Abstract
Kidney stone disease is a polygenic and multifactorial disorder with a worldwide distribution, and its incidence and prevalence are increasing. Although significant progress has been made in recent years towards identifying the specific factors that contribute to the formation of kidney stone, many questions on the pathogenesis of kidney stones remain partially or completely unanswered. However, none of the proposed mechanisms specifically consider the role(s) of the trace elements and, consequently, the contribution of trace constituents to the pathogenesis of kidney stones remains unclear and under debate. The findings of some studies seem to support a role for some major and trace elements in the initiation of stone crystallization, including as a nucleus or nidus for the formation of the stone or simply as a contaminant of the stone structure. Thus, the analysis of kidney stones is an important component of investigations on nephrolithiasis in order to understand the role of trace constituents in the formation of kidney stones and to formulate future strategies for the treatment and prevention of stone formation and its recurrence. The aim of this review is to compare and evaluate the methods/procedures commonly used in the analysis of urinary calculi. We also highlight the role of major and trace elements in the pathogenesis of kidney stones.
Keywords: Kidney stones, Trace elements, Pathogenesis, Spectroscopy, Techniques
Introduction
In the last few decades, it has been widely documented that kidney stone disease is a global health problem that seriously affects human health. Kidney stone disease is common in the populations of industrialized countries. It is also considered to be a serious socio-medical problem (Pak 1998). Kidney stones are the products of a pathological biomineralization process in the urinary system (Bazin and Daudon 2012; Bazin et al. 2012) and are mostly mixtures of two or three or more components. This disorder is multifactorial in origin and is influenced by the physical–chemical conditions of the urinary system (Daudon et al. 1993). Randall (1936) was the first to describe the appearance of what are now known as “Randall’s plaques”, and although important aspects of the process of stone formation in humans have been explored over the years (Carpentier et al. 2011), there is not yet a complete and satisfactory explanation for the pathogenesis of stone formation. To determine the pathogenesis of kidney stones and to formulate future strategies for the treatment and prevention of these stones, it is important to analyze kidney stones at both the elemental and molecular levels.
Thus, the aim of our review is to summarize the most important aspects of kidney stones, paying our attention to the major and trace element composition of these stones. We also briefly discuss the most common methods of kidney stone analysis at both the elemental and molecular levels and include those details of the types of kidney stones with their characteristics which will be useful to the researchers. Our primary emphasis is to explore the role of major and trace elements in the pathogenesis of kidney stones.
Trace elements and pathogenesis of kidney stones
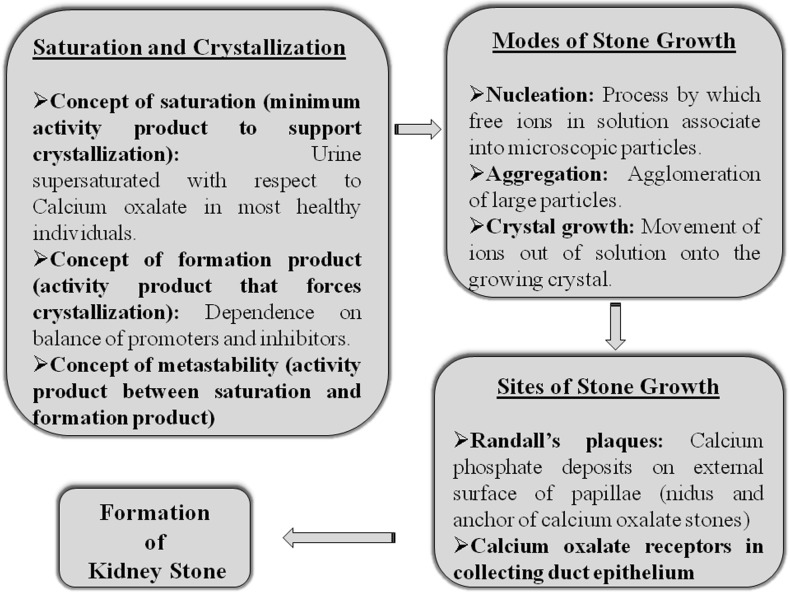
Various mechanisms are involved in kidney stone formation, all of which can only occur in urine, which is supersaturated with respect to the ionic constituents of the specific stone. Possible steps occurring in the formation of kidney stones are presented in Fig. 1. The inhibitory activity of some urinary components, such as pyrophosphate, citrate, magnesium, glycosaminoglycan and kidney proteins (e.g.nephrocalcin, Tamm–Horsfall mucoprotein, uropontin), is reported, but little attention has been paid to the role of trace elements (Trinchieri et al. 1991; Verkoelen et al. 2000; Bihl and Meyers 2001; Marangella et al. 2004; Basavaraj et al. 2005; Moe 2006). In kidney stone disease inhibitory crystallization deficit plays an important role together with supersaturated levels of different salts, promoters and inhibitors of crystallization. The process of crystallization of supersaturated urine components and the establishment of solid concretions can be modified by the activity of promoters and inhibitors and by some morphoanatomic, dietary and environmental factors (Trinchieri et al. 1991; Moe 2006). The lithogenic risk factors that impact urinary stone formation are shown in Table 1 (Bihl and Meyers 2001; Basavaraj et al. 2005). Inhibitors work by absorption on the crystal surface, thereby interfering with the formation of the crystal lattice and retarding the attachment of new ions and ultimately inhibiting nucleation, growth and aggregation into larger crystals. The effects of inhibitors of stone formation have been primarily studied in calcium oxalate stones. Most inhibitors are anionic and seem to exert their effects by binding to the calcium oxalate surface, although the specific structural mechanisms of this process are not completely known (Bihl and Meyers 2001).
Fig. 1.
Multistep physiochemical processes and mechanism of kidney stone formation
Table 1.
Urinary stone promoters and inhibitors
| Promoters | Inhibitors | |
|---|---|---|
| Inorganic | Organic | |
| Calcium (Ca) Oxalate Urate Cystine Low urine pH Low urine flow Bacterial products |
Magnesium (Mg) Citrate Pyrophosphate |
Nephrocalcin Tamm-Horsfall protein (THP) Urinary prothrombin fragment 1 Protease inhibitor: inter-alfa-inhibitor Glycosaminoglycans Osteopontin (uropontin) Other bikunin, calgranulin High urine flow |
Trace elements are essential components of biological structures and are necessary for a multitude of vital functions in the human body. On the other hand, these elements can be toxic at concentrations exceeding those necessary for their biological functions (Welshman and McGeown 1972). The precise role of trace constituents in the pathogenesis of renal calculus is still unclear and under debate, but researchers are increasingly focusing on the role of trace elements in lithogenesis (Parsons 1953; Bird and Thomas 1963; Sutor 1969; Meyer and Angino 1977; Scott et al. 1980; Joost and Tessadri 1987; Oka et al. 1987; Durak et al. 1990, 1992; Komleh et al. 1990; Hofbauer et al. 1991; Rodgers et al. 1994; Perk et al. 2002; Munoz and Valiente 2005; Trinchieri et al. 2005; Atakan et al. 2007; Bazin et al. 2007). Trace elements such as zinc (Zn), copper (Cu), nickel (Ni), aluminium (Al), strontium (Sr), cadmium (Cd) and lead (Pb) form poorly soluble salts with phosphate and oxalate ions and therefore play an important role in kidney stone formation. Trace elements also affect the speed of the crystallization process and influence the external morphology of growing crystals (Welshman and McGeown 1972; Meyer and Angino 1977; Scott et al. 1980; Munoz and Valiente 2005). The inhibitory effect of the elements magnesium (Mg), Al, Zn, iron (Fe) and Cu on calcium oxalate growth at very low concentrations has been demonstrated (Meyer and Angino 1977; Munoz and Valiente 2005; Bazin et al. 2007). Bazin et al. (2007) studied various important trace and heavy elements in a large number of urinary stones and discussed the role of these elements in the formation of urinary stones. Munoz and Valiente (2005) investigated the effects of a number of trace elements on the inhibition of calcium oxalate crystallization, resulting in a great step forward towards elucidating the pathogenesis of urinary stones. Meyer and Angino (1977) analyzed trace metal contents in calcium oxalate and a mixture of calcium oxalate and calcium phosphate and tested the inhibitory effect of each of these trace metals on the crystal growth of calcium oxalate and calcium phosphate., Other researchers have focused on the effects of trace elements on solubility and crystallization, and the determination of trace element content in humans has attracted increasing attention (Levinson et al. 1978). A few studies have determined the concentration of elements in the materials studied, and other have investigated the interactions of elements with promoters or inhibitors (Bird and Thomas 1963; Sutor 1969; Meyer and Angino 1977; Levinson et al. 1978; Scott et al. 1980; Durak et al. 1990, 1992; Komleh et al. 1990; Hofbauer et al. 1991; Rodgers et al. 1994; Munoz and Valiente 2005; Trinchieri et al. 2005; Słojewski et al. 2010). The inference derived from these studies is that knowledge of the relative concentrations of the different elements present in the different parts of the stones would lead to an understanding of the mechanism of stone nucleation and growth. In this context, higher concentrations of trace metals have been found in the core than in the peripheral layers of the stones, suggesting a possible lithogenic effect of trace and heavy metal elements (Durak et al. 1992; Hobarth et al. 1993; Perk et al. 2002; Bazin et al. 2007; Singh et al. 2009). However, currently available data on the role of trace elements in the pathogenesis of urinary stones are still insufficient.
Types of kidney stones
Kidney stones differ in terms of composition and pathogenesis, as shown in Table 2 (Barbas et al. 2002; Stoller and Meng 2007; Carpentier et al. 2009; Daudon et al. 2009; Le Bail et al. 2013). It is important to study the chemical composition and morphology of kidney stones as elemental composition of the stone as well as the function of the different elements are affected by the stone type. Thus, the basic characteristics of the different kinds of kidney stones are discussed in the following sections.
Table 2.
Classification of kidney stones by their location and chemical composition, their corresponding risk factors and their X-ray characteristics and appearancea
| Kidney stone type [chemical formula] | Specification/appearance | Risk factors | Crystal shape in urine | X-ray characteristics |
|---|---|---|---|---|
| Calcium oxalate (75 %) [CaC2O4⋅H2O] | Black, gray, white and small, dense, and sharply circumscribed on radiographs. The cross-sectional views often appear to grow in radial fashion from a nidus with wedges rounding off at their extremities. | Low urine volume, hypercalciuria, hyperuricosuria, hyperoxaluria, hypocitraturia | Envelope | Radiopaque, spherical |
| Calcium phosphate (Brushite) (5 %) [Ca10 (PO4)6⋅2H2O] | Black, gray, or white and small, dense, and sharply circumscribed on radiographs. On cross-section, the stones often appear to grow in radial fashion from a nidus with wedges rounding off at their extremities. | Hypercalciuria, hyperphosphaturia, raised urine pH | Amorphous | Radiopaque, spherical |
| Uric acid (10 %) [C5H4N4O3] | Spherical with a smooth yellow–orange surface. The interior of the stone appears as orange concentric rings. | Hyperuricosuria, low urine pH, low urine volume | Diamond or barrel | Radiolucent |
| Struvite or triple phosphate (10 %) [MgNH4⋅PO4⋅6H2O] | Off-white to light-brown with a rough textured surface. The cross-sectional view of the stone contains white concentric rings, and occasionally with white porous granulated material. | Urinary tract infection (urease splitting organism) | Coffin-lid | Radiopaque, grows in staghorn shape. |
| Cystine (1 %) [C6H12N2O4S2] | Greenish-yellow, flecked with shiny crystallites, with a rounded appearance. | Rare genetic disorder, autosomal recessive disorder | Hexagonal | Faintly radiopaque |
| Protease-related stone | On gross appearance, pure indinavir stones are brown and have a pliable, puttylike consistency. | Used in the treatment of the human immunodeficiency virus (HIV), indinavir competitively inhibits HIV protease. | Pure indinavir calculi are radiolucent. |
aAccording to Stoller and Meng 2007
Most stones contain calcium (Ca) combined with oxalate, phosphate or occasionally uric acid in the form of calcium oxalate (CaC2O4⋅H2O), calcium phosphate [Ca10 (PO4)6⋅2H2O], calcium carbonate (CaCO3), brushite (CaHPO4⋅2H2O), gypsum (CaSO4⋅2H2O) and/or dolomite [CaMg (CO3)2] (Barbas et al. 2002; Carpentier et al. 2009; Daudon et al. 2009; Le Bail et al. 2013). Calcium oxalate is a Ca salt of a dicarboxylic acid and oxalic acid, and it crystallizes into two different chemical and crystallographic forms, namely, calcium oxalate monohydrate (i.e. whewellite, CaC2O4⋅H2O) and calcium oxalate dihydrate (i.e. weddellite, CaC2O4⋅2H2O). All Ca stones are radiopaque (Stoller and Meng 2007).
Uric acid stones are radiographically transparent unless mixed with Ca crystals or struvite and, in contrast to the radiopaque Ca stones, they are radiolucent. Uric acid salts out calcium oxalate and can precipitate out in acid urine even in the absence of raised serum or urinary uric acid concentrations. As with all stones, certain drugs may enhance stone formation, and in the case of uric acid stones, such drugs include hyperuricosuric agents, such as low-dose salicylates, probenecid and thiazides.
Struvite (triple phosphate stones), also known as infection stones, is a crystalline substance composed of magnesium ammonium phosphate (Carpentier et al. 2009). Radiography studies have revealed that struvite stones are large, gnarled and laminated. Admixed struvite/apatite stones are usually light brown in color with a coarse, granular surface. The interior of these stones is normally intermixed with white and light-brown layers (Stoller and Meng 2007). Struvite stones develop if alkaline urine, which has a raised concentration of ammonium, contains trivalent phosphate and urease produced by bacteria. Kajander and Ciftcioglu (1998) reported that not only struvite stones, but also Ca-based stones may have an infectious origin.
The formation of cystine stones is the only clinical expression of cystinuria, and these are associated with a genetically determined defect in the renal transport of certain amino acids, including cystine. Pure L-cystine stones are homogeneously composed of very small yellow spheroids (Stoller and Meng 2007).
Protease-related stones are one of the “newest” types of stone. The increasing incidence of human immunodeficiency virus (HIV)-positive patients has led to widespread use of indinavir sulphate, which competitively inhibits the action of the HIV protease and has been associated with urolithiasis in some patients (Yagisawa et al. 2000; Norman 2001; Barbas et al. 2002; Daudon et al. 2009). Thus, calcium oxalate stones may coexist or form a nidus for indinavir stones or vice versa. Indinavir stones were first described in 1995 (Daudon et al. 2009). Pure indinavir calculi are unique in that they are not visualized on spiral computed tomography scans.
Techniques used for the analysis of kidney stones
To learn all there is to know on kidney stone formation, it is important to have a full understanding of the variations in stone composition. All types of stones should be analyzed to determine their crystalline composition, elemental distribution and, in particular, the different hydrate forms, the urates and purine derivatives of the several calcium phosphates (Bazin et al. 2006; Daudon 2013). Analysis of the kidney stone is of importance to the therapy and metaphylaxis of residual and recurrent stones (Bazin et al. 2006; Daudon 2013). Also, precise analysis of a stone is a basic requirement for an effective metaphylaxis.
The current techniques utilized for the analysis of kidney stones are: (1) chemical analysis, (2) thermogravimetry, (3) polarization microscopy, (4) scanning electron microscopy (SEM), (5) powder X-ray diffraction (XRD) and (6) spectroscopy. In this review we first describe in brief the principles of these methods and then we compare their accuracy and practical application based on our review of the literature.
Wet chemical analysis
The wet chemical technique is one of the most widely used approaches for stone analysis. However, it can only determine the presence of individual ions and radicals—not differentiate mixtures (Kasidas et al. 2004). It is possible to improve its performance by using a quantitative wet chemical approach in which the same routine quantitative methods used for the chemical analysis of blood and urine are used for a suitably prepared solution of the stone (Kasidas et al. 2004).
Thermogravimetry
Since the 1970s, thermogravimetric analysis (TG or TGA) has been extensively applied for the analysis of kidney stones (Lee et al. 2012). This technique is based on the continuous recording of both the temperature and weight loss of the material sampled during a progressive temperature increase to 1,000 °C in an oxygen atmosphere. As each substance has its own specific transformation properties, the starting and ending temperature of the transformation, the amount of the weight change, enthalpy, the nature of the substance and the magnitude of this change indicates the proportion of the elements present (Kasidas et al. 2004).
Polarization microscopy
Polarization microscopy is based on the interaction of polarized light with crystals of the kidney stones. After the stone is fractured, the material to be tested is removed from various locations and assessed under the polarizing microscope using a drop of the appropriate refractive index liquid (Douglas and Tonks 1979). The color, refraction of light and double refraction are the parameters used to identify the stone minerals (Schubert 2006).
Scanning electron microscopy
Scanning electron microscopy is a precise technique used to study the morphology and texture of urinary calculi. This technique is used to determine the properties of stones varying in size from 1 to 5 nm, without changing the specific morphology of the components (Kasidas et al. 2004; Daudon et al. 2008). SEM produces very high-resolution images of a sample surface, and it has recently been proposed as a diagnostic tool for primary hyperoxaluria (Daudon et al. 2008).
Powder X-ray diffraction
In the powder X-ray diffraction method, monochromatic X-rays are used to identify the constituents of a renal stone. This technique is based on the unique diffraction patterns produced by a crystalline material (Kasidas et al. 2004) and is capable of distinguishing and measuring simultaneously all of the different crystal types in a particular mixture.
Different techniques of spectroscopy
Spectroscopy is the study of the interaction between matter and electromagnetic radiation. Here we report the principles and practical applications of the most commonly used methods of spectroscopy.
Infrared spectroscopy
Infrared (IR) spectroscopy was first applied to stone analysis by (Beischer (1955) and has since developed into a popular and reliable method for in vitro quantitative stone analysis (Singh 2008). In IR spectroscopy, the sample is irradiated by IR laser pulses to to cause atomic vibrations; the absorption spectra of the stone samples are then recorded and analyzed to determine the elemental composition of the stone (Nguyen Quy and Daudon 1997; Kasidas et al. 2004). A more recent technique involving IR spectroscopy is the attenuated total reflection technique (ATR), which is suitable for analyzing soft samples (Schubert 2006). Sample preparation for this technique is very easy, as it does not require mixing the sample with an IR inactive material, such as potassium bromide, prior to analysis. Even in difficult cases in which only one water molecule separates the two compounds (weddelite and whewellite), calcium oxalate identification is remarkable easy by IR spectroscopy (Bazin et al. 2012).
Energy dispersive X-ray analysis
Energy dispersive X-ray analysis (EDX or EDAX) is an X-ray analysis technique which is used to obtain the percentage composition of all the elements of stone sample and is significantly useful for recognizing unknown crystals not identified by ordinary light microscopy or SEM alone. The EDX system is generally attached to the SEM system where the imaging capability of the microscope is applied to identify the specimen of interest. The information generated by EDX analysis consists of spectra showing peaks corresponding to the elements, thereby revealing the true composition of the stone samples (Fazil Marickar et al. 2009a).
Laser-induced breakdown spectroscopy
Laser-induced breakdown spectroscopy (LIBS) is a sensitive and rapid optical technique that can be used to make a multi-elemental analysis of solid, gaseous and liquid samples ,including kidney stones (Singh and Rai 2011). The LIBS experimental setup used to examine kidney stone samples has been described in detail by Singh et al. (2009). In brief, the LIBS technique is based on the formation of short-lived (nanoseconds) microplasma following irradiation of the sample with an intense high-power laser pulse. Target molecules are dissociated into their respective atomic and ionic form, and the emission spectra from the microplasma are characteristic of the excited-state—relaxation transitions from atoms, ions, and molecular fragments of the LIBS-generated microplasma (Parigger et al. 2003). The laser-induced plasma contains the spectral signature of the constituents present in the sample, and thus important information on the identification, composition and concentrations of trace elements can be derived from the analysis of LIBS emission spectra. Recently, Singh et al. (2009) used the LIBS technique to analyze kidney stone samples. Their results clearly indicate that LIBS is quite a suitable technique to analyze the kidney stones, especially when the aim is to obtain further information on the spatially distributed elements with the stone sample.
Laser ablation inductively coupled plasma-mass spectrometry
Laser ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS) is a powerful analytical technology that enables highly sensitive elemental and isotopic analyses to be performed directly on solid samples, including biological and environmental samples. Laser ablation ICP-MS begins with a laser beam focused on the sample surface to generate fine particles, following which the ablated particles are then transported to the secondary excitation source of the ICP-MS for digestion and ionization of the sampled mass. The excited ions in the plasma torch are subsequently introduced into a mass spectrometer detector for both elemental and isotopic analysis (Durrant and Ward 2005).
X-Ray absorption spectroscopy
During the last few decades, X-ray absorption spectroscopy (XAS) has made major contributions to a wide variety of biochemical research topics. For example, it has raised important questions on the correlation between structure and function of the metal sites in metallo-proteins (Koningsberger and Prins 1988; Yano and Yachandra 2009). This technique allows us to analyze the local structure of the target element without interference from absorption by the protein matrix, water or air. XAS has also been employed for the study of kidney stones (Bazin et al. 2009; Daudon 2013).
The advantages and disadvantages of these techniques based on the reviewed studies are summarized in Table 3. The different techniques described above for stone analysis cannot be used interchangeably, even though they appear to be equally effacious. IR spectroscopy is used as a practical application in clinical laboratories (for kidney stone analysis), which techniques such as wet chemical analysis are only of historical importance and should no longer be used, and LIBS is generally only used in research laboratories. However, LIBS has proven its effacicy for in vivo stone analysis. The elemental analysis techniques, such as LA ICP-MS, EDX, among others, and other technique such as SEM provide better accuracy and precision and are able to clearly distinguish features of the inorganic and organic substances present in the stones. These techniques may be applied in clinical research laboratories for the elemental and molecular studies required to understand the precise role of factors in kidney stone formation and its development.
Table 3.
Comparison of kidney stone analysis techniques
| Techniques | Advantages | Disadvantages |
|---|---|---|
| Wet chemical analysis | -Easy | -Time consuming and requires large amounts of stone sample |
| -Low cost | -Unable to distinguish calcium oxalate monohydrate and dihydrate stones | |
| Thermogravimetry (TG, TGA) | -Fast | -Requires large amount of stone sample |
| -Simple | -Non-recovery of samples | |
| -Does not provide definitive stone composition | ||
| -Difficult to identify compounds which closely resemble each other | ||
| Polarization microscopy | -Cost effective | -Experienced viewer is needed |
| -Fast examinations | -Quantitative analysis in a mixture is difficult | |
| -Capability of analyzing samples of very small amounts | -Does not provide definitive stone composition | |
| -Ability to detect very small contents of components of the stone | -Differentiation of components is difficult in the groups of uric acid, purine derivatives and calcium phosphates | |
| Scanning electron microscopy (SEM) | -Non-destructive | -Expansive and large |
| -Possibility to visualize the components without altering their spatial orientation and specific morphology | -Experienced viewer is needed | |
| -Does not provide definitive stone composition | ||
| Powder X-ray diffraction (XRD) | -Easy preparation and quantitative measurement | -Expansive |
| -Automatic measurement using a sample chamber | -Unable to detect noncrystalline or amorphous substances | |
| -Possibility of exact differentiation of all crystalline components | ||
| Infrared (IR) spectroscopy | -Cost effective | -Differentiation and qualitative analyses are difficult in the case of uric acid and purines and calcium phosphates |
| -The major advantage of IR spectroscopy is that a limited number of main features exist in the IR spectra of biological samples | -Difficulty in detecting some components due to overlapping of their absorption bands, such as carbonate in stuvite stones or cystine in whewellite or uric acid stones | |
| -Quantitative measurements of small samples are possible | -Resolution of the apparatus and reproducibility of the spectrum bands may affect its reliability | |
| -Preparation is very easy using the attenuated total internal reflection (ATR) technique | ||
| -Useful for detection of organic components | ||
| -Exact differentiation of all crystalline components is possible | ||
| Energy dispersive X-ray analysis (EDX) | -Non-destructive | -Low count rates |
| -Little or no sample preparation is required | -Poor collection efficiencies | |
| -Provides the spatial distribution of elements through mapping which is important in case of stone analysis | -Small detector areas | |
| Laser-induced breakdown spectroscopy (LIBS) | -Simplicity and lack of tedious sample preparation | -Detection limits are generally not as good as those of the conventional techniques |
| -Simultaneous multi-element detection | -Precision is poor as compared to conventional techniques | |
| -Ability to detect high and low atomic number elements | -Safety measures are required to avoid ocular damage by the high-energy laser pulses | |
| -Point detection capability which provides the spatial information of elements in stone samples | ||
| Laser ablation inductively coupled plasma mass spectrometry (LA ICP-MS) | -It provides versatile solid sampling schemes including bulk analysis, local inclusion, defect analysis, depth profiling, elemental and isotope mapping | -Low spatial resolution compared to some other techniques like electron microprobe, ion probe, proton probe) |
| -Fast and high sensitivity | -Requires well-characterized, homogeneous standards | |
| -Multi-elemental capability with good precision and accuracy | -Requires prior knowledge of internal standard concentrations in samples and standards | |
| X-ray absorption spectroscopy (XAS) | -It is element specific and is not limited by the state of the sample | -Difficult to distinguish between scattering atoms with little difference in atomic number (C, N, O or S, Cl, or Mn, Fe) |
| -The local structural information around the element can be obtained even from disordered samples such as powders and liquids | Difficult to determine the coordination numbers or number of backscatterers. | |
| - XAS experiments require a lower X-ray dose than X-ray crystallography and radiation damage can be precisely monitored |
Analysis of kidney stones using different techniques
A study of the chemical composition of kidney stones is important for understanding how they are formed. The choice of medical therapy for kidney stone disease is usually based on an analysis of the stones, which enables proper management of the disease and prevents its recurrence. The chemical composition of urinary calculi was first reported at the end of the 18th century, with the most important chemical components of urinary calculi identified as uric acid and cystine. Uric acid was first isolated from the urine in 1776 by Karl Wilhelm Scheele (Stoller and Meng 2007) and cystine was first isolated from a urinary calculus in 1810 by William Hyde Wollaston (Wollaston 1810; Stoller and Meng 2007). Following systematic studies by Heller (1847) and Ultzmann (1882), the chemical analysis of urinary calculi was established as a routine clinical procedure (Hesse and Sanders 1988). Wet chemical methods are sensitive, but they can only identify the components of the kidney stone as chemical radicals. As a result, the various polymorphs of calcium oxalate, apatite and uric acid salts cannot be separately distinguished. In 1947, Prein and Frondel (1947) introduced polarizing microscopy for the identification of the crystalline constituents of urinary calculi. Silva et al. (2010) compared chemical and morphological analyses of kidney stones and reported that unlike the morphological analysis, chemical analysis can detect only Ca and oxalate separately and not differentiate crystalline type. Identifying the crystalline form is very useful for planning therapy; for example, calcium oxalate dihydrate is associated with hypercalciuria, while calcium oxalate monohydrate is more closely related to hyperoxaluria. Thus, these authors suggested using both types of analysis routinely for a better understanding of the mechanisms involved in lithogenesis (Silva et al. 2010).
TGA has been used by several authors to analyze stones, with the respective authors confirming its ability to produce fast and accurate quantitative results (Rose and Woodfine 1976; Rose 1982). It is considered to be the best physical method to analyze calcium oxalate monohydrate and dihydrate salt, and it may also provide data that helps determine the age of the stones (Rose and Woodfine 1976; Rose 1982). This method provides an alternative method to the use of XRD and Fourier transform infrared spectroscopy (FT-IR) for the quantitative determination of each hydrate of the calcium oxalate when present together with uric acid or magnesium ammonium phosphate (Kaloustian et al. 2003). Similar ignition temperatures and disintegration rates of a number of closely related compounds, such as purines, may also make the identification of stone constitutents difficult. As calcium phosphate, silica and pyrophosphate display very little changes in weight upon heating, TGA cannot convincingly separately identify these materials (Kasidas et al. 2004). According to D’Ascenzo et al. (1983), the components of the nuclei of renal stones are chemically different from those of the outer shell. TGA analysis of urinary stones suggests that some stones contain <80 % calcium oxalate and that the level of calcium oxalate monohydrate and dihydrate differs between the core and the surface layers of the calculi (D'Ascenzo et al. 1983; Lee et al. 2012;). Many early researchers in the field (Strates 1966; Berenyi and Liptay 1971; Berényi et al. 1972) performed thermal analyses with the aim to quantitatively determine the two types of calcium oxalate in urinary stones. Recently, Materazzi et al. (1995) coupled the thermo-balance and FT-IR techniques by using a heat transfer line to analyze urinary calculi in the case of complex mixtures.
The efficacy of XRD technology to characterize and identify different urinary calculi was first explored by Prien and Frondel (1947). Fifteen years later Herring reported using this technique to study 10,000 urinary calculi (Herring 1962). The advantages of XRD technology is that it can be used to determine the proportional rate of particular crystalline components responsible for the formation of the calculus and, additionally, it provides a well-organized and reliable tool for the analysis of urinary stones (Nalbandyan 2008; Orlando et al. 2008). XRD in combination with the crystal optical method has been used to analyze the core and shell regions of urinary stones (Schubert et al. 1983). However, Sutor (1968) reported some difficulty in identifying the different components of mixed urinary calculi using only XRD. The internal standard method and powder XRD have also been used to quantitatively analyze the constituents of urinary stones. Taken together, these studies indicate that XRD analysis on its own merits cannot be regarded as a routine technique for quantitative characterization of urinary calculi, but for semi-quantitative analyses, XRD provides accurate quantitative elemental data which can be used in further studies aimed at precise determination of the true composition of the stone (Wandt and Rodgers 1988; Kumar et al. 2006). Girija et al. (2007) recently used XRD in a mineralogical study of urinary stones collected from patients in southern India. A recent study in China by Ouyang (2006) confirmed the reliability and accuracy of XRD for quantitative and qualitative analysis of urinary stones .
IR spectroscopy is a useful tool for studying kidney stones, particularly for the identification of non-crystalline materials, including amorphous substances and drug metabolites that are not detectable with other techniques (Lehmann et al. 1988). From the medical point of view, quantitative analysis of each mineral component of kidney stones and of their spatial distribution provides information that is very important in terms of determining the origin of the stone and its treatment. Bazin et al. (2012) recently analyzed a very large kidney stone (approx. 18 mm in diameter) and determined the the spatial distribution of the chemical phases from the core to its surface, which in turn provided information on the chronological succession of the different abnormalities. These authors found that the core of the kidney stone consisted of ammonium urate induced by diarrhea, with FT-IR spectroscopy revealing the presence of whewellite. They also found that the surface of the kidney stone was made up of a mixture of carbonated apatite and weddelite related to hypercalciuria of dietetic origin. Using FT-IR spectroscopy, Bhatt and Paul (2008) found that calcium oxalate was the most common constituent in the presence of phases of hydroxyl and carbon apatite. Gould et al. (1995) reported upgraded IR techniques for analyzing urinary calculi where, for example, the use of partial least squares regression in the analysis program enables better quantitation of stone components by use of FT-IR spectrophotometer coupled with a computer and photoacoustic detector. Some authors have focused on FT-IR analysis of a large number of urinary calculi (Estepa and Daudon 1997; Volmer et al. 2001). Gulley-Stahl et al. ( 2009) investigated a new quantitative approach requiring minimal sample preparation for the analysis of kidney stone components utilizing ATR-FTIR.
The combined application of IR and Raman spectroscopy technique to the analysis of urinary calculi was reviewed by Carmona et al. (1997). The authors documented that some of the characteristic bands are very useful for analytical purposes, further suggesting the adaptability of spectroscopy methods to the routine analysis of stones. Paluszkiewicz et al. (1997) linked the structural composition of renal stones, as determined by FT-IR and FT-Raman spectroscopy, with the elemental composition of the stones determined using proton-induced X-ray emission and atomic emission spectroscopy. Based on this study and other published studies, it can be concluded that IR and Raman spectroscopy are the best spectroscopic methods for the identification and quantitative analysis of kidney stones (Nguyen Quy and Daudon 1997). Both techniques are fast and simple to use, and they only require a small quantity of matter for testing. Raman spectroscopy is used alone or as complementary to IR spectroscopy to achieve the same objective. Both XRD and IR spectroscopy have also been employed as reference techniques for stone analysis, and many studies subsequently were designed to compare the quality of these methods (Hesse et al. 1972; Schneider et al. 1973). It is also well documented that the analytical results obtained with both the XRD and IR spectroscopy methods are comparable and highly acceptable; consequently, these two techniques can be considered to be reference methods for the analysis of urinary calculi (Rebentisch 1993).
Charafi et al. (2010) used SEM and FT-IR methods to analyze several renal calculi. In this study, both methods clearly showed that calcium oxalate monohydrate (COM) was the predominant component (54 %) of renal calculi from the study region and that calcium oxalate dihydrate (COD) accounted for only 13.5 %. Purine calculi were almost as frequent as phosphate calculi (24.3 %), and struvite calculi comprised 8.1 %. SEM enabled authentic COM calculi, which are derived from crystalline transformation of the COD form, to be differentiated. Crystalline conversions which are sometimes not evident under a binocular stereoscopic microscope or with FT-IR may be distinguishable using SEM. Thus, the presence of etching on the surface of COD calculi and the large crystals composed of COM suggest a high degree of transformation of COD into COM as a consequence of the “permanency” of the calculus in the kidney. SEM provides information on the nature of the crystalline compounds, shape of the crystals, internal structure, location of components, crystalline conversions, crystallite size distribution and characteristics of the aggregates, as well as some data on the close associations between crystals and the organic matrix and/or on the relationships between different crystalline species (Charafi et al. 2010). The appearance of urinary calculi determined by SEM enables the identification of the stone type based on textural grounds (Rodgers et al. 1984; Rodríguez-Miñón Cifuentes et al. 2006; Oswald et al. 2011). Using SEM as a diagnostic tool for primary hyperoxaluria, Daudon et al. (2008) confirmed that (1) the primary hyperoxaluria type 1 stone has a crystalline structure and (2) this structure was distinct from that of the common type of whewellite stone. This unique morphological characteristic and the ultrastructure of primary hyperoxaluria stones suggest a fundamental difference in the mechanism of stone formation, reflecting the very rapid and permanent crystal formation induced by genetic hyperoxaluria. The unique morphological characteristics of stones consistently observed in patients with primary hyperoxaluria type 1 appear to be pathognomonic of this condition. Therefore, the appearance of such stones might be a valuable indicator of primary hyperoxaluria type 1, prompting early comprehensive laboratory evaluation, including measurements of urinary oxalate, glycolate and glycerate in order to achieve a definitive diagnosis.
The EDX technique has been employed to investigate the composition of different types of urinary calculi (Rodríguez-Miñón Cifuentes et al. 2006; Oswald et al. 2011), namely, calcium oxalate and/or calcium phosphate-containing stones, infection stones, uric acid-containing stones, cystine-containing stones and stones containing rare substances (brushite, whitlockite, xanthine, etc.) and elements (C, N, O, Na, S, Mg, Al, Si, Cl, K, Ca, Mn, Fe, Ni and Zn).
LIBS is an analytical technique which allows real time qualitative and quantitative identification of the major and trace elements present in the biological samples being tested, including stones (Singh and Rai 2011). The first report on the application of laser pulses on kidney stones appeared in 1987 (Hofmann et al. 1989) and the first publication of the application of LIBS for the analysis of kidney stones was reported by Fang et al. (2005). Singh et al. ( 2009) used the LIBS technique to study kidney stones and quantified a number of elements (Cu, Zn, Sr, Mg) by drawing calibration curves. They were also able to identify the constituents in different parts (center, shell, surface) of the stone samples. The elemental concentration determined by LIBS for different kidney stones correlated well with that determined usingICP-MS techniques. Both LIBS spectra and ICP-MS analysis showed that the major constituent of the kidney stones was Ca. Anzano et al. (2009) identified different types of stones using the LIBS technique. More recently, Oztoprak et al. (2012) used LIBS, XRD and X-ray fluorescence techniques for the analysis and classification of heterogeneous kidney stones. These authors detected phosphorus (P), sulfur (S), silicon, titanium (Ti) and Zn as minor elements in the stones and also found that the ratio of hydrogen to carbon was an important indicator of organic compounds, such as uric acid. They employed principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) of broadband LIBS spectra to classify the different types of kidney stones. Stepankova et al. (2013) applied laser ablation methods (LIBS and LA combined with mass or optical ICP spectrometry) to analyze urinary calculi. In doing so, they demonstrated the calibration capabilities and limitations of LA-based techniques applied for spatially resolved elemental mapping on urinary calculi cross-sections. Chaudhri et al. (2007) used LA ICP-MS to analyze several kidney stones in order to determine the spatial distribution of major and trace elements (i.e. Ca, Mn, Li, B, Mg, Al, P, Cr, Zn, Rb, Sr, Ba and Pb) in kidney stones.
It can therefore be concluded that there are many different methods and spectroscopic techniques available which provide capable tools for the analysis of kidney stones. However, in the majority of cases reported, no single technique could provide the complete battery of information on the chemical composition of stones, indicating that various combinations of these techniques are needed for stone analysis. Marickar et al. (2009b) reported that the combination of optical microscopy and IR spectroscopy is an accurate and reliable method for studies of the core, cross section and surface of calculi, with the aim of determining the exact composition of calculi. The combination of XRD or IR spectrometry and wet chemistry may be suitable as reference methodology (Uldall 1981). Other combinations of methods, such as TGA with XRD, TG with FT-IR, FT-IR with SEM and SEM with EDX, have also been mentioned a suitable approaches for stone analysis (Barbas et al. 2002; Basiri et al. 2012). More importantly, it has been widely accepted that different areas of a calculus should be analyzed separated for useful results to be obtained. Since major and trace elements play significant roles in kidney stone formation and also influence the growth of the stone and thus the elemental analysis of different parts of the kidney stone, studies using LIBS, LA ICP-MS and EDX, among other methods, are also needed. Hence, analysis of different parts of a kidney stone separately at the elemental and molecular levels is of utmost importance.
Role of major and trace elements in the pathogenesis of kidney stones
Published reports clearly indicate that all types of kidney stones contain several trace elements. This has given to investigations on the importance of these elements in stone pathogenesis (Sutor 1969; Meyer and Angino 1977; Scott et al. 1980). However, the trace element contents of different stones have been found to be variable. Trace elements also form poorly soluble salts with phosphate and oxalate ions, suggesting a distinct role in lithogenesis. Basavaraj et al. (2005) studied the effect of different factors on stone formation in detail, and the presence of trace elements in calculi and the mechanism of their formation have been studied for more than 35 years (Meyer and Angino 1977). These studied generally take the approach of first determining the concentration of trace elements in the stones under analysis and then analyzing the impact of different factors on the relation between trace elements and calculi formation. Some trace elements, such as Zn and Mg, have an an inhibitory effect on urolithiasis while other ones act as a promoter (Atakan et al. 2007). Food intake, metabolism and the effect of the environment all explain the presence of trace elements in calculi. The effects of trace elements on calculi formation are not unambiguous, and the results of various studies are in some cases contrary. Thus, toxic metals at trace levels cannot be considered urolithiasis-promoting factors, and their presence in calculi would reflect contamination of the organism rather than other factors. In such a case, calculi can be used for biomonitoring purposes, similar to other biological matrices, such as blood, urine, hair, teeth, among others. Thus, there is a great need to know the effects of trace elements in the formation of kidney stones. The possible/proposed role(s) of some of the major and trace elements involved in the formation of kidney stones together with some of the more recent references associated with these roles are presented in Table 4.
Table 4.
Role of major and trace elements in the formation of kidney stones with some references of recent studies
| Elements | Issue studied related to kidney stones | References | Role in lithogenesis |
|---|---|---|---|
| Calcium (Ca) | -High Ca intake has been associated with a lower risk for kidney stones. | Bihl and Meyers 2001; Stoller and Meng 2007 | Ca plays an important role in lithogenesis. |
| -Ca is the predominant element in stones which influences the distribution trace elements. Ca ion promotes the formation and growth of intrarenal crystals. | Basavaraj et al. 2005 | ||
| -Higher concentration of Ca has been observed in the center, shell and surface parts of the kidney stones. | Joost and Tessadri 1987; Barbas et al. 2002; Schubert 2006; Chaudhri et al. 2007 | ||
| Magnesium (Mg) | -Mg is the fourth most abundant element in the body; only 1 % of total Mg is found in blood and the rest is present in combination with Ca and P. | Escott-Stump 2007 | Mg acts as a potential inhibitor in formation of kidney stones. |
| -In vitro studies have demonstrated decreased nucleation and growth of calcium oxalate crystals in the presence of superphysiological concentrations of Mg. Supplementation with Mg and vitamin B6 significantly lowers the risk of kidney stone formation. | Kohri et al. 1988; Johansson et al. 1980; Lindberg et al. 1990 | ||
| -Low concentration of Mg in urine is a risk factor for kidney stone formation. | Durak et al. 1990; Meyer and Angino 1977; Scott et al. 1980 | ||
| -Dietary Mg deficiency causes experimental urolithiasis, and high levels of this element in urine reduce the concentration of oxalate available for calcium oxalate precipitation. | Robertson 1969 | ||
| -Mg inhibits calcium oxalate crystallization in human urine and it also inhibits absorption of dietary oxalate from the gut lumen. | Massey 2005 | ||
| Manganese (Mn) | -Mn is associated with bone development and plays an important role in the metabolic pathway of amino acids, lipids, carbohydrates and proteins. | Fraga 2005 | Role of Mn in kidney stone formation is still not clear. |
| -Mn levels have been found to be lower in the serum and urine of stone patients than in healthy people. | Trinchieri et al. 1991; Komleh et al. 1990 | ||
| -Low level of Mn might interfere with the fragility of urinary stones in extracorporeal shockwave lithotripsy (ESWL) therapy. | Turgut et al. 2008 | ||
| -It was suggested that Mn as well as other elements, such as Ni, Li and Cd, could be of significance in the pathological mechanism of stone formation. | Hofbauer et al. 1991 | ||
| Copper (Cu) | -Cu is an antioxidant and its highest concentration is found in the liver, kidney, heart and brain. Chronic (long-term) effect of Cu exposure can damage the liver and kidney. | New Hampshire Department of Environmental Services 2005 | Cu shows its inhibitory activity against growth of calcium phosphate crystals but not on oxalate. |
| -It was firstly pointed out by Bird et al. (1963) that Cu shows the inhibitory effect on the mineralization process of rachitic rats’ cartilage. | Bird and Thomas 1963 | ||
| -Cu and Mn urinary levels were found to be lower in stone formers than in normal subjects. | Komleh et al. 1990 | ||
| -The inhibitory activity of Cu against growth of calcium phosphate crystals was observed, but not on oxalate. | Meyer and Angino 1977 | ||
| -It has been observed that the amount of Cu stored in the stones is more relevant when compared to Zn, especially in oxalate stones. | Joost and Tessadri 1987 | ||
| Iron (Fe) | -Fe is considered to be an inhibitor of oxalate stones. However, more extensive research is needed to elucidate its role in lithogenesis. | Lieu et al. 2001; Elliot and Ribeiro 1973 | Fe is considered to be an inhibitor of oxalate stones. However, more extensive research is needed to elucidate its role in lithogenesis. |
| -Non-Ca stones have beens reported to have a lower content of Fe than Ca-containing stones. | Bazin et al. 2007; Joost and Tessadri 1987; Levinson et al. 1978 | ||
| -The Fe3+ ions have the ability to establish stable chemical interactions with oxalate ions on the surface of calcium oxalate crystals. | Durak et al. 1992; Munoz and Valiente 2005 | ||
| -An increased 24-h urinary Fe excretion was reported in calcium oxalate stone patients compared with healthy persons. | Atakan et al. 2007; Meyer and Angino 1977 | ||
| Potassium (K) | -K is important for many functions in the human body and has a role in maintaining normal Ca balance in the body as K decreases urinary loss of Ca. It is reported that the lower the K intake below 74 mmol/day, higher the relative risk of stone formation. Such an effect can be ascribed to an increase in urinary Ca and a decrease in urinary citrate excretion induced by a low K intake. | Curhan et al. 1993; Lemann et al. 1991 | K together with Mg can help prevent calcium oxalate kidney stones. |
| -A low–normal K intake and a higher NaCl intake were also observed in stone formers as compared to healthy people. | Borsatti 1991 | ||
| -A sgnificantly higher urine Na/K ratio was observed to increase the risk of stone formation. | Martini et al. 1998; Cirillo et al. 1994 | ||
| -Higher K intake was recommended in order to prevent kidney stone formation. Potassium magnesium citrate reduces the risk of developing calcium oxalate stones. | Ettinger et al. 1997 | ||
| Zinc (Zn) | -Zn is an essential trace element and its deficiency can enhance the expression of angiotensis II that constricts the blood vessels in kidneys and further aggravates the condition of individuals with obstructive kidney disease. | Yanagisawa et al. 2000 | More extensive research is needed to determine the exact role of Zn in lithogenesis. |
| -It has been described as an inhibitor of urinary stone formation but its exact role is divergent. | Hofbauer et al. 1991; Komleh et al. 1990 | ||
| -The higher content of Zn in weddellite stones than whewellite stones is reported, which may be linked to the fact that weddellite is formed during the first phase of the crystallization process and subsequently transforms to whewellite. | Bazin et al. 2007; Joost and Tessadri 1987; Hofbauer et al. 1991; Levinson et al. 1978 | ||
| -It was found in higher concentrations in Ca-containing stones but also in organic urinary stones compared to other trace elements. | Atakan et al. 2007; Bazin et al. 2007; Joost and Tessadri 1987; Levinson et al. 1978 | ||
| -Its inhibitory effect was observed only on calcium phosphate stones but not on calcium oxalate growth. | Meyer & Angino 1977 | ||
| -calcium phosphate stones were reported to have a higher Zn content than calcium oxalate stones. By contrast, brusite stones contain significantly less Zn than carbonate apatite stones. | Bazin et al. 2007; Levinson et al. 1978 | ||
| -Significantly higher amounts of Zn have also been observed in urinary excretion of stone patients. | Trinchieri et al. 1991; Komleh et al. 1990; Rangnekar and Gaur 1993; Ozgurtas et al. 2004; Kumar et al. 1984; Francois et al. 1986; Anke and Schneider 1973; Elliot and Ribeiro 1973) | ||
| -Some authors detected no differences relating to Zn urinary excretion between healthy people and stone patients. | Welshman & McGeown 1972; Hofbauer et al. 1991; Cohanim and Yendt 1975 | ||
| -In some studies, serum Zn is lower in stone patients than in healthy controls. | Pak 1998; Moe 2006; Parsons 1953 | ||
| -Healthy controls were found to have significantly high urinary and serum Zn levels than stone patients. Study documented that mean Zn levels have an inhibitory effect on calcium oxalate stone formation. | Atakan et al. 2007 | ||
| -Trace elements like Zn, Fe, and Mn appear to interfere with whewellite stone fragility. | Turgut et al. 2008 | ||
| Cadmium (Cd) | -Cd is a widely disseminated metal that can be accumulated in living cells, thereby drastically interfering with their biological mechanisms. Increased concentrations of urinary beta-2 microglobulin can be an early indicator of renal dysfunction in persons chronically exposed to low but excessive levels of environmental Cd. The urinary beta-2 microglobulin test is an indirect method of measuring Cd exposure. The long-term exposure of Cd leads to renal damage due to massive low weight tubular proteinuria. | http://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=10; Järup et al. 2000; Kjellström et al. 1977; Iwata et al. 1993 | The experimental findings clearly reveal that Cd plays an important role in kidney formation. |
| -A study of almost 2,700 renal cadaver samples showed that subjects who had died of renal disease had lower Cd concentrations. | Lyon et al. 1999 | ||
| -Its inhibitory effect on calcium oxalate crystallization was also suggested. | Hofbauer et al. 1991 | ||
| -Cd exposure has been found associated with a greater risk of kidney stone formation. | Ferraro et al. 2011 | ||
| -Recently, Swaddiwudhipong et al. (2011) found an increase in stone prevalence with increasing urinary Cd levels observed in both genders. A positive association was found between urinary Cd levels and stone prevalence. Finally, they documented that elevated calciuria induced by Cd might increase the risk of urinary stone formation in environmentally exposed population. | Swaddiwudhipong et al. 2011 | ||
| -Some authors have also noted the increased prevalence of kidney stones in workers exposed to Cd which is possibly related to the increased urinary excretion of Ca or tubular damage. | Järup et al. 1998 | ||
| Boron (B) | -B is an ultra-trace element which is essential for healthy bone and joint function and effects on the balance and absorption of Ca, Mg and P. It is efficiently absorbed and excreted in the urine. Its deficiency seems to affect Ca and Mg metabolism, and affects the composition, structure and strength of bone. Due to its effects on Ca and Mg metabolism, B deficiency may also contribute to the formation of kidney stones. | McCoy et al. 1994; Nielsen 1994 | More extensive research is needed to elucidate its role in lithogenesis. |
| -Hunt et al. (1997) reported low calcium oxalate urine excretion in postmenopausal women as a metabolic response to dietary B supplementation during low Mg intake. It is well reported that sodium borate is the most common form of supplement. B is increasingly used in Ca and bone replenishing nutritional formulas. It may be particularly useful in those whose Mg intake is low. This effect may be useful in the prevention of kidney stones. | Hunt et al. 1997 | ||
| -Low concentration of B has been observed in patients with cystine stones. | Słojewski 2011 | ||
| Selenium (Se) | -Se is an essential trace element in humans and its deficiency has been found to induce renal calcification. Experimental and clinical studies have shown that Se has an inhibitory effect on urolithiasis. | Rayman 2000; Fujieda et al. 2007; Kumar & Selvam 2003 | The experimental findings indicate that Se act as an inhibitor to the stone formation. |
| -Supplementation of Se and vitamin E prevents hyperoxaluria in experimental urolithic rats by decreasing the level of lipid peroxidation and the activities of oxalate synthesizing enzymes such as xanthine oxidase. | Kumar & Selvam 2003 | ||
| -The oxalate and Ca concentration can also be reduced and the process of crystallization can be inhibited by Se which acts as nephroprotective antioxidant. | Lahme et al. 2004 | ||
| -Recently, Sakly et al. (2003) documented that Se is one of the stone formation inhibitors which could adhere to the crystal surface and would inhibit the induction of new crystals, growth and aggregation. | Sakly et al. 2003 |
A large number of trace elements have been quantitatively determined in kidney stones, urine and serum, and in few cases attempts have been made to link the measurements with stone formation (Escott-Stump 2007; Kohri et al. 1988; Johansson et al. 1980; Lindberg et al. 1990; Robertson 1969; Massey 2005; Fraga 2005; Turgut et al. 2008; New Hampshire Department of Environmental Services 2005; Lieu et al. 2001; Curhan et al. 1993; Lemann et al. 1991; Borsatti 1991; Martini et al. 1998; Cirillo et al. 1994; Ettinger et al. 1997; Yanagisawa et al. 2000; Rangnekar and Gaur 1993; Ozgurtas et al. 2004; Kumar et al. 1984; Francois et al. 1986; Anke and Schneider 1973; Elliot and Ribeiro 1973; Cohanim and Yendt 1975; http://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=10; Järup et al. 2000; Kjellström et al. 1977; Iwata et al. 1993; Lyon et al. 1999; Ferraro et al. 2011; Swaddiwudhipong et al. 2011; Järup et al. 1998; McCoy et al. 1994; Nielsen 1994; Hunt et al. 1997; Słojewski 2011; Rayman 2000; Fujieda et al. 2007; Kumar and Selvam 2003; Lahme et al. 2004; Sakly et al. 2003; Barceloux 1993; Grant 2009; Rubin and Strayer 2008; Ekong et al. 2006; Wright et al. 1984; Lin and Huang 1994; Huel et al. 2002; Samachson et al. 1966; Vezzoli et al. 1998; Li et al. 2010; Vezzoli et al. 2003; Blaschko et al. 2013; Wandt and Underhill 1988; Kuta et al. 2012; Strübel et al. 1990; Nagy et al. 1963; Eusebio and Elliot 1967; Küpeli et al. 1993). Vanadium (V) is a trace element which is mostly present at low levels in plant and animal tissues. The highest concentrations of V in mammalian tissues are in kidney, spleen, liver, bone, testes and lung (Barceloux 1993). Słojewski et al. (2010) reported a positive correlation between Vanadium level and the content of magnesium phosphates and phosphate salts, clearly suggesting that V plays a role in the crystallization of phosphate-containing crystals in the human urinary tract.
Pb toxicity in humans is well known condition caused by increased levels of the heavy metal Pb in the body. Pb interferes with a variety of body processes and is toxic to many organs and tissues, including the heart, bones, intestines, kidneys and reproductive and nervous systems. There is evidence that both low and high levels of Pb are the main cause of renal damage (Rubin and Strayer 2008; Grant 2009). Pb poisoning inhibits the excretion of the waste product urate and predisposes the body to gout, in which urate accumulates in the body (Wright et al. 1984; Lin and Huang 1994; Ekong et al. 2006). Inorganic stones contain increased levels of Pb, while Pb has been reported to be completely absent in uric acid and cystine stones (Levinson et al. 1978; Joost and Tessadri 1987). Bazin et al. (2007) reported a higher amount of Pb in Ca-containing stones than in the organic phases. These authors also observed that a decreased level of Pb in stones correlated with a decrease in the available Pb in the environment. The presence of Pb is strongly associated with environmental pollution (Huel et al. 2002). Its role in kidney stone formation is unknown, but some authors have found a correlation between Pb in stones and Pb in the urine, possibly indicating that Pb may play some role in the process of crystallization in the urinary tract (Słojewski et al. 2010).
Sr is a heavy metal which commonly occurs in nature in the form of the sulfate mineral celestite (SrSO4) and the carbonate strontianite (SrCO3). It forms poorly soluble complexes with phosphates and oxalates that can be incorporated into kidney stones. Intestinal absorption and renal filtration studies have clearly demonstrated that Sr is processed by the human body in much the same way as Ca (Samachson et al. 1966; Vezzoli et al. 1998). Sr has been observed to substitute for Ca during the process of biomineralization in bone studies, incorporating into hydroxyapatite in bones by replacing a proportion of the Ca ions (Li et al. 2010). The observation that Sr absorption was higher in hypercalciuric stone formers than in normocalciuric patients (Vezzoli et al. 2003) has led to investigations focusing on Sr incorporation into uroliths. In a recent study, Blaschko et al. (2013) found that 80 % of Sr in kidney stones was present as strontium apatite and 20 % as strontium carbonate. Although studies on the role of Sr in urolithiasis are still in their infancy, available results suggest that strontium hydroxyapatite may serve as a nidus for the formation of stones containing Ca. Relatively higher concentrations of Sr have been reported in calcium phosphate stones (Joost and Tessadri 1987; Wandt and Underhill 1988; Bazin et al. 2007; ). Varying amounts of Sr have been reported in the center, shell and surface parts of the stones as well as in whole stones (Fang et al. 2005; Chaudhri et al. 2007; Singh et al. 2009). Taken together, these studies indicate that Sr has the potential to serve as a valuable marker in studies on renal stone pathogenesis.
Mercury (Hg) is a naturally occurring heavy metal which can cause serious health problems, including kidney damage. Kuta et al. (2012) recently analyzed a large number of urinary stones for their mineralogical and elemental content, with a specific focus on Hg. These authors observed an association between Hg and whewellite mineral as well as a distinctive association with Se. These results confirm the role of Se in Hg excretion. Strübel et al. ( 1990) determined the significance of Hg, together with that of other heavy metals, such as cadmium (Cd), Pb, chromium (Cr) and nickel (Ni) in pathogenesis.
There is an increased presence of trace elements in Ca-containing stones, and only few elements, such as arsenic (As) and antimony (Sb), accumulate in uric acid stones (Joost and Tessadri 1987). In a study carried out in an area of Germany, Hesse et al. (1977, 1978) observed that the concentrations of fluorine (F) in whewellite and weddellite stones were higher when the drinking water was fluoridated than when it was not fluoridated. In addition, the concentration of F was higher in weddellite than in whewellite stones and it increased in carbonate apatite, struvite and uric acid stones in the fluoridated area. The increased F content resulted in an increased degree of crystallization and hence in an increased strength of carbonate apatite (Hesse et al. 1977, 1978). The authors concluded that certain specific urinary components were necessary for the stabilization of the weddellite and uric acid dihydrate (Hesse et al. 1975, 1976; Bazin et al. 2007). As pure chemical compounds, these urinary stone substances are very unstable and transform into the stable crystal phases whewellite or uric acid, respectively, with the elimination of water. High-molecular-weight substances also contribute to the stabilization of weddellite and uric acid dihydrate. Hofbauer et al. (1991) determined the concentration of urinary Ni and serum Ni in stone patients and reported that the urinary Ni level was very low in stone formers. These authors found that there is a significant difference in the level of urinary and serum Ni levels between stone patients and healthy individuals, with stone patients having the lower Ni level. These investigations show that Ni play a significant role in the pathological mechanism of stone formation.
In more recent investigations, other elements have been reported in urinary stones, such as bromine (Br), cobalt (Co) and samarium (Sm), in addition to Ca, sodium (Na), potassium (K), manganese (Mn), Cr, Zn, and chloride (Cl) (Srivastava et al. 2012). Zirconium (Zr) was recently reported in urinary stones along with the other elements such as Cu, Zn, Sr and Cl (Zarasvandi et al. 2013). Abboud (2008) reported a large number of major and minor elements, such as Na, Mg, Al, Si, Ca, K, Fe, barium (Ba), Mn, P, S, Zr, Mo, Cl, Sr, Ni, Zn, Cr, Co, F, Pb, Cd and As in urinary stones. Significantly elevated concentrations of some rare-earth elements, such as europium (Eu), terbium (Tb) and lutetium (Lu), among others, have also been seen in urinary stones (Hobarth et al. 1993).
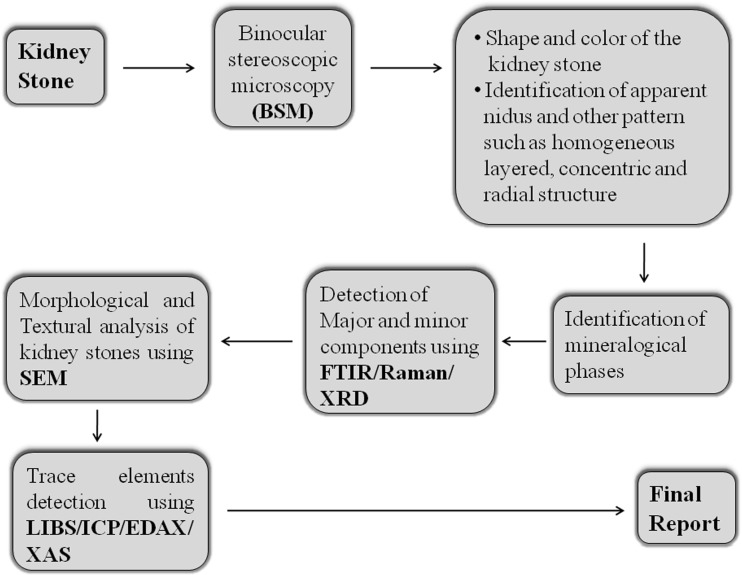
Because most of the stones are multicomponent, the techniques used to analyze kidney stone should be capable of resolving all components of the stones, especially the crystalline components. Laboratory analyses of kidney stones generate different data types, such as quantitative, mineralogical and elemental data, and by combining these different data into a comprehensive report, it is possible to make safe diagnostic and therapeutic decisions. A flow pattern similar to that shown in Fig. 2 may be one approach to analyze kidney stones in order to provide the necessary information for clinical decision-making and patient care. The pathway shown in Fig. 2 is suggested because to date no single technique or method has been found suitable of providing all information on the structure and composition of urinary stones. Only a combination of refined morphological and structural examinations of stones with optical and scanning electron microscopy, completed with a compositional analysis can provide a precise and reliable method for the identification of the stone type. The suggested pathway (Fig. 2) includes all of the necessary techniques and steps which will provide a complete set of useful information on the structure, texture, morphology, mineralogical phases and chemical composition (elemental and molecular level).
Fig. 2.
Flow pattern for the analysis of kidney stones. SEM Scanning electron microscopy, FTIR Fourier transform infrared spectroscopy, XRD X-ray diffraction, LIBS laser-induced breakdown spectroscopy, ICP inductively coupled plasma, EDAX, energy dispersive X-ray analysis, XAS X-ray absorption spectroscopy
Discussion and conclusion
Major and trace elements are naturally present in the human body and essential to human health if taken during eating, drinking or breathing. A large number of trace elements are essential for specific metabolic processes, temporarily stored and then excreted via the kidneys (Hesse et al. 2013). This can result in the accidental incorporation of trace elements into urinary stones, but also affect crystal formation or change the properties of urinary stones. The aim of this review is to assess the role of major and trace elements in the pathogenesis of kidney stones. The first paper on trace elements in urinary stones was published in 1963 by Nagy et al. (1963), who detected a large number of trace elements in the stone samples (Ag, Al, Ba, Bi, Cd, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Si, Sr and Zn). The first study of the influence of trace elements on the crystallization process of calcium oxalate was published by Sutor (1969) and Eusebio and Elliot (1967) who reported that trace elements, particularly Co, Ni, Pb, tin (Sn), V and Zn, could inhibit the crystallization process of calcium oxalate. Joost and Tessadri (1987) found significantly higher concentrations of Fe, Sb, Sr and Zn in calcium oxalate stones, of Fe, As and Zn in phosphate stones and of Sb and As in uric acid stones. This observation can be explained by the effect of heterogenic isomorphism, which is the insertion of a foreign element into a crystal lattice of a salt. The same phenomenon is observed in crystals of apatite, in which P can be replaced by the As ion. Bazin et al. (2007) showed a high proportion of Zn and Sr in phosphate stones and a lower proportion of these elements in calcium oxalate stones. Słojewski et al. (2010) was found a positive correlation between Zn and Sr concentrations in calcium phosphate stones, but not in calcium oxalate stones. Durak et al. (1990) studied the distribution of five metals, particularly Fe, Cu, Cd, Zn, and Mg, in stones and hair and found significant differences among the element levels in the stones, patients’ hair and control patients’ hair. The role of Zn in lithogenesis remains unclear. Early studies by Bird and Thomas (1963) and the recent study by Atakan et al. ( 2007) showed that a low Zn level in the urine of stone-formers suggests its potential inhibitor activity against kidney stone formation. Turgut et al. (2008) reported that low concentrations of Zn, Mn and Mg in calcium oxalate monohydrate stones appear to make them resistant to extracorporeal shock wave lithotripsy. There are similar data on Cu, Fe, Mg, and Zn (Küpeli et al. 1993). Scott et al. (1980) found a high concentration of Mg and K in phosphate stones and a relatively low concentration of Na in calcium oxalate stones.
Separate analyses of the core and shell of urinary stones revealed higher concentrations of Zn in the core of mixed calcium oxalate/apatite stones than in pure calcium oxalate or struvite stones, respectively (Lin et al. 1987; Singh et al. 2009; Hesse et al. 2013). Based on the the higher Zn content of the stone core, these authors concluded that Zn and other trace elements (Cu, Sr) could play a role in the formation of the nucleus. Some heavy metals, including Pb, Cd, Ni and Al, have also been found at higher concentrations in the nuclei than in the crust (Perk et al. 2002). This finding indicates that these heavy metals may take part in the initiation of stone crystallization, for example as a nucleus or nidus for the formation of the stone or, alternatively, they may simply be contaminants of the stone. Trace elements, such as Zn, Cu, Fe, Ni and Sr, form poorly soluble salts with oxalate and phosphate ions (Hesse et al. 2013). No pure urinary stones consisting of these compounds are known so far. However, zinc phosphate has been found in the layers of struvite stones. Some trace elements have also been found in small amounts in uric acid stones. Higher concentrations of Zn and other trace elements, such as Sr, have been found in calcium phosphate stones as compared to calcium oxalate stones. Recently, Giannossi et al. ( 2013)1 analyzed a large set of kidney stone samples of different kinds using ICP spectrometry to determine the specific content of various elements (i.e. Ca, Mg, K, Zn, Fe, Cu and Mn) involved either in the crystallisation process of kidney stones or as potentially toxic agents, such as Pb and Cr. These authors found that most urinary stones had high concentrations of K, Cu and Mg and a low content of Fe as compared to previously reported results. Significant amounts of these elements were found in calcium oxalate and phosphates, whereas a higher content of Zn was found in uric acid and cystine stones. Higher amounts of trace elements were found in calcium phosphate stones than in calcium oxalate stones, and in the latter, more trace elements were observed in weddellite than whewellite.
In conclusion, depending on the stone type and according to their occurrence in urine, trace elements are incorporated into a kidney stone and can affect its properties. Significant variations of some trace elements in blood, urine and serum of stone patients and healthy persons were observed which clearly indicate their role in nephrolithiasis. Some correlations were clearly observed between the elements in each group of stones. A correlation between Ca and other trace elements has also been found which shows that trace elements are relevant to the process taking place during the stone formation and its growth. In vitro and in vivo studies are further required to understand the exact role of elements in kidney stone formation and its growth. It is clear from the review of literature that kidney stones are multicomponent, including crystalline components. Thus, the choice of the technique for the analysis of kidney stones should be capable of resolving all components of the stones. Published studies support the use of FT-IR, Raman or XRD as the preferred analytical methods. The different areas of the stones must also be analyzed separately on both the elemental and molecular levels to obtain useful results regarding its pathogenesis. To determine the elements in each portion of the kidney stone under study, LIBS is a quite suitable method since it has a point detection capability. LIBS has the capability to rapidly and simultaneously perform a multi-elemental analysis of stones samples, urine, blood and serum samples, providing qualitative information within a very short time period on the spatial distribution of elements inside the kidney stone. The other great advantage of LIBS is that it does not damage the sample during the experiments, thereby enabling the sample to be used in other molecular analyses with other methods, such as IR spectroscopy, Raman spectroscopy and XRD.
Acknowledgments
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Vivek K. Singh declares that he has no conflict of interest. Pradeep K. Rai declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This is a very recent publication on trace elements in urinary stones. Giannossi et al. (2013) analyzed a large set of kidney stone samples of different kinds and determined the trace and heavy elements either involved in the crystallization process or potentially toxic. They also compared the elemental contents in these stones and discussed their presence and role in the formation of kidney stones.
References
- Abboud IA. Mineralogy and chemistry of urinary stones: patients from North Jordan. Environ Geochem Health. 2008;30:445–463. doi: 10.1007/s10653-007-9128-7. [DOI] [PubMed] [Google Scholar]
- Anke M, Schneider HJ (1973) Die bedeutung der spurenelemente in der pathogenese and Therapie der urolithiasis. III. Jenaer Harnsteinsymposium, pp 116–126.
- Anzano J, Lasheras RJ. Strategies for the identification of urinary calculus by laser induced breakdown spectroscopy. Talanta. 2009;79:352–360. doi: 10.1016/j.talanta.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Atakan IH, Kaplan M, Seren G, Aktoz T, Gül H, Inci O. Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol. 2007;39:351–356. doi: 10.1007/s11255-006-9050-4. [DOI] [PubMed] [Google Scholar]
- Barbas C, Garca A, Saavedra L, Muros M. Urinary analysis of nephrolithiasis markers. J Chromatogr B. 2002;781:433–455. doi: 10.1016/s1570-0232(02)00557-3. [DOI] [PubMed] [Google Scholar]
- Barceloux DG. Vanadium. J Toxicol Clin Toxicol. 1993;37:265–278. doi: 10.1081/clt-100102425. [DOI] [PubMed] [Google Scholar]
- Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. Eur Assoc Urol Eur Board Urol (EAU-EBU) Update Ser. 2005;5:126–136. [Google Scholar]
- Basiri A, Teheri M, Taheri F. What is the state of the stone analysis technique in Urolithiasis? Urol J. 2012;9:445–454. [PubMed] [Google Scholar]
- Bazin D, Daudon M. Pathological calcifications and selected examples at the medicine-solid-state physics interface. J Phy D: Appl Phys. 2012;45:383001–383010. [Google Scholar]
- Bazin D, Daudon M, Chevallier P, Rouzière S, Elkaim E, Thiaudière D, Fayard B, Foy E, Albouy PA, André G, Matzen G, Veron E. Ann Biol Clin. 2006;64:125. [PubMed] [Google Scholar]
- Bazin D, Chevallier P, Matzen G, Jungers P, Daudon M. Heavy elements in urinary stones. Urol Res. 2007;35:179–184. doi: 10.1007/s00240-007-0099-z. [DOI] [PubMed] [Google Scholar]
- Bazin D, Carpentier X, Brocheriou I, Dorfmuller P, Aubert S, Chappard C, Thiaudière D, Reguer S, Waychunas G, Jungers P, Daudon M. Revisiting the localisation of Zn2+ cations sorbed on pathological apatite calcifications made through XAS. Biochimie. 2009;91:1294–1300. doi: 10.1016/j.biochi.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bazin D, Daudon M, Combes C, Rey C. Characterization and some physicochemical aspects of pathological microcalcifications. Chem Rev. 2012;112:5092–5120. doi: 10.1021/cr200068d. [DOI] [PubMed] [Google Scholar]
- Beischer DE. Analysis of renal calculi by infrared spectroscopy. J Urol. 1955;73:653–659. doi: 10.1016/S0022-5347(17)67449-4. [DOI] [PubMed] [Google Scholar]
- Berenyi M, Liptay G. The use of thermal analysis in medical science with special reference to nephroliths. J Thermal Anal. 1971;3:437–443. [Google Scholar]
- Berényi M, Frang D, Légrády J. Theoretical and clinical importance of the differentiation between the two types of calcium oxalate hydrate. Int Urol Nephrol. 1972;4:341–345. doi: 10.1007/BF02108137. [DOI] [PubMed] [Google Scholar]
- Bhatt PA, Paul P. Analysis of urinary stone constituents using powder X-ray diffraction and FT-IR. J Chem Sci. 2008;120:267–273. [Google Scholar]
- Bihl G, Meyers A. Recurrent renal stone disease-advances in pathogenesis and clinical management. Lancet. 2001;358:651–656. doi: 10.1016/S0140-6736(01)05782-8. [DOI] [PubMed] [Google Scholar]
- Bird ED, Thomas WC. Effect of various metals on mineralization in vitro. Proc Soc Exp Biol Med. 1963;112:640–643. doi: 10.3181/00379727-112-28126. [DOI] [PubMed] [Google Scholar]
- Blaschko SD, Miller J, Chi T, Flechner L, Fakra S, Kahn A, Kapahi P, Stoller ML. Micro-composition of human urinary calculi using advanced imaging techniques. J Urol. 2013;189:726–734. doi: 10.1016/j.juro.2012.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsatti A. Calcium oxalate nephrolithiasis: defective oxalate transport. Kidney Int. 1991;39:1283–1298. doi: 10.1038/ki.1991.162. [DOI] [PubMed] [Google Scholar]
- Carmona P, Bellanato J, Escolar E. Infrared and Raman spectroscopy of urinary calculi: A review. Biospectroscopy. 1997;3:331–346. [Google Scholar]
- Carpentier X, Daudon M, Traxer O, Jungers P, Mazouyes A, Matzen G, Veron E, Bazin D. Relationship between the carbonate rate of carbapatite, morphological characteristics of calcium phosphate stones and etiology. Urology. 2009;73:968. doi: 10.1016/j.urology.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Carpentier X, Bazin D, Combes C, Mazouyes A, Rouziere S, Albouy A, Foy E, Daudon M. High Zn content of Randall’s plaque: A μ-X-ray fluorescence investigation. J Trace El Med Biol. 2011;25:160–165. doi: 10.1016/j.jtemb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Charafi S, Mbarki M, Bauza AC, Prieto RM, Oussama A, Grases F. A comparative study of two renal stone analysis methods. Int J Nephrol Urol. 2010;2:469–475. [Google Scholar]
- Chaudhri MA, Watling J, Khan FA. Spatial distribution of major and trace elements in bladder and kidney stones. J Radioanal Nucl Chem. 2007;271:713–720. [Google Scholar]
- Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease. Kidney Int. 1994;46:1133–1139. doi: 10.1038/ki.1994.376. [DOI] [PubMed] [Google Scholar]
- Cohanim M, Yendt ER. The effects of thiazides on serum and urinary zinc in patients with renal calculi. Johns Hopkins Med J. 1975;136:137–141. [PubMed] [Google Scholar]
- Curhan GC, Willet WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo G, Curini R, de Angelis G, Cardarelli E, Magri A, Miano L. Renal calculi analysis. Application of thermal analytical techniques. Thermochim Acta. 1983;62:149–169. [Google Scholar]
- Daudon M. Bazin D (2013) When the Synchrotron radiations highlight the Randall's plaques and kidney concretions. J Phys: Conference Series. 2013;425:022006. [Google Scholar]
- Daudon M, Bader CA, Jungers Urinary calculi: Review of classification methods and correlations with etiology. Scan Microsc. 1993;7:1081–1106. [PubMed] [Google Scholar]
- Daudon M, Junger P, Bazin D. Peculiar Morphology of stones in Primary hyperoxaluria. New Eng J Med. 2008;359:100. doi: 10.1056/NEJMc0800990. [DOI] [PubMed] [Google Scholar]
- Daudon M, Bazin D, Jungers P, Andre G, Cousson A, Chevallier P, Veron E, Matzen G. Opportunities offered by Scanning electron microscopy, powder neutron diffraction in the study of whewellite kidney stones. J App Cryst. 2009;42:109. [Google Scholar]
- Douglas DE, Tonks DB. The qualitative analysis of renal calculi with the polarising microscope. Clin Biochem. 1979;12:182–183. doi: 10.1016/s0009-9120(79)80086-7. [DOI] [PubMed] [Google Scholar]
- Durak I, Kilic Z, Perk H, Sahin A, Yurtarslani Z, Yaşar A, Küpeli S, Akpoyraz M. Iron, copper, cadmium, zinc and magnesium contents of urinary tract stones and hair from men with stone disease. Eur Urol. 1990;17:243–247. doi: 10.1159/000464048. [DOI] [PubMed] [Google Scholar]
- Durak I, Kilic Z, Sahin A, Akpoyraz M. Analysis of calcium, iron, copper and zinc contents of nucleus and crust parts of urinary calculi. Urol Res. 1992;20:23–26. doi: 10.1007/BF00294330. [DOI] [PubMed] [Google Scholar]
- Durrant SF, Ward NI. Recent biological and environmental applications of laser ablation inductively coupled plasma mass spectrometry (LA ICP-MS) J Anal At Spectrom. 2005;20:821–829. [Google Scholar]
- Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- Elliot JS, Ribeiro ME. The urinary excretion of trace metals in patients with calcium oxalate urinary stone. Invest Urol. 1973;10:253–255. [PubMed] [Google Scholar]
- Escott-Stump S (2007) Nutritional review. In: Nutrition and diagnosis-related care. Wolters Kluwer, Lippincot Williams and Wilkins, Philadelphia, pp 842–862
- Estepa L, Daudon M. Contribution of Fourier transform infrared spectroscopy to the identification of urinary stones and kidney crystal deposits. Biospectrosc Biospectrosc. 1997;3:347–369. [Google Scholar]
- Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A. Potassium magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- Eusebio E, Elliot JS. Effect of trace metals on the crystallization of calcium oxalate. Invest Urol. 1967;4:431–435. [PubMed] [Google Scholar]
- Fang X, Ahmad SR, Mayo M, Iqbal S. Elemental analysis of urinary calculi by laser induced plasma spectroscopy. Lasers Med Sci. 2005;20:132–137. doi: 10.1007/s10103-005-0356-8. [DOI] [PubMed] [Google Scholar]
- Fazil Marickar YM, Lekshmi PR, Varma L, Koshy P. Elemental distribution analysis of urinary crystals. Urol Res. 2009;37:277–282. doi: 10.1007/s00240-009-0203-7. [DOI] [PubMed] [Google Scholar]
- Fazil Marickar YM, Lekshmi PR, Varma L, Koshy P. EDAX versus FTIR in mixed stones. Urol. 2009;39:271–276. doi: 10.1007/s00240-009-0202-8. [DOI] [PubMed] [Google Scholar]
- Ferraro PM, Bonello M, Frigo AC, D'Addessi A, Sturniolo A, Gambaro G. Cadmium exposure and kidney stone formation in the general population—an analysis of the National Health and Nutrition Examination Survey III data. J Endourol. 2011;25:875–880. doi: 10.1089/end.2010.0572. [DOI] [PubMed] [Google Scholar]
- Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med. 2005;26:235–244. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Francois B, Cahen R, Pascal B. Inhibitors of urinary stone formation in 40 recurrent stone formers. Br J Urol. 1986;58:479–483. doi: 10.1111/j.1464-410x.1986.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Fujieda M, Naruse K, Hamauzu T, Miyazaki E, Hayashi Y, Enomoto R, Lee E, Ohta K, Yamaguchi Y, Wakiguchi H, Enza H. Effect of seleniumdeficient diet on tubular epithelium in normal rats. Pediatr Nephrol. 2007;22:192–201. doi: 10.1007/s00467-006-0266-4. [DOI] [PubMed] [Google Scholar]
- Giannossi ML, Summa V, Mongelli G. Trace element investigations in urinary stones: A preliminary pilot case in Basilicata (Southern Italy) J Trace Elem Med Biol. 2013;27:91–97. doi: 10.1016/j.jtemb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Girija EK, Kalkura SN, Sivaraman PB, Yokogawa Y. Mineralogical composition of urinary calculi from southern India. J Sci Ind Res. 2007;66:632–639. [Google Scholar]
- Gould N, Hallson PC, Kasidas GP, Samuell CT, Weir TB. Rapid computer-assisted infrared analysis of urinary calculi using photoacoustic detection. Urol Res. 1995;23:63–69. doi: 10.1007/BF00298853. [DOI] [PubMed] [Google Scholar]
- Grant LD. Lead and compounds. In: Lippmann M, editor. Environmental toxicants: human exposures and their health effects. 3. Hoboken: John Wiley & Sons; 2009. pp. 757–810. [Google Scholar]
- Gulley-Stahl HJ, Haas JA, Schmidt KA, Evan AP, Sommer AJ. Attenuated total internal reflection Fourier transform infrared spectroscopy: a quantitative approach for kidney stone analysis. Appl Spectrosc. 2009;63:759–766. doi: 10.1366/000370209788701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring LC. Observations on the analysis of ten thousand urinary calculi. J Urol. 1962;88:545–562. doi: 10.1016/S0022-5347(17)64842-0. [DOI] [PubMed] [Google Scholar]
- Hesse A, Sanders G. Atlas of infrared spectra for the analysis of urinary concrements. Stuttgart: Georg Thieme Verlag; 1988. [Google Scholar]
- Hesse A, Schneider HJ, Hienzsch E. Infra-red spectroscopy of urinary calculi. Dtsch Med Wochenschr. 1972;97:1694–1701. doi: 10.1055/s-0028-1107632. [DOI] [PubMed] [Google Scholar]
- Hesse A, Schneider HJ, Berg W, Hienzsch E. Uric acid dihydrate as urinary calculus component. Invest Urol. 1975;12:405–409. [PubMed] [Google Scholar]
- Hesse A, Berg W, Schneider HJ, Hienzsch E. A contribution to the formation mechanism of calcium oxalate urinary calculi. II. In vitro experiments concerning the theory of the formation of whewellite and weddellite urinary calculi. Urol Res. 1976;4:157–160. doi: 10.1007/BF00262348. [DOI] [PubMed] [Google Scholar]
- Hesse A, Dietze HJ, Berg W, Hienzsch E. Mass spectrometric trace element analysis of calcium oxalate uroliths. Eur Urol. 1977;3:359–361. doi: 10.1159/000472136. [DOI] [PubMed] [Google Scholar]
- Hesse A, Müller R, Schneider HJ, Taubert F. Analytic experiments on the significance of the fluorine content of urinary calculi (German) Urologe A. 1978;17:207–210. [PubMed] [Google Scholar]
- Hesse A, Siener R, et al. Trace Elements in Urolithiasis. In: Talati JJ, et al., editors. Urolithiasis. London: Springer; 2013. pp. 227–230. [Google Scholar]
- Hobarth K, Koeberl C, Hofbauer J. Rare-earth elements in urinary calculi. Urol Res. 1993;21:261–264. doi: 10.1007/BF00307707. [DOI] [PubMed] [Google Scholar]
- Hofbauer J, Steffan I, Höbarth K, Vujicic G, Schwetz H, Reich G, Zechner O. Trace elements and urinary stone formation: new aspects of the pathological mechanism of urinary stone formation. J Urol. 1991;145:93–96. doi: 10.1016/s0022-5347(17)38256-3. [DOI] [PubMed] [Google Scholar]
- Hofmann R, Hartung R, Schmidt-Kloiber H, Reichel E. First clinical experience with a Q-switched Nd:YAG laser for urinary calculi. J Urol. 1989;141:275–279. doi: 10.1016/s0022-5347(17)40739-7. [DOI] [PubMed] [Google Scholar]
- Huel G, Fréry N, Takser L, Jouan M, Hellier G, Sahuquillo J, Giordanella JP. Evolution of blood lead levels in urban French population (1979–1995) Rev Epidemiol Sante Publique. 2002;50:287–295. [PubMed] [Google Scholar]
- Hunt CD, Herbel JL, Nielsen FH. Metabolic responses of postmenopausal women to supplemental dietary boron and aluminum during usual and low magnesium intake: boron, calcium, and magnesium absorption and retention and blood mineral concentrations. Am J Clin Nutr. 1997;65:803–813. doi: 10.1093/ajcn/65.3.803. [DOI] [PubMed] [Google Scholar]
- Iwata K, Saito H, Moriyama M, Nakano A. Renal tubular function after reduction of environmental cadmium exposure: a ten-year follow-up. Arch Environ Health. 1993;48:157–163. doi: 10.1080/00039896.1993.9940814. [DOI] [PubMed] [Google Scholar]
- Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24:1–51. [PubMed] [Google Scholar]
- Järup L, Hellström L, Alfvén T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spång G, Schütz A, Elinder CG. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000;57:668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G, Backman U, Danielson BG, Fellstrom B, Ljunghall S, Wikstrom B. Biochemical and clinical effects of the prophylactic treatment of renal calcium stones with magnesium hydroxide. J Urol. 1980;124:770–774. doi: 10.1016/s0022-5347(17)55655-4. [DOI] [PubMed] [Google Scholar]
- Joost J, Tessadri R. Trace element investigations in kidney stone patients. Eur Urol. 1987;13:264–270. doi: 10.1159/000472792. [DOI] [PubMed] [Google Scholar]
- Kajander EO, Ciftcioglu N. Nanobacteria: An alternating mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci USA. 1998;95:8274–8279. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloustian J, El-Moselphy TF, Portugal H. Determination of calcium oxalate (mono and dehydrate) in mixture with magnesium ammonium phosphate or uric acid: The use of simultaneous thermal analysis in urinary calculi. Clin Chim Acta. 2003;334:117–129. doi: 10.1016/s0009-8981(03)00228-6. [DOI] [PubMed] [Google Scholar]
- Kasidas GP, Samuell CT, Weir TB. Renal stone analysis: why and how? Ann Clin Biochem. 2004;41:91–97. doi: 10.1258/000456304322879962. [DOI] [PubMed] [Google Scholar]
- Kjellström T, Evrin PE, Rahnster B. Dose–response analysis of cadmiuminduced tubular proteinuria: a study of urinary beta2-microglobulin excretion among workers in a battery factory. Environ Res. 1977;13:303–317. doi: 10.1016/0013-9351(77)90106-2. [DOI] [PubMed] [Google Scholar]
- Kohri K, Garside J, Blacklock NJ. The role of magnesium in calcium oxalate urolithiasis. Br J Urol. 1988;61:107–112. doi: 10.1111/j.1464-410x.1988.tb05057.x. [DOI] [PubMed] [Google Scholar]
- Komleh K, Hada P, Pendse AK, Singh PP. Zinc, copper and manganese in serum, urine and stones. Int Urol Nephrol. 1990;22:113–118. doi: 10.1007/BF02549826. [DOI] [PubMed] [Google Scholar]
- Koningsberger DC, Prins R. X-ray absorption: principles, applications, techniques of EXAFS. New York: SEXAFS and XANES, Wiley; 1988. [Google Scholar]
- Kumar M, Selvam R. Supplementation of vitamine E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem. 2003;14:306–313. doi: 10.1016/s0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gupta A, Shrivastava DK. Role of zinc in nephrolithiasis. J Indian Med Assoc. 1984;82:235–237. [PubMed] [Google Scholar]
- Kumar N, Singh P, Kumar S. Physical, X-ray diffraction and scanning electron microscopic studies of uroliths. Indian J Biochem Biophys. 2006;43:226–232. [PubMed] [Google Scholar]
- Küpeli S, Arikan N, Durak I, Sarica K, Akpoyraz M, Karalezli G. Efficiency of extracorporeal shockwave lithotripsy on calcium-oxalate stones: role of copper, iron, magnesium and zinc concentrations on disintegration of the stones. Eur Urol. 1993;23:409–412. doi: 10.1159/000474640. [DOI] [PubMed] [Google Scholar]
- Kuta J, Jiri M, Daniela B, Rostislav C, Tamara K. Urinary calculi-a typical source of information on mercury in human biomonitoring. Cent Eur J Chem. 2012;10:1475–1483. [Google Scholar]
- Lahme S, Feil G, Strohmaier WL, Bichler KH, Stenzl A. Renal tubular alteration by crystalluria in stone disease an experimental study by means of MDCK cells. Urol Int. 2004;72:244–251. doi: 10.1159/000077124. [DOI] [PubMed] [Google Scholar]
- Le Bail A, Daudon M, Bazin D. A new compound in kidney stones? Powder X-ray diffraction study of calcium glycinate trihydrate. Acta Cryst. 2013;C69:734–737. doi: 10.1107/S0108270113015709. [DOI] [PubMed] [Google Scholar]
- Lee HP, Leong D, Heng CT. Characterization of kidney stones using thermogravimetric analysis with electron dispersive spectroscopy. Urol Res. 2012;40:197–204. doi: 10.1007/s00240-011-0428-0. [DOI] [PubMed] [Google Scholar]
- Lehmann CA, McClure GL, Smolens I. Identification of renal calculi by computerized infrared spectroscopy. Clin Chim Acta. 1988;173:107–116. doi: 10.1016/0009-8981(88)90248-3. [DOI] [PubMed] [Google Scholar]
- Lemann JJ, Pleuss JA, Gray RW, Hoffmann RG. Potassium administration increases and potassium deprivative reduces urinary calcium excretion in healthy adults. Kidney Int. 1991;39:973–983. doi: 10.1038/ki.1991.123. [DOI] [PubMed] [Google Scholar]
- Levinson AA, Nosa M, Davidman M, Prien EL, Sr, Prien EL, Jr, Stevenson RG. Trace elements in kidney stones from three areas in the United States. Invest Urol. 1978;15:270–274. [PubMed] [Google Scholar]
- Li C, Paris O, Siegel S, Roschger P, Paschalis EP, Klaushofer K, Fratzl P. Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res. 2010;25:968–975. doi: 10.1359/jbmr.091038. [DOI] [PubMed] [Google Scholar]
- Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Asp Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Lin JL, Huang PT. Body lead stores and urate excretion in men with chronic renal disease. J Rheumatol. 1994;21:705–709. [PubMed] [Google Scholar]
- Lin SM, Tseng CL, Yang MH. Determination of major, minor and trace elements in urinary stones by neutron activation analysis. Int J Rad Appl Instrum A. 1987;38:635–639. doi: 10.1016/0883-2889(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Lindberg J, Harvey J, Pak CY. Effect of magnesium citrate and magnesium oxide on the crystallization of calcium salts in urine: changes produced by food-magnesium interaction. J Urol. 1990;143:248–251. doi: 10.1016/s0022-5347(17)39924-x. [DOI] [PubMed] [Google Scholar]
- Lyon TD, Aughey E, Scott R, Fell GS. Cadmium concentrations in human kidney in the UK: 1978–1993. J Environ Monit. 1999;1:227–231. doi: 10.1039/a901366k. [DOI] [PubMed] [Google Scholar]
- Marangella M, Bagnis C, Bruno M, Vitale C, Petrarulo M, Ramello A. Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int. 2004;1:6–10. doi: 10.1159/000076583. [DOI] [PubMed] [Google Scholar]
- Martini LA, Cuppari L, Cunha NA, Schor N, Heilberg Potassium and sodium intake and excretion in calcium stone forming patients. J Renal Nutr. 1998;8:127–131. doi: 10.1016/s1051-2276(98)90003-6. [DOI] [PubMed] [Google Scholar]
- Massey L. Magnesium therapy for nephrolithiasis. Magnes Res. 2005;18:123–126. [PubMed] [Google Scholar]
- Materazzi S, Curini R, D’Ascenzo G, Magri AD. TG-FTIR coupled analysis applied to the studies in urolithiasis: characterization of human renal calculi. Thermochim Acta. 1995;264:75–93. [Google Scholar]
- McCoy H, Kenney MA, Montgomery C, Irwin A, Williams L, Orrell R. Relation of boron to the composition and mechanical properties of bone. Environ Health Perspect. 1994;102:49–53. doi: 10.1289/ehp.94102s749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JL, Angino EE. The role of trace metals in calcium urolithiasis. Invest Urol. 1977;14:347–350. [PubMed] [Google Scholar]
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- Munoz JA, Valiente M. Effects of trace metals on the inhibition of calcium oxalate crystallization. Urol Res. 2005;33:267–272. doi: 10.1007/s00240-005-0468-4. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Szabó E, Kelenhegyi M. Spektralanalytische Untersuchung von Nierensteien auf metallische Spurenelemente. Z Urol. 1963;56:185–190. [Google Scholar]
- Nalbandyan VB. X-ray diffraction analysis of urinary calculi: need for heat treatment. Urol Res. 2008;36:247–249. doi: 10.1007/s00240-008-0148-2. [DOI] [PubMed] [Google Scholar]
- New Hampshire Department of Environmental Services (2005) Copper: health information summary. Environmental Fact Sheet. ARD-Department of Environment and Heritage Protection (EHP)-9. Available at: http://des.nh.gov/organization/commissioner/pip/factsheets/ard/documents/ard-ehp-9.pdf
- Nguyen Quy D, Daudon M. Infrared and Raman spectra of calculi. Paris: Elsevier; 1997. [Google Scholar]
- Nielsen FH. Biochemical and physiologic consequences of boron deprivation in humans. Environ Health Perspect. 1994;102:59–63. doi: 10.1289/ehp.94102s759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RW. Metabolic evaluation of stone disease patients: a practical approach. Curr Opin Urol. 2001;11:347–351. doi: 10.1097/00042307-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Oka T, Yoshioka T, Koide T, Takaha M, Sonoda T. Role of magnesium in the growth of calcium oxalate monohydrate and calcium oxalate dihydrate crystals. Urol Int. 1987;42:89–93. doi: 10.1159/000281861. [DOI] [PubMed] [Google Scholar]
- Orlando MTD, Kuplich L, de Souza DO, Belich H, Depianti JB, Orlando CGP, Medeiros EF, da Cruz PCM, Martinez LG, Corrêa HPS, Ortiz R. Study of calcium oxalate monohydrate of kidney stones by X-ray diffraction. Powder Diffraction Suppl. 2008;23:S59–S64. [Google Scholar]
- Oswald I, Cavalu S, Maghiar TT, Osvat D. Identification of the urinary stone composition upon extracorporeal shock wave lithotripsy. Romanian J Biophys. 2011;21:107–112. [Google Scholar]
- Ouyang JM. The application of X-ray diffraction in the study of urinary stones. Gang Pu Xue Yu Guang Pu Fen Xi. 2006;26:170–174. [PubMed] [Google Scholar]
- Ozgurtas T, Yakut G, Gulec M, Serdar M, Kutluay T. Role of urinary zinc and copper on calcium oxalate stone formation. Urol Int. 2004;72:233–236. doi: 10.1159/000077122. [DOI] [PubMed] [Google Scholar]
- Oztoprak BG, Gonzalez J, Yoo J, Gulecen T, Mutlu N, Russo RE, Gundogdu O, Demir A. Analysis and classification of heterogeneous kidney stones using laser induced breakdown spectroscopy (LIBS) Appl Spectrosc. 2012;66:1353–1361. doi: 10.1366/12-06679. [DOI] [PubMed] [Google Scholar]
- Pak CY. Kidney stones. Lancet. 1998;351:1797–1801. doi: 10.1016/S0140-6736(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Paluszkiewicz C, Galka M, Kwiatek W, Parczewski A, Walas S. Renal stone studies using vibrational spectroscopy and trace element analysis. Biospectroscopy. 1997;3:403–407. [Google Scholar]
- Parigger CG, Guan G, Hornkohl JO. Measurement and analysis of OH emission spectra following laser-induced breakdown. Appl Opt. 2003;42:5986–5991. doi: 10.1364/ao.42.005986. [DOI] [PubMed] [Google Scholar]
- Parsons J. Zinc phosphate identified as a constituent of urinary calculi. Science. 1953;118:217–218. doi: 10.1126/science.118.3060.217. [DOI] [PubMed] [Google Scholar]
- Perk H, Serel TA, Kosar A, Deniz N, Sayin A. Analysis of the trace element contents of inner nucleus and outer crust parts of urinary calculi. Urol Int. 2002;68:286–290. doi: 10.1159/000058452. [DOI] [PubMed] [Google Scholar]
- Prien EL, Frondel C. Studies in urolithiasis: I. The composition of urinary calculi. J Urol. 1947;57:949–991. doi: 10.1016/S0022-5347(17)69732-5. [DOI] [PubMed] [Google Scholar]
- Randall A. An hypothesis for the origin of renal calculus. N Engl J Med. 1936;214:234–242. [Google Scholar]
- Rangnekar GV, Gaur MS. Serum and urinary zinc levels in urolithiasis. Br J Urol. 1993;71:527–529. doi: 10.1111/j.1464-410x.1993.tb16019.x. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Rebentisch G. Results of external quality assessment of analysis of urinary calculi by various methods. Scand J Clin Lab Invest Suppl. 1993;212:54–55. doi: 10.3109/00365519309085457. [DOI] [PubMed] [Google Scholar]
- Robertson WG. Measurement of ionized calcium in biological fluids. Clin Chim Acta. 1969;24:149–157. doi: 10.1016/0009-8981(69)90152-1. [DOI] [PubMed] [Google Scholar]
- Rodgers AL, Scaillet, Wandt MA (1984) Urinary stone. In: Livingstone C, Ryall RL (eds) Proc 2nd Int Urinary Stone Conf. New York
- Rodgers A, Barbour L, Pougnet B, Lombard C, Ryall R. Urinary element concentrations in kidney stone formers and normal controls: the week-end effect. J Trace Elem Electrolytes Health Dis. 1994;8:87–91. [PubMed] [Google Scholar]
- Rodríguez-Miñón Cifuentes JL, Salvador E, Traba Villameytide ML. Usual elements in kidney stones. Acta Urol Esp. 2006;30:57–62. doi: 10.1016/s0210-4806(06)73397-6. [DOI] [PubMed] [Google Scholar]
- Rose G. Stone analysis of thermogravimetric technique: In Urinary stones: Chemical and laboratory aspects. Lancaster: MTP Press; 1982. pp. 77–85. [Google Scholar]
- Rose GA, Woodfine C. The thermogravimetric analysis of renal stones (in clinical practice) Br J Urol. 1976;48:403–412. doi: 10.1111/j.1464-410x.1976.tb06668.x. [DOI] [PubMed] [Google Scholar]
- Rubin R, Strayer DS. Rubin's pathology: clinicopathologic foundations of medicine. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Sakly R, Chaouch A, El Hani A, Najjar MF. Effects of intra peritoneally administered vitamine E and selenium on calcium oxalate renal stone formation: experimental study in rat. Ann Urol. 2003;37:47–50. doi: 10.1016/s0003-4401(03)00007-x. [DOI] [PubMed] [Google Scholar]
- Samachson J, Scheck J, Spencer H. Radiocalcium absorption at different times of day. Am J Clin Nutr. 1966;18:449–451. doi: 10.1093/ajcn/18.6.449. [DOI] [PubMed] [Google Scholar]
- Schneider HJ, Berenyi M, Hesse A, Tscharnke J. Comparative urinary stone analyses. Quantitative chemical, x-ray diffraction, infrared spectroscopy and thermo-analtytical procedures. Int Urol Nephrol. 1973;5:9–17. doi: 10.1007/BF02081747. [DOI] [PubMed] [Google Scholar]
- Schubert G. Stone analysis. Urol Res. 2006;34:146–150. doi: 10.1007/s00240-005-0028-y. [DOI] [PubMed] [Google Scholar]
- Schubert G, Brien G, Bick C. Separate examinations on core and shell of urinary calculi. Urol Int. 1983;38:65–69. doi: 10.1159/000280865. [DOI] [PubMed] [Google Scholar]
- Scott R, East BW, Janczyszyn J, Boddy K, Yates AJ. Concentration of some minor and trace elements in urinary tract stones: a preliminary study. Urol Res. 1980;8:167–169. doi: 10.1007/BF00256412. [DOI] [PubMed] [Google Scholar]
- Silva SFR, Matos DC, Silva SL, Daher EDF, Campos HH, Silva CAB. Chemical and morphological analysis of kidney stones: a double-blind comparative study. Acta Cir Bras. 2010;25:444–448. doi: 10.1590/s0102-86502010000500011. [DOI] [PubMed] [Google Scholar]
- Singh I. Renal geology (quantitative renal stone analysis) by Fourier transform infrared spectroscopy. Int Urol Nephrol. 2008;40:595–602. doi: 10.1007/s11255-007-9327-2. [DOI] [PubMed] [Google Scholar]
- Singh VK, Rai AK. Prospects of laser induced breakdown spectroscopy for biomedical applications: A review. Lasers Med Sci. 2011;26:673–687. doi: 10.1007/s10103-011-0921-2. [DOI] [PubMed] [Google Scholar]
- Singh VK, Rai AK, Rai PK, Jindal PK. Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Lasers Med Sci. 2009;24:749–759. doi: 10.1007/s10103-008-0635-2. [DOI] [PubMed] [Google Scholar]
- Słojewski M. Major and trace elements in lithogenesis. Central Eur J Urol. 2011;64:58–61. doi: 10.5173/ceju.2011.02.art1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słojewski M, Czerny B, Safranow K, Jakubowska K, Olszewska M, Pawlik A, Gołąb A, Droździk M, Chlubek D, Sikorski A. Microelements in stones, urine, and hair of stone formers: a new key to the puzzle of lithogenesis? Biol Trace Elem Res. 2010;137:301–316. doi: 10.1007/s12011-009-8584-6. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Swain KK, Ajith N, Wagh DN, Acharya R, Reddy AVR, Mete U. Trace element study of kidney stones from subjects belonging to stone belt region of India. J Radioanal Nucl Chem. 2012;294:425–428. [Google Scholar]
- Stepankova K, Novotny K, Vasinova Galiova M, Kanicky V, Kaiser J, Hahn DW. Laser ablation methods for analysis of urinary calculi: comparison study based on calibration pellets. Spectrochim Acta Part B. 2013;81:43–49. [Google Scholar]
- Stoller ML, Meng MV. Urinary stone disease: The practical guide to medical and surgical management. Totowa: Humana Press; 2007. [Google Scholar]
- Strates BS. Use of thermal gravimetry in the study of nephroliths. Experimentia. 1966;22:574–575. [Google Scholar]
- Strübel G, Rzepka-Glinder V, Grobecker KH, Jarrar K. Heavy metals in human urinary calculi. Fresenius J Anal Chem. 1990;337:316–319. [Google Scholar]
- Sutor DJ. Difficulties in the identification of components of mixed urinary calculi using the X-ray powder method. British J Urol. 1968;40:29–32. doi: 10.1111/j.1464-410x.1968.tb11809.x. [DOI] [PubMed] [Google Scholar]
- Sutor DJ. Growth studies of calcium oxalates in the presence of various ions and compounds. Br J Urol. 1969;41:171–178. doi: 10.1111/j.1464-410x.1969.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. An association between urinary cadmium and urinary stone disease in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ Res. 2011;111:579–583. doi: 10.1016/j.envres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Trinchieri A, Mandressi A, Luongo P, Longo G, Pisani E. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. Br J Urol. 1991;67:230–236. doi: 10.1111/j.1464-410x.1991.tb15124.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri A, Castelnuovo C, Lizzano R, Zanetti G. Calcium stone disease: a multiform reality. Urol Res. 2005;33:194–198. doi: 10.1007/s00240-004-0459-x. [DOI] [PubMed] [Google Scholar]
- Turgut M, Unal I, Berber A, Demir TA, Mutlu F, Aydar Y. The concentration of Zn, Mg and Mn in calcium oxalate monohydrate stones appears to interfere with their fragility. Urol Res. 2008;36:31–38. doi: 10.1007/s00240-007-0133-1. [DOI] [PubMed] [Google Scholar]
- Uldall A. Analysis of urinary calculi; a quality control programme. Scand J Clin Lab Invest. 1981;41:1339–1345. doi: 10.3109/00365518109092055. [DOI] [PubMed] [Google Scholar]
- Verkoelen CF, Van Der Boom BG, Romijn JC. Identification of hyaluronan as a crystal-binding molecule at the surface of migrating and proliferating MDCK cells. Kidney Int. 2000;58:1045–1054. doi: 10.1046/j.1523-1755.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Vezzoli G, Baragetti I, Zerbi S, Caumo A, Soldati L, Bellinzoni P, Centemero A, Rubinacci A, Moro G, Bianchi G. Strontium absorption and excretion in normocalciuric subjects: relation to calcium metabolism. Clin Chem. 1998;44:586–590. [PubMed] [Google Scholar]
- Vezzoli G, Rubinacci A, Bianchin C. Intestinal calcium absorption is associated with bone mass in stone-forming women with idiopathic hypercalciuria. Am J Kidney Dis. 2003;42:1177–1183. doi: 10.1053/j.ajkd.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Volmer M, de Vries JCM, Goldschmidt HMJ. Infrared analysis of urinary calculi by a single reflection accessory and a neural network interpretation algorithm. Clin Chem. 2001;47:1287–1296. [PubMed] [Google Scholar]
- Wandt MA, Rodgers AL. Quantitative X-ray diffraction analysis of urinary calculi by use of the internal-standard method and reference intensity ratios. Clin Chem. 1988;34:289–293. [PubMed] [Google Scholar]
- Wandt MAE, Underhill G. Covariance biplot analysis of trace element concentrations in urinary stones. Br J Urol. 1988;61:474–481. doi: 10.1111/j.1464-410x.1988.tb05083.x. [DOI] [PubMed] [Google Scholar]
- Welshman SG, McGeown MG. A quantitative investigation of the effects on the growth of calcium oxalate crystals on potential inhibitors. Br J Urol. 1972;44:677–680. doi: 10.1111/j.1464-410x.1972.tb10141.x. [DOI] [PubMed] [Google Scholar]
- Wollaston WH. On cystic oxide: a new species of urinary calculus. Philos Trans R Soc Lond. 1810;100:223–230. [Google Scholar]
- Wright LF, Saylor RP, Cecere FA. Occult lead intoxication in patients with gout and kidney disease. J Rheumatol. 1984;11:517–520. [PubMed] [Google Scholar]
- Yagisawa T, Hayashi T, Yoshida A, Kobayashi C, Okuda H, Ishikawa N, Toma H. Comparison of metabolic risk factors in patients with recurrent urolithiasis stratified according to age and gender. Eur Urol. 2000;38:297–301. doi: 10.1159/000020296. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Moridaira K, Wada O. Zinc deficiency further increases the enhanced expression of endothelin-1 in glomeruli of the obstructed kidney. Kidney Int. 2000;58:575–586. doi: 10.1046/j.1523-1755.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- Yano J, Yachandra VK. X-ray absorption spectroscopy. Photosynth Res. 2009;102:241–254. doi: 10.1007/s11120-009-9473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarasvandi A, Heidari M, Sadeghi M, Mousapoor E. Major and trace element composition of urinary stones, Khuzestan province, southwest, Iran. J Geochem Explor. 2013;131:52–58. [Google Scholar]