Abstract
A growing number of nanoparticle systems, termed “nanomedicines”, are being developed for diagnostic and therapeutic applications. Nanoparticles can employ various cellular entry pathways and trafficking mechanisms to effectively deliver drugs, biomolecules, and imaging agents to precise sub-cellular locations. However, the dynamic transport of nanoparticles through the complex intracellular environment is not well understood, having been primarily studied with static or bulk averaged methods in the past. Such techniques do not provide detailed information regarding the transport mechanism and rates of individual nanoparticles, where understanding of the interaction of nanoparticles with the cellular environment remains incomplete. Recent advances in live-cell fluorescence microscopy and real-time multiple particle tracking (MPT) have facilitated an improved understanding of cell trafficking pathways. Understanding the dynamic transport of nanoparticles as they are delivered into complex cellular components may lead to rational improvements in the design of nanomedicines. This review discusses different cellular uptake and trafficking pathways of nanomedicines, briefly highlights current fluorescence microscopy tools, and provides examples from the recent literature on the use of MPT and its applications.
Keywords: Fluorescence microscopy, Live-cell imaging, Particle tracking, Endocytosis, Nanomedicine, Nanoparticles
Introduction
Nanoparticle systems hold tremendous promise for early disease diagnosis and targeted drug and gene delivery (Duncan 2003). A growing number of these “nanomedicines” are being developed in research and clinical applications. They are expected to drastically improve early diagnosis through molecular imaging and to enhance the efficacy of delivered therapeutics by targeting small molecules, peptides, proteins, and nucleic acids to selective organelles within target cell populations or organs. Effective delivery of nanomedicine requires nanoparticle systems to undergo intracellular trafficking directed to specific compartments inside cells (Rajendran et al. 2010). For example, DNA intended for gene therapy must be delivered to the nucleus in order for the protein to be optimally expressed. Some therapeutics, such as RNAi, must target the cytosol in order to interfere with cellular mRNA. In other cases, pro-apoptotic drugs must be selectively targeted to the mitochondria where they exert their actions (Mossalam et al. 2010). This necessitates a thorough understanding of the cell biology of endocytic pathways and the dynamics of intracellular trafficking of nanoparticles.
Endocytosis is a term that encompasses multiple methods of cellular internalization of macromolecules, including nanoparticles. Endocytosis is generally divided into phagocytosis (uptake of large particles) and pinocytosis (uptake of fluids and solutes). Phagocytosis is a process by which professional phagocytes engulf particles as large as 20 μm. Pinocytosis, in contrast, is present in all cell types and has multiple forms. Pinocytosis is classified as clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CvME), clathrin- and caveolae-independent endocytosis, or macropinocytosis, based on the proteins involved in the pathways (Conner and Schmid 2003). Each endocytic pathway brings opportunities for selective delivery as well as particular challenges for nanomedicine, depending on the delivery system and the nature of its cargo therapeutics.
Effective delivery of nanomedicines is often limited by inefficient transport through the complex intracellular environment, which presents a multitude of barriers. Most significantly, endolysosomal trafficking is a classic consequence of CME in which internalized cargo passes from early endosomes, to recycling endosomes or late endosomes, and finally to lysosomes (Lechardeur et al. 2005). This can result in degradation of therapeutic molecules, including proteins, chemotherapeutic agents, and DNA, by a low pH environment rich in acid hydrolases and DNaseII. Although CME is the predominant endocytosis mechanism, cells possess a host of alternative internalization mechanisms that lead to processing outside the traditional endolysosomal pathway. For example, non-enveloped simian virus 40 (SV40) exploits CvME for trafficking to the endoplasmatic reticulum (ER) independent of CME (Pelkmans et al. 2002). Many viruses and toxins, such as influenza and Sendai viruses, can also infect cells via non-clathrin and non-caveolae pathways (Sieczkarski and Whittaker 2002). It has been suggested that the clathrin-independent pathways may facilitate more efficient delivery of many forms of nanomedicine, since they reduce delivery to lysosomes (Medina-Kauwe 2007).
Live-cell microscopy enables direct imaging of the intracellular transport of nanoparticles with high spatial resolution and sensitivity. However, the investigation of nanoparticle trafficking in complex intracellular environments has focused largely on static or bulk particle transport properties, which may not reveal individual particle interactions with their cellular environments. Following the landmark paper by Suh and coworkers (Suh et al. 2003), several investigators have applied real-time multiple-particle tracking (MPT) to study the dynamic transport of nanoparticles in live cells (Bausinger et al. 2006; Bergen and Pun 2008; de Bruin et al. 2007; Lai et al. 2008; Payne 2007; Ruthardt and Brauchle 2010; Ruthardt et al. 2011; Suk et al. 2007). MPT is valuable in obtaining quantitative (such as diffusivity and velocity) and qualitative (such as directionality and transport mode classification) information at the individual particle level, which can be critical in revealing cellular processes that control the overall bulk transport properties.
In this review, we briefly outline different endocytic pathways utilized by nanomedicines and their dynamic intracellular transport mechanisms. In view of the great diversity of nanoparticle systems and the complexity of cellular pathways, this review cannot be complete. We will highlight the current fluorescence microscopy tools used to quantify the transport of nanomedicines, followed by examples from the recent literature and our own work with real-time MPT.
Endocytosis and its regulation
Cells employ highly organized endocytic pathways to control the entry of extracellular materials, including nanoparticles (Fig. 1). Endocytosis is a process of uptake of extracellular cargos via invagination and internalization of the plasma membrane. Different endocytic pathways utilize different lipids and/or protein compositions. The delivery of nanomedicine could be enhanced by determining the most effective internalization pathway, and harnessing that pathway to target selective subcellular structures. Below is a summary of our current understanding of the endocytosis of nanomedicines; for a more comprehensive survey, we refer readers to a recent review (Sahay et al. 2010).
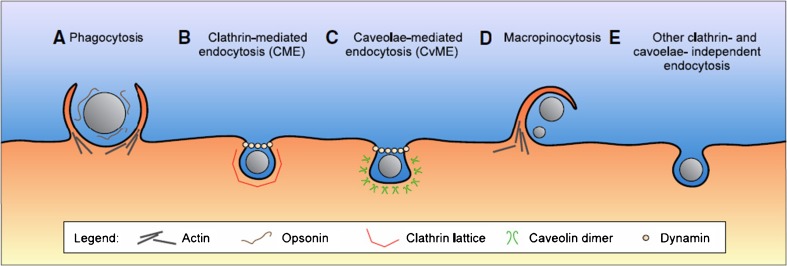
Fig. 1.
Nanoparticle internalization pathways. A Phagocytosis is an actin-based mechanism occurring primarily in professional phagocytes, and closely associated with opsonization. B CME is a widely shared pathway of nanoparticle internalization, associated with the formation of a clathrin lattice and depending on the GTPase dynamin. C CvME occurs in flaskshaped invaginations of the membrane coated with caveolin dimers, also depending on dynamin. D Macropinocytosis is an actin-based pathway, engulfing nanoparticles and the extracellular milieu with a poor selectivity. E Other endocytosis pathways can be involved in the nanoparticle internalization, independent of both clathrin and caveolae. Reprinted with kind permission from Hillaireau and Couvreur (2009)
Phagocytosis
Phagocytosis is employed by specialized cells, such as macrophages, monocytes, neutrophils, dendritic cells and microglia, to clear pathogens, cell debris, or large particles (larger than ~300 nm). Phagocytosis can be described by three distinct steps: recognition by opsonization, adhesion of the opsonized particles to the macrophages, and ingestion of the particles. The phagosome may have different sizes depending on the size of the engulfed particles, which can range from a few hundred nanometers to as large as 20 microns (Hillaireau and Couvreur, 2009). The phagosome and its contents undergo maturation through a series of fusion and fission events, resulting in transfer of the cargo to late endosomes and, ultimately, lysosomes to form a phagolysosome. Model polystyrene particles between 250 nm and 3 μm have been shown to have an optimal in vitro phagocytosis rate, whereas particles smaller than 250 nm were less efficiently internalized (Korn and Weisman 1967). Particles based on other polymers (e.g. modified cellulose, poly(methylmetacrylate), and poly(alkyl cyanoacrylates)) also exhibited a similar trend (Schafer et al. 1992). Liposomes and polymeric nanoparticles with positively or negatively charged surfaces displayed a higher uptake by macrophages as compared to neutrally charged particles (Tabata and Ikada 1988). Particle shape is also receiving increasing attention to control phagocytosis. A recent study demonstrated that local particle shape, measured by tangent angles, at the point of initial contact to the macrophage plays the determining role in phagocytosis, whereas particle size was not a crucial factor (Champion and Mitragotri 2006). The authors found that the local particle shape defined the complexity of actin structures that would need to be rearranged to initiate phagocytosis (Fig. 2).
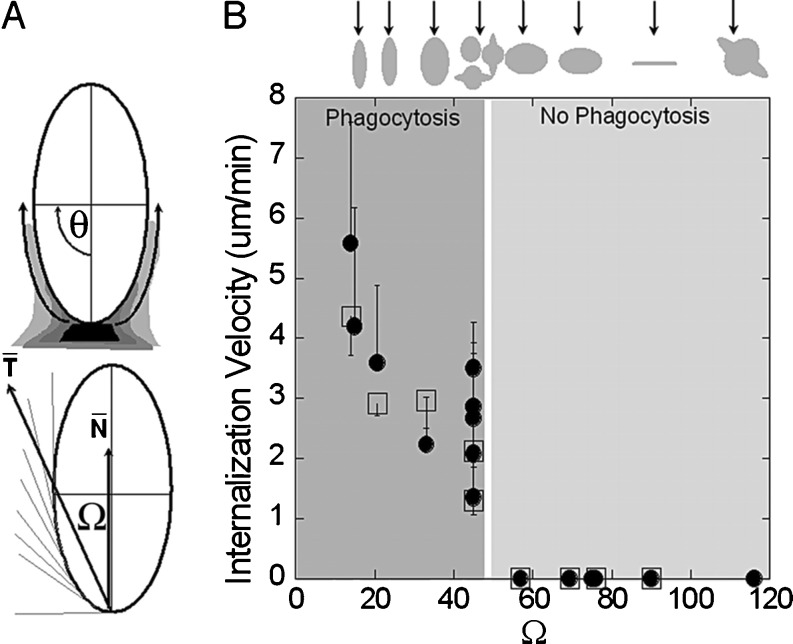
Fig. 2.
Role of particle geometry on phagocytosis. a Entry of nanoparticles depends on the angle between the membrane normal at the point of initial contact and the line defining the particle curvature at this point (Ω). b Internalization velocity is positive at Ω ≤ 45°, which indicates that the particle undergoes internalization. As the angle exceeds critical value (~45°), the macrophages lose the ability to entrap particles and start spreading over the particle. Reprinted with kind permission from Champion and Mitragotri (2006)
Clathrin-mediated endocytosis
Clathrin-mediated endocytosis (CME) is the “classical route” of cellular entry for macromolecules and plasma membrane constituents. It is responsible for the uptake of nutrients, regulation of signaling molecules on the membrane, cell homeostasis, and transmission of synaptic signals. This pathway represents the most common route of cellular entry for internalized ligands, such as transferrin and epidermal growth factor (EGF), and is utilized by a number of viruses, such as adenovirus and Semliki Forest virus, for infection. This mode of endocytosis is thus of paramount importance for nanoparticles bearing these targeting ligands on their surface. Invagination of coated pits results in the formation of clathrin-coated vesicles (100–200 nm), which are pinched off from the plasma membrane by a small GTPase, dynamin (Pucadyil and Schmid 2009). Clathrin-coated vesicles lose their clathrin coat upon internalization and fuse with early endosomes, where they are sorted to late endosomes/lysosomes (pH 5.0–6.0), to the trans-Golgi network, or to recycling endosomes to be transported back to plasma membrane (Rappoport 2008). Contrary to phagocytosis, it is difficult to describe a thorough and consistent profile of nanoparticles uptake for CME. Nanoparticle size is a relevant parameter for the CME pathway, although its significance may depend on cell type. Internalization of polystyrene nanoparticles having a diameter below 200 nm was found to employ CME in non-phagocytic murine melanoma B16 cells (Rejman et al. 2004). Polymeric nanoparticles and lipoplexes possessing a positively charged surface generally display better association and internalization in several cell lines via the CME pathway (Harush-Frenkel et al. 2007). Other examples of nanomedicines that have been shown to employ CME include poly(ethylene glycol)-polylactide (PEG-PLA) nanoparticles, poly(lactic-co-glycolic acid) (PLGA) nanoparticles, silica-based nanoparticles, and chitosan nanoparticles (Sahay et al. 2010).
Caveolae-mediated endocytosis
Caveolae are flask-shaped invaginations (60–80 nm) in the plasma membrane enriched in cholesterol and sphingolipids, and marked by the integral membrane protein caveolin-1 (Simons and Toomre 2000). Caveolae-mediated endocytosis (CvME) is utilized for cellular entry of molecular complexes (albumin), pathogens (cholera toxin B and SV40), and antibody-clustered glycosylphosphatidylinositol (GPI)-anchored proteins. Internalized cargoes can integrate into the classic endosome–lysosome pathway, or can be transported in caveolin-positive vesicles, called caveosomes. Caveosomes differ from early endosomes by their neutral pH and delivery of their cargo to different intracellular organelles, namely the Golgi apparatus or the ER. The uptake kinetics of CvME is known to occur at a much slower rate than that of CME. Several pathogens exploit the caveolae-mediated pathway to prevent lysosomal degradation; hence, this pathway is of considerable interest in nanomedicine (Pelkmans et al. 2001; Rejman et al. 2006). Surprisingly, in one report, 500-nm microspheres underwent slow caveolae-mediated uptake in non-phagocytic murine melanoma B16 cells, whereas microspheres smaller than 200 nm entered cells through CME (Rejman et al. 2004). However, the typical endosome sizes reported (i.e., 100 nm for CME, 50–80 nm for CvME) do not always match with the sizes of the nanoparticles. Therefore, more studies are needed to understand the CvME uptake of larger nanoparticles. Other examples of nanomedicines that have been shown to employ CvME include poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) micelles, self-assembled poly(3-aminopropyl)siloxane (PAPS) nanoparticles, and the FDA approved nanoparticle formulations, DOXIL and Abraxane (Sahay et al. 2010).
Clathrin- and caveolae-independent endocytosis
Clathrin- and caveolae-independent endocytosis has been shown to carry different cargoes including extracellular fluid, SV40 (which can also be taken up in caveolae-mediated endocytosis), cholera toxin subunit B (CTB), GPI-linked proteins, interleukin-2, and growth hormones. For these vesicles (~90 nm), multiple entry pathways appear to exist, and all these pathways require specific lipid compositions and are dependent on cholesterol, but are dynamin-independent (Doherty and McMahon 2009). While their later stages are not clearly identified, they appear to bypass the rab5-positive early endosomes. Ricin and Shiga toxins utilize clathrin- and caveolae-independent mechanisms for transport to the Golgi apparatus and, subsequently, to the ER (Sandvig et al. 2002). There are not many nanoparticle systems known to utilize clathrin- and caveolae-independent endocytosis. We recently reported that 24-nm polystyrene nanoparticles entered HeLa cells via a cholesterol-independent and non-clathrin and non-caveolae mechanism, whereas the same but larger nanoparticles (43 nm) entered via CME (Lai et al. 2007).
Macropinocytosis
Macropinocytosis is the active intake of large amounts of extracellular milieu by closure of membrane ruffles. It is initiated by the activation of receptor tyrosine kinases by growth factors (Mercer and Helenius 2009). Macropinosomes are formed by actin-driven ruffling of the plasma membrane, followed by folding and pinching off of irregularly sized vesicles. The macropinosomes are larger (0.5–10 μm) and distinct compared to other vesicles formed during pinocytosis. Macropinosomes can acidify but do not intersect with lysosomes, thus representing a potential alternative cellular entry route for nanomedicines to avoid lysosomal degradation. This endocytic pathway does not seem to display any selectivity, but is involved in the uptake of various nanoparticles. Particles such as bacteria, apoptotic bodies, necrotic cells, and viruses can induce the ruffling behavior independently of specific signaling molecules and internalize in macropinosomes (Mercer and Helenius 2009). There is no consensus as to the final fate of macropinosomes, and their trafficking seems to depend on cell type and mode of induction.
Dynamics of nanoparticle trafficking in cells
Cellular internalization
Receptor-mediated endocytosis is a common mechanism for cellular internalization of nanoparticles. To understand how nanoparticles interact with the cell surface, the motion of polyplexes on the surface of HuH-7 cells was studied using single-particle tracking (SPT) (Bausinger et al., 2006). The observed particle motion displayed free diffusion with diffusion constants between 10−2 and 10−4 μm2/s, which was attributed to the diffusion of proteoglycans within the plasma membrane. Two-color SPT showed that polyplexes and antibodies against proteoglycans were colocalized during internalization, suggesting that polyplexes bound to proteoglycans are internalized as a unit into the cells (Payne et al. 2007). Recently, it has been suggested that the actin cytoskeleton might be involved in the cellular uptake of polyplexes after binding to the cell surface. After attachment to the cell membrane, EGFR-targeted polyplex displayed slow directed transport with a velocity of 0.01 μm/s (Fig. 3), which was attributed to the movement of the underlying actin cytoskeleton mediated by the EGF receptor and linker proteins (de Bruin et al. 2007). A similar observation was reported for untargeted polyplexes, where cell-surface proteoglycans have been suggested as “unspecific receptors” for electrostatic interaction (Bausinger et al. 2006). In another study, it was shown that caveolae were retained on the cell surface by cortical actin filaments after endocytosis. Disruption of the actin filaments by lantrunculin A facilitated the pinching off and internalization of caveolar vesicles, which then trafficked along microtubules at speeds ranging from 0.3–2 μm/s (Mundy et al. 2002).
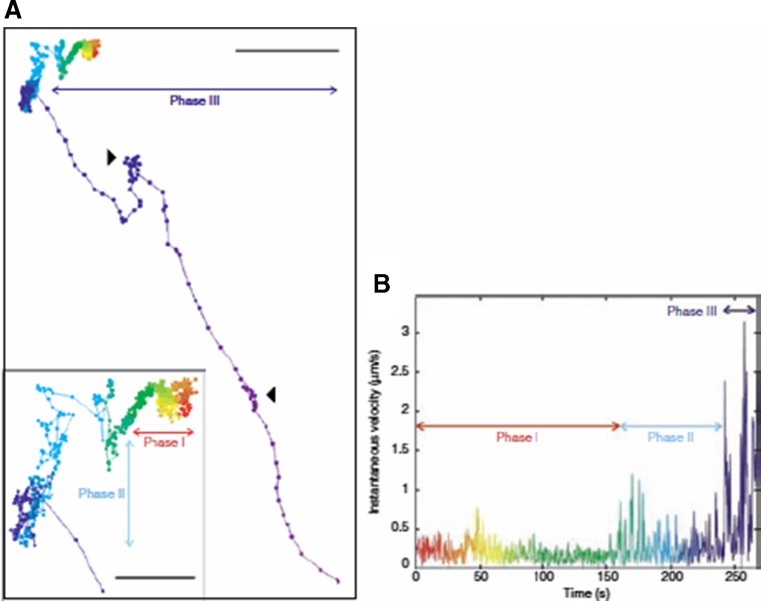
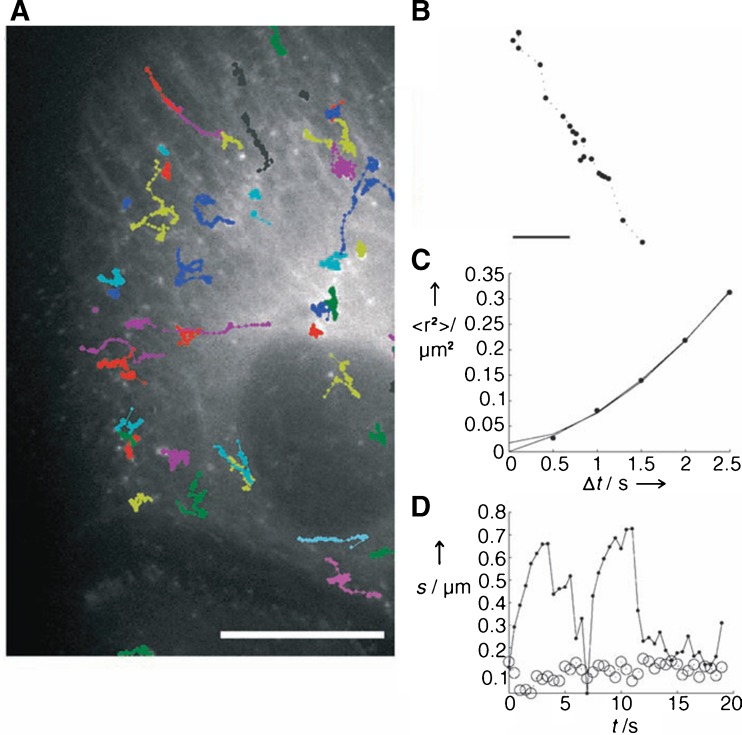
Fig. 3.
Particle transport of EGF-targeted polyplex. a Particle trajectories immediately after the polyplex attaches to the surface of HuH-7 cells. Inset an enlargement of the first part of the trajectory. Scale bar 3 μm; scale bar inset 1 μm. b Instantaneous velocity of EGF-targeted polyplex versus time. The color coding corresponds to matching time points in the trajectory. Phase I slow directed motion, phase II diffusion, phase III directed motion along microtubules. Reprinted with kind permission from de Bruin et al. (2007)
Diffusion in cell cytoplasm
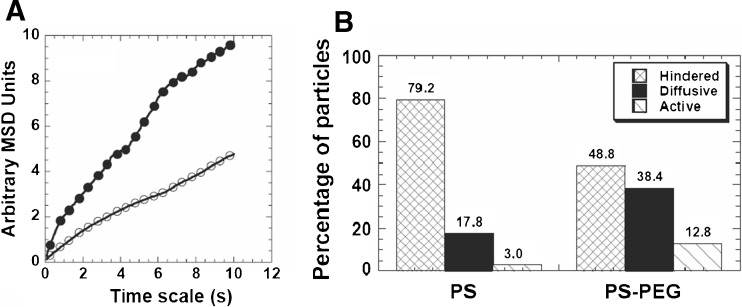
After entering into the cytoplasm, nanomedicines may interact with their target (e.g., a signal transduction protein or receptor) or traffic to subcellular compartments (e.g., nucleus, mitochondria, or peroxisome). The cell cytoplasm is highly crowded with membrane-bounded compartments (organelles such as the ER and Golgi) and macromolecules (mostly proteins), which pose steric and/or adhesive obstacles to the efficient diffusion of nanoparticles (Lechardeur et al., 2005; Suh et al., 2005). The motility of DNA microinjected into the cell cytoplasm was dependent on DNA size. The diffusion of small nucleic acid fragments (<250 bp) was only modestly slowed down, whereas the diffusion of larger DNA (6,000 bp) was greatly reduced (Lukacs et al. 2000). The actin cytoskeleton, with an average mesh size of 50 nm, was the principal determinant of this size-dependent DNA mobility in the cytoplasm (Janson et al. 1996). In a separate study, to reduce potential adhesive interactions with intracellular components, nanoparticles surface-coated with polyethylene glycol (PEG) were microinjected into the cytoplasm of HeLa cells (Suh et al. 2007). MPT revealed that PEGylation increased average diffusivities of 100 nm nanoparticle by 100% compared to unmodified particles (Fig. 4), which correlated with a marked decrease in the number of nanoparticles that underwent hindered transport (from 79.2 to 48.8%). These studies suggest that nanomedicines that have successfully escaped endosomes may still face a formidable task in the cytoplasm to achieve efficient therapeutics delivery to subcellular compartments.
Fig. 4.
Cytoplasmic transport rates of PS or PS-PEG nanoparticles microinjected into live HeLa cells. a Ensemble averaged MSD of PS (open circles, n = 101) or PS-PEG (closed circles, n = 86) nanoparticles. b Overall transport modes of PS (n = 101) or PS-PEG (n = 86) nanoparticles. The hindered group is composed of immobile and sub-diffusive particles. Reprinted with kind permission from Suh et al. (2007)
Role of cytoskeleton and motor proteins
The cytoskeleton provides a highway for transport of vesicles and their encapsulated cargo from one organelle to another. Directed active transport is mediated by the microtubule family of molecular motors, which includes kinesin (towards the cell membrane) and dynein (towards the nucleus), along the cytoskeletal elements. Microtubule-based active transport is an important cellular mechanism that facilitates the delivery of nanomedicine to the nucleus, a strategy used by some DNA viruses. Internalized vesicles emerging from the clathrin-mediated pathway translocate along the microtubules via dynein motors, with an average velocity of 0.7 μm/s (King and Schroer 2000). Active transport of adeno-associated virus (AAV) was found to depend on the presence of intact microtubules and exhibited velocities between 1.8 and 3.7 μm/s, which are on the same order of magnitude as dynein-mediated active transport. MPT revealed that polyethylenimine nanocomplexes (PEI/DNA) achieved rapid perinuclear accumulation due to their active transport along microtubules in COS-7 cells (Suh et al. 2003). Disruption of the microtubule network by nocodazole completely inhibited the active transport of nanocomplexes. In another study, PEI/DNA polyplexes exhibited rapid, microtubule-dependent transport in HuH-7 cells that resulted in a maximum velocity of 0.65 μm/s (Fig. 5), in agreement with the expected values for kinesin and dynein (Bausinger et al. 2006). Polyarginine-coated quantum dots (QDs) have been used to probe the movement of intracellular vesicles along microtubules (Nan et al. 2005). High-resolution particle-tracking (with spatial precision of 1.5 nm) revealed 8-nm steps both towards and away from the nucleus, indicative of dynein and kinesin motor proteins moving along the microtubules.
Fig. 5.
Active transport of PEI/DNA polyplexes in HuH-7 cells. a Fluorescence image of EGFP-labeled microtubules with PEI/DNA complexes (scale bar 10 μm). b Trajectory (scale bar 1 μm) and c MSD plot for an actively transported particle along a microtubule. d Bidirectional movement along microtubules. Time dependence of the particle coordinates along (dots, solid line) and perpendicular to the filament (open circles, dashed line). Reprinted with kind permission from Bausinger et al. (2006)
Dynamics of vesicle trafficking
Vesicles are known to rapidly fuse and bud to achieve proper sorting of various cellular cargoes and for signal transduction. As nanoparticles are internalized by endocytosis, their characteristic motion resembles the movement of the formed vesicles inside the cell. Surprisingly, in primary neurons, MPT revealed that the transport properties of PEI/DNA nanocomplexes were similar to that of adenovirus, although their trafficking pathways were substantially different (Suk et al. 2007). The nanocomplexes trafficked through the endolysosomal pathway to end up in late endosomes/lysosomes, whereas adenoviruses efficiently escaped endosomes after endocytosis. In COS-7 cells, MPT revealed that the intracellular transport of internalized nanocomplexes depend on spatial location and time after transfection (Fig. 6). Within 30 min, the largest fraction of nanocomplexes accumulated near the nucleus. The transport mechanism over 22.5 h after transfection showed that the nanocomplexes in the peripheral region of the cells displayed active transport, whereas nanocomplexes close to the nucleus were largely subdiffusive (Suh et al. 2003). Within axons, QDs conjugated to a single nerve growth factor (NGF) receptor (QD-NGF) displayed active transport along microtubules with a stop-and-go motion towards the cell body (Rajan et al. 2008). Interestingly, the average speed of particle transport varied considerably between axons, suggesting that the endosomal trafficking may differ between individual axons.
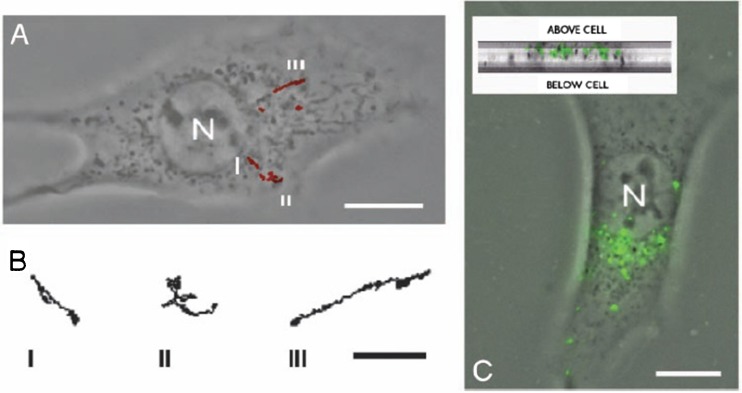
Fig. 6.
Transport of PEI/DNA nanocomplexes in COS-7 cells. a 20-s trajectories (obtained at 33-ms intervals) 4 h after transfection. Three of six complexes shown displayed active transport with linear or curvilinear trajectories. Their detailed trajectories are shown in (b). c PEI/DNA nanocomplexes accumulated in the perinuclear region. Inset cross-section of the COS-7 cell to demonstrate that PEI/DNA nanocomplexes were inside the cell. N the nucleus Scale bars (a, c) 10 μm, (b) 2 μm. Reprinted with kind permission from Suh et al. (2003)
Defining the endocytic pathway by vesicle transport
The mechanisms contributing to the differences in vesicle transport between different endocytic pathways remain unknown. A plausible explanation is that differences exist between different vesicles with regard to the surface density and types of membrane associated proteins (MAPs) that bind to microtubule-associated motor proteins. Alternatively, a higher association affinity of different vesicles to other cytoskeletal networks, such as actins and intermediate filaments, may also lead to the reduced vesicle transport (Lai and Hanes 2008). Polymeric nanoparticles (24 nm) that entered HeLa cells by a non-clathrin- and non-caveolae-dependent mechanism exhibited a 4-fold slower transport rate than that of acidic vesicles carrying 43-nm nanoparticles (Lai and Hanes 2008). Recently, an octaarginine (R8)-modified liposome (R8-Lip) that was taken up via macropinocytosis displayed a slower directional transport rate of 0.21 ± 0.19 μm/s, compared to adenovirus that moved with 0.56 ± 0.24 μm/s (Akita et al. 2010). We recently found that clinically tested DNA nanoparticles composed of poly-L-lysine and PEG, CK30PEG10k, move within live human bronchial epithelial (BEAS-2B) cells at average velocities between 0.09 and 0.11 μm/s, in good agreement with reported values for caveolae (Kim et al. 2011). The active transport rates of CK30PEG10k DNA nanoparticles were up to 8-fold slower, when compared to the transport of 100 nm PS nanoparticles that employed CME.
Biophysical methods to study trafficking
Live-cell fluorescence microscopy is now well established for studying trafficking of nanoparticle systems, and it is the method that arguably causes the least artifacts (Payne, 2007). Real-time multiple particle tracking (MPT) has been used by several investigators to study the endocytosis of nanomedicines and reveal new insights into their complex transport (Bausinger et al. 2006; Lai and Hanes 2008; Payne 2007; Suh et al. 2003; Suk et al. 2007). Other fluorescence microscopy methods, including Forster resonance energy transfer (FRET), fluorescence recovery after photobleaching (FRAP), and fluorescence correlation spectroscopy (FCS), are widely used to study intracellular trafficking of nanomedicines within living cells (Remaut et al. 2007). While FRAP and FCS measure averaged transport properties of a large ensemble of nanoparticles, MPT provides detailed dynamic transport information of each individual nanoparticle. Furthermore, investigation of the transport data from hundreds of individual particles provides important statistical insights of the population as a whole.
Basics of multiple particle tracking
MPT involves resolving dynamic motions of hundreds of individual particles in a given environment, using fluorescence video microscopy. Although the resolution of optical microscopes is typically around 250 nm, the position of nanoparticles can be determined with sub-resolution accuracy by finding the center of the diffraction-limited spot. Due to current limitations in microscopy, the motions of nanoparticles are typically tracked in two-dimensions (2D), which provides accurate information on particle behavior if the biological environment (e.g., cell cytoplasm) is locally isotropic, but not necessarily homogeneous. In this case, the displacements of a particle along the x, y, and z axes (Δx, Δy, Δz) are uncorrelated; hence, <Δx 2 > = < Δy 2 > = < Δz 2 >, so the mean squared displacement (MSD) in 2D is given by
| 1 |
which is equal to two-thirds of the MSD in 3D,
| 2 |
Transport modes
Particle transport in complex cellular environments is highly heterogeneous. Similarly transporting particles can be grouped into useful classes, called transport modes, which provide important insights into particle transport mechanisms and limitations. The Brownian motion of particles in a purely viscous (i.e., not elastic) environment tracked in 2D can be described by
| 3 |
where the time scale-independent diffusivity D o is governed by the Stokes Einstein relationship D o = k B T/6πηa, where k B is Boltzmann’s constant, T is the temperature, η is the fluid viscosity, and a is the particle radius. The key feature of unobstructed diffusion is that D o is independent of time scale, τ. Thus, D o can be obtained from D o = <Δr 2(τ) > 2D/4τ. Purely diffusive Brownian motion can be identified by a slope of 1 on an MSD versus τ log–log plot. The hindered transport arising from steric and adhesive interactions can be characterized by
| 4 |
where the value of α quantifies the extent of impediment (0 < α < 1; smaller α values represent more strongly hindered transport). In the case of strong interactions between nanoparticles and their microenvironment, the impediment may lead to displacements below the microscope’s detection limit, which we often refer to as immobile. Nanoparticles may also utilize motor proteins to undergo energy-dependent active transport, which is characterized by large, directed displacements and saltatory motions. The mean velocity of active transport is determined from the measured MSD of a particle through the relation
| 5 |
By fitting Equation 5 to an MSD curve, the mean velocity υ of the directed motion and the diffusion coefficient Do are obtained. In the absence of facilitated transport mechanisms, Equation 5 can be used to describe displacements influenced by convection, perhaps due to fluid flow.
Classification of transport modes
In complex cellular environments, individual particles may experience episodes of hindered transport, free diffusion, and facilitated active transport. To dissect various types of motion displayed by a moving particle, the MSD must be analyzed over subregions of the trajectory. Despite the ambiguity, it is possible to distinguish particles that move substantially faster or slower than diffusive particles by analyzing the Relative Change (RC) in effective diffusivity. For more information on MPT and the concept of RC, the reader is referred to previous reviews (Lai and Hanes 2008; Suh et al. 2005).
Conclusion and perspectives
Understanding the cellular entry and trafficking of nanoparticle systems is essential to the field of nanomedicine. Future nanomedicines may utilize specific endocytic trafficking mechanisms to deliver therapeutics to selective intracellular destinations. Using real-time MPT, the dynamics of intracellular transport of nanomedicines has begun to be uncovered. In-depth understanding of these transport processes will lead to greater success in directing endocytosed therapeutics to their desired intracellular targets. However, a number of challenges remain. Studying such complex biological barriers with reliable in vitro systems remains difficult, and it is futher complicated by disparities in the experimental conditions used by different research groups. Another challenge is that our curerent understanding is based mostly on in vitro studies. Development of novel imaging techniques to study in vivo intracellular trafficking of nanomedicines remains a key challenge and opportunity for the field.
Acknowledgments
The project described was supported by Grant Numbers P01HL51811 and F32HL103137 (for A.J.K.) from the National Heart, Lung, And Blood Institute and R01EB003558 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute of Biomedical Imaging and Bioengineering, or the National Institutes of Health. We thank Nick Boylan, Himat Patel, Benjamin Schuster, and Jungsoo Suk for helpful comments.
Conflict of Interest
None
Contributor Information
Anthony J. Kim, Email: akim61@jhu.edu
Justin Hanes, Phone: +1-410-6146512, Email: hanes@jhu.edu.
References
- Akita H, Enoto K, Masuda T, Mizuguchi H, Tani T, Harashima H. Particle tracking of intracellular trafficking of octaarginine-modified liposomes: a comparative study with adenovirus. Mol Ther. 2010;18:955–964. doi: 10.1038/mt.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausinger R, von Gersdorff K, Braeckmans K, Ogris M, Wagner E, Brauchle C, Zumbusch A. The transport of nanosized gene carriers unraveled by live-cell imaging. Angew Chem Int Ed Engl. 2006;45:1568–1572. doi: 10.1002/anie.200503021. [DOI] [PubMed] [Google Scholar]
- Bergen JM, Pun SH. Analysis of the intracellular barriers encountered by nonviral gene carriers in a model of spatially controlled delivery to neurons. J Gene Med. 2008;10:187–197. doi: 10.1002/jgm.1137. [DOI] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- de Bruin K, Ruthardt N, von Gersdorff K, Bausinger R, Wagner E, Ogris M, Brauchle C. Cellular dynamics of EGF receptor-targeted synthetic viruses. Mol Ther. 2007;15:1297–1305. doi: 10.1038/sj.mt.6300176. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- Hillaireau H, Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson LW, Ragsdale K, Luby-Phelps K. Mechanism and size cutoff for steric exclusion from actin-rich cytoplasmic domains. Biophys J. 1996;71:1228–1234. doi: 10.1016/S0006-3495(96)79367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Boylan NJ, Suk JS, Lai SK, Hanes J (2011) Non-degradative intracellular trafficking of highly compacted polymeric DNA nanoparticles. J Control Release. doi:10.1016/j.jconrel.2011.10.031 [DOI] [PMC free article] [PubMed]
- Korn ED, Weisman RA. Phagocytosis of latex beads by Acanthamoeba. II. Electron microscopic study of the initial events. J Cell Biol. 1967;34:219–227. doi: 10.1083/jcb.34.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Hanes J. Real-time multiple particle tracking of gene nanocarriers in complex biological environments. Methods Mol Biol. 2008;434:81–97. doi: 10.1007/978-1-60327-248-3_6. [DOI] [PubMed] [Google Scholar]
- Lai SK, Hida K, Chen C, Hanes J. Characterization of the intracellular dynamics of a non-degradative pathway accessed by polymer nanoparticles. J Control Release. 2008;125:107–111. doi: 10.1016/j.jconrel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Hida K, Man ST, Chen C, Machamer C, Schroer TA, Hanes J. Privileged delivery of polymer nanoparticles to the perinuclear region of live cells via a non-clathrin, non-degradative pathway. Biomaterials. 2007;28:2876–2884. doi: 10.1016/j.biomaterials.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Lechardeur D, Verkman AS, Lukacs GL. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv Drug Deliv Rev. 2005;57:755–767. doi: 10.1016/j.addr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- Medina-Kauwe LK. "Alternative" endocytic mechanisms exploited by pathogens: new avenues for therapeutic delivery? Adv Drug Deliv Rev. 2007;59:798–809. doi: 10.1016/j.addr.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Mossalam M, Dixon AS, Lim CS. Controlling subcellular delivery to optimize therapeutic effect. Ther Deliv. 2010;1:169–193. doi: 10.4155/tde.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- Nan X, Sims PA, Chen P, Xie XS. Observation of individual microtubule motor steps in living cells with endocytosed quantum dots. J Phys Chem B. 2005;109:24220–24224. doi: 10.1021/jp056360w. [DOI] [PubMed] [Google Scholar]
- Payne CK. Imaging gene delivery with fluorescence microscopy. Nanomedicine (Lond) 2007;2:847–860. doi: 10.2217/17435889.2.6.847. [DOI] [PubMed] [Google Scholar]
- Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan SS, Liu HY, Vu TQ. Ligand-bound quantum dot probes for studying the molecular scale dynamics of receptor endocytic trafficking in live cells. ACS Nano. 2008;2:1153–1166. doi: 10.1021/nn700399e. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discovery. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- Rappoport JZ. Focusing on clathrin-mediated endocytosis. Biochem J. 2008;412:415–423. doi: 10.1042/BJ20080474. [DOI] [PubMed] [Google Scholar]
- Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. J Liposome Res. 2006;16:237–247. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut K, Sanders NN, De Geest BG, Braeckmans K, Demeester J, De Smedt SC. Nucleic acid delivery: Where material sciences and bio-sciences meet. Mat Sci Eng R. 2007;58:117–161. doi: 10.1016/j.mser.2007.06.001. [DOI] [Google Scholar]
- Ruthardt N, Brauchle C. Visualizing uptake and intracellular trafficking of gene carriers by single-particle tracking. Top Curr Chem. 2010;296:283–304. doi: 10.1007/128_2010_66. [DOI] [PubMed] [Google Scholar]
- Ruthardt N, Lamb DC, Brauchle C (2011) Single-particle tracking as a quantitative microscopy-based approach to unravel cell entry mechanisms of viruses and pharmaceutical nanoparticles. Mol Ther (in press) [DOI] [PMC free article] [PubMed]
- Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, Iversen TG. Pathways followed by ricin and Shiga toxin into cells. Histochem Cell Biol. 2002;117:131–141. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- Schafer V, von Briesen H, Andreesen R, Steffan AM, Royer C, Troster S, Kreuter J, Rubsamen-Waigmann H. Phagocytosis of nanoparticles by human immunodeficiency virus (HIV)-infected macrophages: a possibility for antiviral drug targeting. Pharm Res. 1992;9:541–546. doi: 10.1023/A:1015852732512. [DOI] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Suh J, Choy KL, Lai SK, Suk JS, Tang BC, Prabhu S, Hanes J. PEGylation of nanoparticles improves their cytoplasmic transport. Int J Nanomed. 2007;2:735–741. doi: 10.2217/17435889.2.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Suh J, Wirtz D, Hanes J. Efficient active transport of gene nanocarriers to the cell nucleus. Proc Natl Acad Sci USA. 2003;100:3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Suh J, Lai SK, Hanes J. Quantifying the intracellular transport of viral and nonviral gene vectors in primary neurons. Exp Biol Med (Maywood) 2007;232:461–469. [PubMed] [Google Scholar]
- Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9:356–362. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]