacy trials
To date, three randomized efficacy trials have been conducted to determine the effects of iron-biofortified staple food crops on iron status, including rice, beans, and pearl millet. Characteristics of these studies are presented in Table 1.
Table 1.
Characteristics of randomized efficacy feeding trials of iron-biofortified crops
| Setting | Manila, Philippines [12] | Maharashtra, India [13] | Huye, Rwanda [14] |
| Population | Adult female (18–45 years) religious sisters in nine convents |
Male and female adolescents (12–16 years) living in three hostels |
Adult female (18–27 years) university students |
| Study Design | Randomized efficacy trial | Randomized efficacy trial | Randomized efficacy trial |
| Randomization | By individual | By individual | By individual |
| Sample size | n = 192 | n = 246 | n = 195 |
| Intervention | Iron-biofortified rice | Iron-biofortified pearl millet as Bhakri | Iron-biofortified beans |
| High iron: n = 92 Control: n = 100 |
High iron: n = 122 Control: n = 124 |
High iron: n = 94 Control: n = 101 |
|
| Length of feeding | 9 months | 6 months | 4.5 months |
The efficacy of consuming iron-biofortified rice (Oryza sativa) was examined during a 9-month double-blind randomized feeding trial in 192 religious sisters living in nine convents in metro-Manila, Philippines [12]. Participants were randomized to consume either iron-biofortified rice (3.21 mg/kg Fe) or a local variety of conventional rice (0.57 mg/kg Fe). The iron-biofortified rice contributed 1.79 mg of iron per day to the diet, compared to 0.37 mg of iron per day from the control rice. At baseline, 28% of women were anemic (hemoglobin <120 g/L) and 34% were iron deficient (serum ferritin <15.0 μg/L). In analyses among non-anemic women, iron-biofortified rice increased serum ferritin concentrations (P = 0.02) and total body iron (P = 0.05), compared to conventional rice. Findings represented a 20% increase after controlling for baseline values and daily rice consumption. Overall, the greatest improvements in iron status were observed in non-anemic women who had the lowest baseline iron status and in individuals who consumed the most iron from rice.
The second randomized efficacy trial was conducted to determine the effects of consuming iron-biofortified pearl millet (Pennisetum glaucum) on iron status, compared to conventional pearl millet, among 246 children (12–16 years) for six months in rural Maharashtra, India [13••]. The iron-biofortified pearl millet contained 87 mg per kilogram of iron, compared to 30 mg per kilogram of iron in the conventional pearl millet. All children received 200–300 g of (dry) pearl millet per day in the form of Bhakri flatbread during lunch and dinner. Bhakri was prepared two times per day by seven cooks who used only one of two types of pearl millet flour and followed a protocol to standardize bhakri diameter, weight, and consistency. Iron status, including hemoglobin, serum ferritin (SF), soluble transferrin receptor (sTfR), and total body iron (TBI), inflammatory biomarkers C-reactive protein (CRP) and alpha-1 acid glycoprotein (AGP), and anthropometric indices were evaluated at enrollment, at four months, and at six months. At baseline, 43% of children were iron deficient (serum ferritin <15.0 μg/L) and 28% were anemic (hemoglobin <120 g/L). Iron-biofortified pearl millet significantly increased serum ferritin concentrations and total body iron after four months compared to the conventional pearl millet. Among children who were iron deficient at baseline, those who received iron-biofortified pearl millet were 1.64 times more likely to become iron replete by six months compared to those received the control pearl millet (RR: 1.64, 95% CI: 1.07, 2.49, P = 0.02). The effects of iron-biofortified pearl millet on iron status were greater among children who were iron deficient at baseline than children who were not iron deficient at baseline, suggesting a greater potential to benefit. This trial was registered at clinicaltrials.gov as NCT02152150.
The third randomized controlled trial was conducted to determine the efficacy of iron-biofortified beans (Phaseolus vulgaris) on improving iron status in women of reproductive age in Huye, Rwanda [14••]. A total of 195 female university students (aged 18–27 years) who were iron insufficient (serum ferritin <20.0 μg/L) at baseline were randomly assigned to receive either iron-biofortified beans containing 86 mg of iron per kilogram, or standard unfortified beans, containing 50 mg of iron per kilogram, two times per day for 128 days. Random serial sampling was used to collect blood during each of the eight middle weeks of the randomized feeding trial. A total of 86% of participants were iron deficient (serum ferritin <15.0 μg/L) and 36% were anemic (hemoglobin <120 g/L) at baseline. The intervention group receiving the iron-biofortified beans consumed 14.5 mg of iron per day, whereas the control group receiving conventional beans consumed 8.6 mg of iron per day (mean ± SD, 14.5 ± 1.6 vs. 8.6 ± 0.8, P < 0.05). The intervention group receiving iron-biofortified beans had significantly greater increases in hemoglobin (3.8 g/L), serum ferritin (1.10 μg/L), and total body iron (0.5 mg/kg), compared to the group consuming conventional beans after 128 days of follow-up. This trial was registered at clinicaltrials.gov as NCT01594359.
In summary, the three randomized efficacy trials published to date demonstrate that biofortification is an efficacious strategy to improve iron status in diverse settings including the Philippines, India, and Rwanda, and in at-risk populations such as women of reproductive age and school-age adolescent children. Findings also indicate that the effects of biofortified staple crops were highest among individuals who were iron deficient at baseline and among participants who consumed the greatest amount of the biofortified crop, suggesting the greatest potential to benefit.
Meta-analyses
Based on the demonstrated efficacy of biofortification as a strategy to improve iron status in the three above-described randomized trials, we conducted a meta-analysis to synthesize evidence for the efficacy of iron-biofortified interventions on iron status. In the analyses below, we used a meta-analyses approach to estimate a summary measure for the potential benefit that may be observed with different iron-biofortified crops in different age groups to inform future trials and effectiveness studies.
The primary outcomes evaluated are presented in Table 2. The primary outcomes evaluated included hemoglobin, serum ferritin, and sTfR concentrations, total body iron, anemia, and iron deficiency. Anemia was defined as hemoglobin less than 120 g/L, in accordance with World Health Organization criteria. Total body iron (TBI) was estimated with the approach originally proposed by Cook et al. [15]. Iron deficiency was defined as serum ferritin less than 15.0 μg/L for the primary analyses and as TBI less than 0 mg/kg or sTfR greater than 8.3 mg/L in additional analyses.
Table 2.
Primary outcomes
| Continuous | Categorical |
|---|---|
| Hemoglobin, g/L | <120 g/L |
| Serum ferritin, μg/L | <15.0 μg/L |
| sTfR, mg/L | >8.3 mg/L |
| Total body iron, mg/kga | <0.0 mg/kg |
Total body iron (TBI) = −[log10 (sTfR (mg/L) × 1000/SF (μg/L) − 2.8229]/0.1207 (Cook’s equation) [15].
Meta-analyses were conducted using random effects models (DerSimonian and Laird method), and the weights used are reported in the figures. Models were tested for heterogeneity and analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Iron deficiency and anemia were common in these populations: at baseline, 31% of participants were anemic (Hb <120 g/L) and 54% were iron deficient (serum ferritin <15.0 μg/L) in the overall sample. The prevalence of iron deficiency ranged from 34% in the Philippines to 86% in Rwanda (43% in India), and the prevalence of anemia ranged from 28% in the studies in the Philippines and India to 36% in Rwanda.
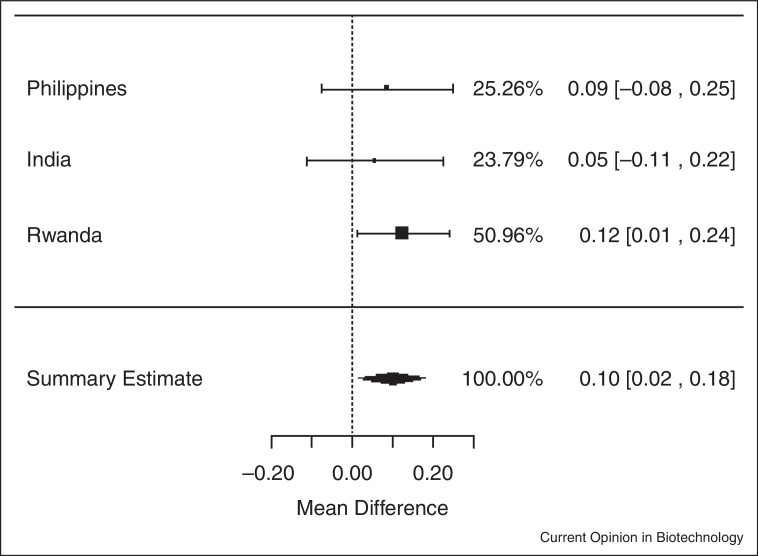
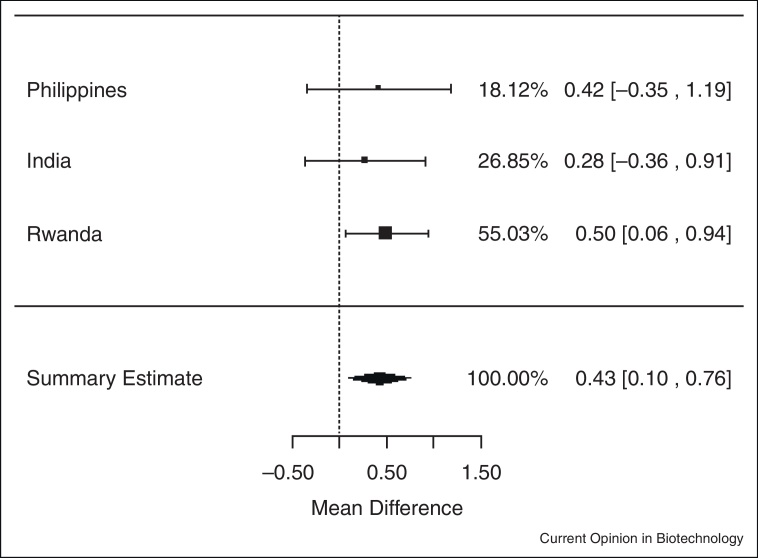
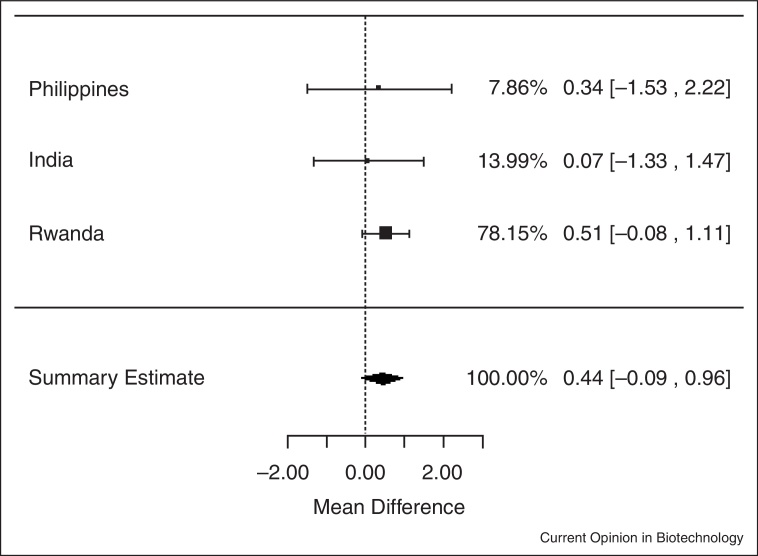
In meta-analyses of data from the three trials, iron-biofortified crop interventions significantly increased serum ferritin concentrations (Figure 1.1 ), compared to conventional crops with a mean increase of 1.10 μg/L of serum ferritin (ln (SF): 0.10 μg/L; 95% CI: 0.02, 0.18; P = 0.02). Similarly, iron-biofortified crop interventions significantly increased total body iron (Figure 2.1 ) during the feeding trials, with a mean increase of 0.43 mg/kg (95% CI: 0.10, 0.76; P = 0.01). However, there were no statistically significant effects of iron-biofortified interventions on hemoglobin (Figure 3.1 ; P = 0.25) or sTfR (data not shown; P > 0.05) concentrations, compared to conventional crops.
Figure 1.1.
Effect of iron-biofortified crop interventions on changes in serum ferritin concentrations (μg/L; natural logarithmically transformed).
Figure 2.1.
Effect of iron-biofortified crop interventions on changes in total body iron (mg/kg).
Figure 3.1.
Effect of iron-biofortified crop interventions on changes in hemoglobin concentrations (g/L).
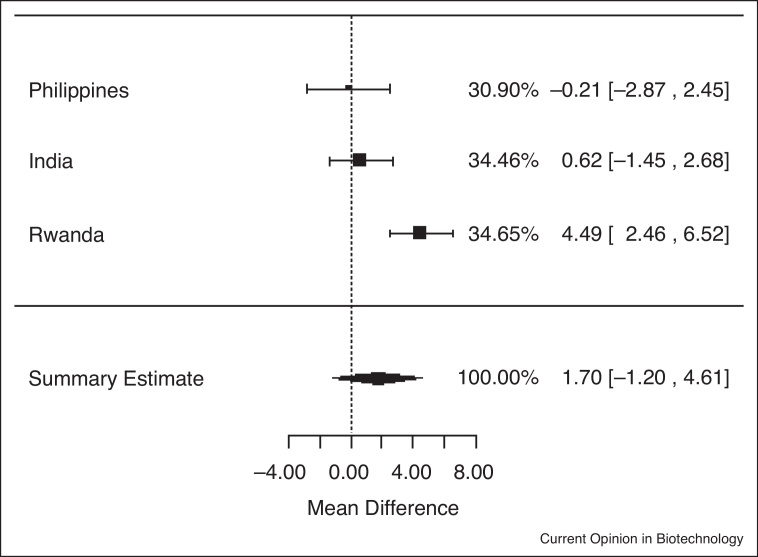
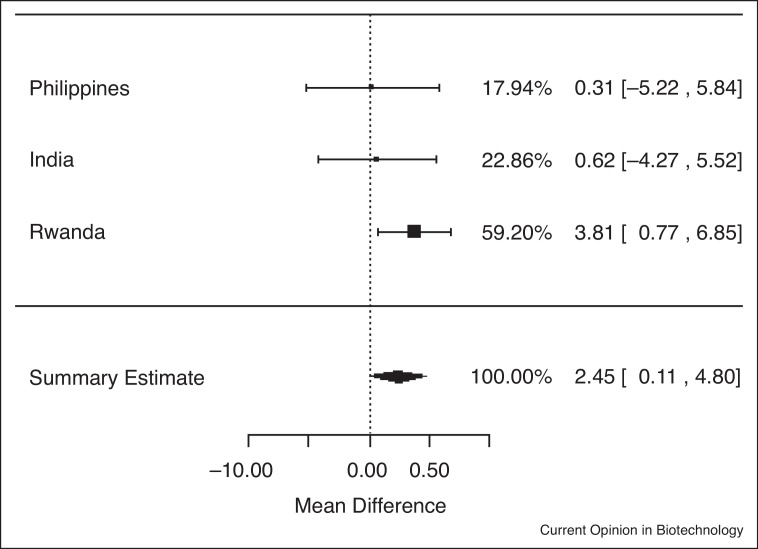
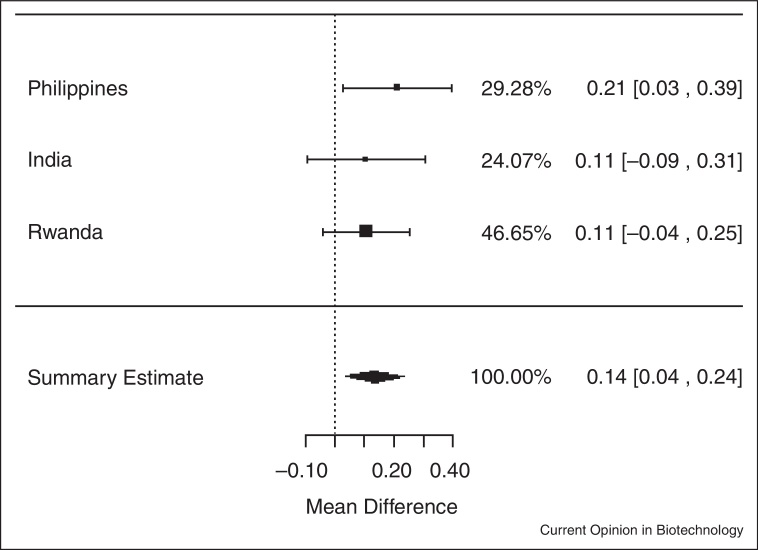
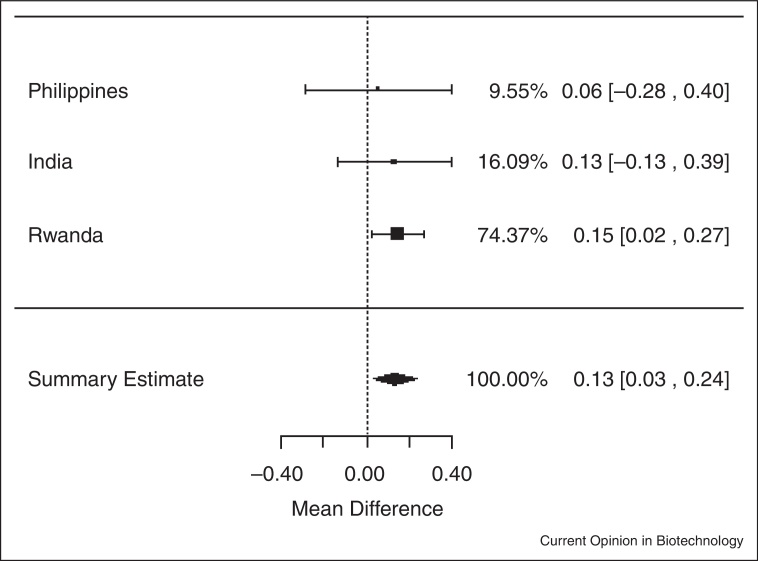
In analyses of data from the three trials among individuals who were iron deficient (serum ferritin <15.0 μg/L) at baseline, iron-biofortified crop interventions significantly increased serum ferritin concentrations (Figure 1.2), compared to conventional crops (ln(SF): 0.13; 95% CI: 0.03, 0.24; P = 0.01). In analyses among individuals who had low total body iron (<0 mg/kg) at baseline, there were no significant increases in TBI in individuals consuming the iron-biofortified crops compared to conventional crops (Figure 2.2; P = 0.10). In analyses among individuals who were anemic (Hb <120 g/L) at baseline, iron-biofortified crop interventions significantly increased hemoglobin concentrations (Figure 3.2 ) during the feeding trials, compared to conventional crops, with a mean increase of 2.45 g/L (P = 0.04).
Figure 2.2.
Effect of iron-biofortified crop interventions on changes in total body iron (mg/kg) among individuals who were iron deficient (TBI <0 mg/kg) at baseline.
Figure 3.2.
Effect of iron-biofortified crop interventions on changes in hemoglobin concentrations (g/L) among individuals who were anemic (Hb <120 g/L) at baseline.
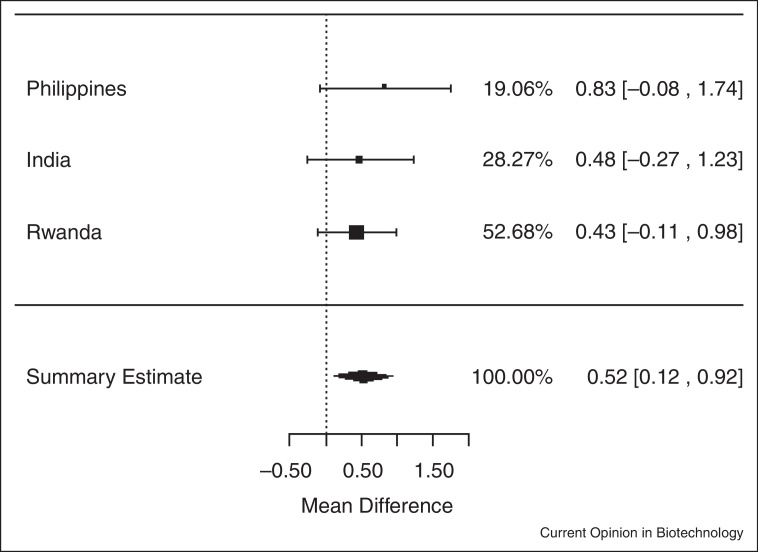
In analyses of data among individuals who were not anemic at baseline (Hb ≥120 g/L) iron-biofortified crops significantly increased both serum ferritin concentrations and total body iron, with a mean increase of 1.15 μg/L of serum ferritin (ln(SF): 0.14; 95% CI: 0.04, 0.24; P < 0.01) (Figure 4.1 ) and 0.52 mg/kg of total body iron (0.52 mg/kg; 95% CI: 0.12, 0.92; P = 0.01) (Figure 4.2 ), respectively.
Figure 4.1.
Effect of iron-biofortified crop interventions on changes in serum ferritin concentrations, among individuals who were not anemic (Hb ≥120 g/L) at baseline (μg/L; natural logarithmically transformed).
Figure 4.2.
Effect of iron-biofortified crop interventions on changes in total body iron (mg/kg) among individuals who were not anemic (Hb ≥120 g/L) at baseline.
Findings from these meta-analyses provide insights into the efficacy of biofortification as a strategy to improve iron status, and may inform future trials and effectiveness studies of the potential benefit of iron-biofortified crops on iron status in different age groups. In particular, findings suggest that the benefits of biofortified stable crops were greatest among individuals who were iron deficient at baseline. Meta-analyses among non-anemic individuals also indicated that non-anemic individuals had a greater benefit in terms of iron status (serum ferritin and TBI), consistent with evidence that absorbed iron is preferentially allocated to iron stores after hemoglobin concentrations are repleted.
This analysis has several limitations which warrant caution in the interpretation of findings. For example, the diversity of the populations and the interventions in the combined randomized trials represents a potential limitation. Other limitations include only consideration of baseline and endline data for these analyses, as a different sampling scheme was used across the three different randomized trials. This precludes our ability to detect changes between baseline and midline, as observed in the India pearl millet trial, and more sensitive time-to-event analyses within the duration of the follow-up period across randomized trials.
Conclusions and future directions
Findings to date from randomized trials suggest that iron-biofortified crops are an efficacious intervention to improve iron status, including serum ferritin and total body iron. In particular, findings from all three trials suggest that the benefits of biofortified stable crops were greatest among individuals who were iron deficient at baseline and among participants who consumed the greatest amount of the biofortified crop. Assessment of functional outcomes and consideration of other high-risk populations such as young children, are warranted along with effectiveness studies to scale-up the use of iron-biofortified staple food crops to improve human health.
Conflict of interest
All authors have received competitive grant funding for conducting randomized efficacy trials of biofortified crops from HarvestPlus. SM also has an equity interest in a diagnostic startup planning to commercialize his work on point of care methods of nutritional assessment.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Figure 1.2.
Effect of iron-biofortified crop interventions on changes in serum ferritin concentrations, among individuals who were iron deficient (SF <15.0 μg/L) at baseline (μg/L; natural logarithmically transformed).
References
- 1.Stevens G.A., Finucane M.M., De-Regil L.M., Paciorek C.J., Flaxman S.R., Branca F., Pena-Rosas J.P., Bhutta Z.A., Ezzati M., Nutrition Impact Model Study G Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson M. Anaemia in adolescent girls: effects on cognitive function and activity. Proc Nutr Soc. 1996;55:359–367. doi: 10.1079/pns19960035. [DOI] [PubMed] [Google Scholar]
- 3.Stivelman J.C. Benefits of anaemia treatment on cognitive function. Nephrol Dial Transplant. 2000;15(Suppl. 3):29–35. doi: 10.1093/oxfordjournals.ndt.a027973. [DOI] [PubMed] [Google Scholar]
- 4.McClung J.P., Murray-Kolb L.E. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr. 2013;33:271–288. doi: 10.1146/annurev-nutr-071812-161205. [DOI] [PubMed] [Google Scholar]
- 5.Murray-Kolb L.E., Beard J.L. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- 6.Haas J.D., Brownlie T.t. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. discussion 688S–690S. [DOI] [PubMed] [Google Scholar]
- 7.Bouis H.E., Hotz C., McClafferty B., Meenakshi J.V., Pfeiffer W.H. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull. 2011;32:S31–S40. doi: 10.1177/15648265110321S105. [DOI] [PubMed] [Google Scholar]
- 8•.Garcia-Casal M.N., Peña-Rosas J.P., Giyose B., consultation working groups Staple crops biofortified with increased vitamins and minerals: considerations for a public health strategy. Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.13293. December 9. [DOI] [PubMed] [Google Scholar]; This publication summarizes findings from the recent technical consultation entitled “Staple Crops Biofortified with Vitamins and Minerals: Considerations for a Public Health Strategy”, which was convened by the World Health Organization in collaboration with the Food and Agriculture Organization of the United Nations and the Sackler Institute for Nutrition Science at the New York Academy of Sciences (Apr 2016).
- 9.Vasconcelos M.W., Gruissem W., Bhullar N.K. Iron biofortification in the 21st century: setting realistic targets, overcoming obstacles, and new strategies for healthy nutrition. Curr Opin Biotechnol. 2016;44:8–15. doi: 10.1016/j.copbio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 10•.Petry N., Olofin I., Boy E., Donahue Angel M., Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the First 1000 days of life: a systematic review and meta-analysis. Nutrients. 2016;8 doi: 10.3390/nu8120773. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review was conducted to examine the efficacy of low-dose iron and zinc intake during the first 1,000 days of life. No studies have been conducted to date to determine the efficacy of iron or zinc biofortification interventions during this window on maternal and child health outcomes. Findings suggest that consideration of high-risk populations such as pregnant women and young children are warranted in the design of biofortification interventions in resource-limited settings.
- 11.De Moura F.F., Palmer A.C., Finkelstein J.L., Haas J.D., Murray-Kolb L.E., Wenger M.J., Birol E., Boy E., Pena-Rosas J.P. Are biofortified staple food crops improving vitamin A and iron status in women and children? New evidence from efficacy trials. Adv Nutr. 2014;5:568–570. doi: 10.3945/an.114.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas J.D., Beard J.L., Murray-Kolb L.E., del Mundo A.M., Felix A., Gregorio G.B. Iron-biofortified rice improves the iron stores of nonanemic Filipino women. J Nutr. 2005;135:2823–2830. doi: 10.1093/jn/135.12.2823. [DOI] [PubMed] [Google Scholar]
- 13••.Finkelstein J.L., Mehta S., Udipi S.A., Ghugre P.S., Luna S.V., Wenger M.J., Murray-Kolb L.E., Przybyszewski E.M., Haas J.D. A randomized trial of iron-biofortified pearl millet in school children in India. J Nutr. 2015;145:1576–1581. doi: 10.3945/jn.114.208009. [DOI] [PubMed] [Google Scholar]; This was the first randomized efficacy trial of iron-biofortification in school-aged children. Findings demonstrated that iron-biofortified pearl millet significantly increased serum ferritin concentrations and total body iron, and increased the likelihood of resolution of iron deficiency. The effects of iron-biofortified pearl millet on iron status were greater among children who were iron deficient at baseline, suggesting a greater potential to benefit.
- 14••.Haas J.D., Luna S.V., Lung’aho M.G., Wenger M.J., Murray-Kolb L.E., Beebe S., Gahutu J.B., Egli I.M. Consuming iron biofortified beans increases iron status in Rwandan women after 128 days in a randomized controlled feeding trial. J Nutr. 2016;146:1586–1592. doi: 10.3945/jn.115.224741. [DOI] [PubMed] [Google Scholar]; This study was the first randomized efficacy trial of iron-biofortified beans on improving iron status in women of reproductive age. The intervention group receiving iron-biofortified beans had significantly greater increases in hemoglobin, serum ferritin, and total body iron, compared to the group consuming conventional beans after 128 days of follow-up.
- 15.Cook J.D., Flowers C.H., Skikne B.S. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]