Abstract
The correct control of cell fate decisions is critical for metazoan development and tissue homeostasis. It is established that the integrin family of cell surface receptors regulate cell fate by mediating cell–cell and cell–extracellular matrix (ECM) interactions. However, our understanding of how the different family members control discrete aspects of cell biology, and how this varies between tissues and is temporally regulated, is still in its infancy. An emerging area of investigation aims to understand how integrins translate changes in tension in the surrounding microenvironment into biological responses. This is particularly pertinent due to changes in the mechanical properties of the ECM having been linked to diseases, such as cancer. In this review, we provide an overview of the roles integrins play in important developmental processes, such as proliferation, polarity, apoptosis, differentiation and maintenance of “stemness”. We also discuss recent advances in integrin mechanobiology and highlight the involvement of integrins and aberrant ECM in cancer.
Keywords: Integrins, Extracellular matrix, Cancer, Cell fate, Stem cells, Mechanobiology
Introduction
Integrins are evolutionarily conserved heterodimeric cell surface receptors that are composed of an α and β subunit. In mammals there are 18 α-subunits and eight β-subunits that combine to form a total of 24 different receptors (Anderson et al. 2013). Integrins recruit a myriad of effector molecules, such as Talin, Paxillin, ILK, FAK and various GTPases, to supramolecular signalling platforms, known as focal adhesions, to propagate signals bi-directionally across the plasma membrane. Integrin-dependent adhesions also have structural functions, linking the internal cytoskeleton to the external environment through cell–cell and cell–ECM interactions. Given the location of integrins at this nexus of molecular activity, it is understandable that they are key players in the regulation of processes important for normal development. In this review we will discuss the roles of integrins during development and the implications of aberrant integrin signalling in cancer.
Integrins in tissue development
Integrins play essential roles during tissue development, as demonstrated by a number of genetic deletion studies using mouse knockout models (Bouvard et al. 2001). Almost all known integrin subunits, as well as many of their extracellular matrix (ECM) ligands have been genetically deleted, resulting in a range of phenotypes, including embryonic lethality or defects during the development of a particular tissue and organ (Hynes 1996; Bouvard et al. 2001; Hynes 2002; Docheva et al. 2007; Prowse et al. 2011). Integrins and their ligands are required early on in embryogenesis to regulate a number of processes, including fertilisation, implantation and early blastula formation (De Arcangelis and Georges-Labouesse 2000; Tarone et al. 2000). Embryos from homozygous β1 integrin null mice die shortly after invading the uterine basement membrane (Fassler and Meyer 1995; Stephens et al. 1995), whereas constitutive deletion of integrin α2, α3, α6 and β4 subunits resulted in mice dying just after birth. Indeed, some of the different integrin subunit knockout mice show similar phenotypes, suggesting that critical processes require a combination of multiple receptors (Kreidberg et al. 1996; DiPersio et al. 1997; Ryan et al. 1999; Chen et al. 2002). Deletion of fibronectin, a major component of the ECM and an integrin ligand, resulted in embryonic death at embyronic day 8.5, indicating that specific integrin ligands also play an essential role during development (George et al. 1993).
The defects observed in integrin null mice can be related back to the function of the integrin subunits examined; for example, β1 integrin knockout mice have the most severe embryonic lethal phenotype (Fassler and Meyer 1995; Stephens et al. 1995), which is likely due to β1 integrin’s role as a component of 12 of the 24 known integrin heterodimers. Subsequent studies utilised chimeric or “floxed” mice to further investigate the effect of the loss of β1 integrin in different cell and tissue types, such as haematopoietic, cardiovascular and epithelial systems. Some of the defects observed following β1 ablation included defective haematopoietic stem cells homing and migration (Hirsch et al. 1996; Potocnik et al. 2000), delayed and impaired formation of complex vasculature in mouse embryonic stem cells (Bloch et al. 1997) and cardiac muscle defects (Baudoin et al. 1998; Keller et al. 2001). Since these initial studies, extensive interrogation of β1 integrin and other integrin subunit function in specific tissue types has been undertaken using conditional mouse knockout models (Brakebusch et al. 2000; Raghavan et al. 2000; Naylor et al. 2005; Akhtar et al. 2009; Moran-Jones et al. 2012). However, although it has been long appreciated that integrins are essential for many aspects of development, it is testament to the complexity of integrin function that the mechanisms by which integrins control cell fate are still the subject of intense interest.
Integrins and cell fate
The formation of the different tissue types that make up complex organisms, such as mammals, stem from a myriad of cell fate decisions. Integrins play a key role in mediating these cell fate decisions by providing the spatial cues required by cells to respond to the temporal cues, which in turn are provided by soluble factors produced at distinct times during development. Integrins also provide the cell with information on the composition of the ECM and the surrounding cellular mechanical tension. Together, this information helps with the development and regulation of a wide range of cell phenotypes in a context-dependent manner.
Integrins and stem cells
Integrins have been one of the cell surface markers commonly used during fluorescent activated cell sorting to distinguish different stem and progenitor cell populations. For example, in skin and mammary gland, isolation of stem cells has been performed based on a high expression of α6 integrin (CD49f) (Tani et al. 2000; Stingl et al. 2006). High β1 integrin (CD29) expression also enables enrichment of mammary stem cells, and β3 integrin (CD61) is a marker of lineage-specific progenitor cells (Shackleton et al. 2006; Asselin-Labat et al. 2007). However, despite this, there is still relatively little known about the role of integrins in stem cell biology.
The stem cell niche is an area in which spatial information provided by integrins is likely to be of great importance. High levels of integrin expression may aid the physical interaction between a stem cell and the niche in which it resides, ensuring that the stem cell remains following proliferation, while the daughter cells exit the stem cell niche (Chen et al. 2013). In the Drosophila midgut, integrins are required for both the maintenance and proliferation of intestinal stem cells (ISCs) (Lin et al. 2013). An important mechanism for maintenance of the stem cell pool is the control of asymmetric cell division. Goulas et al. (2012) reported that Drosophila ISCs required integrin-dependent adhesion to the ECM to regulate cell polarity and ensure the asymmetric segregation of proteins into daughter cells. Similar defects have been observed in mammalian epithelial tissues following integrin ablation. Taddei et al. (2008) found that genetic deletion of β1 integrin from the basal epithelial cell compartment in the mammary gland, which is believed to contain the mammary stem cells, disrupted the orientation of cell division and reduced stem cell numbers.
In addition to regulating stem cell numbers, integrins are also implicated in maintaining stem cell pluripotency. Characterisation of integrin expression in mouse embryonic stem (ES) cells suggests that α5β1, αvβ5, α6β1 and α9β1 integrins may be involved in regulating stemness (Lee et al. 2010). Using integrin-specific peptides and hydrogel scaffold, Lee and colleagues demonstrated that simultaneous stimulation of the four integrin heterodimers was necessary to maintain transcription of stem cell-related genes. Furthermore, their precisely engineered scaffold induced a pattern of gene expression similar to ES cells cultured on fibroblast feeder layers (Lee et al. 2010). α2β1 integrin has also been implicated in maintaining mouse ES cells in their undifferentiated state through binding to full-length collagen-I (Suh and Han 2011). However, in addition to α2β1 integrin, this effect of full-length collagen-I on ES cells also required collagen-I binding to the discoidin domain receptor 1.
Human bone marrow-derived mesenchymal stem cells (hMSCs) have been found to express a wide selection of integrin subunits, with the expression levels of specific subunits changing as the cells differentiated down specific lineages (Frith et al. 2012). The contribution of hMSC integrins to cell differentiation was then examined using immobilised peptides. To maintain hMSC survival, the cells required a small amount of RGD peptide to be present, which suggested a role for α5β1, αVβ3 and αVβ5 integrins in hMSC survival. When cultured in the osteogenic or adipogenic differentiation media, the presence of laminin-derived IKVAV peptides increased differentiation down the respective lineages, compared with RGD peptide alone (Frith et al. 2012). These studies in embryonic and tissue-specific stem cells demonstrate the importance of integrins in the regulation of stem cell phenotype. They also indicate that manipulation of integrin signalling may be an attractive target for the optimisation of stem cell-based therapeutics.
Integrins in differentiation
Integrin expression changes during cell differentiation, suggesting that different integrin-dependent signals regulate cellular differentiation. In the epidermis, analyses of integrin expression across the different cell layers have revealed that specific integrin expression is lost from the basal layer during differentiation, which is accompanied by a loss in adhesion from the basement membrane (BM) and decreased proliferation (Watt 2002). In the prostate and the skin, the greatest change in integrin expression is observed in the β4 integrin, which when paired with α6 integrin forms the primary constituents of hemidesmosomes that attach basal epithelial cells to the BM. Loss of other integrin heterodimers, such as α2β1 and α3β1, is also observed during differentiation. The reduction in α6β4 expression is accompanied initially by proteolytic degradation of both the α6 and β4 subunits before subsequent suppression of integrin gene expression, suggesting that posttranscriptional regulation of α6β4 is a direct effect of a commitment to differentiation (Tennenbaum et al. 1996). Supporting this concept, neoplastic keratinocytes delay maturation, promote proliferation and prevent downregulation of α6β4 (Tennenbaum et al. 1993; Watt 2002).
β1 integrin has been extensively characterised during epithelial differentiation in the mammary gland (Taddei et al. 2003; Naylor et al. 2005; Taddei et al. 2008). A main function of mammary epithelial cells is milk production to facilitate lactation. This process is controlled by the hormone prolactin (Prl), which following binding to its receptor activates the nuclear translocation of STAT5 and subsequent milk gene expression. However, STAT5 activation by Prl requires β1 integrin to be bound to laminin-1 in the BM, as shown by Naylor et al. (2005) who observed that deletion of β1 integrin resulted in impaired alveologenesis and lactation. This example highlights an increasing common phenomenon where cross-talk between integrins and other receptors are required for signal propagation to occur. Interestingly, conditional deletion of β1 integrin in prostate epithelium resulted in a relatively mild phenotype, with an expansion of the p63-positive basal cell population and decreased differentiation (Moran-Jones et al. 2012). The reduced phenotype in prostate epithelium compared to breast epithelium is either due to potential compensation from other integrins or because β1 integrin does not play as critical a role following the formation of the appropriate BM.
Analysis of downstream integrin adaptor molecules has shown that integrin-linked kinase (ILK)—not focal adhesion kinase—is the critical downstream effector involved in mediating β1 integrin’s function in the mammary gland (Akhtar et al. 2009). The link between Prl and integrin signalling is provided by the Rho family GTPase—Rac1—which was shown to rescue the expression of milk protein genes in β1 integrin-null cells while, conversely, a dominant negative Rac1 mutant prevented Prl signalling and milk protein production (Akhtar and Streuli 2006). This body of work has helped define how integrins cooperate with hormone signalling in the control of mammary cell fate determination.
Integrins in proliferation
It has long been established that integrin-mediated adhesion signals regulate cell cycle progression through regulation of cyclin-dependent cell cycle checkpoints (Guadagno et al. 1993; Zhu et al. 1996) (Mettouchi et al. 2001; Hirsch et al. 2002). Integrin–ECM engagement is also necessary in many cases for growth factor-induced mitogenic signals. Adherent cells require attachment to the ECM for a mitogenic signal from a growth factor to activate proliferation through a range of signalling pathways, including PI3K, JNK, STAT5, MAPK and Rho-family GTPases (DeMali et al. 1999; Assoian and Schwartz 2001; Cascone et al. 2005; Defilippi et al. 2005; Mahabeleshwar et al. 2007). However, the mechanisms by which integrins control cell cycle progression and the precise roles of distinct heterodimers are still the subject of much investigation (Wang et al. 2011; Jeanes et al. 2012). In mammary epithelial cells lacking β1 integrin, epidermal growth factor (EGF) stimulation was sufficient to activate the ERK signalling pathway, but proliferation rates were reduced compare to wildtype controls. In this system, β1 integrin was required to facilitate Rac1 activation, which enabled ERK translocation to the nucleus to drive proliferation (Jeanes et al. 2012). Rac1 activation downstream of β1 integrin may be facilitated by the integrin-binding protein, Talin, which is necessary for the recruitment of adhesion proteins that drive cell cycle progression (Wang et al. 2011).
Integrins and cell polarity
Cellular polarity is an essential feature of epithelial organs and is initiated following the formation of tight junctions. This process allows cells to orientate themselves and form both basal (facing the basement membrane) and apical (facing the lumen) surfaces (Carmosino et al. 2010). Integrins help form the axis of polarity, with early studies in Madin–Darby canine kidney (MDCK) cells showing that β1 integrin mediates the orientation of the apical pole of polarised MDCK cysts through activation of Rac1 (O’Brien et al. 2001; Yu et al. 2005). In vivo, deletion of α6 and β4 integrins, which form components of hemidesmosomes, results in a loss of epidermal polarity (Dowling et al. 1996; Georges-Labouesse et al. 1996; Raghavan et al. 2000). Furthermore, in endothelial cells β1 integrin is required in vivo to maintain polarity by controlling the expression of the polarity protein Par3 (Zovein et al. 2010).
Recently, significant insight into the molecular mechanism by which integrins establish polarity in glandular epithelium has been made (Akhtar and Streuli 2013). Using in vivo and three-dimensional primary culture models, these authors showed that β1 integrin/ ILK is required for the endocytic removal of apical proteins from the BM surface of the cell and for correct Golgi positioning. Prior to polarisation, the epithelial cells contained fragmented Golgi and apical proteins localised on their basolateral surface. Following β1 integrin engagement with the BM at the basal surface, ILK interacted with microtubules via their plus-ends and polarised the microtubules along the apicobasal axis. This drove the endocytosis of apical-destined proteins and their re-distribution to the new apical surface opposing the BM orientating the cell (Akhtar and Streuli 2013).
Integrins and apoptosis
The importance of the cell–ECM interaction during cell survival was first discovered using human endothelial cells which rapidly underwent apoptosis in the absence of ECM attachment (Meredith et al. 1993). The term “anoikis” was then coined by Frisch and Francis (1994) to describe this form of apoptosis which occurs when there is a loss of adhesion signalling. Importantly, it is the composition of the surrounding ECM that heavily influences cell survival, i.e. it is not just detachment that initiates anoikis, but also a lack of correct adhesion signalling. For example, mammary epithelial cells cultured on collagen I undergo apoptosis within a few days, whereas a laminin-rich substrate inhibits cell death (Pullan et al. 1996). Furthermore, this integrin–laminin–cell–ECM interaction also cooperates with insulin to suppress apoptosis in mammary epithelial cells (Farrelly et al. 1999). Integrins signal via molecules such as FAK and PI3K to tightly regulate cell sensitivity to apoptosis by controlling the dynamics of the association between apoptotic proteins and mitochondria (Schellenberg et al. 2013).
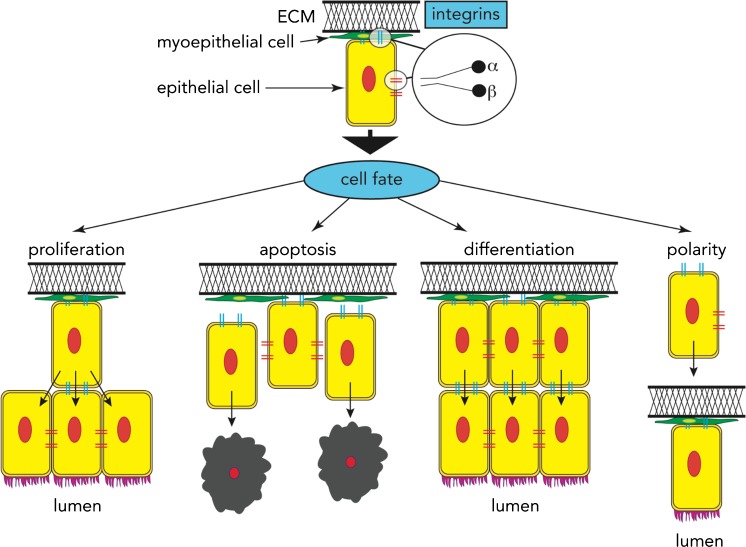
Together, a picture emerges of how integrin signalling controls a variety of cellular phenotypes, including survival, proliferation, differentiation and polarity (Figure 1). However, these effects on cell fate are dependent not only on the contact between integrins and the ECM, but also the mechanical forces and tension of the local environment (Engler et al. 2006). For integrins to regulate cell fate in response to changes in force/tension in the extracellular environment, they must be able to detect these changes and then translate them into signals to elicit a biological response.
Fig. 1.
The role of integrins in cell fate. Integrins are unique in their ability as transmembrane receptors that control cell fate decisions such as whether a cell should undergo proliferation, apoptosis or differentiation, or become polarised. The cellular context also influences these cell fate decisions, with integrins acting as sensors that communicate information from the surrounding microenvironment [extracellular matrix (ECM)] to the cell
Integrins role in mechanobiology
The mechanobiology of integrin signalling is evident from the initiation of integrin engagement, with a tension of approximately 40 pN applied to individual integrin-ligand interactions during the initial engagement (Wang and Ha 2013). Increasing force then drives a shift in the integrin to a high-affinity conformation that stabilises the interaction with ligand (Chen et al. 2012). This higher affinity interaction in response to increasing force suggests a catch–bond model of integrin engagement, in which bond lifetime initially increases with increasing tension (Kong et al. 2009). However, the complexity of the mechanics of integrin–ligand interactions has increased with the recent description of cyclic mechanical reinforcement (CMR) (Kong et al. 2013). In CMR, the cyclic application of tension to the integrin–-ligand interaction greatly increases the bond lifetime. Once bonds have been established, further cellular tension may then stimulate the recruitment of additional integrins to adhesion sites to facilitate a phenotypic response (Thodeti et al. 2009).
In the last few years, the mechanisms by which integrin-dependent changes in tension elicit a biological response have begun to be elucidated. The focal adhesion protein Talin, which links β-integrin tails to the actin cytoskeleton, contains cryptic binding sites for vinculin that are exposed upon the stretching of Talin (del Rio et al. 2009). Myosin-dependent stretching of Talin in vivo is proposed to expose these sites, allowing vinculin recruitment to be regulated by cycles of stretching and relaxation (Margadant et al. 2011). These studies shed light on the molecular basis of tension sensing at the cell surface, but in order for cellular force to regulate cell fate decisions, the signals emanating from the sites of contact with the ECM need to be communicated to the nucleus to drive changes in gene expression. In endothelial cells, the focal adhesion protein zyxin translocates to the nucleus in response to cell stretching. Nuclear zyxin then adopts the role of a transcription factor to promote the expression of stretch-sensitive genes with roles in inflammation, apoptosis and proliferation (Wojtowicz et al. 2010).
In an elegant study, Dupont and colleagues recently demonstrated that the cell fate regulators YAP and TAZ were activated in cells plated on stiff ECM (Dupont et al. 2011). Notably, the tension-dependent differentiation of mesenchymal stem cells (MSCs) was disrupted by YAP/TAZ siRNA. On stiff ECM, YAP/TAZ knockdown blocked MSC differentiation into osteoblasts and remarkably facilitated differentiation into adipocytes. Furthermore, overexpression of active YAP rescued cell survival and proliferation in human microvascular endothelial cells plated on confined ECM that limits cell spreading. Thus, this study demonstrates that the control of cell fate decisions by cellular tension can be circumvented by manipulating downstream transcription factors. Such knowledge may be of critical importance in combating pathologies associated with altered ECM-stiffness, such as cancer. Whilst there is still much to be learned about the mechanisms governing mechanobiology, recent advances in nano-engineering that enable precise control of substrate compositions and mechanical stimulation will enable integrin mechanobiology to be studied in detail in the future (Subramony et al. 2013).
Integrins and cancer
Defective integrin signalling results in a number of pathologies associated with the formation of incorrect tissue architecture, adhesion, migration, proliferation and apoptosis. One such pathology associated with disrupted control of cell fate decisions is cancer. Significant progress in understanding integrin function in tumorigenesis has been made though the use of knockout mouse models combined with spontaneous cancer models.
Integrins and tumorigenesis
β1 integrin influences several aspects of epithelial tumorigenesis. Conditional deletion of β1 integrin in the mammary gland of PyMT-driven breast cancer dramatically attenuates tumour progression (White et al. 2004). In this study β1 integrin both regulated tumour initiation and maintained cancer cell proliferation (White et al. 2004). In contrast, β1 integrin expression was not required for tumour initiation in the ErbB2/neu-driven breast cancer model—but it did influence metastasis (Huck et al. 2010). Interestingly, deletion of the integrin signalling molecule ILK reduced tumour incidence in the ErbB2/neu model (Pontier et al. 2010), indicating that perhaps while other integrins can compensate for the loss of β1 integrin, a surrogate for ILK’s function downstream of multiple integrin hetrodimers is less readily available.
Further work in the ErbB2/neu model of tumorigenesis showed that inhibition of α2β1 integrin expression (achieved by deletion of the α2-subunit), moderately delayed tumour incidence but had no effect on primary tumour growth. However, there were significantly more, and larger, lung metastases in the animals lacking α2 integrin. Ramirez et al. (2011) observed that tumour cells lacking α2 integrin exhibited increased anchorage-independent growth and migration in vitro, and they detected a significant increase in circulating tumour cells in vivo, suggesting the absence of α2 integrin promoted intravasation. Significantly, in this same study, reduced α2 integrin expression in human cancer patients correlated with metastasis incidence and a worse prognosis in breast and prostate cancer. Data on human patients strongly suggest a suppressive function of α2 integrin during cancer progression. It is possible that loss of α2 integrin expression allows β1 integrin to contribute to cancer progression by partnering with other α-subunits, as β1 integrin expression was found to be required for metastasis in the ErbB2 tumour model (Huck et al. 2010).
Recently, β1 integrin was conditionally deleted in prostate epithelium, where in contrast to the phenotypes observed in the breast, β1 integrin ablation resulted in decreased survival in the TRAMP cancer model, as well as increased tumour progression and proliferation (Moran-Jones et al. 2012). These studies are similar to the developmental studies mentioned earlier, in which deletion of β1 integrin during development in breast and prostate had quite different effects, thereby further highlighting the contextual and tissue-specific effects of integrins.
Conditional deletion of β4 integrin in the ErbB2/neu mouse breast cancer model suppressed tumour formation and metastasis (Guo et al. 2006), while targeted expression of α6β4 integrin in the suprabasal layer of the mouse epidermis increased tumour formation and metastasis (Owens et al. 2003). In both of these cases, increased β4 integrin expression correlated with cancer progression. Other studies have reported similar correlations of increased α6β4 integrin expression in thyroid, bladder, colorectal and gastic cancers, suggesting that α6β4 integrin has an important role in epithelial carcinogenesis (Mercurio and Rabinovitz 2001). α6β4 integrin is predominantly found in hemidesmosome structures which form the rigid adhesive structures on the basal cell surface and link the actin cytoskeleton to laminin in the basement membrane. However, α6β4 has also been implicated in mediating epithelial cell migration (Mercurio et al. 2001). This role in migration may account for the observation that α6β4 integrin levels are often high in invasive carcinomas yet hemidesmosome formation is absent (Mercurio and Rabinovitz 2001). EGF stimulation can mobilise α6β4 integrins from hemidesmosomes into lamellipodia in squamous cell carcinoma cells to facilitate migration (Mainiero et al. 1996; Rabinovitz et al. 1999; Mercurio and Rabinovitz 2001). Rabinovitz et al. (1999) proposed that the mechanism of this mobilisation involves activation of PKCα and the phosphorylation of β4. However, further studies are required to determine whether this is the case across other cancer types.
In prostate cancer, loss of androgen receptor (AR) expression is a well-known hallmark of androgen-resistance cancer and a more aggressive phenotype. A link between α6β4 integrin expression and loss of AR expression was made in a study where AR expression in prostate cancer cells was shown to suppress α6β4 integrin expression. This finding suggests that loss of AR may increase α6β4 integrin expression and promote a more invasive phenotype in prostate cancer cells (Bonaccorsi et al. 2000; Cinar et al. 2001).
As integrins activate multiple signalling pathways, it will be critical to delineate the roles that each pathway plays in cancer progression, along with the compensatory mechanisms elicited in response to inhibition of downstream mediators—if integrins are to be targeted as cancer therapeutics. Inhibition of the individual components of the integrin adhesome may be insufficient to prevent tumorigenesis driven by integrin expression (Deakin and Turner 2011). It will also be necessary to understand how integrin receptors that bind to the same substrate, but elicit different responses, operate, so that only the specific heterodimer of interest is targeted (Schiller et al. 2013). However, the finding that blocking integrin function with inhibitory antibodies sensitised breast cancer cells to apoptosis induced by radiotherapy is encouraging (Park et al. 2008) as it demonstrates that integrins may be a useful therapeutic target.
Integrins and cancer stem cells
A current challenge to cancer therapy is the existence of the so-called, cancer stem cells (CSCs; also known as tumour initiating cells) that are thought to contribute to disease progression and recurrence (Owens and Naylor 2013). There is growing evidence that integrins contribute to the CSC phenotype and may therefore provide another area to explore for novel therapeutic intervention strategies.
In human triple negative breast cancer cell lines, neuropilin-2 (NRP-2) expressed on CSCs signals through α6β1 integrin to upregulate GLI-1, which subsequently induces further expression of NRP2. Disruption of this autocrine signalling loop has been found to delay tumour progression in mice (Goel et al. 2013). In the mouse Her2 breast cancer model, CSCs express high levels of α6 and β3 integrins (Lo et al. 2012). Significantly, knockdown of β3 integrin suppressed CSC self-renewal by impairing tumour growth factor-beta signalling (Lo et al. 2012). In prostate cancer, β4 integrin expression was observed to be higher in tumour samples from bone metastases and from patients who had undergone androgen ablation therapy, compared with naïve primary tumours (Yoshioka et al. 2013). In this same study, in mouse prostate cancer models, deletion of the β4 integrin signalling domain delayed tumorigeneis. β4 integrin signalling was required for the expansion of prostate cancer stem cells via cross-talk with ErbB2 and cMet receptors (Yoshioka et al. 2013). β4 integrin may prove to be an attractive therapeutic target as it only pairs with the α6 integrin subunit, therefore enabling the design of β4 integrin-specific antagonists that would not interfere with other integrin signalling pathways, hopefully reducing side effects.
Mechanobiology in cancer
Cancer progression is typically associated with increased tissue rigidity, and there is growing evidence that the tensile strength of the ECM surrounding a cell can induce carcinogenesis (Butcher et al. 2009; Samuel et al. 2011). Remodelling of the ECM by tumour cells perturbs normal integrin function and promotes tumourigenesis by inducing inappropriate cellular functions, such as migration, proliferation and differentiation. Indeed, cellular force or matrix composition has been shown to influence cell fate in ES cells. Engler et al. demonstrated this by growing ES cells on a wide spectrum of matrices ranging from soft to stiff to rigid matrices, representing different tissue types (Engler et al. 2006). These authors showed that soft matrices similar to brain tissue directed stem cells down neurogenic lineages, stiffer matrices similar to muscle tissue directed stem cells down myogenic lineages and rigid matrices that resemble bone tissue directed stem cells down osteogenic lineages (Engler et al. 2006) (Figure 2).
Fig. 2.
Tensile strength and cell fate of the ECM. The increasing rigidity of tissue can have dramatic effects on cell fate. In normal embryonic stem cells the rigidity of the matrix they are exposed to can influence their cell fate, with the resulting cells becoming either neurogenic (brain), myogenic (muscle) or osteogenic (bone) with increasing matrix stiffness, respectively. In cancer, increasing matrix rigidity leads to increased deposition of ECM components (collagen I & V) as well as increased degradation to help facilitate tumour growth. The increased ECM stiffness results in increased recruitment of integrins to focal adhesions, the upregulation of downstream integrin signalling pathways and increased Rho kinase (ROCK) activation, resulting in proliferation and cell survival
In the breast, the first indicators that ECM matrix composition may play a role in cancer originated from studies examining mammographic density in the breast, which have shown that high mammographic density is a strong predictor of breast cancer risk (Boyd et al. 1998; Boyd et al. 2001; Ursin et al. 2005; Stone et al. 2006; Ginsburg et al. 2008). A link has also been established between breast density and tumour initiation, as breast tumours tend to arise in dense areas of the breast (Martin and Boyd 2008). This would also suggest that women with a genetic predisposition to having increased fibrous breast tissue also have an increased risk of developing breast cancer.
Cells can influence the stiffness of the surrounding ECM by depositing or altering ECM proteins, such as collagen. Increased collagen I and V deposition occurs in a process known as desmoplasia, which is observed following the invasion of tumour cells through stromal tissue (Barsky et al. 1982). The deposited collagen can be altered in both its structure and its organisation by tumour cells. For example, there are higher levels of cross-linked collagen in tumour stroma, which increases the stiffness of the ECM. Lysyl oxidase is responsible for cross-linking collagen fibrils, and elevated levels of this enzyme are frequently observed in tumours (Peyrol et al. 1997; Decitre et al. 1998; Erler et al. 2009; Levental et al. 2009). Collagen is also organised into tracks of parallel and perpendicular fibres, which can facilitate the invasion of tumour cells into the surrounding tissue (Provenzano et al. 2006, 2008). Together, the effects of increased collagen deposition, altered structure through cross-linking and altered collagen organisation contribute to tissue fibrosis, which in turn increases the risk of carcinogenesis (Colpaert et al. 2003; van der Slot et al. 2005).
Integrins and ECM signalling in cancer
Epithelial cells can utilise two methods to detect matrix stiffness, namely by Rho-mediated contractility and by integrins acting as mechanosensors via outside–in signalling in response to increasing cellular force from a stiffening matrix. The result of these processes is increased integrin expression, activation and focal adhesion assembly (Riveline et al. 2001; Tzima et al. 2001; Galbraith et al. 2002). In both of these methods, matrix stiffness leads to the activation of a number of integrin signalling pathways, such as tyrosine kinases/phosphatases, MAP kinases and small GTPases involved in cell growth, viability, differentiation and motility (Chrzanowska-Wodnicka and Burridge 1996; Choquet et al. 1997; Geiger and Bershadsky 2002; Paszek et al. 2005; Sawada et al. 2006). The mechanism of Rho-mediated contractility involves Rho activation of its effector Rho kinase (ROCK), which phosphorylates the regulatory light chain of myosin and results in myosin–actin-mediated contraction (Totsukawa et al. 2000). When a matrix becomes too stiff to contract, the increased isometric tension results in the formation of integrin-based focal adhesions, increased activation of FAK and sustained Rho activation. This results in elevated proliferation, an increased invasive phenotype and a loss in cellular morphology (Paszek et al. 2005; Sawada et al. 2006). Interestingly, Rho is in fact regulated by matrix stiffness, with studies showing that in a compliant (soft) matrix Rho exists in an inactive GDP-bound form, while in a non-compliant (stiff) matrix Rho is activated in the GTP-bound form (Wozniak et al. 2003). A recent study has also demonstrated similar results in skin, where ROCK activation functioned to promote tumour growth and progression, while ROCK inhibition attenuated these effects, thus demonstrating that actomyosin-mediated cellular tension and collagen deposition are significant tumour promoters (Samuel et al. 2011). While active Rho GTPases have been associated with stiff tumours (Fritz et al. 1999) and ROCK activation induces tumour migration (Croft et al. 2004), it still remains to be determined whether either of these processes can actually initiate a tumour or if they merely act to aid oncogenes during carcinogenesis.
Future perspectives
Over the past 15 years the tissue microenvironment has emerged as a critical player in mediating important cellular processes, such as tissue development, homeostasis and cell fate, through receptors such as integrins. Integrins do not operate in isolation, but instead mediate cross-talk between cell–ECM, cell–cell contacts, growth-factor signals and mechanical stimuli. Therefore, it will be necessary to study these factors in combination to create a complete picture of integrin function in development and disease. An emerging theme in integrin signalling is the influence of matrix composition and rigidity, both on normal tissue development and cancer progression. However, it remains to be determined whether tissue rigidity and cellular force directly initiate carcinogenesis, or whether they act later to promote tumour progression. Understanding how signals emanating from integrins at the cell surface result in transcriptional responses will aid in the discovery of new therapeutics targeted at disrupting the integrin signals that drive the cancer cell phenotype. The apparent variable roles of integrins in different cancers or between different models of cancer in the same tissue, as well as the relative contributions of downstream signalling molecules, compounded by the added complication of redundancy, exemplifies the necessity to build a comprehensive picture of the role of integrins in vivo—a picture that despite many significant insights is a long way from complete.
Acknowledgements
Financial support was provided by the Cancer Council NSW, Cancer Institute NSW, National Breast Cancer Foundation of Australia, National Health & Medical Research Council of Australia, Prostate Cancer Foundation of Australia and the Sydney Medical School, University of Sydney.
Conflict of interest
None.
Footnotes
Luke R. Anderson and Thomas W. Owens contributed equally to this work.
References
- Akhtar N, Streuli C. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Streuli C. An integrin-ILK-microtubule network orients cellpolarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027. doi: 10.1242/dev.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LR, Owens TW, Naylor MJ (2013) Structural and mechanical functions of integrins. Biophys Rev. doi:10.1007/s12551-013-0124-0 [DOI] [PMC free article] [PubMed]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Assoian R, Schwartz M. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/S0959-437X(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Barsky S, Rao C, Grotendorst G, Liotta L. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol. 1982;108:276–283. [PMC free article] [PubMed] [Google Scholar]
- Baudoin C, Goumans M, Mummery C, Sonnenberg A. Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W, Forsberg E, Lentini S, Brakebusch C, Martin K, Krell H, et al. Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–278. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi L, Carloni V, Muratori M, Salvadori A, Giannini A, Carini M, et al. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology. 2000;141:3172–3182. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszódi A, Bengtsson T, Berna A, et al. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Boyd N, Lockwood G, Byng J, Tritchler D, Yaffe M. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Orev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- Boyd N, Martin L, Stone J, Greenberg C, Minkin S, Yaffe M. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano J, Pirro A, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher D, Alliston T, Weaver V. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmosino M, Valenti G, Caplan M, Svelto M. Polarized traffic towards the cell surface: how to find the route. Biol Cell. 2010;102:75–91. doi: 10.1042/BC20090134. [DOI] [PubMed] [Google Scholar]
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Diacovo T, Grenache D, Santoro S, Zutter M. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/S0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Lou J, Evans EA, Zhu C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J Cell Biol. 2012;199:497–512. doi: 10.1083/jcb.201201091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld D, Sheetz M. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/S0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar B, Koeneman K, Edlund M, Prins G, Zhau H, Chung L. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res. 2001;61:7310–7317. [PubMed] [Google Scholar]
- Colpaert C, Vermeulen P, Fox S, Harris A, Dirix L, Van Marck E. The presence of a fibrotic focus in invasive breast carcinoma correlates with the expression of carbonic anhydrase IX and is a marker of hypoxia and poor prognosis. Breast Cancer Res Treat. 2003;81:137–147. doi: 10.1023/A:1025702330207. [DOI] [PubMed] [Google Scholar]
- Croft D, Sahai E, Mavria G, Li S, Tsai J, Lee W, et al. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 2004;64:8994–9001. doi: 10.1158/0008-5472.CAN-04-2052. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/S0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2011;22:327–341. doi: 10.1091/mbc.E10-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Investig. 1998;78:143–151. [PubMed] [Google Scholar]
- Defilippi P, Rosso A, Dentelli P, Calvi C, Garbarino G, Tarone G, et al. {beta}1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J Cell Biol. 2005;168:1099–1108. doi: 10.1083/jcb.200405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali K, Balciunaite E, Kazlauskas A. Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J Biol Chem. 1999;274:19551–19558. doi: 10.1074/jbc.274.28.19551. [DOI] [PubMed] [Google Scholar]
- DiPersio C, Hodivala-Dilke K, Jaenisch R, Kreidberg J, Hynes R. Alpha3 beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu Q, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler A, Sen S, Sweeney H, Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Erler J, Bennewith K, Cox T, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly N, Lee Y, Oliver J, Dive C, Streuli C. Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J Cell Biol. 1999;144:1337–1348. doi: 10.1083/jcb.144.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Frisch S, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith JE, Mills RJ, Hudson JE, Cooper-White JJ. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012;21:2442–2456. doi: 10.1089/scd.2011.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(SICI)1097-0215(19990531)81:5<682::AID-IJC2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Galbraith C, Yamada K, Sheetz M. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/S0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- George E, Georges-Labouesse E, Patel-King R, Rayburn H, Hynes R. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Ginsburg O, Martin L, Boyd N. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. 2008;99:1369–1374. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, et al. GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5:488–508. doi: 10.1002/emmm.201202078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T, Ohtsubo M, Roberts J, Assoian R. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller W, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–991. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik A, Hartmann U, Fässler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Barberis L, Brancaccio M, Azzolino O, Xu D, Kyriakis J, et al. Defective Rac-mediated proliferation and survival after targeted mutation of the beta1 integrin cytodomain. J Cell Biol. 2002;157:481–492. doi: 10.1083/jcb.200111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck L, Pontier S, Zuo D, Muller W. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci USA. 2010;107:15559–15564. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jeanes AI, Wang P, Moreno-Layseca P, Paul N, Cheung J, Tsang R, et al. Specific β-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells. J Biol Chem. 2012;287:24103–24112. doi: 10.1074/jbc.M112.360834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Shai S, Babbitt C, Pham C, Solaro R, Valencik M, et al. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol. 2001;158:1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, García A, Mould A, Humphries M, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Li Z, Parks WM, Dumbauld DW, Garcia AJ, Mould AP, et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol Cell. 2013;49:1060–1068. doi: 10.1016/j.molcel.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J, Donovan M, Goldstein S, Rennke H, Shepherd K, Jones R, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials. 2010;31:1219–1226. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Levental K, Yu H, Kass L, Lakins J, Egeblad M, Erler J, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Zhang X, Ren J, Pang Z, Wang C, Xu N, et al. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol. 2013;377:177–187. doi: 10.1016/j.ydbio.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q, et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene. 2012;31:2614–2626. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar G, Feng W, Reddy K, Plow E, Byzova T. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti F. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9:e1001223. doi: 10.1371/journal.pbio.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Boyd N. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio A, Rabinovitz I. Towards a mechanistic understanding of tumor invasion–lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Mercurio A, Rabinovitz I, Shaw L. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/S0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Meredith J, Fazeli B, Schwartz M. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick J, et al. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/S1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Moran-Jones K, Ledger A, Naylor MJ. Beta1 integrin deletion enhances progression of prostate cancer in the TRAMP mouse model. Sci Rep. 2012;2:526. doi: 10.1038/srep00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Owens TW, Naylor MJ. Breast cancer stem cells. Front Physiol. 2013;4:225. doi: 10.3389/fphys.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D, Romero M, Gardner C, Watt F. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M, Zahir N, Johnson K, Lakins J, Rozenberg G, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud J, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150:497–507. [PMC free article] [PubMed] [Google Scholar]
- Pontier SM, Huck L, White DE, Rayment J, Sanguin-Gendreau V, Hennessy B, et al. Integrin-linked kinase has a critical role in ErbB2 mammary tumor progression: implications for human breast cancer. Oncogene. 2010;29:3374–3385. doi: 10.1038/onc.2010.86. [DOI] [PubMed] [Google Scholar]
- Potocnik A, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/S1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- Provenzano P, Eliceiri K, Campbell J, Inman D, White J, Keely P. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P, Inman D, Eliceiri K, Trier S, Keely P. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse A, Chong F, Gray P, Munro T. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Pullan S, Wilson J, Metcalfe A, Edwards G, Goberdhan N, Tilly J, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109(Pt 3):631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Toker A, Mercurio A. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O’Rear LD, Nashabi A, et al. The alpha(2)beta(1) integrin is a metastasis suppressor in mouse models and human cancer. J Clin Investig. 2011;121:226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban N, Schwarz U, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Lee K, Miyashita Y, Carter W. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M, Lopez J, McGhee E, Croft D, Strachan D, Timpson P, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler B, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble J, Rodriguez-Enriquez R, Walker S, Owens T, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Stephens L, Sutherland A, Klimanskaya I, Andrieux A, Meneses J, Pedersen R, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Stone J, Dite G, Gunasekara A, English D, McCredie M, Giles G, et al. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15:612–617. doi: 10.1158/1055-9965.EPI-05-0127. [DOI] [PubMed] [Google Scholar]
- Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–1953. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226:3422–3432. doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- Taddei I, Faraldo M, Teulière J, Deugnier M-A, Thiery J, Glukhova M. Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia. 2003;8:383–394. doi: 10.1023/B:JOMG.0000017426.74915.b9. [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, et al. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725–731. [PubMed] [Google Scholar]
- Tennenbaum T, Weiner A, Belanger A, Glick A, Hennings H, Yuspa S. The suprabasal expression of alpha 6 beta 4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res. 1993;53:4803–4810. [PubMed] [Google Scholar]
- Tennenbaum T, Belanger A, Quaranta V, Yuspa S. Differential regulation of integrins and extracellular matrix binding in epidermal differentiation and squamous tumor progression. J Investig Dermatol. 1996;1:157–161. [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne D, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, del Pozo M, Shattil S, Chien S, Schwartz M. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G, Hovanessian-Larsen L, Parisky Y, Pike M, Wu A. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:8. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Slot A, van Dura E, de Wit E, De Groot J, Huizinga T, Bank R, et al. Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta. 2005;1741:95–102. doi: 10.1016/j.bbadis.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol. 2011;195:499–513. doi: 10.1083/jcb.201104128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ha T. Defining single molecular forces required to activate integrin and Notch signaling. Science. 2013;340:991–994. doi: 10.1126/science.1231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wojtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res. 2010;107:898–902. doi: 10.1161/CIRCRESAHA.110.227850. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Desai R, Solski P, Der C, Keely P. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Otero J, Chen Y, Kim YM, Koutcher JA, Satagopan J, et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. J Clin Investig. 2013;123:682–699. doi: 10.1172/JCI60720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien L, Mak G, Jou T-S, et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Böhmer R, Roberts J, Assoian R. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A, Luque A, Turlo K, Hofmann J, Yee K, Becker M, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]