Abstract
Integrins are ubiquitously expressed cell surface receptors that play a critical role in regulating the interaction between a cell and its microenvironment to control cell fate. These molecules are regulated either via their expression on the cell surface or through a unique bidirectional signalling mechanism. However, integrins are just the tip of the adhesome iceberg, initiating the assembly of a large range of adaptor and signalling proteins that mediate the structural and signalling functions of integrin. In this review, we summarise the structure of integrins and mechanisms by which integrin activation is controlled. The different adhesion structures formed by integrins are discussed, as well as the mechanical and structural roles integrins play during cell migration. As the function of integrin signalling can be quite varied based on cell type and context, an in depth understanding of these processes will aid our understanding of aberrant adhesion and migration, which is often associated with human pathologies such as cancer.
Keywords: Integrins, Extracellular matrix, Inside–out signalling, Outside–in signalling, Focal adhesion, Migration
Introduction
The local environment surrounding a cell plays an important role in maintaining normal cellular homeostasis as well as influencing cell fate. Cells create these environments by laying down extracellular matrix (ECM) components to support the development of various tissue types. Integrins are ubiquitously expressed in all metazoan cell types and are key mediators of interactions with other cells or the surrounding ECM. As such, integrins can influence a wide variety of cellular phenotypes, including adhesion, migration, proliferation, survival, differentiation, mechano-sensing and cytoskeletal organisation, thereby implicating integrins in processes such as tissue development and repair, angiogenesis, immune response and haemostasis. Consequently, deregulation of integrin signalling is associated with various pathological processes, including autoimmunity, inflammation and cancer. In this review we first summarise the structural aspects of the integrin α- and β-subunits, heterodimer regulation, activation and affinity for both intra- and extracellular ligands and then we describe the mechanical functions of integrins, such as their role during cell adhesion and migration.
Integrin structure
The integrin family contains 18 α- and eight β-subunits that bind noncovalently to form 24 distinct αβ integrin heterodimers with each β subunit binding several α-subunits. The α- and β-subunits are both type I transmembrane receptors and share structural similarities, such as a large extracellular domain, a single transmembrane domain and a cytoplasmic tail (Xiao et al. 2004).
α-Subunit
The mammalian integrin α-subunits can be grouped according to whether or not they contain an inserted (I) domain (‘αI’ domain). The αI domain is a 200-amino acid region that shares similarity with the von Willebrand factor ‘A’ domain and contains a metal ion-dependent adhesion site (MIDAS) that forms the ligand recognition component of these receptors (Lee et al. 1995). Integrin α-subunits containing this inserted αI domain are the collagen receptors (α1, α2, α10 and α11) and the leukocyte receptors (αE, αL, αM, αD and αX) (Heino 2007; Popova et al. 2007; Rose et al. 2007). The α-subunits that lack the αI domain are classified into four subfamilies based on their evolutionary lineage. The first is the laminin receptors (α3, α6 and α7), also known as the PS1 cluster due to their evolutionary similarity to the drosophila PS1 proteins (Gotwals et al. 1994). These receptors pair predominantly with β1 and are required for tissue integrity in organs such as muscle, kidney and skin. The second subgroup is the Arg-Gly-Asp (RGD) sequence receptors (αIIb, αv, α5 and α8), also known as the PS2 cluster due to their structural similarity to drosophila PS2 proteins. These α-subunits form heterodimers with β1 and β3 subunits and bind ECM ligands that contain RGD sequences, such as fibronectin or latent transforming growth factor-beta (Munger et al. 1999). A third subgroup, known as PS3, is only found in invertebrates, specifically in insects (Huhtala et al. 2005). The fourth and final subgroup is known as the α4/α9 cluster and comprises the α4 and α9 subunits. These α-subunit pairs with the β1 and β7 subunits and share similar ligands to the α-subunits containing the αI domain, such as vascular cell-adhesion molecules (VCAMs) and intercellular adhesion molecules (ICAMs), soluble ligands in blood, such as fibrinogen and complement, or pathogens (Vlahakis et al. 2005, 2007).
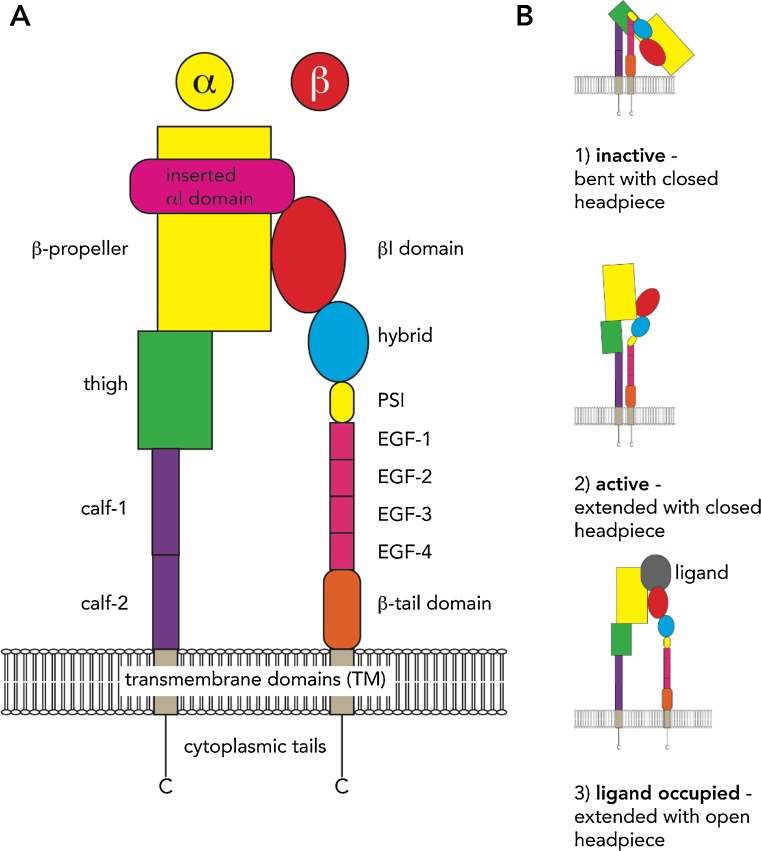
The structure of the α-subunit consists of a seven-bladed β-propeller domain which forms the head, a thigh domain, two calf domains, a single transmembrane domain and a short cytoplasmic tail (Fig. 1). When present, the αI domain in α-subunits forms the major ligand-binding site and is inserted between β-sheets 2 and 3 in the β-propeller (Xiong et al. 2001, 2004).
Fig. 1.
Integrin structure and activation model. a Schematic of the domain structure of the α- and β-subunit. b The model of integrin heterodimer activation from resting to ligand occupied. EGF Epidermal growth factor, PSI plexin–semaphorin–integrin domain
β-Subunit
The integrin β-subunits can be classified into three phylogenetic branches: vertebrate group A, vertebrate group B and the invertebrate group. Group A contains the β1, β2 and β7 domains while group B consists of the β3–β6 and β8 domains (Huhtala et al. 2005). Despite some similarities, the structural composition of the β-subunit is more complex compared to that of the α-subunit. The β-subunit also contains a βI domain that is homologous to the inserted αI domain found in the α-subunits. This highly conserved region of about 240 residues also contains two additional sections that either play a role in ligand binding—the specificity-determining loop—or in forming a critical interface with the β-propeller of the α-subunit (Huang et al. 2000; Xiong et al. 2001). The βI domain also contains a MIDAS site similar to the αI domain, and this site is important in mediating ligand binding to negatively charged amino acid residues. In addition to the MIDAS site, the βI domain contains two other metal ion-binding sites called the adjacent metal ion-dependent adhesion site (ADMIDAS) and the synergistic metal ion-binding site (SyMBS) (Xiao et al. 2004). The βI domain functions to either bind ligands directly in integrins lacking the inserted αI domain or to regulate the binding activity of α-subunits containing the αI domain (Xiong et al. 2001; Xiao et al. 2004).
The structure of each β-subunit consists of a head region, a stalk/leg section, a transmembrane (TM) domain and a cytoplasmic tail. The head region is composed of a βI domain, which is inserted into a hybrid domain that attaches to the plexin–semaphorin–integrin (PSI) domain (Fig. 1). This is followed by the β-subunit stalk/leg section that contains four cysteine-rich integrin epidermal growth factor-like (I-EGF) modules, before the β-ankle TM domain and a cytoplasmic tail (Shi et al. 2005; Zhu et al. 2008). Both the α- and β-cytoplasmic tails have no actin-binding or enzymatic activity and instead act as a hub for adaptor protein complex assembly (Zhu et al. 2008). Interestingly, the β-tails are more highly conserved that the α-tails and are the primary moderator of intracellular ligand interactions. They contain a phosphotyrosine-binding (PTB) domain which consists of a membrane proximal NPxY motif and a membrane distal NxxY motif (‘x’ represents any amino acid) (Calderwood et al. 2003). This PTB domain is important for binding multiple integrin adaptor proteins, in particular kindlin and talin. In this regard, the β-tails function as a hub for the ‘adhesome’ interactions that help mediate ‘outside–in’ signalling (Zaidel-Bar et al. 2003).
Heterodimer conformational changes—integrin activation models
Integrin activation involves major conformational changes between the integrin α- and β-subunit, with these changes representing different stages of integrin activation. Three phases of integrin activation have been proposed, with each reflecting the level of affinity of integrin heterodimers for their cognate ligand, as depicted by different conformational states (Fig. 1). These phases are (1) inactive, i.e. bent with a closed headpiece (low affinity); (2) active, i.e. extended with a closed headpiece (high affinity); (3) ligand occupied, i.e. extended with an open headpiece (Xiong et al. 2001, 2002; Shimaoka et al. 2003; Xiao et al. 2004). At the present time, two models are proposed to describe the process of integrin activation: the ‘deadbolt’ model and the ‘switchblade’ model (Xiong et al. 2003; Luo et al. 2007). The ‘deadbolt’ model proposes that integrin activation and extension occur after ligand binding, whereas the ‘switchblade’ model suggests that ligand binding only occurs once the integrin heterodimer is in the active extended conformation (Takagi et al. 2001; Beglova et al. 2002; Shimaoka et al. 2002). Debate is still ongoing on which model is correct although the ‘switchblade’ model is the most widely adopted despite examples still existing of integrin activation occurring while in the bent or partially bent conformation (Adair et al. 2005; Arnaout et al. 2007).
Integrin regulation
Integrin expression
The regulation of integrins can occur via many different mechanisms, one of which is through the regulation of integrin expression. The number of integrin receptors displayed on the cell surface often does not correlate with expression levels of integrins as the production of α- and β-subunits may not be balanced (Heino et al. 1989). For example, the αv and β1 subunits are often produced in abundance relative to other subunits (Sheppard et al. 1992), possibly due to their ability to promiscuously pair with multiple α- and β-subunits. The pairing of integrin with their binding partners to form heterodimers occurs in the endoplasmic reticulum (ER), and only intact heterodimeric αβ integrins appear on the surface of the cell. Excess unpaired α- or β-subunits are retained in the ER and degraded (Johnson et al. 2009). This strategy limits the pairing combination of α- and β-subunits and is dynamically regulated such that the composition of integrins at the cell surface can be quickly altered (Johnson et al. 2009).
Bidirectional signalling
Integrins are unique when compared with other TM receptors in their ability to signal bidirectionally. This is partly how they derive their name—by integrating the extracellular and intracellular environments, either by binding ligands outside the cell or signalling molecules and cytoskeletal components inside the cell (Hynes 1992, 2002). This bidirectional signalling influences the adhesiveness of integrins for their ligands via two mechanisms, namely ‘inside–out’ and ‘outside–in’ signalling (Kim et al. 2003). This dynamic control of integrin receptor affinity for their cognate ligands is used to carefully regulate the function of these receptors.
Inside–out signalling involves an internal signal binding to the cytoplasmic tail of integrins, which then promotes conformational changes in the heterodimer and influences the affinity of integrin for its ECM ligand (Takagi et al. 2001; Vinogradova et al. 2002). Both the TM domains and cytoplasmic tails play a role during inside–out signalling. The TM domains exist in a coiled-coil structure in the resting or inactive state, and this structure keeps the integrin α- and β-subunits in close proximity (Gottschalk 2005). Separation of these TM domains is a prerequisite for integrins to enter the active state. The function of the cytoplasmic tails during integrin activation is to facilitate the binding of integrin adaptor proteins, such as talin and kindlin, via NxxY motifs (Calderwood et al. 1999). Upon adaptor protein binding, the cytoplasmic tails separate along with the TM domains, which destabilises the tail–head interface and facilitates the ‘switchblade’-like opening, causing the hybrid domain to swing out and integrin to enter the high-affinity or active conformation (Takagi et al. 2002; Vinogradova et al. 2002; Xiao et al. 2004; Luo et al. 2007). In this ‘active’ extended conformation with open headpiece, integrins are able to bind extracellular ligands, which further stabilises the integrin heterodimer and eventually leads to integrin clustering, intracellular kinase recruitment and activation of downstream signalling pathways (Luo et al. 2007).
Outside–in signalling, on the other hand, involves integrin binding to ECM ligands to induce conformational changes first, followed by integrin clustering and the assembly of large intracellular adhesion complexes (Arnaout et al. 2005; Chen et al. 2006; Zhu et al. 2007). Protein tyrosine phosphorylation is an important event in outside–in signalling and is mediated through Src and FAK family protein tyrosine kinases (Arias-Salgado et al. 2003, 2005; Jiang et al. 2003; Cluzel et al. 2005). Src family kinases (SFKs) and their inhibitor Csk are permanently bound to integrin β-tails, but upon ligand binding, Csk dissociates to facilitate SFK activation and recruitment of tyrosine phosphatases (Arias-Salgado et al. 2005). Interestingly, mutational studies of β-tails have revealed that SFK binding to integrin tails is not required for inside–out signalling and, conversely, mutation of the talin binding motif in β-tails does not affect the initiation of outside–in signalling by SFKs, thus providing functional distinction between these two integrin signalling processes (Arias-Salgado et al. 2005).
Integrin activation states
Expression of integrins in an inactive state comprises another level of integrin regulation and normally occurs when integrin activation results in detrimental effects if initiated in the wrong context. For example, the fibrinogen receptors on platelet cells—αIIbβ3 integrin—are constantly exposed to fibrinogen in the blood; however, as their activation results in platelet aggregation and thrombosis they must be kept in the inactive state unless blood vessel injury occurs. Inappropriate activation of these integrins results in pathologies, such as bleeding disorders (Shattil and Ginsberg 1997; Shattil and Newman 2004; Petrich et al. 2007). The β2 integrin subfamily found in leukocytes and T cells are also expressed in the inactive state (Scharffetter-Kochanek et al. 1998; Baker and Koretzky 2008). Upon activation by cytokines, these receptors bind their ligands and facilitate adhesion to facilitate inflammation, cytotoxic killing, phagocytosis or lymphocyte recruitment (Rosenkranz and Mayadas 1999). The ligands of leukocyte-specific integrins comprise the immunoglobulin superfamily of counter-receptors, such as ICAMs, found on the endothelial cells that make up blood vessel walls. Human pathologies due to deficient β2 integrin activation in leukocytes result in recurring bacterial infections and wound healing defects, collectively known as leukocyte adhesion deficiencies (Abram and Lowell 2009). αIIbβ3 integrin and β2 integrin are both examples of integrins that predominantly exist in the inactive state, however, α6β4 and α3β1 are two examples of integrins that are generally active and form components of hemidesmosomes or link the ECM to the actin cytoskeleton, respectively (Carter et al. 1990; Xia et al. 1996; DiPersio et al. 1997; Hodivala-Dilke et al. 1998). Using these different activation states, integrins can regulate the biophysical properties of cells to respond to injury or infection or provide positional identity to a cell through cell–ECM or cell–cell adhesion.
Integrin ligands and downstream integrin binding partners
Integrin ligands
The different pairing combinations of α- and β-subunits determine integrin ligand specificity. Interestingly, as a number of integrin heterodimers can bind the same ligands, both the regulation of integrin expression and activation and ligand availability are important factors in determining integrin function. Thus, integrin-mediated intracellular signalling is dependent on a variety of factors, such as the type of integrin heterodimer involved and the nature and mechanical properties of the ECM ligand. In addition, integrins also participate in signalling ‘crosstalk’, where in addition to the activation of their own downstream signalling cascades, they interact with and regulate the activation of growth hormones or G-protein coupled receptor-induced signalling pathways. Integrin ligands consist of either (1) ECM ligands, such as glycoproteins that make up the ECM or (2) non-ECM ligands such as molecules on the surface of other cells. ECM ligands can comprise collagen, laminins, fibronectin and proteoglycans (e.g. chondroitin sulfate and keratan sulfate) and are secreted by cells to create their extracellular niche (Humphries et al. 2006). Non-ECM ligands include counter-receptors (ICAMs and VCAMs), plasma proteins (fibrinogen and von Willebrand factor), complement factors, cytokines and pathogens (viruses, bacteria and toxins) (Laurie et al. 1982; Humphries et al. 2006).
The binding of integrins to extracellular ligands can be clustered into four main groups of integrin–ligand combinations, and these groups are based on the structural nature of the molecular interaction. The first group consists of RGD-binding integrins that recognise ligands containing an RGD tripeptide active site. These integrins are the most promiscuous in the family as they bind to a large number of extracellular and soluble ligands. The second group comprises LDV-binding integrins that bind to an acidic amino acid motif (referred to as ‘LDV’), which is similar and functionally related to the RGD motif. It is postulated that these LVD ligands bind integrin in a similar fashion as RGD ligands. The third group is the A-domain β1 integrins, which comprises α-subunits containing the inserted αI domain that pair with β1 integrins to form a specific subfamily of laminin/collagen-binding receptors. The fourth group is the non-αA-domain-containing laminin-binding integrins, which is composed of α-subunits that lack the inserted αI domain and pair with β1 integrins to form specific laminin receptors (Humphries et al. 2006). Integrins can also bind to a number of other ligands (for review, see van der Flier and Sonnenberg 2001; Humphries et al. 2006).
Downstream integrin binding partners
Integrin cytoplasmic tails are relatively short (β-tails are 40–60 amino acids long) and have no catalytic activity, so they recruit and bind a number of accessory proteins with catalytic activity and scaffold or structural function. Analysis of the sequence of β-integrin tails has revealed three ‘hot-spots’ or regions that represent preferred binding sites for adaptor proteins. The first of these hot-spots is a membrane proximal HDRK motif, which has been shown to bind paxillin, FAK and Fyn (Schaller et al. 1995; Reddy et al. 2008). The second and third hot-spots are the membrane distal NxxY and the membrane proximal NPxY motifs (Calderwood et al. 2003). These second and third motifs bind to adaptor proteins that contain PTB domains, such as talin, kindlin 1, kindlin 2 and Shc (Calderwood et al. 2002; Kloeker et al. 2004; Shi et al. 2007). The binding of talin to β-integrin tails via its structurally conserved PTB-like domain results in the separation of the α and β cytoplasmic tails and subsequent integrin activation (Wegener et al. 2007; Wegener and Campbell 2008).
Once recruited to integrin tails, adaptor proteins form structures that are collectively referred to as focal adhesions (FA) that are responsible for facilitating the signalling and mechanical properties of integrins. As many proteins can bind to the cytoplasmic tails of integrins and the composition of proteins bound to integrins influences the signalling pathways which integrins activate, post-translational modification of the integrin tails is a mechanism used to regulate protein binding. For example, serine/threonine phosphorylation of integrin tails by kinases, such as ERK2, AKT, protein kinase C isoforms and PDK1, can regulate the binding of adaptor proteins such as 14-3-3 isoforms and filamin to influence cell phenotypes (Freed et al. 1989; Hibbs et al. 1991; Kirk et al. 2000; Calderwood et al. 2001; Han et al. 2001; Fagerholm et al. 2005; Lerea et al. 2007).
Integrin adaptor proteins can be loosely grouped into three categories based on their function, namely, (1) adaptors that have catalytic activity, (2) adaptors that have a structural function and (3) adaptors that form scaffolds for other adaptors to interact and bind with. There is sometimes functional overlap within these three categories with some focal adhesion proteins playing a role in two or sometimes three of these categories depending on the context. These adaptors usually contain identifiable folded protein domains, such as LIM, PTB, pleckstrin homology (PH), Src homology 2 (SH2) and Src homology 3 (SH3). Catalytic adaptors are responsible for communicating the signal from adhesion sites into the correct downstream signalling pathway and include Src, integrin linked kinase (ILK) and focal adhesion kinase (FAK) (Schaller et al. 1995; Hannigan et al. 1996; Chen et al. 2000; Ahmed et al. 2002; Eliceiri et al. 2002; Pasquet et al. 2002; Arias-Salgado et al. 2003; Arias-Salgado et al. 2005). Structural adaptors, which include filamin, tensin, α-actinin and talin, bind F-actin and form a direct link between integrins and the actin cytoskeleton (Otey et al. 1993; Calderwood et al. 1999, 2001, 2003). Scaffold adaptors, such as paxillin, kindlin-2 and -3 and 14-3-3 proteins, provide binding sites with which other focal adhesion proteins interact (Han et al. 2001; Fagerholm et al. 2005; Ma et al. 2008; Moser et al. 2008). For a detailed review of adaptor proteins that bind β-integrin cytoplasmic tails, the reader is referred to Legate and Fässler (2009). Collectively the composite of all the proteins, such as kinases, phosphatases and proteases, known to interact with integrins to assemble into FAs are loosely termed the integrin ‘adhesome’ (Zaidel-Bar et al. 2007).
Integrin adaptor proteins function to link integrin to the actin and microtubule cytoskeletal network, which helps integrins to modulate the regulation and organisation of the cytoskeleton. This in turn can influence cell behaviour induced by intracellular signals, such as cell cycle progression via ERK and cyclin D1, cell survival via PI3K and AKT or morphology, polarity and motility moderated via protein tyrosine kinases, phosphatases and members of the Ras and Rho family of small GTPases (Kirk et al. 2000; Ahmed et al. 2002; Jaffe and Hall 2005; Legate and Fässler 2009). The use of genetic deletion studies has helped to further elucidate the role of these integrin adaptor proteins which link integrin to the cytoskeleton and are involved in signal transduction, such as talin, α-actinin, vinculin, paxillin, FAK, ILK and p130 (Ilic et al. 1995; Monkley et al. 2000; Hagel et al. 2002; Sakai et al. 2003). These deletion studies also enable the mechanism of integrin signalling to be delineated. For example, ILK deletion in the mammary gland recapitulated the integrin deletion phenotype, whereas removal of FAK in the same system had no effect (Naylor et al. 2005; Akhtar et al. 2009).
In addition to the positive regulators of integrin signalling, there are a growing number of molecules being identified that inhibit integrin activation (Bouvard et al. 2013). Filamin, DOK1, ICAP1, SHARPIN and MDGI bind the cytoplasmic tails of integrins to prevent interactions with integrin-activating molecules, such as Talin. SHARPIN and MDGI bind to α-subunits, while filamin, DOC1 and ICAP1 bind to specific β-subunits through interactions with the NPxY and NxxY motifs. These integrin inactivators likely contribute to the regulation of integrin recruitment to adhesions, adhesion maturation and turnover. For instance, ICAP1 and SHARPIN associate with integrins in membrane ruffles—but outside of adhesion sites—thereby perhaps facilitating integrin transport around the cell surface, while preventing unwanted adhesions forming (Rantala et al. 2011; Fournier et al. 2002). In osteoblasts, loss of ICAP1 has been found to impair maturation of fibrillar adhesions and to result in reduced fibronection deposition (Brunner et al. 2011). Together, the combination of positive and negative regulators of integrin structural and signalling functions enables integrins to control many cell phenotypes, including adhesion and migration.
Mechanical functions of integrins: mechanisms of integrin-mediated adhesion and migration
Integrin-mediated adhesion
Results from the initial studies investigating integrin-mediated adhesion and migration led to the hypothesis that integrin activation simply occurred when an integrin receptor came into contact with its correct ligand. The functional diversity of the integrin family with 24 distinct αβ heterodimers, all with different ligand affinities, implied that activation and the strength of this interaction would be based on the affinity for that particular integrin with its ligand. Early studies using platelet models on integrin receptors, such as αIIbβ3, originally indicated this process occurred through passive diffusion, i.e. integrin passing by its ligand led to activation and subsequent adhesion and migration (Shattil and Ginsberg 1997). However, the presence of glycocalyx, an extracellular substance consisting of glycoproteins, polysaccharides and proteoglycans which coats cells in multicellular organisms, suggests that the process is more complicated. The thickness of glycocalyx can vary from 7 nm on red blood cells to >100 nm on epithelial and endothelial cells (Ito 1969; Sabri et al. 2000; Martins and Bairos 2002). This argues against the passive diffusion model of integrin binding as, for example, the proposed thickness of glycocalyx on leukocytes is approximately 40 nm and activated integrin receptors are only 20 nm in length (Cox et al. 1977; Xiong et al. 2009; Boettiger and Wehrle-Haller 2010). These values indicate that glycocalyx is physically preventing integrins from passively diffusing and binding to their cognate ligands (Boettiger and Wehrle-Haller 2010). To overcome this physical barrier, the application of force is required to compress the glycocalyx and allow ligand binding (Sabri et al. 2000). This mechanism also encourages integrin clustering, as higher numbers of integrins increase the strength of the adhesion (Shimaoka et al. 2003; Bunch 2010). Furthermore, the cluster size can also reflect the stiffness and thickness of the surrounding glycocalyx, as well as the membrane rigidity, with larger clusters being required in more rigid cellular microenvironments.
The physical barrier of glycocalyx is overcome by structures called filopodia through two mechanisms—their physical size and the curvature of the leading edge (Sabri et al. 2000). These two factors mean that only a relatively small amount of force is required to expose integrin receptors through glycocalyx to their extracellular substrates (Atilgan and Ovryn 2009; Boettiger and Wehrle-Haller 2010). The adhesive strength of exposed integrins with their substrates is influenced by the mechanism of activation, i.e. integrin clustering or mechanical allostery. Boettiger (2012) proposed these two mechanisms as a means to describe the dynamic behaviour of stable or unstable integrin interactions. The basis of these theories stems from the collective adhesive bond strength of integrins, i.e. the more the integrins are bound, the stronger and more permanent the adhesion. However, the type of bond formed between an integrin and its ligand will also influence whether the integrin remains activated and bound. Two types of bonds are proposed. The first is the ‘slip bond’ where the application of force increases the distance between the integrin and its ligand, resulting in bond instability and decreased integrin binding (Bell 1978). The second is the ‘catch bond’ where the application of force strengthens the bond interface between integrin and its ligand, resulting in increased bond strength and a more stable adhesion complex (Kong et al. 2009; Puklin-Faucher and Vogel 2009; Boettiger 2012).
Integrin-mediated migration
One of the fundamental mechanical functions of integrins is their involvement in migration. The state of adhesion complexes during migration is divided into three phases: assembly, dynamic and disassembly. The assembly phase involves an increase in the concentration of the lipid second messengers, phosphatidylinositol(4,5)P2 (PIP2) and phosphatidylinositol(4,5)P3 (PIP3) at the leading edge of the cell, in conjunction with filamentous (F-) actin protrusions and the subsequent recruitment and activation of the integrin adaptor proteins talin, vinculin and FAK or kindlin and ILK (Chen and Guan 1994; Goksoy et al. 2008; Legate et al. 2011). This creates a positive feedback loop and causes cytoplasmic changes, such as actin cytoskeleton reorganisation, whereby cellular morphology is adjusted to prepare for migration. This step is associated with integrins binding to F-actin in preparation for migration. Characteristics of this phase are the formation of lamellipodia/filopodia, glycocalyx repulsion, formation of nascent adhesions and focal complexes, catch bond and affinity regulation (Schürpf and Springer 2011).
The dynamic phase involves the maturation of the adhesion complexes, which is induced by actomyosin-dependent contraction and causes the downregulation of the Rac1 activation pathway (Kuo et al. 2011). Increasing local contractile pressure results in the maturation of adhesion sites, which then grow in size (valency regulation). This phase is characterised by increased tension or mechanical stress, integrin clustering and the formation of stress fibres and fibrillar adhesions (Legate et al. 2011; Qu et al. 2011). As a result, cells can remain in this phase for long periods of time and play a role in mechano-sensing and polarity.
An imbalance between integrin-dependent adhesion and tension typifies the beginning of the disassembly phase and, in combination with extracellular proteolysis and integrin-adapter mediated endocytosis, results in the progressive disassembly of adhesion sites to allow controlled rear contraction and cell migration. This phase involves the coordinated rapid integrin recruitment to the inner edge of adhesions, while integrins are removed from the distal edge, resulting in adhesion site sliding and rear retraction. The lipid second messengers play an important role in this retraction process, with PIP2 levels remaining high at the cell front to ensure that adapters such as talin are recruited to facilitate the adapter-mediated endocytosis of integrins (Chao et al. 2010a). Conversely, to prevent the recruitment of integrin adaptors involved in adhesion site initiation and assembly at the rear of the cell, such as ILK and kindlin, the levels of PIP3 are kept low (Chao and Kunz 2009; Ezratty et al. 2009; Chao et al. 2010b).
Cellular protrusions during migration
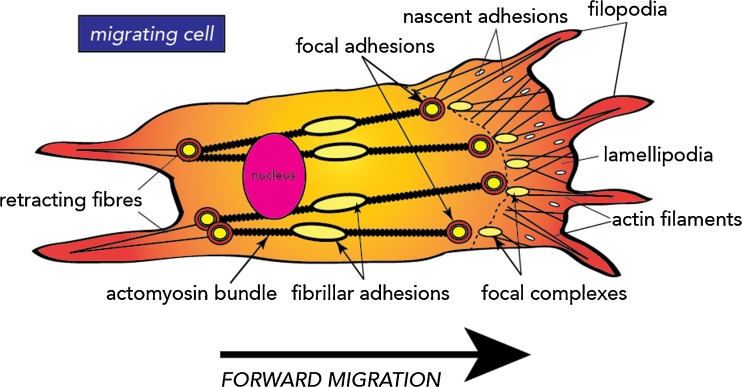
Migrating cells predominantly use two different types of cellular protrusions to initiate migration, namely, lamellipodia and filopodia, although two other types of actin-based protrusions also exist which are called podosomes and invadopodia (Mattila and Lappalainen 2008; Murphy and Courtneidge 2011). These F-actin rich protrusions contain high levels of integrins ready to be brought in contact with ECM ligands to initiate adhesion (Wehrle-Haller 2012). Lamellipodia form sheet-like protrusions at the leading edge of the cell, while filopodia form finger-like protrusions that branch out from within lamellipodia (Fig. 2). Both are similar in diameter (0.1–0.2 μm), although the filopodia are much longer (3–10 μm). Investigations into the type of integrins found within lamellipodia and filopodia have revealed that β1 integrins are preferentially concentrated in clusters at the ends of both (Galbraith et al. 2007), whereas β3 integrins require activation before they can be assembled in filopodia or lamellipodia. This assembly can occur through either the binding of intracellular adaptor proteins to initiate inside–out activation or by binding of the extracellular ligand to initiate outside–in activation (Zhu et al. 2008). In contrast, podosomes and invadopodia are cylindrical structures that are usually larger in diameter (0.5–2 μm) than lamellipodia and filopodia and found on the ventral cell surface either behind the leading edge (podosomes) or beneath the nucleus (invadopodia) (Chhabra and Higgs 2007; Murphy and Courtneidge 2011). Podosomes are typified by containing FA proteins that help form the central ‘core’ structure, such as paxillin, cortactin, gelsolin and dynamin (Chellaiah et al. 2000; Ochoa et al. 2000; Luxenburg et al. 2012), and are commonly found in leukocytes, osteoclasts and macrophages, whereas invadopodia are only found in malignant tissue (Murphy and Courtneidge 2011).
Fig. 2.
Overview of structures involved in cell adhesion and migration. Schematic of the different cellular structures used during adhesion and migration
In addition to cellular protrusions, initial binding of integrins to the ECM results in the integrin molecules clustering into focal complexes or nascent adhesions (Choi et al. 2008) that can eventually mature into larger FAs or fibrillar adhesions (Geiger et al. 2001). There are three different types of cell-matrix adhesions, namely, focal complexes/nascent adhesions, FAs and fibrillar adhesions, which have been so named due to their differing morphology, cell location and size (Fig. 2) (Wehrle-Haller 2012). Focal complexes/nascent adhesions consist of integrin clusters that form under lamellipodia or filopodia where they facilitate the mechanical attachment of the advancing F-actin cytoskeleton to the ECM substrate (Fournier et al. 2010). They are usually located on the edge of lamellipodium, have a ‘dot-like’ morphology and are about 1 μm in size along their long axis. Focal complexes/nascent adhesions are usually associated with cell migration and are formed as a result of Rac1 activation (Nobes and Hall 1995; Rottner et al. 1999; Kiosses et al. 2001). They typically contain all of the components of a mature FAs and can form without acto-myosin-dependent force (Choi et al. 2011). These dynamic focal complexes/nascent adhesions (precursor FAs) can therefore either dissipate or mature into FAs with mechano-sensing or adhesion roles depending on the cellular context.
FAs form at the base of lamellipodia, have an ‘elongated, oval’ morphology and are larger in size (about 2–μm) than focal complexes/nascent adhesions (Murphy and Courtneidge 2011). Focal adhesions are predominantly found in vitro in cultured cells; however, examples of similar structures have been reported in vivo (Kano et al. 1996). Further back from the leading edge, FAs can mature into fibrillar adhesions, which have a ‘fibrillar’ or ‘beaded’ morphology and vary in size from 1 to 10 μm along their long axis (Zamir et al. 1999, 2000; Geiger et al. 2001; Zamir and Geiger 2001). These are characterised by containing extracellular fibronectin fibrils, the fibronectin receptor (integrin α5β1) and the cytoplasmic protein tensin (Zamir et al. 2000; Geiger et al. 2001). In addition, they lack paxillin and a number of other focal adhesion proteins; however, ILK is a common component of both fibrillar adhesions and FAs (Zamir et al. 1999, 2000; Pankov et al. 2000).
Conclusions and future perspectives
The essence of integrin function is defined through its structural and mechanical roles, which are involved in mediating cell–cell or cell–ECM contacts. Through these contacts, integrins communicate with the surrounding microenvironment by signalling bidirectionally, a function unique to integrin receptors. Key questions relating to the control of integrin activity include how is the intracellular location of distinct adhesion complexes regulated and (2) how do cells utilise different integrin receptors, which bind the same substrate, to elicit distinct cellular responses. Our understanding of the latter was recently advanced in a comprehensive comparison of the specific functions of αv- and β1-integrins, in response to binding fibronection (Schiller et al. 2013). In addition to the culture models used to study integrins, it is imperative that we also determine how tissue-specific functions of integrins are regulated in vivo. The combination of in vitro and in vivo knowledge of how integrins control cell migration, adhesion, mechano-sensing, proliferation, apoptosis, among others will allow us to understand diseases associated with dysregulated integrin signalling, such as cancer.
Acknowledgements
Financial support was provided by the Cancer Council NSW, Cancer Institute NSW, National Breast Cancer Foundation of Australia, National Health & Medical Research Council of Australia and the Prostate Cancer Foundation of Australia.
Conflict of interest
None.
References
- Abram C, Lowell C. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair B, Xiong J-P, Maddock C, Goodman S, Arnaout M, Yeager M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J Cell Biol. 2005;168:1109–1118. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Niu J, Dorahy D, Gu X, Andrews S, Meldrum C, et al. Direct integrin alphavbeta6–ERK binding: implications for tumour growth. Oncogene. 2002;21:1370–1380. doi: 10.1038/sj.onc.1205286. [DOI] [PubMed] [Google Scholar]
- Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027. doi: 10.1242/dev.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado E, Lizano S, Sarkar S, Brugge J, Ginsberg M, Shattil S. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado E, Lizano S, Shattil S, Ginsberg M. Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J Biol Chem. 2005;280:29699–29707. doi: 10.1074/jbc.M503508200. [DOI] [PubMed] [Google Scholar]
- Arnaout M, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–791. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Arnaout M, Goodman S, Xiong J-P. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilgan E, Ovryn B. Nucleation and growth of integrin adhesions. Biophys J. 2009;96:3555–3572. doi: 10.1016/j.bpj.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Koretzky G. Regulation of T cell integrin function by adapter proteins. Immunol Res. 2008;42:132–176. doi: 10.1007/s12026-008-8047-8. [DOI] [PubMed] [Google Scholar]
- Beglova N, Blacklow S, Takagi J, Springer T. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9:282–287. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- Bell G. Models for the specific adhesion of cells to cells. Science (New York, N.Y.) 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012;24:592–599. doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Boettiger D, Wehrle-Haller B. Integrin and glycocalyx mediated contributions to cell adhesion identified by single cell force spectroscopy. J Phys. 2010;22:194101. doi: 10.1088/0953-8984/22/19/194101. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14:430–442. doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- Brunner M, Millon-Frémillon A, Chevalier G, Nakchbandi IA, Mosher D, Block MR, et al. Osteoblast mineralization requires β1 integrin/ICAP-1-dependent fibronectin deposition. J Cell Biol. 2011;194:307–322. doi: 10.1083/jcb.201007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch T. Integrin alphaIIbbeta3 activation in Chinese hamster ovary cells and platelets increases clustering rather than affinity. J Biol Chem. 2010;285:1841–1849. doi: 10.1074/jbc.M109.057349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D, Zent R, Grant R, Rees D, Hynes R, Ginsberg M. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Calderwood D, Huttenlocher A, Kiosses W, Rose D, Woodside D, Schwartz M, et al. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol. 2001;3:1060–1068. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- Calderwood D, Yan B, de Pereda J, Alvarez B, Fujioka Y, Liddington R, et al. The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- Calderwood D, Fujioka Y, de Pereda J, García-Alvarez B, Nakamoto T, Margolis B, et al. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W, Kaur P, Gil S, Gahr P, Wayner E. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W-T, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W-T, Ashcroft F, Daquinag A, Vadakkan T, Wei Z, Zhang P, et al. Type I phosphatidylinositol phosphate kinase beta regulates focal adhesion disassembly by promoting beta1 integrin endocytosis. Mol Cell Biol. 2010;30:4463–4479. doi: 10.1128/MCB.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W-T, Daquinag A, Ashcroft F, Kunz J. Type I PIPK-alpha regulates directed cell migration by modulating Rac1 plasma membrane targeting and activation. J Cell Biol. 2010;190:247–262. doi: 10.1083/jcb.200911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska K. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol. 2000;148:665–678. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Guan J. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang W, Kim M, Carman C, Springer T. Regulation of outside–in signaling and affinity by the beta2 I domain of integrin alphaLbeta2. Proc Natl Acad Sci USA. 2006;103:13062–13067. doi: 10.1073/pnas.0605666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra E, Higgs H. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Choi C, Vicente-Manzanares M, Zareno J, Whitmore L, Mogilner A, Horwitz A. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Zareno J, Digman M, Gratton E, Horwitz A. Cross-correlated fluctuation analysis reveals phosphorylation-regulated paxillin-FAK complexes in nascent adhesions. Biophys J. 2011;100:583–592. doi: 10.1016/j.bpj.2010.12.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof B, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S, Baur P, Haenelt B. Retention of the glycocalyx after cell detachment by EGTA. J Histochem Cytochem. 1977;25:1368–1372. doi: 10.1177/25.12.411829. [DOI] [PubMed] [Google Scholar]
- DiPersio C, Hodivala-Dilke K, Jaenisch R, Kreidberg J, Hynes R. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri B, Puente X, Hood J, Stupack D, Schlaepfer D, Huang X, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty E, Bertaux C, Marcantonio E, Gundersen G. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S, Hilden T, Nurmi S, Gahmberg C. Specific integrin alpha and beta chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J Cell Biol. 2005;171:705–715. doi: 10.1083/jcb.200504016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier HN, Dupe-Manet S, Bouvard D, Lacombe ML, Marie C, Block MR, et al. Integrin cytoplasmic domain-associated protein 1alpha (ICAP-1alpha) interacts directly with the metastasis suppressor nm23-H2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. J Biol Chem. 2002;277:20895–20902. doi: 10.1074/jbc.M200200200. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sauser R, Ambrosi D, Meister J-J, Verkhovsky A. Force transmission in migrating cells. J Cell Biol. 2010;188:287–297. doi: 10.1083/jcb.200906139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E, Gailit J, van der Geer P, Ruoslahti E, Hunter T. A novel integrin beta subunit is associated with the vitronectin receptor alpha subunit (alpha v) in a human osteosarcoma cell line and is a substrate for protein kinase C. EMBO J. 1989;8:2955–2965. doi: 10.1002/j.1460-2075.1989.tb08445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith C, Yamada K, Galbraith J. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science (New York) 2007;315:992–995. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada K. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Goksoy E, Ma Y-Q, Wang X, Kong X, Perera D, Plow E, et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell. 2008;31:124–133. doi: 10.1016/j.molcel.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk K-E. A coiled-coil structure of the alphaIIbbeta3 integrin transmembrane and cytoplasmic domains in its resting state. Structure (London) 2005;13:703–712. doi: 10.1016/j.str.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Gotwals P, Fessler L, Wehrli M, Hynes R. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci USA. 1994;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Rodriguez L, Guan J. Identification of a novel interaction between integrin beta1 and 14-3-3beta. Oncogene. 2001;20:346–357. doi: 10.1038/sj.onc.1204068. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- Heino J, Ignotz R, Hemler M, Crouse C, Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989;264:380–388. [PubMed] [Google Scholar]
- Hibbs M, Jakes S, Stacker S, Wallace R, Springer T. The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1 beta subunit: sites required for binding to intercellular adhesion molecule 1 and the phorbol ester-stimulated phosphorylation site. J Exp Med. 1991;174:1227–1238. doi: 10.1084/jem.174.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke K, DiPersio C, Kreidberg J, Hynes R. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zang Q, Takagi J, Springer T. Structural and functional studies with antibodies to the integrin beta 2 subunit. A model for the I-like domain. J Biol Chem. 2000;275:21514–21524. doi: 10.1074/jbc.M002286200. [DOI] [PubMed] [Google Scholar]
- Huhtala M, Heino J, Casciari D, de Luise A, Johnson M. Integrin evolution: insights from ascidian and teleost fish genomes. Matrix Biol. 2005;24:83–95. doi: 10.1016/j.matbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Humphries J, Byron A, Humphries M. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3904. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969;28:12–25. [PubMed] [Google Scholar]
- Jaffe A, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley D, Fukumoto E, Sheetz M. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Johnson M, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim Biophys Acta. 2009;1788:779–789. doi: 10.1016/j.bbamem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Kano Y, Katoh K, Masuda M, Fujiwara K. Macromolecular composition of stress fiber-plasma membrane attachment sites in endothelial cells in situ. Circ Res. 1996;79:1000–1006. doi: 10.1161/01.res.79.5.1000. [DOI] [PubMed] [Google Scholar]
- Kim M, Carman C, Springer T. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science (New York) 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Kiosses W, Shattil S, Pampori N, Schwartz M. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- Kirk R, Sanderson M, Lerea K. Threonine phosphorylation of the beta 3 integrin cytoplasmic tail, at a site recognized by PDK1 and Akt/PKB in vitro, regulates Shc binding. J Biol Chem. 2000;275:30901–30906. doi: 10.1074/jbc.M001908200. [DOI] [PubMed] [Google Scholar]
- Kloeker S, Major M, Calderwood D, Ginsberg M, Jones D, Beckerle M. The Kindler syndrome protein is regulated by transforming growth factor-beta and involved in integrin-mediated adhesion. J Biol Chem. 2004;279:6824–6833. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- Kong F, García A, Mould A, Humphries M, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J-C, Han X, Hsiao C-T, Yates J, Waterman C. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G, Leblond C, Martin G. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982;95:340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Rieu P, Arnaout M, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Legate K, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–285. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Legate K, Takahashi S, Bonakdar N, Fabry B, Boettiger D, Zent R, et al. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 2011;30:4539–4553. doi: 10.1038/emboj.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerea K, Venjara A, Olson S, Kelly M. Threonine phosphorylation of integrin beta3 in calyculin A-treated platelets is selectively sensitive to 5'-iodotubercidin. Biochim Biophys Acta. 2007;1773:185–191. doi: 10.1016/j.bbamcr.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Luo B-H, Carman C, Springer T. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–666. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C, Winograd-Katz S, Addadi L, Geiger B. Involvement of actin polymerization in podosome dynamics. J Cell Sci. 2012;125:1666–1672. doi: 10.1242/jcs.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-Q, Qin J, Wu C, Plow E. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Bairos V. Glycocalyx of lung epithelial cells. Int Rev Cytol. 2002;216:131–173. doi: 10.1016/s0074-7696(02)16005-0. [DOI] [PubMed] [Google Scholar]
- Mattila P, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Monkley S, Zhou X, Kinston S, Giblett S, Hemmings L, Priddle H, et al. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Munger J, Huang X, Kawakatsu H, Griffiths M, Dalton S, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Murphy D, Courtneidge S. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ochoa G, Slepnev V, Neff L, Ringstad N, Takei K, Daniell L, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C, Vasquez G, Burridge K, Erickson B. Mapping of the alpha-actinin binding site within the beta 1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz B, Matsumoto K, Lin D, Lin S, et al. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet J-M, Noury M, Nurden A. Evidence that the platelet integrin alphaIIb beta3 is regulated by the integrin-linked kinase, ILK, in a PI3-kinase dependent pathway. Thromb Haemost. 2002;88:115–122. [PubMed] [Google Scholar]
- Petrich B, Marchese P, Ruggeri Z, Spiess S, Weichert R, Ye F, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiol (Oxford) 2007;190:179–187. doi: 10.1111/j.1748-1716.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- Puklin-Faucher E, Vogel V. Integrin activation dynamics between the RGD-binding site and the headpiece hinge. J Biol Chem. 2009;284:36557–36568. doi: 10.1074/jbc.M109.041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Tu Y, Shi X, Larjava H, Saleem M, Shattil S, et al. Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J Cell Sci. 2011;124:879–891. doi: 10.1242/jcs.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala JK, Pouwels J, Pellinen T, Veltel S, Laasola P, Mattila E, et al. SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol. 2011;13:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K, Smith D, Plow E. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J Cell Sci. 2008;121:1641–1648. doi: 10.1242/jcs.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Alon R, Ginsberg M. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz A, Mayadas T. Leukocyte-endothelial cell interactions—lessons from knockout mice. Exp Nephrol. 1999;7:125–136. doi: 10.1159/000020593. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small J. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sabri S, Soler M, Foa C, Pierres A, Benoliel A, Bongrand P. Glycocalyx modulation is a physiological means of regulating cell adhesion. J Cell Sci. 2000;113(Pt 9):1589–1600. doi: 10.1242/jcs.113.9.1589. [DOI] [PubMed] [Google Scholar]
- Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Otey C, Hildebrand J, Parsons J. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- Schürpf T, Springer T. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30:4712–4727. doi: 10.1038/emboj.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S, Ginsberg M. Integrin signaling in vascular biology. J Clin Invest. 1997;100:5. [PubMed] [Google Scholar]
- Shattil S, Newman P. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- Sheppard D, Cohen D, Wang A, Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- Shi M, Sundramurthy K, Liu B, Tan S-M, Law S, Lescar J. The crystal structure of the plexin-semaphorin-integrin domain/hybrid domain/I-EGF1 segment from the human integrin beta2 subunit at 1.8-A resolution. J Biol Chem. 2005;280:30586–30593. doi: 10.1074/jbc.M502525200. [DOI] [PubMed] [Google Scholar]
- Shi X, Ma Y-Q, Tu Y, Chen K, Wu S, Fukuda K, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Takagi J, Springer T. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu J-H, Yang Y, Dong Y, Jun C-D, et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Erickson H, Springer T. C-terminal opening mimics ‘inside–out’ activation of integrin alpha5beta1. Nat Struct Biol. 2001;8:412–416. doi: 10.1038/87569. [DOI] [PubMed] [Google Scholar]
- Takagi J, Petre B, Walz T, Springer T. Global conformational rearrangements in integrin extracellular domains in outside–in and inside–out signaling. Cell. 2002;110:599–11. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, et al. A structural mechanism of integrin alpha(IIb)beta(3) “inside–out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Vlahakis N, Young B, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis N, Young B, Atakilit A, Hawkridge A, Issaka R, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- Wegener K, Campbell I. Transmembrane and cytoplasmic domains in integrin activation and protein-protein interactions (review) Mol Membr Biol. 2008;25:376–387. doi: 10.1080/09687680802269886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener K, Partridge A, Han J, Pickford A, Liddington R, Ginsberg M, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol. 2012;24:569–581. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Xia Y, Gil S, Carter W. Anchorage mediated by integrin alpha6beta4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kD protein. J Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller B, Wang J-H, Springer T. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott D, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science (New York) 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J-P, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman S, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science (New York, N.Y.) 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- Xiong J-P, Stehle T, Goodman S, Arnaout M. New insights into the structural basis of integrin activation. Blood. 2003;102:1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]
- Xiong J-P, Mahalingham B, Alonso J, Borrelli L, Rui X, Anand S, et al. Crystal structure of the complete integrin alphaVbeta3 ectodomain plus an alpha/beta transmembrane fragment. J Cell Biol. 2009;186:589–600. doi: 10.1083/jcb.200905085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz B, Aota S, Yamada K, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112(Pt 11):1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada K, Katz B, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zhu J, Carman C, Kim M, Shimaoka M, Springer T, Luo B-H. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside–in signaling. Blood. 2007;110:2475–2483. doi: 10.1182/blood-2007-03-080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo B-H, Xiao T, Zhang C, Nishida N, Springer T. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]