Abstract
Myosin filaments in muscle, carrying the ATPase myosin heads that interact with actin filaments to produce force and movement, come in multiple varieties depending on species and functional need, but most are based on a common structural theme. The now successful journeys to solve the ultrastructures of many of these myosin filaments, at least at modest resolution, have not been without their false starts and erroneous sidetracks, but the picture now emerging is of both diversity in the rotational symmetries of different filaments and a degree of commonality in the way the myosin heads are organised in resting muscle. Some of the remaining differences may be associated with how the muscle is regulated. Several proteins in cardiac muscle myosin filaments can carry mutations associated with heart disease, so the elucidation of myosin filament structure to understand the effects of these mutations has a clear and topical clinical relevance.
Keywords: Myosin filaments, Myosin heads, Muscle, Heart disease
Introduction
The present day ideas about how muscle works were based on two fundamentally important papers from the 1950s (Huxley and Hanson 1953; Huxley and Niedergerke 1953) which showed that the changing length of the fundamental building block of muscle, the sarcomere (Fig. 1), is associated with the sliding of relatively inextensible filaments, the myosin and actin filaments. This so-called ‘sliding filament model’ of muscle contraction is the framework on which all subsequent ideas about muscle contraction have been based.
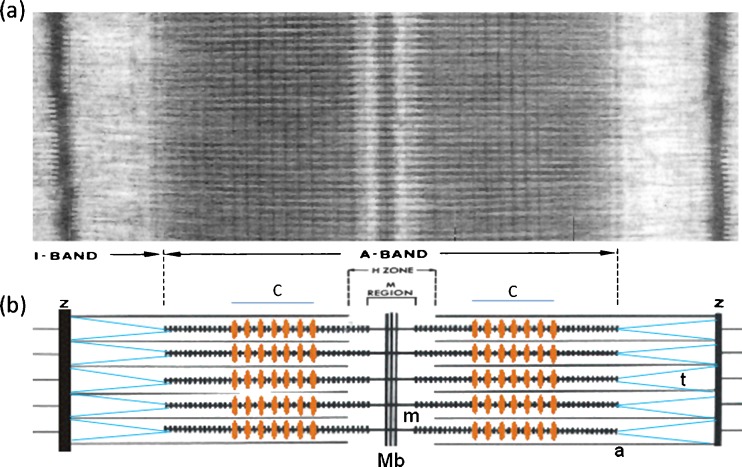
Fig. 1.
a Electron micrograph of a longitudinal section from frog muscle showing the A-band where the bipolar myosin filaments are (see b). The actin filaments run through the I-band from the Z-line and into the A-band where they interdigitate with the myosin filaments. The actin filaments end at the edge if the H-zone. b Schematic diagram of the overlapping myosin (m) and actin (a) filaments. The protein titin (t; blue) runs from the M-band (Mb) along the myosin filaments and then across the I-band to the Z-line (Z). C-protein (MyBP-C) forms a set of stripes in the two halves of the A-band at the positions marked C (orange stripes). C-protein binds to the myosin filament backbone and in some conditions extends out to bind to actin. Figure modified from Fig. 5 of Squire et al. (2005)
In order to understand how a particular muscle works, it is necessary to know the structures of the actin, myosin and other filaments and assemblies in the muscle; here, we concentrate on the structures of myosin filaments. The myosin filaments carry projections which interact with the adjacent actin filaments to produce muscular force and movement. Myosin filaments have been shown to occur in different forms in different muscle types, but they are all variations on a common theme. In this short review, the structures and symmetries of different myosin filament types are briefly described. Since other reviews of the field have been written recently (e.g. Barral and Epstein 1999; Squire et al. 2005; Craig and Woodhead 2006), the present review approaches the topic slightly differently in that it attempts to provide an overview of the fundamentally important conceptual and technical advances that have taken the field forward since 1953.
The early years—bipolar myosin filaments—two heads are better than one
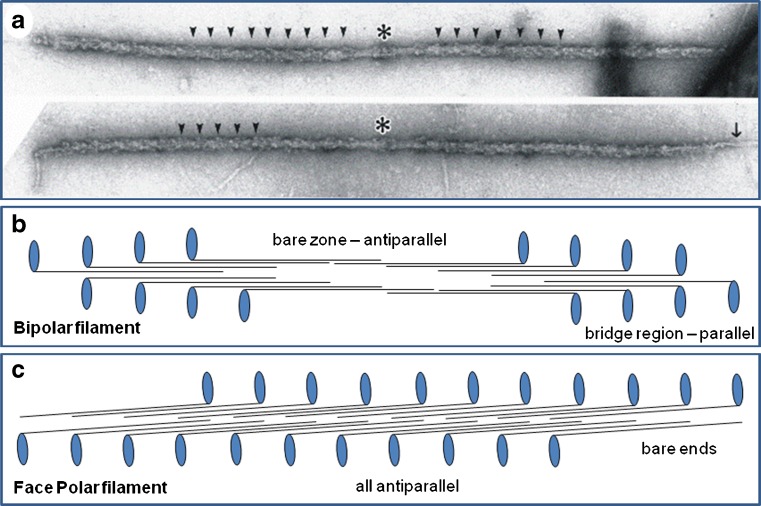
In his early pioneering work on muscle myosin filaments with the electron microscope and the application of negative staining to isolated biological particles, Huxley (1963) defined the underlying packing scheme for bipolar myosin filaments. Myosin molecules, about 150 nm long, had been seen to consist of a long narrow rod on one end of which was a 10- to 15-nm globular domain (Fig. 2b, c). Images of myosin filaments from vertebrate striated muscles showed them to be about 1.6 µm long with a bare central region called the bare zone and outer ends apparently covered in projecting masses (Fig. 2a). Huxley therefore proposed that the rods of the myosin molecules were packed in an antiparallel fashion in the middle of the filament (the bare zone on isolated filaments or the M-region in muscle; Figs. 1 and 2b) and that additional myosin molecules would then grow the filament outwards from the middle using parallel interactions (Fig. 2a, b). This scheme has not been faulted and appears to apply to most (not all) myosin filament types whatever their origin and size.
Fig. 2.
a Electron micrographs of isolated myosin filaments from bony fish muscle, adapted from AL-Khayat et al. (2008). The M-band is centrally located (asterisk) and some of the 43 nm crown repeats are indicated by arrowheads. The single arrow on the right indicates so-called end filaments which are part of the titin assembly at the tip of the myosin filaments (Trinick 1981). b Schematic diagram showing how myosin molecules pack together to give bipolar myosin filaments. Each myosin molecule is represented as a black line with a blue globular region on one end. Each blue oval represents the myosin head pair of one molecule. Rod packing is antiparallel in the middle of the filaments and parallel at each end. The parallel packing continues for very many crowns on each side of the central bare zone (the head-free region). Myosin molecules in one half of the filament have opposite polarity to those in the other half. This compares with the different packing scheme in (c) for the face polar myosin filaments in vertebrate smooth muscle. Here, the packing is antiparallel throughout and the molecules on opposite faces of the filaments point in opposite directions
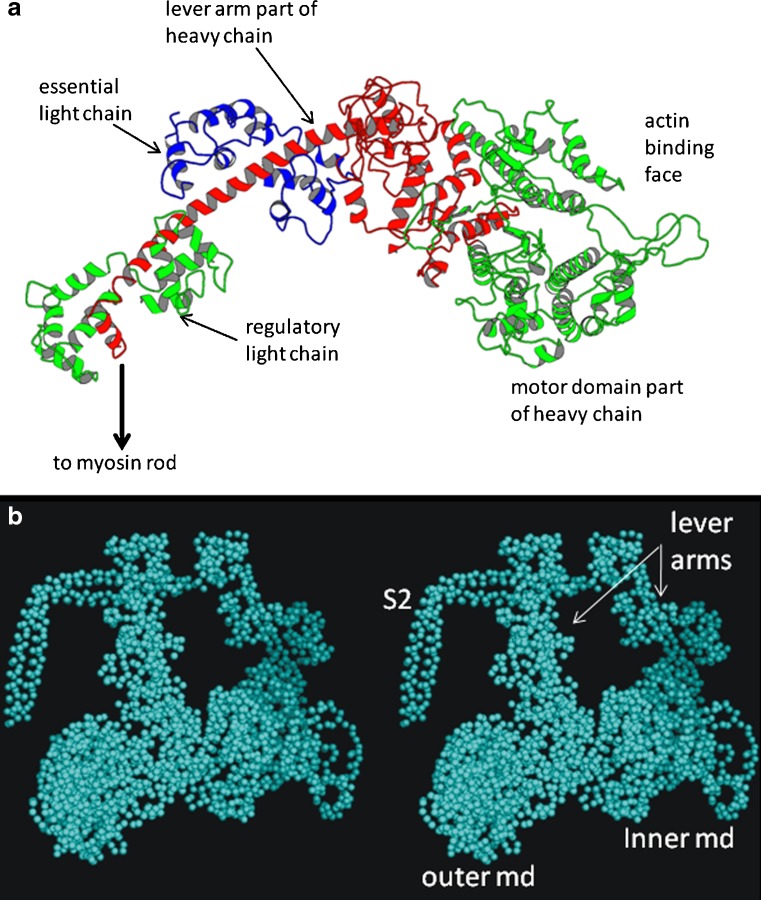
Further studies of myosin molecules showed them to consist of parallel, two-chain, coiled-coil, α-helical rods with globular domains on the ends of each rod (see Squire 1986b, Ch. 4; Sellers 2000). These two globular domains, originally seen as one mass by Huxley, are now known to carry the enzymatic active site of the protein; they are ATPases which interact with and are activated by actin filaments in the muscle sarcomere (Fig. 1). Each globular domain is termed a myosin head, the structure of which has been determined by protein crystallography (Rayment et al. 1993a, b; Fig. 3a). The main part of the head, often termed the motor domain, consists of a globular domain with an actin-binding face and housing the ATP binding site. The rest of the head is a narrower neck region linking back to the myosin rod. The motor domain, the central α-helical part of the neck region and one half of the myosin rod are formed by a single ‘heavy’ chain of myosin. In addition, in muscle, the neck region of the head carries two light chains, the essential light chain and the regulatory light chain (Fig. 3a). Muscle myosin is part of a diverse superfamily; it is Myosin II. Some non-muscle myosins carry many more light chains (Sellers 2000; Kendrick-Jones et al. 2009) and have very long lever arms, but many do not form filaments, so they will not be mentioned further here.
Fig. 3.
a Ribbon diagram of the structure of the myosin head determined by Rayment et al. (1993a, b), showing the key components of the head; the motor domain shown in green on the right, the continuation of the heavy chain in red going back to link to the myosin rod, and the two light chains, the essential light chain (blue) and the regulatory light chain (green left). b Stereo pair of a head coupling scheme similar to the Wendt et al. (2001) structure for vertebrate smooth muscle HMM. The motor domain (md) of the inner head (right) binds to the junction between the essential light chain and the back of the motor domain of the outer head. S2 is part of subfragment 2 of the rod part of the myosin molecule. The whole of S2 together with two heads is known as heavy meromyosin (HMM)
Early ideas about how muscle force and movement are produced, especially before the myosin head structure was determined, suggested that once bound to an actin filament in an active muscle the myosin heads might change their tilt or structure on actin thus moving the actin filament past the myosin filament (e.g. Reedy et al. 1965; Huxley 1969; Lymn and Taylor 1971). With the availability of different crystal structures of the head showing the neck domain in different configurations on the motor domain (e.g. Rayment et al. 1993a, b; Dominguez et al. 1998; Houdusse et al. 2000), this story developed to the idea that the motor domains would interact relatively rigidly with actin and that the movement would be produced by the neck regions of the myosin heads swinging on the motor domains. In this scenario, the neck would act as a kind of lever moving the actin filament past the myosin filament so it came to be known as the lever arm of the myosin head.
Detailed knowledge of myosin filament structure will show how the myosin filaments are assembled, what their important components are, how the myosin heads are organised in resting muscle and how the heads need to move in order to generate force. It might also shed light on the mechanisms of regulation in different muscles. There are four main structural aspects to this understanding of myosin filaments. The first is to know the lattice arrangement of the heads on the filament surface. The second is to define the configuration of each myosin head pair in space on each of these lattice points. The third is to discover how the myosin rods are packed in the myosin filament backbone to produce the observed surface lattices. The fourth is to discover how the non-myosin parts of the filament are organised; for example, titin and C-protein in vertebrate striated muscle filaments. Here, we consider the first two aspects in some detail and briefly discuss backbone packing and non-myosin proteins.
Development: repeats and early models for two striated muscle myosin filaments
X-ray diffraction
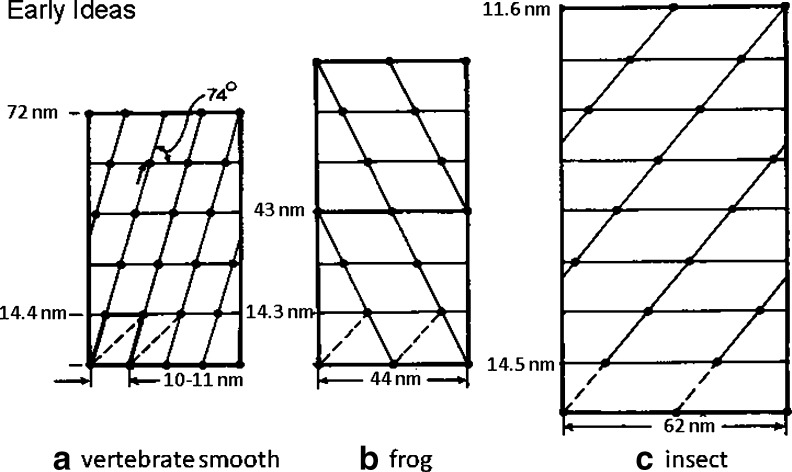
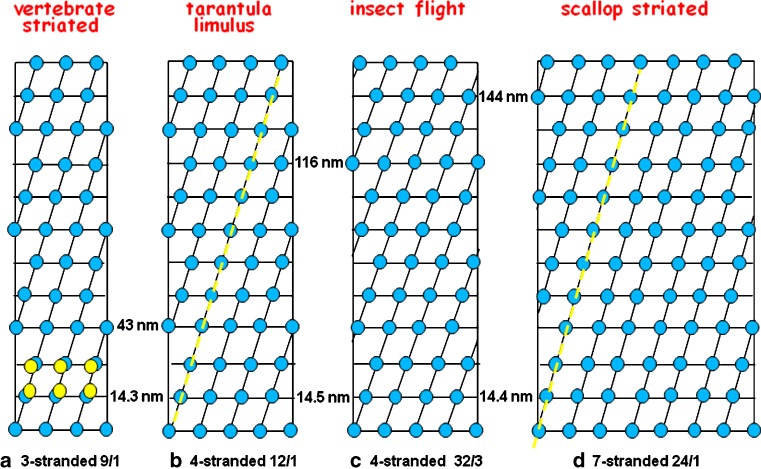
The first ideas about the myosin head surface lattices on myosin filaments came from Huxley again (Huxley and Brown 1967) studying frog muscle structure by X-ray diffraction. The proposed structure is illustrated in Fig. 4b; it can be described as a 2-start helix with the heads of two myosin molecules diametrically opposed across the myosin filament (i.e. 180° apart around the filament surface) at the same axial level; a so-called crown of heads. The term 2-start describes the number of parallel co-axial helices that can be drawn through the surface array of myosin heads (see Fig. 4). In frog muscle myosin filaments, the next crown was proposed to be about 14.3 nm along the myosin filaments, but rotated around the filament axis by 60°. The kind of illustration in Fig. 4b is known as a radial or helical net. One can imagine it being produced by wrapping a piece of paper around the myosin filament to produce a cylinder and marking on it where each of the myosin head pairs is located. When the paper is opened out again the helical net is seen. The left hand and right hand edges are the same position on the filament; this is the seam where the cylinder would join. Tilted straight lines on the helical net represent separate helical strands on the filament. In the case of frog muscle, the head pairs 180° apart on the first crown and subsequent rotation of crowns by 60° makes the fourth crown the same as the first one (Fig. 4b). There is therefore an axial repeat of 3 × 14.3 nm ∼ 43 nm. Helical structures are often described in terms of the number of repeating units (in this case myosin head pairs) in one pitch of the helix on which they lie. The pitch is the axial movement in one turn of the helical strand when the strand has rotated around the filament axis by 360°. The model of Huxley and Brown (1967) has six head pairs in one turn of the helix and there are two helical strands, so this is therefore a 2-start or 2-stranded, 6/1 or 61 helix. Molecular packing models for such a 2-stranded structure were suggested by Pepe (1975).
Fig. 4.
The early published ideas about the arrangements of myosin heads on myosin filaments in a vertebrate smooth muscle (Small and Squire 1972), b frog sartorius muscle (Huxley and Brown 1967) and c insect (Lethocerus) flight muscle (Reedy 1968). The marked differences in the way that the myosin molecules would have to pack to make these structures prompted Squire (1971) to propose an alternative set of structures for (b) and (c)
Electron microscopy
The next filament type to be studied and for which a symmetry model was proposed was based on using electron microscopy of sections of the beautifully ordered flight muscles of the insect Lethocerus (the giant water bug). This pioneering study by Reedy (1968) also gave the insect muscle myosin filaments a 2-stranded helical structure, once again with an axial crown repeat of around 14.5 nm, now found to be a fundamental spacing in all myosin filaments. However, in this case the long repeat of the multistrand helix (the axial repeat) was found to be after 8 crowns, giving the filament an axial repeat of 8 × 14.5 nm ∼ 116 nm. By 1968, then, there were two bipolar myosin filament models, both 2-stranded, both with a crown spacing of 14.3–14.5 nm, but with different axial repeats. Note that, since the myosin rods are very much longer than the crown repeat, a cross-section of a myosin filament at a single crown, where there might be just a few (here two) head pairs in the crown, will have many more myosin rods in the backbone with the heads on different rods appearing at different crown levels along the filament.
The next type of filament to have its symmetry modelled was from a completely different myosin filament type, namely from vertebrate smooth muscle. Rather than having the myosin heads pointing in opposite directions in the two halves of a myosin filament as in bipolar filaments (Fig. 2b), in this case what was found was heads pointing in opposite directions on opposite faces of a rather flat ribbon-shaped structure (Fig. 2c); the myosin ribbon structure was said to be face polar (Small and Squire 1972). The original study was under rather non-physiological conditions and gave rise to myosin ribbons of constant thickness but variable width as though the myosin molecules could assemble against the filament edges to make a wider ribbon. Later studies suggested that under normal conditions vertebrate muscle myosin filaments were still face polar but were rather narrower than seen by Small and Squire. Craig and Megerman (1977) also showed that such filaments could be produced synthetically. They termed their filaments side-polar, but the meaning and the symmetry is the same as the ribbons and the original name seems preferable since the myosin heads still emanate from the faces of the filaments where they will have a defined lateral packing arrangement and the sides of the filament are bare.
The Small and Squire study used electron microscopy of sections and in these sections some of the face polar myosin filaments could be viewed face on. Even though unusually wide, the ribbons are still perfectly good aggregates of myosin. Because of their width one could extract from these views preliminary evidence about the lattice of myosin heads. What was deduced at the time is shown in Fig. 4a. Once again there is a 14.4-nm axial spacing in the surface lattice, something that was very clear in the face views, but this time the lateral separations of adjacent heads, not so easy to determine, only appeared to be about 10–12 nm. Although it was hard to define, the axial repeat appeared to be 5 × 14.4 nm ∼ 72 nm.
This result led to a puzzle. Since the myosin molecules in all of these muscles, vertebrate striated, insect flight and vertebrate smooth, appeared to be similar, why would they pack so differently in different myosin filaments? The lateral separation of heads in vertebrate smooth muscle face polar ribbons appeared to be about 10–12 nm, in the 2-stranded Huxley and Brown model for a frog muscle filament of around 7 nm radius the equivalent separation would be about 22 nm and for the insect flight muscle myosin filaments of radius about 8–10 nm, it would be around 25–31 nm. Could similar myosin molecules pack in such different ways?
2-strands are not enough. Is there a common theme?
It was this dichotomy which led Squire (1971, 1972) to propose some different models for myosin filaments. These new models were based on the suggestion that the surface packing arrangements for all myosin filaments are probably very similar. Based on this, the frog muscle myosin filaments might be 3-stranded or 4-stranded and the insect flight muscle myosin filaments might be up to 6-stranded. All these structures would have the same crown repeat of around 14.3–14.5 nm, but they would have different helical pitches for the strands to keep the frog muscle axial repeat at 43 nm and the insect flight muscle axial repeat at 115 nm. After this suggestion, there was considerable activity over an extended period trying to decide what the true symmetries are.
The main problem in trying to study vertebrate striated muscle myosin filaments by electron microscopy was that it was very difficult to preserve the order in the myosin heads known to be present from X-ray diffraction studies of intact muscle. Although the rotational symmetry of these myosin filaments could be seen clearly and unambiguously in muscle sections to be 3-fold (Luther et al. 1981), as was also inferred from X-ray diffraction studies (Squire 1981), it was Kensler and Stewart (1983) who were the first to preserve these filaments well enough to see the ordered 3-stranded myosin head array in electron micrographs (e.g. Fig. 2a). By contrast, myosin filaments in some invertebrate muscles were relatively easy to obtain with the myosin heads still ordered (see below), and these were found to have different rotational symmetries.
To summarise the work of many authors, in the end it was shown by electron microscopy and X-ray diffraction that vertebrate striated muscle myosin filaments have 3-strands of heads (i.e. the filament has 3-fold symmetry around its long axis; Luther et al. 1981; Kensler and Stewart 1983) and insect flight muscle myosin filaments have 4-strands (4-fold symmetry; Wray 1979a; Reedy et al. 1981; Morris et al. 1991). The symmetries of other invertebrate myosin filament types have also been determined, of particular note being Limulus telson muscle and tarantula leg muscle myosin filaments which are both 4-stranded (Wray et al. 1974; Stewart et al. 1981, 1985; Crowther et al. 1985) and the filaments of scallop striated adductor muscle which are 7-stranded (Vibert and Craig 1983; Craig et al. 1991). All these filaments have the characteristic 14.3–14.5 nm myosin crown spacing, but they have varying axial repeats. Their characteristics are summarised in Table 1.
Table 1.
Myosin filament symmetries
| Muscle type | Rotational symmetry | Axial symmetry | Lattice type | Crown repeat | Full repeat | Lateral repeat |
|---|---|---|---|---|---|---|
| Vertebrate smooth muscle | N/a | Face polar | Planar | 14.5 nm | 72 nm? | 10–12 nm |
| Tarantula | 4-stranded | Bipolar | Helical | 14.5 nm | 43 nm | 15–16 nm |
| Limulus | 4-stranded | Bipolar | Helical | 14.5 nm | 43.5 nm | 15–16 nm |
| Insect flight muscle | 4-stranded | Bipolar | Helical | 14.5 nm | 116 nm | 14 nm |
| Scallop striated muscle | 7-stranded | Bipolar | Helical | 14.4 nm | 144 nm | 14 nm |
| Vertebrate striated muscle | 3-stranded | Bipolar | Quasi-helical | 14.3 nm | 43 nm | 14–15 nm |
Figure 5 shows helical nets of the known myosin filament lattices for bipolar myosin filaments adjusted for their diameters. The similarities between the basic cells in each of these surface lattices are evident; myosin molecules certainly do appear to like to pack in a particular way. The slight variation in tilt angle of the helical strands between species generates the variable long repeat that has been seen.
Fig. 5.
The accepted symmetries for the myosin filaments in a vertebrate striated muscle, b tarantula and Limulus myosin filaments, c insect flight muscle (Lethocerus) and d scallop striated adductor muscle. These structures all have the myosin head pairs grouped in very similar surface arrays; a common axial repeat of about 14.3–14.5 nm and a common lateral spacing of around 10–15 nm). The slightly different tilt angles of the helical strands give rise to the different observed long repeats. The yellow dashed lines in (b) and (d) indicate the long-pitched helices along which the Class III head interactions (Fig. 6) were originally thought to occur
Note that all the invertebrate muscle myosin filaments contain the protein paramyosin (Kantha et al. 1990), which is like the rod part of the myosin molecule without the two heads. The presence of paramyosin appears to enable the building of large diameter filaments with higher rotational symmetry (4-fold or greater) than in vertebrate striated muscle filaments where paramyosin is absent. The paramyosin forms a core which fills part or all of the hollow centre of these larger diameter filaments. The very large myosin-containing filaments in molluscan smooth muscles have a variable diameter and are largely made of paramyosin with a narrow ‘crust’ of myosin on the surface (Squire 1971; Bennett and Elliott 1984).
Helical Reconstruction and X-ray Diffraction Modelling
In the early 1970s, the ability to determine filamentous structures from electron micrographs was greatly enhanced by the introduction of the new technique of helical reconstruction (Moore et al. 1970). Originally applied to actin filament structure, the new method was soon applied to images of isolated myosin filaments, particularly those from invertebrate muscles where the myosin head arrays appeared to be perfectly helical and were relatively easily preserved. One of the problems in analysing the myosin filaments from vertebrate striated muscles, apart from the difficulty of preserving the head array, is that the helix is not quite as in Fig. 5b since there is a regular perturbation from this structure, as pointed out by Huxley and Brown (1967) and indicated in one repeat of the figure by yellow circles. This means that in relaxed muscle the head arrangements on the three crowns in one 43-nm repeat are not quite the same. The implications of this perturbed structure will be discussed later.
Going back to the perfectly helical invertebrate filaments, 3-dimensional reconstruction was applied to electron micrograph images of negatively-stained filaments from tarantula muscle (Crowther et al. 1985), Limulus telson muscle (Stewart et al. 1985), insect flight muscle (Morris et al. 1991) and scallop striated adductor muscle (Vibert 1992), all in the relaxed state. These not only helped to confirm the rotational symmetries of the filaments, as summarised earlier, but they also gave the first indications of the resting myosin head configurations on the filament surface. For the latest structures and other earlier references, see Zhao et al. (2009) for Limulus, Alamo et al. (2008) for tarantula and Al-Khayat et al. (2009) for scallop.
Ideas about head conformations in resting muscle
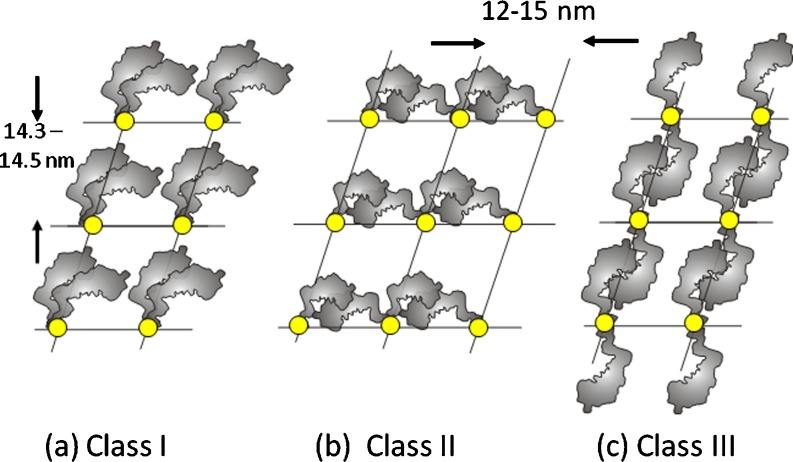
In considering how the myosin heads might be organised on the filament surface, Squire et al. (2005) chose to conceptualise the possible configurations as in Fig. 6. Here, if the helical structures are stabilised by head to head interactions in resting muscle, then the two heads of one myosin molecule might interact with each other in a parallel fashion (Class I), they might separate laterally and interact with a head from an adjacent molecule in the same crown (Class II), or the two heads might separate and interact up and down the filament with head interactions between successive crowns (Class III). Note that if the heads were not stabilising each other in some way in resting muscle as in one of these three classes, it is questionable whether regularly organised relaxed myosin head structures would ever occur. However, strictly, there should be a Class IV, where the heads do not interact with each other at all.
Fig. 6.
The three Classes of head coupling described by Squire et al. (2005). Invertebrate myosin filaments were originally thought to be Class III, with heads interacting between adjacent crowns along the long-pitched helices shown in the helical nets in Fig. 4. Now, they have been shown to be Class I structures (e.g. Woodhead et al. 2005; heads within the same myosin molecule interacting). The Class II structure with heads from two different myosin molecules interacting around a single crown has been proposed for insect flight muscle myosin filaments (Lethocerus; AL-Khayat et al. 2003). An unlikely fourth possibility is that the heads do not interact with each other at all (Class IV)
Helical reconstructions from several invertebrate myosin filaments in negatively-stained images, usually achieving a resolution usually in the range of 5–7 nm, always seemed to show a helical ridge of mass running along one of the long-pitched helices on the different filament surfaces as indicated by yellow dashed lines in Fig. 5; for many years, it was thought that invertebrate myosin molecules interact with heads above and below their own crown as in the Class III structure of Fig. 6c (Crowther et al. 1985; Stewart et al. 1985; Vibert 1992; Offer et al. 2000). The only exception to this for the invertebrates was insect flight muscle where the myosin head mass appeared to be restricted to axially narrow shelves around the filament backbone on each crown (Reedy 1968; Reedy and Garrett 1977; Clarke et al. 1986; Morris et al. 1991). Helical reconstruction in this case suggested a Class II structure. Modelling of IFM filaments by X-ray diffraction later appeared to support this type of model (AL-Khayat et al. 2003).
The problem with myosin filaments from vertebrate striated muscle came from the presence of the perturbation in the myosin head array. This perturbation precluded the use of helical reconstruction, which presumed that every crown was the same, except as a means of generating an ‘average’ crown structure (e.g. Eakins et al. 2002). The perturbation problem was tackled initially by an ingenious but complicated procedure of separating various Bessel order contributions on different parts of the diffraction pattern (different myosin layer-lines in computed Fourier transforms from tilt series of myosin filaments; see Stewart and Kensler 1986). In this way, the full 43-nm repeat could be reconstructed instead of the structure being averaged onto a 14.3-nm-spaced crown. This same problem was then approached using the rich low-angle X-ray diffraction patterns from bony fish muscle which, with a novel computer program called MOVIE, were modelled with a Class I head arrangement for vertebrate striated muscle filaments to a nominal resolution of about 7 nm, but an effective positional sensitivity of about 1 nm since the known very high resolution myosin head crystal structure was being used (Hudson et al. 1997; AL-Khayat and Squire 2006). This showed slightly different structures on the three crowns of the 43-nm repeat, with two crowns similar but different from the third.
Single particle analysis, filaments in ice and everything Tete-a-tete
The real breakthrough in defining the positions of myosin heads on myosin filaments came with two novel approaches. The first was the technique of single particle analysis applied to isolated negatively-stained periodic but non-helical filaments. The idea here is that if there are many different images available of identical particles viewed in different directions, then their 3D structure can be determined, providing there is enough computer power available which was not true in earlier years. However, the conventional single particle approach of van Heel et al. (1996, 2000), until then applied successfully to spherical particles like viruses or globular objects like ribosomes which could be assumed to lie in all possible orientations on an electron microscope grid, had to be modified for elongated particles like myosin and actin filaments which lie parallel with the grid surface and thus only show rotations about one major axis. The application of single particle analysis is still advantageous over helical reconstruction even with helical filaments; isolated filaments can show slight deviations from being truly straight and there may also be very slight variations in pitch. The resolution in helical reconstructions of such filaments will be compromised by these irregularities, whereas with single particle analysis chopping the filament images into short segments and then independently aligning these segments reduces the effects of disorder. Egelman (2000) generated a successful single particle method for reconstructing helical filaments. However, both vertebrate striated muscle myosin filaments and actin filaments with troponin are periodic but non-helical structures. A single particle method for solving such non-helical periodic filaments was evaluated by Paul et al. (2004) and successfully applied to 3D reconstructions of negatively-stained vertebrate striated muscle myosin filaments (AL-Khayat et al. 2004, 2006).
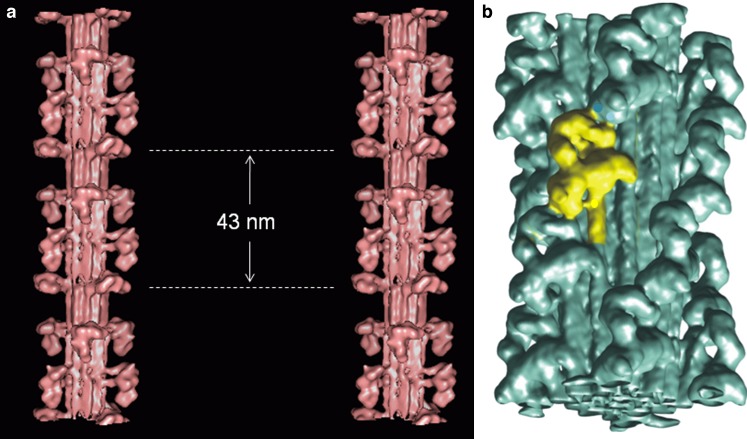
The second novel approach as far as myosin filaments are concerned, which was also in itself a ‘great leap forward’, was to preserve them in ice and study them frozen-hydrated in the electron microscope thus producing very much higher resolution than possible with negative stain. This approach had been tried for several years (Menetret et al. 1990; Vibert 1992), but initially little new information was forthcoming. However, the remarkable result of viewing tarantula muscle filaments in ice and also applying single particle analysis to the micrographs (Woodhead et al. 2005) was to generate a reconstruction with unprecedented resolution (about 2.5 nm) which, for the first time, clearly separated the positions of the two heads in each myosin molecule and even revealed some of the path of the rod part of myosin close to the heads (Fig. 7b). It was then found that the two heads were in close contact with each other, forming a head couplet in which the motor domain of one head could be seen to interact with the motor/ lever arm junction of the second head of the same molecule (Fig. 3b). Remarkably this pairing was very similar indeed to what had been seen by Wendt et al. (2001) in reconstructions from 2D crystals of part of the vertebrate smooth muscle myosin molecule, namely the part consisting of two heads and about one-third of the myosin rod and known as heavy meromyosin (HMM; Fig. 3b). Later, single particle reconstructions from the 4-stranded Limulus myosin filaments in ice (Zhao et al. 2009) showed that the head pairs in this case also adopted the Wendt et al. couplet scheme, while at lower resolution the 7-stranded scallop myosin filaments in negative stain appeared to be consistent with this (AL-Khayat et al. 2009). At the time of writing, the higher resolution cryo-EM reconstruction of scallop thick filaments has yet to be fully published (Zhao et al. 2007).
Fig. 7.
Recent images of myosin filament models from a vertebrate striated muscle determined by modelling the low-angle X-ray diffraction pattern from bony fish muscle and shown in stereo (AL-Khayat and Squire 2006) with the backbone shown as a molecular crystal structure (Squire 1973; Chew and Squire 1995), and b tarantula muscle determined by single particle analysis of filaments viewed frozen-hydrated (Woodhead et al. 2005; the image is inverted from the original to make it consistent with all other figures in the review (M-band towards the bottom). In both cases, the heads are mostly in the Wendt-like pairing in which the two heads of one molecule interact in a parallel fashion (Class 1 in Fig. 6). The exception is the crown in vertebrate striated muscle filaments at the dotted lines in (a) which appears very different
The Wendt et al. head coupling scheme is a Class I interaction scheme which seems to be a common head interaction mode in the off state of many of the invertebrate myosin filaments as has also been demonstrated by studies of isolated myosin molecules (Jung et al. 2008a, b). Many invertebrate muscles are myosin regulated by phosphorylation of the regulatory light chain (Sweeney et al. 1993). It has been suggested that the Class I head interaction in invertebrates may be a feature of myosin-regulated muscles (Alamo et al. 2008). The exception to the Class I structure for invertebrates so far has been the filaments of insect flight muscle where the structure still appears to be Class II (Morris et al. 1991; AL-Khayat et al. 2003), but which also appears to involve an interaction between the motor domain of one head and somewhere on the motor/neck junction of the other head.
Vertebrate striated muscle myosin filaments - do they conform?
What about the heads on vertebrate striated muscle thick filaments? Do they do something different? The answer to this appears to be yes and no. Recent reconstructions of filaments from bony fish muscle (AL-Khayat et al. 2006), from rat heart muscle (Zoghbi et al. 2008) and from rabbit heart muscle (AL-Khayat et al. 2008), none of them yet using frozen-hydrated preparations which have proved troublesome with this filament type, seem to be most consistent with the idea that something like the Wendt et al. (2001) head coupling occurs on two crowns out of the three in the 43-nm repeat, but that the third crown shows something different. This had also been suggested by X-ray diffraction analysis (AL-Khayat and Squire 2006). One reason why these vertebrate filaments might be different is that, as well as myosin, they contain part of the giant protein titin (connectin) which probably runs axially along the filament surface from the M-band (at the middle of the bare zone in the muscle A-band) to the myosin filament tips and beyond to the Z-line (see reviews in Tskhovrebova and Trinick 2003; Granzier and Labeit 2005). Some preliminary surface images of titin on the thick filament have been seen by Cantino et al. (2002) and Zoghbi et al. (2008).
Vertebrate striated muscle myosin filaments also contain the protein C-protein or myosin binding protein C (MyBP-C) which occurs on 7–9 stripes evenly spaced at 43-nm intervals in the so-called C-zone roughly centrally placed in each half of the myosin filaments (Offer et al. 1973; Sjostrom and Squire 1977; Bennett et al. 1986; Luther et al. 2008). It may be the specific titin sequence which helps to locate C-protein in the C-zone on the 43-nm repeat and not on every crown. AL-Khayat et al. (2006, 2008) showed that the presence of C-protein probably helps to create the perturbation in the crossbridge array and that this perturbation seems to be most obvious on one crown on which the heads swing away laterally from their ideal sites on the underlying helix, thus giving the myosin filaments an intrinsic 6-foldness about them in projection down the filament axis rather than the 9-foldness expected from the underlying 3-stranded 9/1 helix of head pairs. The effect on myosin filament structure of the absence of C-protein was reported by Zoghbi et al. (2008), Luther et al. (2008) and Kensler and Harris (2008). According to them and AL-Khayat et al. (2008), in addition to any other role, the presence of C-protein seems to alter the tilts and slews of heads on different crowns on their underlying perfectly helical lattice sites to produce the perturbation in such a way that the myosin filament head configurations are tailored to fit into the hexagonal array of actin filaments. The absence of C-protein reduces the perturbation and makes the myosin head array more helical (Kensler and Harris 2008; Zoghbi et al. 2008). Interestingly, C-protein may also interact with actin filaments (e.g. Kulikovskaya et al. 2003; Kunst et al. 2000; Squire et al. 2003; Whitten et al. 2008; Shaffer et al. 2009). Its role in muscle may not be just structural, C-protein may also make a functional contribution in its effects on the acto-myosin ATPase cycle (Winegrad 2003). C-protein, as well as myosin itself, is also known to occur with many mutations that can give rise to cardiomyopathies (Flashman et al. 2004; Govada et al. 2008; Ababou et al. 2008, and references in these papers), so its study is a hot topic at the present time.
Since the Class I head coupling may occur on two out of three crowns in vertebrate striated muscle thick filaments, even if not on the third, and since these filaments are not myosin regulated except for a modulating effect of light chain phosphorylation after the muscle has been activated through the actin filaments, one might question whether the presence of the Class I structure in invertebrate filaments is really uniquely associated with myosin-linked regulation as has been suggested (Alamo et al. 2008). However, there may be a spectrum of Class I structures ranging from those associated with strong myosin-linked regulation (the invertebrates) to a slightly modified structure in vertebrates which still maintains some rudimentary modulatory myosin-linked regulation through phosphorylation of the light chain.
Conclusions: are we learning from past mistakes?
In summary, all known myosin filaments now appear to have basically the same 2D arrangement of myosin head pair lattice sites on their surface helical nets (Fig. 5). Even the filaments of vertebrate striated muscles seem to conform as well since the perturbation seems to be caused by the presence of extra proteins such as C-protein. Bipolar filaments can come in a variety of rotational symmetries varying from 3-fold up to 7-fold so far, but the head pairs on all crowns usually seem to be coupled in the Class I way discovered by Wendt et al. (2001) for vertebrate smooth muscle. The exceptions are insect flight muscle where there appears to be Class II coupling and vertebrate striated muscle where the Wendt et al. structure may occur on two crowns but not the third. Of particular note is that the Class III coupling favoured for many years for a number of invertebrate myosin filaments does not appear to occur at all. This was presumably a result of the limited resolution (5–7 nm) of the early helical reconstructions from negatively-stained filaments. The higher resolutions (∼2.5 nm) achieved by application of single particle analysis to frozen-hydrated specimens have now clarified this issue and shown that Class III interactions appear not to occur in any of the filaments studied so far.
The next major challenge in understanding myosin filament structure is to know how the myosin rods are packed in the filament backbone to produce the surface lattices seen in Fig. 5. Squire (1973, 1986a) proposed a kind of molecular crystal scheme and he and others have considered the presence of sub-filaments (Wray 1979b; Squire 1986a). This has been tested to 0.5-nm resolution by modelling high-angle X-ray diffraction data from vertebrate muscle (Chew and Squire 1995), where the molecular crystal was the preferred structure. However, Woodhead et al. (2005) and Zhao et al. (2009) favour subfilaments for the tarantula and Limulus thick filament backbones. From X-ray fibre diffraction, it is known that myosin rods are only about 2 nm apart in the filament backbone (see Chew and Squire 1995). So, even though the use of frozen-hydrated specimens has pushed myosin filament reconstructions to about 2.5-nm resolution, this is right on the edge of the available resolution needed to separate myosin rods. The question of the nature of the myosin packing in the filament core remains open, but we should perhaps learn from the mistake of erroneously assigning Class III interactions to most invertebrate myosin filaments, when they are in fact Class I, that one can be misled by interpretations that look obvious but which are really beyond the resolution of the available 3D maps. Nevertheless, great progress is being made and further even higher resolution single particle ‘cryo’ studies or Angstrom resolution high-angle X-ray diffraction modelling should provide the necessary answers. Only then will the true significance of the mutations involved in various cardiomyopathies become apparent (Flashman et al. 2004; Zoghbi et al. 2008; Kensler and Harris 2008).
Acknowledgements
It has been a real pleasure to work over several decades on myosin filament structure and the techniques to study it with excellent colleagues and collaborators including; Vic Small, Michael Sjostrom, Pradeep Luther, Jeff Harford, Graham Fuller, Michael Chew, Richard Denny, Liam Hudson, Robert Kensler, Felicity Eakins, Hind AL-Khayat, Ed Morris, Danielle Paul and Carlo Knupp. My part of this work was supported over the years by the BBSRC, the MRC, the Wellcome Trust, and the British Heart Foundation and is currently funded by the European MYORES network.
References
- Ababou A, Rostkova E, Mistry S, Le Masurier C, Gautel M, Pfuhl M. Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1. J Mol Biol. 2008;384:615–630. doi: 10.1016/j.jmb.2008.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamo L, Wriggers W, Pinto A, Bartoli F, Salazar L, Zhao FQ, Craig R, Padron R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khayat HA, Squire JM. Refined structure of bony fish muscle myosin filaments from low-angle x-ray diffraction data. J Struct Biol. 2006;155:218–229. doi: 10.1016/j.jsb.2006.03.029. [DOI] [PubMed] [Google Scholar]
- AL-Khayat HA, Hudson L, Reedy MK, Irving TC, Squire JM. Myosin head configuration in relaxed insect flight muscle: x-ray modelled resting cross-bridges in a pre-powerstroke state are poised for actin binding. Biophys J. 2003;85:1063–1079. doi: 10.1016/S0006-3495(03)74545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Khayat HA, Morris EP, Squire JM. Single particle analysis: a new approach to solving the 3D structure of myosin filaments. J Muscle Res Cell Motil. 2004;25:635–644. doi: 10.1007/s10974-004-5333-5. [DOI] [PubMed] [Google Scholar]
- AL-Khayat HA, Morris EP, Powell AS, Kensler RW, Squire JM. 3D structure of vertebrate (fish) muscle myosin filaments by single particle analysis. J Struct Biol. 2006;155:202–217. doi: 10.1016/j.jsb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- AL-Khayat HA, Morris EP, Kensler RW, Squire JM. 3D structure of relaxed mammalian (rabbit) cardiac muscle myosin filaments by electron microscopy and single particle analysis. J Struct Biol. 2008;163:117–126. doi: 10.1016/j.jsb.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Khayat HA, Morris EP, Squire JM. 3D structure of relaxed scallop striated muscle myosin filaments by electron microscopy and single particle analysis. J Struct Biol. 2009;166:183–194. doi: 10.1016/j.jsb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Barral JM, Epstein HF. Protein machines and self assembly in muscle organization. BioEssays. 1999;21:813–823. doi: 10.1002/(SICI)1521-1878(199910)21:10<813::AID-BIES3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bennett P, Elliott A. Molecular organization of paramyosin in the core of molluscan thick filaments. J Mol Biol. 1984;176:477–493. doi: 10.1016/0022-2836(84)90173-6. [DOI] [PubMed] [Google Scholar]
- Bennett P, Craig R, Starr R, Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J Muscle Res Cell Motil. 1986;7:550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- Cantino M, Chew MWK, Luther PK, Morris E, Squire JM. Structure and nucleotide-dependent changes of thick filaments in relaxed and rigor plaice fin muscle. J Struct Biol. 2002;137:164–175. doi: 10.1006/jsbi.2002.4474. [DOI] [PubMed] [Google Scholar]
- Chew MW, Squire JM. Packing of alpha-helical coiled-coil myosin rods in vertebrate muscle thick filaments. J Struct Biol. 1995;115:233–249. doi: 10.1006/jsbi.1995.1048. [DOI] [PubMed] [Google Scholar]
- Clarke M, Hoffman W, Wray JS. ATP binding and crossbridge structure in muscle. J Mol Biol. 1986;191:581–585. doi: 10.1016/0022-2836(86)90153-1. [DOI] [PubMed] [Google Scholar]
- Craig R, Megerman J. Assembly of smooth muscle myosin into side polar filaments. J Cell Biol. 1977;75:990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Woodhead J. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Craig R, Padron R, Alamo L. Direct determination of myosin filament symmetry in scallop striated adductor muscle by rapid freezing and freeze substitution. J Mol Biol. 1991;220:125–132. doi: 10.1016/0022-2836(91)90386-K. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Padron R, Craig R. Arrangement of the heads of myosin in relaxed thick filaments from tarantula muscle. J Mol Biol. 1985;182:429–439. doi: 10.1016/0022-2836(85)90292-X. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Freyzon Y, Trybus KM, Cohen C. Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-powerstroke state. Cell. 1998;94:559–571. doi: 10.1016/S0092-8674(00)81598-6. [DOI] [PubMed] [Google Scholar]
- Eakins F, AL-Khayat HA, Kensler RW, Morris EP, Squire JM. 3D Structure of fish muscle myosin filaments. J Struct Biol. 2002;137:154–163. doi: 10.1006/jsbi.2002.4453. [DOI] [PubMed] [Google Scholar]
- Egelman (2000) A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy 85:225–234 [DOI] [PubMed]
- Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- Govada L, Carpenter L, da Fonseca PCA, Helliwell JR, Rizkallah P, Flashman E, Chayen NE, Redwood C, Squire JM. Crystal structure of the C1 domain of cardiac myosin binding protein-C: implications for hypertrophic cardiomyopathy. J Mol Biol. 2008;378:387–397. doi: 10.1016/j.jmb.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Labeit S (2005) Titin and its associated proteins. In: Squire JM, Parry DAD (ed) Muscle & molecular motors. Adv Protein Chemistry 71:89–119 [DOI] [PubMed]
- Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci. 2000;USA 97:11238–11243. doi: 10.1073/pnas.200376897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Harford JJ, Denny RC, Squire JM. Myosin head configuration in relaxed fish muscle: resting state myosin heads must swing axially by up to 150 A or turn upside down to reach rigor. J Mol Biol. 1997;273:440–455. doi: 10.1006/jmbi.1997.1321. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J Mol Biol. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Brown W. The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967;30:383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Hanson EJ. Structural basis of the cross-striations in muscle. Nature. 1953;172:530–532. doi: 10.1038/172530b0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction: interference microscopy of living muscle fibres. Nature. 1953;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Jung SK, Komatsu S, Ikebe M, Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell. 2008;19:3234–3242. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SK, Burgess SA, Billington N, Colegrave M, Patel H, Chalovich JM, Chantler PD, Knight PJ. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc Natl Acad Sci USA. 2008;105:6022–6026. doi: 10.1073/pnas.0707846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantha SS, Watabe S, Hashimoto K. Comparative biochemistry of paramyosin - a review. J Food Biochem. 1990;14:61–88. doi: 10.1111/j.1745-4514.1990.tb00821.x. [DOI] [Google Scholar]
- Kendrick-Jones J et al (2009) http://www.mrc-lmb.cam.ac.uk/myosin/Review/Reviewframeset.html
- Kensler RW, Harris SP. The structure of isolated cardiac myosin thick filaments from cardiac myosin binding protein C knockout mice. Biophys J. 2008;94:1707–1718. doi: 10.1529/biophysj.107.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler RW, Stewart M. Frog skeletal muscle thick filaments are three-stranded. J Cell Biol. 1983;96:1797–1802. doi: 10.1083/jcb.96.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikovskaya I, McClellan G, Flavigny J, Carrier L, Winegrad S. Effect of MyBP-C binding to actin on contractility in heart muscle. J Gen Physiol. 2003;122:761–774. doi: 10.1085/jgp.200308941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst G, Kress KR, Gruen M, Uttenweiler D, Gautel M, Fink RH. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ Res. 2000;86:51–58. doi: 10.1161/01.res.86.1.51. [DOI] [PubMed] [Google Scholar]
- Luther PK, Munro PMG, Squire JM. Three-dimensional structure of the vertebrate muscle A-band III: M-region structure and myosin filament symmetry. J Mol Biol. 1981;151:703–730. doi: 10.1016/0022-2836(81)90430-7. [DOI] [PubMed] [Google Scholar]
- Luther PK, Bennett PM, Knupp C, Craig R, Padrón R, Harris SP, Patel J, Moss RL. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Menetret JF, Schroder RR, Hofmann W. Cryo-electron microscopic studies of relaxed striated muscle thick filaments. J Muscle Res Cell Motil. 1990;11:1–11. doi: 10.1007/BF01833321. [DOI] [PubMed] [Google Scholar]
- Moore PB, Huxley HE, DeRosier DJ. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970;50:279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Morris EP, Squire JM, Fuller GW. The 4-stranded helical arrangement of myosin heads on insect (Lethocerus) flight muscle thick filaments. J Struct Biol. 1991;107:237–249. doi: 10.1016/1047-8477(91)90049-3. [DOI] [Google Scholar]
- Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Offer G, Knight PJ, Burgess S, Alamo L, Padron R. A new model for the surface arrangement of myosin molecules in tarantula thick filaments. J Mol Biol. 2000;298:239–260. doi: 10.1006/jmbi.2000.3664. [DOI] [PubMed] [Google Scholar]
- Paul D, Patwardhan A, Squire JM, Morris EP. Single particle analysis of filamentous and highly elongated macromolecular assemblies. J Struct Biol. 2004;148:236–250. doi: 10.1016/j.jsb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Pepe FA. Structure of muscle filaments from immunohistochemical and ultrastructural studies. J Histochem Cytochem. 1975;23:543–562. doi: 10.1177/23.7.1095653. [DOI] [PubMed] [Google Scholar]
- Rayment I, Holden HM, Whittaker M, Yohn M, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reedy MK. Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968;31:155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Garrett WE (1977) Electron microscope studies of Lethocerus flight muscle in rigor. In: Tregear R (ed) Insect flight muscle. Elsevier, pp 115–136
- Reedy MK, Holmes KC, Tregear RT. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965;207:1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Leonard KR, Freeman R, Arad T. Thick myofilament mass determination by electron scattering measurements with scanning transmission electron microscopy. J Muscle Res Cell Motil. 1981;2:45–64. doi: 10.1007/BF00712061. [DOI] [PubMed] [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000;1496:3–22. doi: 10.1016/S0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Shaffer JF, Kensler RW, Harris SP. The myosin binding protein C motif binds to F-actin in a phosphorylation sensitive manner. J Biol Chem. 2009;284:12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom M, Squire JM. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977;109:49–68. doi: 10.1016/S0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Small JV, Squire JM. Structural basis of contraction in vertebrate smooth muscle. J Mol Biol. 1972;67:117–149. doi: 10.1016/0022-2836(72)90390-7. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model for the structure of all myosin-containing filaments. Nature. 1971;233:457–462. doi: 10.1038/233457a0. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model of myosin filament structure. II. Myosin filaments and cross-bridge interactions in vertebrate striated and insect flight muscles. J Mol Biol. 1972;72:125–138. doi: 10.1016/0022-2836(72)90074-5. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model of myosin filament structure. 3. Molecular packing arrangements in myosin filaments. J Mol Biol. 1973;77:291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]
- Squire JM. The structural basis of muscular contraction. New York: Plenum; 1981. [Google Scholar]
- Squire JM. Muscle myosin filaments: internal structure and crossbridge organisation. Comments Mol Cell Biophys. 1986;3:155–177. [Google Scholar]
- Squire JM. Muscle: design, diversity and disease. Menlo Park, California: Benjamin Cummings; 1986. [Google Scholar]
- Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol. 2003;331:713–724. doi: 10.1016/S0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- Squire JM, Al-Khayat HA, Knupp C, Luther PK (2005) 3D molecular architecture of muscle. In: Squire JM, Parry DAD (ed) Muscle & molecular motors. Adv Protein Chemistry 71:17–87 [DOI] [PubMed]
- Stewart M, Kensler RW. Arrangement of mtyosin heads in relaxed thick filaments from frog skeletal muscle. J Mol Biol. 1986;192:831–851. doi: 10.1016/0022-2836(86)90032-X. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kensler RW, Levine RJ. Structure of Limulus telson muscle thick filaments. J Mol Biol. 1981;153:781–790. doi: 10.1016/0022-2836(81)90418-6. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kensler RW, Levine RJ. Three-dimensional reconstruction of thick filaments from Limulus and scorpion muscle. J Cell Biol. 1985;101:402–411. doi: 10.1083/jcb.101.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Trinick JA. End-filaments: a new structural element of vertebrate skeletal muscle thick filaments. J Mol Biol. 1981;151:309–314. doi: 10.1016/0022-2836(81)90517-9. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Titin: properties and family relationships. Nat Rev Mol Cell Biol. 2003;4:679–689. doi: 10.1038/nrm1198. [DOI] [PubMed] [Google Scholar]
- van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- van Heel M, Gowen B, Matadeen R, Orlova EV, Finn R, Pape T, Cohen D, Stark H, Schmidt R, Schatz M, Patwardhan A. Single-particle electron cryo-microscopy: towards atomic resolution. Q Rev Biophys. 2000;33:307–369. doi: 10.1017/S0033583500003644. [DOI] [PubMed] [Google Scholar]
- Vibert PJ. Helical reconstruction of frozen-hydrated scallop myosin filaments. J Mol Biol. 1992;223:661–671. doi: 10.1016/0022-2836(92)90982-P. [DOI] [PubMed] [Google Scholar]
- Vibert PJ, Craig R. Electron microscopy and image analysis of filaments from scallop striated muscle. J Mol Biol. 1983;165:303–320. doi: 10.1016/S0022-2836(83)80259-9. [DOI] [PubMed] [Google Scholar]
- Wendt T, Taylor D, Trybus KM, Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci USA. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: implications for cardiac function. Proc Natl Acad Sci USA. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Myosin binding protein C (MyBP-C) in cardfiac muscle and contractility. Adv Exp Med Biol. 2003;538:31–40. doi: 10.1007/978-1-4419-9029-7_3. [DOI] [PubMed] [Google Scholar]
- Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- Wray JS. Filament geometry and the activation of insect flight muscles. Nature. 1979;280:325–326. doi: 10.1038/280325a0. [DOI] [Google Scholar]
- Wray JS. Structure of the backbone in myosin filaments of muscle. Nature. 1979;277:37–40. doi: 10.1038/277037a0. [DOI] [PubMed] [Google Scholar]
- Wray JS, Vibert PJ, Cohen C. Cross-bridge arrangements in Limulus muscle. J Mol Biol. 1974;88:343–348. doi: 10.1016/0022-2836(74)90486-0. [DOI] [PubMed] [Google Scholar]
- Zhao F-Q, Woodhead J, Craig R (2007) Head-head interaction characterizes the relaxed state of scallop and Limulus muscle myosin filaments. Biophys J 215a [DOI] [PMC free article] [PubMed]
- Zhao F-Q, Craig R, Woodhead JL. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J Mol Biol. 2009;385:423–431. doi: 10.1016/j.jmb.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci U S A. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]