Abstract
Striated muscle is well known to exist in either of two states—contraction or relaxation—under the regulation of Ca2+ concentration. Described here is a less well-known third, intermediate state induced under conditions of partial activation, known as SPOC (SPontaneous Oscillatory Contraction). This state is characterised by auto-oscillation between rapid-lengthening and slow-shortening phases. Notably, SPOC occurs in skinned muscle fibres and is therefore not the result of fluctuating Ca2+ levels, but is rather an intrinsic and fundamental phenomenon of the actomyosin motor. Summarised in this review are the experimental data on SPOC and its fundamental mechanism. SPOC presents a novel technique for studying independent communication and coordination between sarcomeres. In cardiac muscle, this auto-oscillatory property may work in concert with electro-chemical signalling to coordinate the heartbeat. Further, SPOC may represent a new way of demonstrating functional defects of sarcomeres in human heart failure.
Keywords: SPOC, Auto-oscillation, Cardiac muscle, Skeletal muscle, Sarcomere
Contraction and relaxation in striated muscle
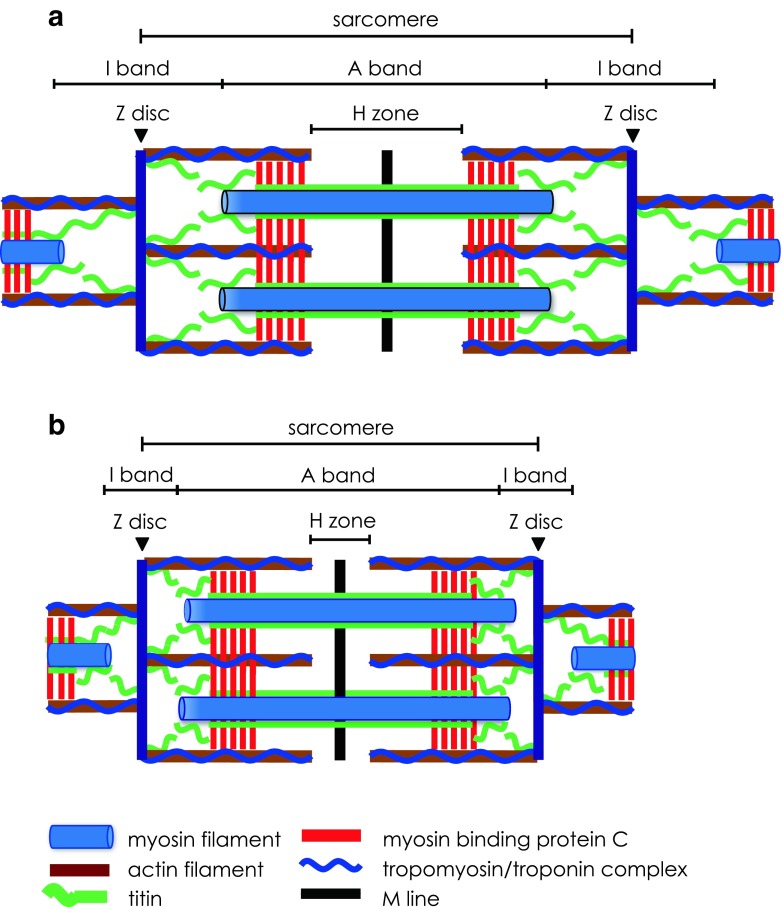
The sarcomere is the fundamental contractile unit of striated muscle cells, or myocytes (Fig. 1a). It is a repeating array of interdigitating structural and contractile filament proteins. Many successive sarcomeres comprise the myofibril, and many thousands of myofibrils are assembled in parallel to comprise a myocyte.
Fig. 1.
Structural and contractile proteins of the striated muscle sarcomere in relaxed (a) and contracted (b) states. a The sarcomere extends between two adjacent Z discs. The Z disc anchors the actin filaments with their overlying tropomyosin/troponin complex. The M line defines the centre of the sarcomere, bisects the myosin filaments and extends across the width of the A band. The I band is the gap between the free ends of adjacent thick filaments in the A band and is bisected by the Z disc. A giant elastic protein, titin, extends from the Z disc through the I band, attaches to the thick filaments of the A band and ends at the M line. Myosin binding protein C (MyBP-C) is located in the part of the A band where cross-bridges are formed. b Graphic representation of how contraction occurs by mutual sliding of the thick and thin filaments while the lengths of the filaments remain unchanged. Thus, during sarcomere shortening, both the H zone and I band progressively narrow while there is no change in the A band
Contraction of the sarcomere occurs by mutual sliding of myosin thick and actin thin filaments (Fig. 1b) (Huxley and Hanson, 1954; Huxley and Niedergerke, 1954; Huxley and Hanson 1959; Huxley 1969). Prior to contraction, ATP binds to myosin and is hydrolysed, forming a myosin-ADP-PI complex. In the relaxed state, tropomyosin (Tm) blocks the myosin-binding sites on actin, preventing cross-bridge formation. Tm is stabilised over these binding sites by troponin (Tn), which comprises three subunits: one that binds Tm (TnT), another that binds actin (TnI) and a third that binds Ca2+ (TnC). Binding of Ca2+ with TnC causes a conformational change that allows the displacement of Tm, and thus actomyosin-ADP-PI (AM-ADP-PI) cross-bridge complexes may be formed. The intracellular sarcoplasmic reticulum membrane system is responsible for the release of Ca2+ that enables formation of these cross-bridges, as well as for Ca2+ recapture during relaxation (Ebashi and Endo 1968). Contact between myosin cross-bridges and actin filaments triggers the power stroke, during which the actin filament is drawn over the myosin filament. PI is released immediately prior to, and ADP immediately following, the power stroke. Contraction is the result of the synchronised sliding of actin filaments over myosin filaments in successive sarcomeres. In the heart, the cardiomyocytes are extensively interconnected via intercalated discs, which facilitate synchronised contraction known as systole (Estigoy et al. 2009).
The myosin cross-bridge detaches from actin when it binds a fresh ATP molecule. If sufficient Ca2+ is still present, the cross-bridge and power stroke cycle will continue, and the sarcomere will contract still further. If Ca2+ is no longer present, the actin filaments can slide back to their original, relaxed position. This sliding is assisted by the elastic protein titin (Fig. 1) and, in the case of the heart, also by ventricular filling during diastole.
SPOC: What is it?
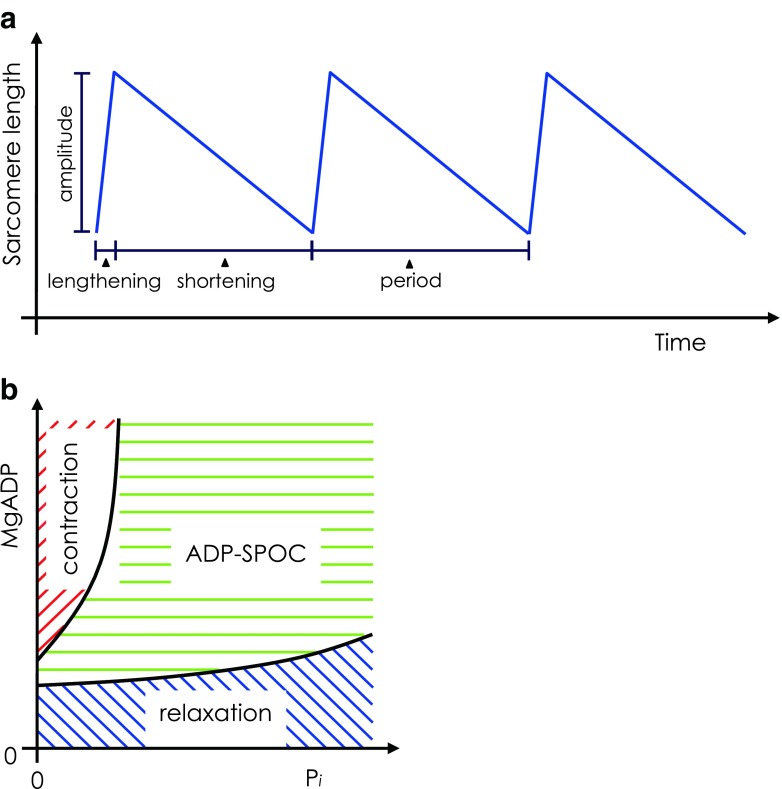
SPontaneous Oscillatory Contraction (SPOC) describes steady, rhythmic cycles of rapid lengthening (relaxation) and subsequent slow shortening (contraction) that can be induced in the sarcomeres of striated muscle, i.e. both cardiac and skeletal muscle types. Thus, SPOC has a characteristic ‘saw-tooth’ waveform of lengthening and shortening (Fig. 2a). Such spontaneous oscillations occur under ionic conditions that maintain the partial activation of sarcomeres so that the sarcomeres exist in a stable, precisely balanced state intermediate to full relaxation and activation. Partial activation of sarcomeres is necessary to induce SPOC and is achieved by precisely balancing the proportion of force-generating and non-force-generating cross-bridges, as discussed further below.
Fig. 2.
a Schematic of oscillations in sarcomere length during SPontaneous Oscillatory Contraction (SPOC). The total SPOC period comprises rapid-lengthening and slow-shortening phases. b Two-dimensional state-diagram of striated muscle showing contraction, relaxation and ADP–SPOC plotted against inorganic phosphate (Pi; x-axis) and MgADP (y-axis) concentrations, where MgATP concentration is fixed. SPOC requires the coexistence of MgATP and MgADP above a certain threshold proportion. Therefore, if the fixed concentration of MgATP is increased, a higher MgADP concentration is required to induce SPOC. Adapted from Shimizu et al. (1992)
Two types of SPOC may be observed. Calcium–SPOC (Ca–SPOC) is induced under conditions of partial activation using precise concentrations of free Ca2+ (pCa defined as −log [Ca2+] in mol/L). In an elegant paper, Fukuda et al. (1998) provided experimental evidence backed up by a thorough mathematical analysis, showing that both ADP and inorganic phosphate (Pi) regulate the state of activation of the thin filaments.
Ca–SPOC is characterised by a short SPOC period, where each sarcomere quickly repeats the rapid-lengthening and slow-shortening phases. ADP–SPOC is induced under conditions of partial activation of sarcomeres resulting from the addition of exogenous ADP and PI in the presence of ATP and the absence of Ca2+. The period of ADP–SPOC is much greater than that of Ca–SPOC. In both types of SPOC, the rapid-lengthening phase propagates smoothly through sarcomeres down the long axis of the myofibrils, defining the characteristic ‘SPOC wave’. The SPOC wave can propagate between cardiomyocytes across their intercalated discs (Sasaki et al. 2005).
Ca–SPOC occurs at fixed concentrations of free Ca2+ (pCa between 5.75 and 6.25) and ADP–SPOC occurs in the absence of Ca2+. Thus, oscillation occurs independent of any fluctuation in the levels of Ca2+, the master regulator of contraction in vivo. And, as Fukuda et al. (1998) suggested, the state of thin filaments is synergistically regulated by both the binding of Ca2+ to troponin and the formation of the actomyosin ± ADP complex.
SPOC is induced near instantaneously and may persist for many minutes. This allows measurements of changes in sarcomere length to be taken and averaged over many SPOC cycles. Thus, precise values for several SPOC parameters may be achieved. These parameters include the total SPOC period, rates of lengthening and shortening, velocity of the SPOC wave and the amplitude of oscillation. SPOC therefore provides a highly reproducible measure of the functional performance of sarcomeres.
A brief history of SPOC
There are several early reports of cyclic oscillations of contraction and relaxation in striated muscle fibres under partial activation with sub-micromolar Ca2+ concentrations (Goodall 1956; Lorand and Moos 1956; Pratt et al. 1976; Mooseker et al. 1977) or high pH (Onodera and Umazume 1984; Onodera 1990).
Fabiato and Fabiato (1975a, b; 1978a) were the first to observe spontaneous tension oscillations in skinned cardiac cells. They attributed the phenomenon to the release and re-uptake of Ca2+ by the sarcoplasmic reticulum. These cyclic contractions were induced under EGTA-buffered conditions that ensure very low free Ca2+ concentrations (Fabiato and Fabiato 1978b). In the relaxation phase, tension is reduced and the sarcomeres elongate. This is immediately followed by the redevelopment of tension and sarcomere shortening. Partial activation using sub-micromolar free Ca2+ (Ca–SPOC) caused oscillations in myocyte tension (Stephenson and Williams 1981) and sarcomere length (Iwazumi and Pollack 1981). This type of oscillation, known as Ca–SPOC, has been observed in slow-type skeletal muscle (Stephenson and Williams 1982), fast-type skeletal muscle (Shimamoto et al. 2008) and cardiac muscle (Sweitzer and Moss 1990; Linke et al. 1993).
The term ‘SPOC’ was coined by Okamura and Ishiwata (1988), who observed spontaneous and steady oscillations in skinned skeletal myofibrils induced in the presence of high concentrations of ADP and PI, together with ATP, and in the absence of free Ca2+ (see also Ishiwata et al. 1987). This form of SPOC, termed ADP–SPOC, is observed irrespective of muscle type.
Just seconds after inducing ADP–SPOC conditions, Okamura and Ishiwata (1988) observed oscillations in the free-floating centres of myofibrils whose two ends adhered to a glass surface. The total length of the myofibril remained constant, and thus any tension developed was not strong enough to displace the attached ends. The oscillations lasted for some minutes, but could be maintained for longer with continuous flow of fresh SPOC solution. Oscillation amplitude reached as much as 15% of the sarcomere length (Ishiwata et al. 1987). Widening of the H zone and I band observed during lengthening and corresponding narrowing during shortening were consistent with mutual sliding of thin and thick filaments during activation. Free-floating myofibrils contracted without oscillation, though at a rate slower than in normal activation.
The dynamics of SPOC
An intermediate state of striated muscle
Okamura and Ishiwata (1988) developed a ‘state’-diagram of myofibrils showing that a balance of ADP and PI concentrations, in the presence of ATP, was required to induce oscillation. Figure 2b schematically illustrates the states of contraction, relaxation and the intermediate SPOC state. These oscillatory contractions occur in a region between relaxation and contraction (see also Ishiwata et al. 1991). SPOC is a dynamic steady state—once induced, the period and amplitude of the saw-tooth oscillation between the rapid-relaxation and slow-contraction phases are consistent and can be maintained periods up to 1 h.
A study by Shimizu et al. (1992) further supports the claim that SPOC is induced by a balanced ratio of ATP and ADP that compete for myosin ATPase sites. As ATP was increased, higher concentrations of ADP were required to induce SPOC. Further, Shimizu et al. (1992) described ADP as an ‘activator’ and PI as an ‘inhibitor’. Addition of exogenous ADP caused the predominant formation of strong-binding AM-ADP complexes, which act allosterically to suppress the inhibition of contraction by regulatory proteins, in a similar manner to the rigor complex. This dis-inhibition of contraction by ADP possibly complements the normal action of Ca2+. The isometric tension induced by ADP reached nearly 90% of that induced by full Ca2+ activation. The addition of PI favoured the formation of AM-ADP-PI complexes and decreased the isometric tension, since these complexes exist in a weak-binding state that allows inhibition of contraction to redevelop and lengthening to occur. Ishiwata et al. (1991) had previously suggested that, by binding to actin directly, exogenous PI may shift thin filaments to an ‘off-state’.
SPOC in half-sarcomeres
Okamura and Ishiwata (1988) noted asymmetric lengthening occurring in the half-sarcomere—that is, lengthening of the sarcomere at the interface between contraction and relaxation occurred first at an I band on one side of an A band, and then on the other.
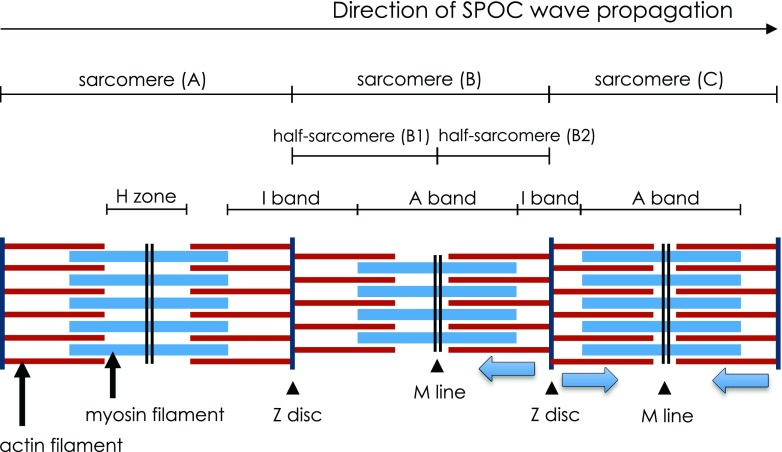
Ishiwata et al. (1991) developed a view of the length–tension relationship of a half-sarcomere during SPOC (see Fig. 3). This figure shows that as half-sarcomere B1 experiences a quick lengthening, the SPOC wave arrives, causing the adjacent half-sarcomere B2 (which is shortening) to experience a quick release. Half-sarcomere B1 then redevelops tension, causing sarcomere B2 to lengthen under the newly imposed strain. This process continues at successive half-sarcomeres along the length of the myofibril, generating the SPOC wave.
Fig. 3.
An illustration of three adjacent sarcomeres (A, B and C) during SPOC. As the SPOC wave progresses from left to right, sarcomere A is in the lengthening phase, and sarcomere C is in the shortening phase. Sarcomere B is at the interface between phases and demonstrates asymmetric length increase in half-sarcomere B1, while half-sarcomere B2 remains in the shortening phase. Lengthening in half-sarcomere B1 results in a quick release of tension at adjacent half-sarcomere B2. Half-sarcomere B1 redevelops tension soon after lengthening, causing half-sarcomere B2 to lengthen under the newly imposed strain. In this way, the SPOC wave propagates along successive half-sarcomeres down the length of the myofibril. Adapted from Ishiwata et al. (1991)
The SPOC wave
By 1992, the observation of SPOC had been restricted to isometric conditions. Here, the oscillations extended over only a small number of sarcomeres and appeared out of phase with oscillations occurring in other parts of the myofibril. To address this, Anazawa et al. (1992) developed a method that allowed tension development during SPOC to be observed under auxotonic conditions, where a contracting muscle shortens against an increasing load. Each end of a single myofibril was wrapped around a glass micro-needle. The stiffer needle fixed one end of the myofibril while tension measurements were taken from the displacement of a flexible needle at the other end.
The result was the observation of waves of SPOCs that propagated steadily and repeatedly over more than ten sarcomeres (originally termed the ‘organised state of SPOC’ but now known as ‘metachronal SPOC’). Here, tension gradually decreased with the propagation of a SPOC wave, then increased when the SPOC wave reached the end of the myofibril.
Using this experimental setup, Anazawa et al. (1992) investigated the appearance of SPOC under increasing and reversibly decreasing loads by stretching the myofibril. This was achieved by repositioning of the stiffer micro-needle. While the pattern of the SPOC wave remained unchanged throughout, the tension, period and amplitude of oscillation increased with load. Thus, SPOC can be induced under increased load (that is, stretch activation). This can be explained by enhanced ATPase activity resulting from increased mechanical strain imposed on cross-bridges and/or thin filaments (Anazawa et al. 1992; Shimizu et al. 1992).
In a subsequent study, Yasuda et al. (1996) successfully observed SPOC under isotonic conditions, where the sarcomeres contracted and relaxed in synchrony. This highly synchronous behaviour supported the concept that a mechanism for communication existed between adjacent sarcomeres.
Actomyosin interaction in SPOC
Fujita and Ishiwata (1998) provided evidence that SPOC is an intrinsic phenomenon of the actomyosin (AM) motor. In this study, the thin filaments of glycerinated cardiac muscle were removed by gelsolin treatment and reconstituted under polymerising conditions with exogenous actin. The reconstituted thin filaments therefore lacked the inhibitory Tm–Tn complex (see Fujita et al. 1996). SPOC was successfully observed in these actin filament-reconstituted fibres. Here, 2,3-butanedione 2-monoxime (BDM, a reversible inhibitor of AM interaction) effectively took on the role of the inhibitory Tm–Tn complex, suggesting that SPOC is an inherent property of the AM motor.
Shimamoto et al. (2007; 2008) demonstrated a non-linear sarcomere length–force relationship in skeletal myofibrils under SPOC conditions in which active force was higher when thick and thin filament overlap was decreased (see also Endo 1972). In other words, active force increased with increased sarcomere length, over sarcomere lengths of approximately 2.4–3 μm. At increased sarcomere lengths, the space between the thin and thick filaments correspondingly decreases, whereas during shortening, the interfilament space increases. Therefore, a lengthened sarcomere may form more cross-bridges since more myosin heads are in close vicinity of the thin filament.
Shimamoto et al. (2007) further confirmed this phenomenon by observing the active force developed in the presence of dextran, a non-polar macromolecule. With addition of 1% (w/v) dextran, myofibril diameter decreased by 4–6% with a concomitant twofold increase in active force. In addition, Yasuda et al. (1996) observed that shortening velocity was fastest at the start of the shortening phase of SPOC, subsequently slowing to a near-constant rate until shortening ceased entirely.
The role of titin
The giant elastic protein titin, or connectin as it was originally called (Maruyama, 1976), extends from the Z disc (where its N-terminus is anchored) to the M line region, passing through the I band and into the A band where it is bound to the myosin rod. Titin extends along the thick filaments to the M line. Thus, titin tethers the myosin filaments to the adjacent two Z discs (Horowits et al. 1986; Granzier et al. 2005) and is probably responsible for the centreing of the thick filaments (and therefore the A band). When sarcomeres are stretched, titin unfolds, and as elongation progresses, titin exerts an increasing resistance, causing passive tension to rise.
However, although it is responsible for resting tension (Funatsu et al. 1990; 1993), the contribution of titin to the lengthening phase of SPOC was argued to be negligible for two main reasons. Firstly, SPOC can occur at very short sarcomere lengths (approx. 2 μm) where the I bands have almost disappeared. Secondly, treatment with the protease trypsin will almost certainly cleave titin but it does not prevent the appearance of SPOC activity (Shimizu et al. 1992; Ishiwata et al. 1996; Yasuda et al. 1996). Even at very low concentrations, trypsin would hydrolyse a giant protein like titin (approx. 2970–3700 kDa; Fukuda et al. 2001), while having essentially no effect on the smaller sarcomeric proteins.
Individual sarcomeres at partial activation
Shimamoto et al. (2009) recently used partial activation, characteristic of SPOC, to investigate the responses of single skeletal sarcomeres to an applied load. The authors pointed out that previous investigations of the mechanical responses of sarcomeres have overlooked the dynamic interactions between individual sarcomeres, since earlier measurements were averages taken from thousands of sarcomeres interconnected both in series along the myofibril and in parallel in the myocyte as a whole.
In this study, the averaged sarcomere response along the myofibril demonstrated an increased change in sarcomere length with increased applied force. Analysis of individual sarcomeres, however, revealed two distinct types of responses. ‘Yielding’ sarcomeres considerably elongated before gradually shortening. ‘Resisting’ sarcomeres slightly elongated and then maintained their length. ‘Yielding’ sarcomeres occurred in clusters along the myofibril. The ability of adjacent sarcomeres to interact and form ‘yielding’ clusters could be disrupted using an antibody (anti-α-actinin) that binds to and alters the mechanical properties of the Z disc. This study provided a unique demonstration of single sarcomere responses and cooperation between sarcomeres independent of Ca2+ regulation.
SPOC therefore seems well suited to experimental investigations of sarcomere interaction and cooperation, both at the level of the individual sarcomere and the myofibril as a whole, where the influence of Ca2+ as the primary regulator can be eliminated.
Modelling the SPOC mechanism
The molecular mechanism of SPOC is still under investigation, and several models have been developed to describe the oscillation phenomenon (e.g. Smith and Stephenson 1994; Vilfan and Duke 2003; Günther and Kruse 2007). Jülicher and Prost (1997) developed a two-state model where motor units interact with an elastic element. Smith and Stephenson (2009) provided a detailed model of the SPOC mechanism in skeletal muscle. Their model considered the structure and function of a contractile system that exists initially in partial overlap and at low levels of activation—that is, low actin–myosin affinity. They also accounted for the elastic properties of titin.
SPOC and the auto-oscillatory heart
As early as 23 days post-fertilisation, the human heart begins to beat in rhythmic cycles of rapid relaxation (diastole) and comparatively slower contraction (systole) (Abdulla et al. 2004). It will beat many thousands of times each day for 70–80 years or more (that is, over 2.5 billion beats in a lifetime). Contraction of the myocardium is initiated wholly within the heart. Impulses generated at the sinoatrial (SA) node spread rapidly through the heart via specialised conduction pathways to produce coordinated contraction. Thus, the heart exists in an enduring state of auto-oscillation for an entire lifetime.
Depolarisation of the cardiomyocyte membrane triggers an influx of Ca2+ through L-type Ca2+ channels located in the sarcolemma. The result is a transient increase in intracellular Ca2+, which in turn induces Ca2+ release from the sarcoplasmic reticulum (SR) via a channel called the cardiac ryanodine receptor (RyR). This process is referred to as Ca2+-induced Ca2+ release (CICR) (see Fabiato, 1983). Ca2+ then binds to TnC, and force-generating cross-bridges are subsequently formed. Following peak contraction, re-uptake of intracellular Ca2+ occurs via several transport systems, including the sarcoplasmic/endoplasmic reticulum Ca2+–ATPase (SERCA) pump, the Na+–Ca2+ exchanger and the sarcolemmal Ca2+–ATPase pump (Fares and Howlett 2010). As a result, intracellular Ca2+ levels decrease, Ca2+ dissociates from TnC, the formation of cross-bridges is inhibited and relaxation/diastole is achieved. Oscillation between systole and diastole occurs as this process repeats. This oscillation is distinct from SPOC, since the latter is independent and occurs in the absence of the SR function.
Fabiato and Fabiato (1978b) first described spontaneous tension oscillations during partial activation (pCa of approx. 6.0) in skinned cardiac cells where the SR had been destroyed. This is consistent with the levels of intracellular Ca2+ (pCa of approx. 6.0) during in vivo contraction/systole. A number of studies have since confirmed the observation of spontaneous oscillations in cardiac muscle (e.g. Sweitzer and Moss 1990; Linke et al. 1993; Fukuda et al. 1996; Fukuda and Ishiwata 1999; Sasaki et al. 2005; 2006). Notably, these oscillations are distinct from those observed in intact cardiac muscle, which are no longer observed following interference with the SR (Fabiato and Fabiato 1975a; Rieser et al. 1979; Lappé and Lakatta 1980; Mulder et al. 1989). In addition, SPOC is reportedly unaffected by addition of ryanodine (Linke et al. 1993; Fukuda et al. 1996).
SPOC in cardiac physiology
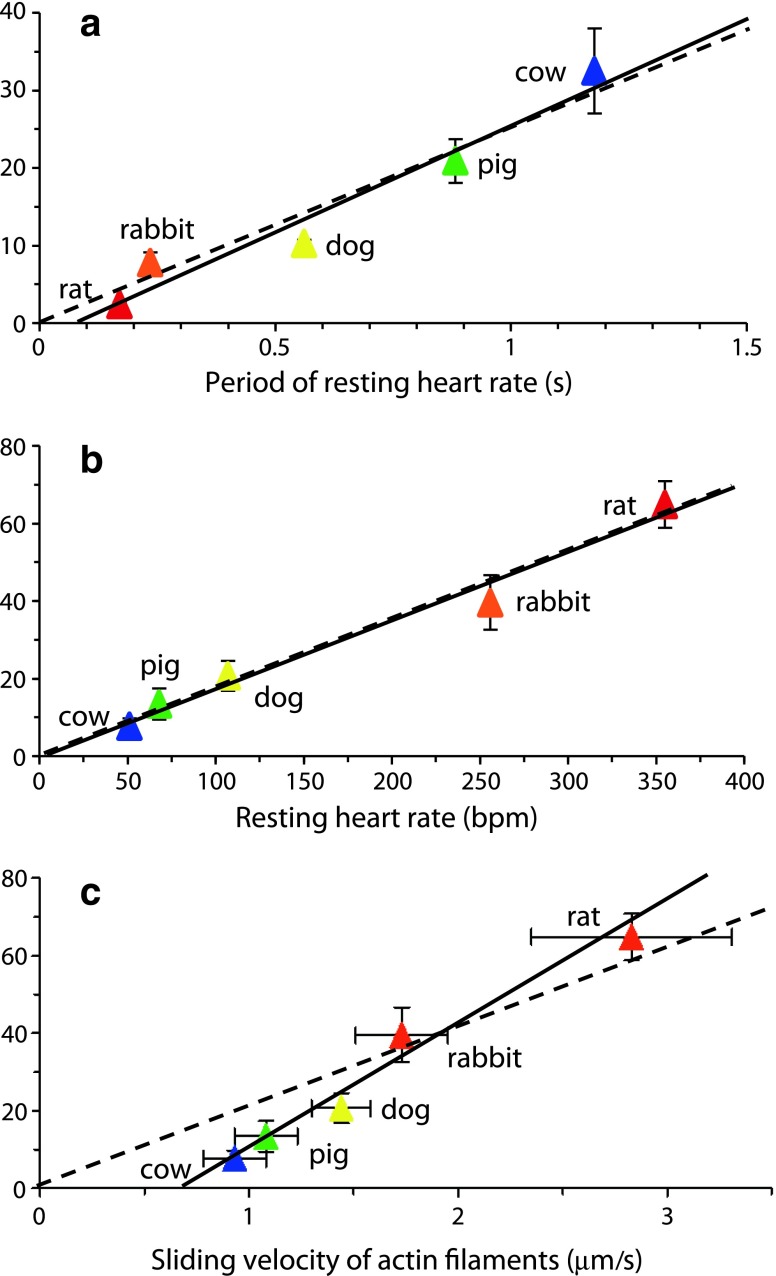
Does SPOC, particularly Ca–SPOC, play a role cardiac muscle in vivo? Sasaki et al. (2005) suspected that the auto-oscillations characteristic of SPOC in cardiomyocytes might be significant in the beating heart. They examined a range of animals (rat, rabbit, dog, pig and cow) and found that the SPOC period strongly correlated with the resting heart rate period (Fig. 4a). The SPOC period is dominated by its shortening phase (Fig 2) and, with the exception of the rat, the amplitudes of oscillation were found to be similar. Thus, it follows that the shortening velocity of SPOC is proportional to resting heart rate (Fig. 4b) (Sasaki et al. 2005). Furthermore, the shortening velocity correlated well with the velocity of the in vitro actin motility assay, i.e. the rate at which actin filaments move on a bed of myosin (Fig. 4c).
Fig. 4.
Relationship between ADP–SPOC and resting heart rate. a Period of ADP–SPOC correlated with the period of resting heart rate across animal species (correlation coefficient 0.976), b shortening velocity of SPOC very closely correlated with resting heart rate (correlation coefficient 0.990), with a near-proportional relationship (bpm beats per minute), c Strong correlation between shortening velocity during ADP–SPOC and the sliding velocity of actin filaments determined by in vitro motility assay (correlation coefficient 0.987), though the relationship was not a proportional one. Data are presented as the mean ± standard deviation (SD). Solid lines Regression lines, broken lines regression lines that cross the origin, indicating a proportional relationship. Graphs adapted from Sasaki et al. (2005)
Differences in sliding velocity between animals were, in part, accounted for by different expression of the two mammalian myosin heavy chain (MHC) isoforms, namely α-MHC (a fast ATPase) and β-MHC (a slow ATPase). This results in the expression of three different myosin isoforms, namely, V1 (αα), V2 (αβ) and V3 (ββ), where the ATPase rate is fastest in V1 and slowest in V3 (Dillmann 1984). In the ventricles of animals with heart rates greater than 300 bpm, V1 predominates, while in those with heart rates less than 300 bpm, the V3 isoform predominates (Hamilton and Ianuzzo 1991). Sasaki et al. (2005) also acknowledged that other sarcomeric proteins, such as troponin (Tn), tropomyosin (Tm) and titin (see Fig. 1) might also influence shortening velocity.
In addition, cardiac myosin binding protein C (MyBP-C) may play a role in actomyosin interaction in SPOC (Fig. 1). Both the phosphorylated and dephosphorylated forms of MyBP-C increased ATPase activity (Winegrad 1999). This increase, however, was prevented by removal of myosin regulatory (or phosphorylatable) light chain (MLC-2) (Margossian 1985). MyBP-C and MLC-2 may therefore work in concert to regulate AM-ATPase activity (Winegrad 1999).
Human cardiac MyBP-C has three phosphorylatable sites (A, B and C) which, when dephosphorylated, cause the myosin heads to appear disordered and to protrude from the myosin backbone at different angles (Oakley et al. 2007). Phosphorylation of site B causes the myosin heads to align along on the thick filament backbone, forming a tight structure. Subsequent phosphorylation of sites A and C results in ordered myosin heads that extend towards the thin filament. Thus, the extent of MyBP-C phosphorylation is linked to the packing of the myosin heads and their ability to interact with the thin filament to form pre-force generating, weak-binding cross-bridges in the relaxed state. This could affect SPOC parameters and may be the basis of future research.
The ADP–SPOC period is approximately 20-fold longer than the period of resting heart rate across animal species (Fig. 4a) (Sasaki et al. 2005). In a subsequent investigation, Sasaki et al. (2006) concluded that high levels of exogenous ADP increased the relative number of non-force-generating cross-bridges, thus creating a drag during shortening (Gorga et al. 2003). This effect does not occur in Ca–SPOC, and it was subsequently shown that the Ca–SPOC period also correlated with resting heart rate and was only two- to fourfold longer than the resting heart rate period at 22 ± 1°C (Sasaki et al. 2006). At 39 ± 1°C, the Ca–SPOC period was reduced to approximately half the period of the resting heart rate in pig. Thus, these researchers concluded that the Ca–SPOC period falls within the broad range of resting heart rates in the above-mentioned animal species (Sasaki et al. 2006).
The SPOC state-diagram (see Fig. 2b) in essence applies to both skeletal and cardiac muscle. SPOC requires the co-existence of weak-binding cross-bridges and force-generating cross-bridges above a certain threshold proportion (Ishiwata and Yasuda 1993). For ADP–SPOC, the high concentration of ADP required clearly does not reflect normal physiological conditions. The region of the SPOC state is broader in cardiac muscle than in skeletal muscle, and thus SPOC can be achieved at lower ADP concentrations (Ishiwata and Yasuda 1993), although still not at levels that reflect in vivo conditions. However, for Ca–SPOC, the required proportion of cross-bridges achieved by partial Ca2+ activation occurs under normal physiological ionic conditions in the in vivo heart. In particular, Fukuda et al. (1996) presented a state-diagram that includes a SPOC region for cardiac muscle where ATP and Ca2+ coexists with ADP and PI, thus resembling physiological conditions. Further, SPOC occurs more frequently at neutral pH (Fukuda and Ishiwata, 1999).
It is important to note that SPOC is distinct from the repeating contraction and relaxation cycle of the beating heart in real life, which is ultimately controlled by depolarisation and repolarisation of the sarcolemma. However, Sasaki et al. (2005, 2006) believe that SPOC may play a part in the molecular mechanism of the beating myocardium. Fukuda and Ishiwata (1999) suggested that SPOC may stabilise the rhythm of the heartbeat and that the intrinsic pacemaker system and SPOC may work in concert to produce coordinated, synchronous contraction in vivo.
Interestingly, a phenomenon similar to the SPOC wave (i.e. propagated sarcomeric lengthening) was observed by Stehle et al. (2002) when Ca2+ was rapidly removed in activated cardiac myofibrils, leading these authors to suggest that SPOC might facilitate the rapid relaxation of the myocardium in vivo. Sasaki et al. (2006) proposed that this propagation phenomenon and SPOC might share a fundamental molecular mechanism.
The future of SPOC
SPOC is a novel experimental tool for studying the coordination and communication between sarcomeres in a partially activated state. Like actin motility assays, SPOC allows us to observe an interaction between actin and myosin filaments in the absence of Ca2+ regulation. Much remains to be investigated regarding the molecular mechanism of the SPOC state and its role in physiology.
SPOC is yet to be characterised in non-failing human heart samples. Given that several SPOC parameters (rate of shortening, rate of lengthening, the SPOC period and SPOC amplitude) can be monitored, it may prove to be a suitable technique for quantifying the contractile performance of sarcomeres, such as in endocardial biopsies. Hypertrophic cardiomyopathy (HCM), for example, is characterised by an abnormal thickening of the ventricular walls and septum (Maron 2002). Histologically, the primary indicators of HCM are cardiomyocyte hypertrophy, disarray and interstitial fibrosis (Hughes 2004). HCM is a familial disorder, where a causal genetic mutation in proteins of the cardiac sarcomere has been identified in more than half the cases (Richard et al. 2003; Alcalai et al. 2008). The question that still remains, however, is whether SPOC can be used to demonstrate in vitro the functional manifestation of defects in sarcomeric proteins in the failing human myocardium. If it can, it may provide an objective and valuable set of parameters that can objectively assess the state of human heart failure.
References
- Abdulla R, Blew GA, Holterman MJ. Cardiovascular embryology. Pediatr Cardiol. 2004;25:191–200. doi: 10.1007/s00246-003-0585-1. [DOI] [PubMed] [Google Scholar]
- Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: From bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Anazawa T, Yasuda K, Ishiwata S. Spontaneous oscillation of tension and sarcomere length in skeletal myofibrils. Microscopic measurement and analysis. Biophys J. 1992;61:1099–1108. doi: 10.1016/S0006-3495(92)81919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann WH. Hormonal influences on cardiac myosin ATPase activity and myosin isoenzyme distribution. Mol Cell Endocrinol. 1984;34:169–181. doi: 10.1016/0303-7207(84)90173-4. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Endo M. Calcium ions and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Endo M. Stretch-induced increase in activation of skinned muscle fibers by calcium. Nat New Biol. 1972;237:211–213. doi: 10.1038/237211a0. [DOI] [PubMed] [Google Scholar]
- Estigoy CB, Pontén F, Odeberg J, Herbert B, Guilhaus M, Charleston M, Ho JWK, Cameron D, dos Remedios CG. Intercalated discs: Multiple proteins perform multiple functions in non-failing and failing human hearts. Biophys Rev. 2009;1:43–49. doi: 10.1007/s12551-008-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975;249:497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscle. J Physiol. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978;72:667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares E, Howlett SE. Effect of age on cardiac excitation-contraction coupling. Clin Exp Pharm Physiol. 2010;37:1–7. doi: 10.1111/j.1440-1681.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- Fujita H, Ishiwata S. Spontaneous oscillatory contraction without regulatory proteins in actin filament-reconstituted fibers. Biophys J. 1998;75:1439–1445. doi: 10.1016/S0006-3495(98)74062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Yasuda K, Niitsu S, Funatsu T, Ishiwata S. Structural and functional reconstitution of thin filaments in the contractile apparatus of cardiac muscle. Biophys J. 1996;71:2307–2318. doi: 10.1016/S0006-3495(96)79465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Ishiwata S. Effects of pH on spontaneous tension oscillation in skinned bovine cardiac muscle. Pflügers Arch. 1999;438:125–132. doi: 10.1007/s004240050889. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Fujita H, Fujita T, Ishiwata S. Spontaneous tension oscillation in skinned bovine cardiac muscle. Pflügers Arch. 1996;433:1–8. doi: 10.1007/s004240050241. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Fujita H, Fujita T, Ishiwata S. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. J. Muscle Res Cell Motil. 1998;19:909–921. doi: 10.1023/A:1005437517287. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle. Role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001;104:1639–1645. doi: 10.1161/hc3901.095898. [DOI] [PubMed] [Google Scholar]
- Funatsu T, Higuchi H, Ishiwata S. Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol. 1990;110:53–62. doi: 10.1083/jcb.110.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T, Kono E, Higuchi H, Kimura S, Ishiwata S, Yoshioka T, Maruyama K, Tsukita S. Elastic filaments in situ in cardiac muscle: Deep-etch replica analysis in combination with selective removal of actin and myosin filaments. J Cell Biol. 1993;120:711–724. doi: 10.1083/jcb.120.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall MC. Auto-oscillations in extracted muscle fibre systems. Nature. 1956;177:1238–1239. doi: 10.1038/1771238b0. [DOI] [PubMed] [Google Scholar]
- Gorga JA, Fishbaugher DE, VanBuren P. Activation of the calcium-regulated thin filament by myosin strong binding. Biophys J. 2003;85:2484–2491. doi: 10.1016/S0006-3495(03)74671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: Physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10:211–233. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- Günther S, Kruse K. Spontaneous sarcomere dynamics. Chaos. 2007;20:045122. doi: 10.1063/1.3523283. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Ianuzzo CD. Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol Cell Biochem. 1991;106:133–141. doi: 10.1007/BF00230179. [DOI] [PubMed] [Google Scholar]
- Horowits R, Kempner ES, Bisher ME, Podolsky RJ. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986;323:160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. doi: 10.1111/j.1365-2559.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1366. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–977. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Hanson J. The structural basis of the contraction mechanism in striated muscle. Ann N Y Acad Sci. 1959;81:403–408. doi: 10.1111/j.1749-6632.1959.tb49323.x. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction: Interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Ishiwata S, Yasuda K. Mechano-chemical coupling in spontaneous oscillatory contraction of muscle. Phase Transitions. 1993;45:105–136. doi: 10.1080/01411599308223720. [DOI] [Google Scholar]
- Ishiwata S, Okamura N, Shimizu H. Spontaneous oscillation of sarcomeres in skeletal myofibril. Observation with a phase-contrast microscope. J Muscle Res Cell Motil. 1987;8:275. [Google Scholar]
- Ishiwata S, Okamura N, Shimizu H, Anazawa T, Yasuda K. Spontaneous oscillatory contraction (SPOC) of sarcomeres in skeletal muscle. Adv Biophys. 1991;27:227–235. doi: 10.1016/0065-227X(91)90022-6. [DOI] [PubMed] [Google Scholar]
- Ishiwata S, Yasuda K, Shindo Y, Fujita H. Microscopic analysis of the elastic properties of connectin/titin and nebulin in myofibrils. Adv Biophys. 1996;33:135–142. doi: 10.1016/0065-227X(96)81669-8. [DOI] [PubMed] [Google Scholar]
- Iwazumi T, Pollack GH. The effect of sarcomere non-uniformity on the sarcomere length-tension relationship of skinned fibers. J Cell Physiol. 1981;106:321–337. doi: 10.1002/jcp.1041060302. [DOI] [PubMed] [Google Scholar]
- Jülicher F, Prost J. Spontaneous oscillations of collective motor units. Phys Rev Lett. 1997;78:4510–4513. doi: 10.1103/PhysRevLett.78.4510. [DOI] [Google Scholar]
- Lappé DL, Lakatta EG. Intensity fluctuation spectroscopy monitors contractile activation in “resting” cardiac muscle. Science. 1980;207:1369–1371. doi: 10.1126/science.7355295. [DOI] [PubMed] [Google Scholar]
- Linke WA, Bartoo ML, Pollack GH. Spontaneous sarcomeric oscillations at intermediate activation levels in single isolated cardiac myofibrils. Circ Res. 1993;73:724–734. doi: 10.1161/01.res.73.4.724. [DOI] [PubMed] [Google Scholar]
- Lorand L, Moos C. Auto-oscillations in extracted muscle fibre systems. Nature. 1956;177:1238–1239. doi: 10.1038/1771239a0. [DOI] [PubMed] [Google Scholar]
- Margossian SS. Reversible dissociation of dog cardiac myosin regulatory light chain 2 and its influence on ATP hydrolysis. J Biol Chem. 1985;260:13747–13754. [PubMed] [Google Scholar]
- Maron BJ. Hypertrophic cardiomyopathy: A systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic protein from myofibrils. J Biochem. 1976;80:405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Pratt M, Kiehart DP, Stephens RE. Cyclic contraction and relaxation of sarcomeres in isolated myofibrils. Biophys J. 1977;17:173a. [Google Scholar]
- Mulder BJM, de Tombe PP, ter Keurs HEDJ. Spontaneous and propagated contractions in rat cardiac trabeculae. J Gen Physiol. 1989;93:943–961. doi: 10.1085/jgp.93.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley CE, Chamoun J, Brown LJ, Hambly BD. Myosin binding protein-C: Enigmatic regulator of cardiac contraction. Int J Biochem Cell Biol. 2007;39:2161–2166. doi: 10.1016/j.biocel.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Okamura N, Ishiwata S. Spontaneous oscillatory contraction of sarcomeres in skeletal myofibrils. J Muscle Res Cell Motil. 1988;9:111–119. doi: 10.1007/BF01773733. [DOI] [PubMed] [Google Scholar]
- Onodera S. Oscillatory contraction waves in skinned skeletal muscle at high pH without Ca2+ Jikeikai Med. 1990;37:447–455. [Google Scholar]
- Onodera S, Umazume Y. Periodic contraction of skinned muscle fiber under high pH. Biophysics (Jpn) 1984;24:S84. [Google Scholar]
- Pratt MM, Mooseker MS, Kiehart DP, Stephens RE. Cyclic contraction and relaxation of glycerinated myofibrils isolated from skeletal muscle. Biol Bull. 1976;151:426. [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet J-P, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Rieser G, Sabbadini R, Paolini P, Fry M, Inesi G. Sarcomere motion in isolated cardiac cells. Am J Physiol. 1979;236:C70–C77. doi: 10.1152/ajpcell.1979.236.1.C70. [DOI] [PubMed] [Google Scholar]
- Sasaki D, Fujita H, Fukuda N, Kurihara S, Ishiwata S. Auto-oscillations of skinned myocardium correlating with heartbeat. J Muscle Res Cell Motil. 2005;26:93–101. doi: 10.1007/s10974-005-0249-2. [DOI] [PubMed] [Google Scholar]
- Sasaki D, Fukuda N, Ishiwata S. Myocardial sarcomeres spontaneously oscillate with the period of heartbeat under physiological conditions. Biochem Biophys Res Commun. 2006;343:1146–1152. doi: 10.1016/j.bbrc.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y, Kono F, Suzuki M, Ishiwata S. Nonlinear force-length relationship in the ADP-induced contraction of skeletal myofibrils. Biophys J. 2007;93:4330–4341. doi: 10.1529/biophysj.107.110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto Y, Suzuki M, Ishiwata S. Length-dependent activation and auto-oscillation in skeletal myofibrils at partial activation by Ca2+ Biochem Biophys Res Commun. 2008;366:233–238. doi: 10.1016/j.bbrc.2007.11.123. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y, Suzuki M, Mikhailenko SV, Yasuda K, Ishiwata S. Inter-sarcomere coordination in muscle revealed through individual sarcomere response to quick stretch. Proc Natl Acad Sci USA. 2009;106:11954–11959. doi: 10.1073/pnas.0813288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Fujita T, Ishiwata S. Regulation of tension development by MgADP and Pi without Ca2+. Role in spontaneous tension oscillation of skeletal muscle. Biophys J. 1992;61:1087–1098. doi: 10.1016/S0006-3495(92)81918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Stephenson DG. Theory and observation of spontaneous oscillatory contractions in skeletal myofibrils. J Muscle Res Cell Motil. 1994;15:369–389. doi: 10.1007/BF00122112. [DOI] [PubMed] [Google Scholar]
- Smith DA, Stephenson DG. The mechanism of spontaneous oscillatory contractions in skeletal muscle. Biophys J. 2009;96:3682–3691. doi: 10.1016/j.bpj.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle R, Krüger M, Pfitzer G. Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid Ca2+ changes. Biophys J. 2002;83:2152–2161. doi: 10.1016/S0006-3495(02)73975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer NK, Moss RL. The effect of altered temperature on Ca2+-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990;96:1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilfan A, Duke T. Synchronization of active mechanical oscillators by an inertial lode. Phys Rev Lett. 2003;91:114101. doi: 10.1103/PhysRevLett.91.114101. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Cardiac myosin binding protein C. Circ Res. 1999;84:1117–1126. doi: 10.1161/01.res.84.10.1117. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Shindo Y, Ishiwata S. Synchronous behavior of spontaneous oscillations of sarcomeres in skeletal myofibrils under isotonic conditions. Biophys J. 1996;70:1823–1829. doi: 10.1016/S0006-3495(96)79747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]