Abstract
The aim of this study was to shown that the photosensitizer in photodynamic therapy (PDT) can contribute to the dark toxicity and phototoxicity of the tumor by binding with copper. This binding process can remove the copper from the body, stopping angiogenesis as well as activating the mechanisms of cell death, such as apoptosis and necrosis. In PDT, this coupling may be considered a new route for fighting cancer in addition to those already known which involve reactive oxygen species.
Keywords: Cancer, Copper, Angiogenesis, Photosensitizer, Photodynamic therapy
Introduction
Cancer is one of the leading causes of death in worldwide (Kohler et al. 2011). Cancer treatments, such as surgery, radiation, cytostatic chemotherapy, and photodynamic therapy (PDT), are generally insufficient to achieve full recovery and are associated with serious side effects. Therefore, cancer treatments, including PDT, can be toxic.
PDT is a complementary non-invasive modality used to treat different types of cancers of the breast, skin, pancreas, lung, neck, brain, head, intraperitoneal cavity, and prostate (Al-Omari and Ali 2009). At the present time, PDT is not only approved for tumor therapy, but it is also used to treat other diseases, including scleroderma, psoriasis, and age-related macular degeneration (Dolmans et al. 2003). PDT involves the use of a photosensitizer (PS), molecular oxygen (O2), and light to selectively destruct unhealthy tissue (Al-Omari et al. 2004; Ermilov et al. 2004a). After PS molecule has been activated with the appropriate wavelength of light, it generates reactive oxygen species (ROS) consisting mainly of singlet oxygen (1O2) (Allison et al. 2004; Al-Omari et al. 2011). This cytotoxic agent damages the tissue only in the local region of the PS, resulting in well-defined damage of the area where light is applied (Röder et al. 2004). However, PDT is more than a localized therapy as it can cause the release of compliment and cytokines. During PDT, the tumor is often primarily destroyed by ROS (Allison et al. 2004; Al-Omari and Ali 2009). 1O2 is considered to be the most significant cytotoxic agent involved in PDT (Kochevar et al. 1996; Ermilov et al. 2004b), but other ROS, such as hydroxyl radicals (OH•) and the superoxide anion (O2 −•), may also contribute to the biological damage (Dolmans et al. 2003; Allison et al. 2004; Ermilov et al. 2004a; Al-Omari and Ali 2009, 2010; Al-Omari et al. 2011).
Two types of photodynamic (PD) mechanisms are possible in the presence of O2 (Dougherty et al. 1998). In this context, the excited PS can react with O2 (mechanism type II) to generate 1O2 or with solvent (mechanism type I). The type I reaction is due to either proton or electron transfer yielding radicals. However, in the absence of light, the cytotoxicity of PS towards cancerous cells can also occur during so-called dark cytotoxicity (Hornung et al. 1999; Coutier 2001; Paul et al. 2003; Ahmed et al. 2004; Wieder et al. 2006; Kuan et al. 2009), which means that neither 1O2 nor other ROS were involved in the tumor destruction mechanisms.
The aim of this study was to reveal and underline PD mechanisms other than those involving ROS species (neither type I nor type II mechanisms) responsible for destroying tumors by PS in the absence or presence of light.
Principles of PDT
In PDT, cancer tissue is selectively destroyed by a combination of PS (photosensitizing drug) and light in the presence of O2 (Dougherty et al. 1998; Dolmans et al. 2003; Allison et al. 2004). The PS is first allowed to accumulate in tissue for a period that typically ranges from 3 to 96 h (Bonnett 1995), and then the unhealthy tissue is illuminated by a suitable wavelength through a specialized optical system, such as an optical fiber. The PS absorbs the light, thereby generating the ROS 1O2 in the presence of O2. This kind of ROS generation results in damage to cellular functions and vital structures and, consequently, the direct destruction of the tumor tissue. Accompanying effects comprise the destruction of the cancer-associated vasculature, which prevents the supply of O2 to the cancer, and infiltration of macrophages, leukocytes, and lymphocytes into PDT-treated tissue due to activation of the immune response (Gollnick et al. 2004).
Most PSs used in PDT are cyclic tetrapyrroles (Berg et al. 2005). They have an extended conjugated p-electron system which is responsible for a number of relevant electronic properties in PDT. A PS has a high intersystem crossing quantum yield and a long triplet lifetime; this results in a high singlet oxygen quantum yield (Ermilov et al. 2005). Various types of tetrapyrrolic compounds, such as phthalocyanines, chlorins, porphyrins, and chlorins, are employed as PSs in PDT (Berg et al. 2005).
Photophysical processes of photosensitization
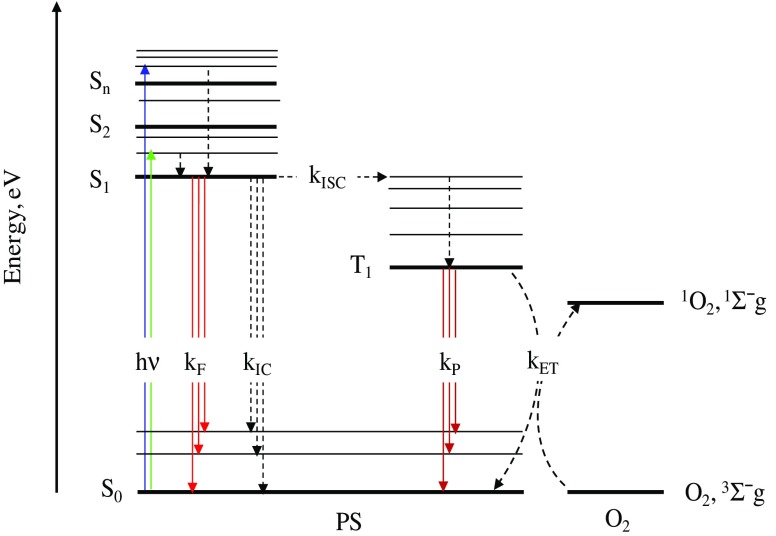
Figure 1 presents the Jablonski diagram of the primary photophysical processes involved in PDT. Upon absorption of a photon of the appropriate energy (hν), the PS molecule is excited from its ground state (S0) to the first excited singlet state (S1) or to higher excited singlet state (Sn) in which a fast relaxation to S1 often occurs (Al-Omari 2010a). The S1 state decays by a fluorescence emission pathway with rate constant of kF or by a radiationless transition (internal conversion) with rate constant of kIC or intersystem crossing (ISC) with a rate constant of kISC to the S0 or first excited triplet state (T1), respectively (Lakowicz 1999). The fluorescence quantum yield (ΦF), which is an important parameter of PS, is calculated by the S1→S0 transition. The ISC of the S1→T1 transition is spin-forbidden. This prohibition for tetrapyrrolic molecules is weak due to enhanced spin-orbit coupling resulting in a high intersystem crossing quantum yield (ΦISC) (Solovyov and Borisevich 2005). The lifetime of the triplet state (τT) is in micro- to millisecond range since the transition from T1 to S0 is spin-forbidden. The long τT is needed for the following photo-induced chemical reactions, which are in competition with phosphorescence emission occurring at rate constant of kp. There are two types of photodynamic reactions (Dougherty et al. 1998). An excited PS in S1 or T1 can react with a solvent or substrate (type I mechanism) or with O2 (type II mechanism) to form 1O2. In type I, hydrogen or electron transfer may occur in both directions yielding radicals or radical ions.
Fig. 1.
Scheme of the primary photophysical processes of the photosensitizer (PS) molecule and the photosensitized generation of singlet oxygen (1 O 2). The other abbreviations are defined in the text (Section Photophysical processes of photosensitization)
The reactive intermediates which are generated, such as hydrogen peroxide, hydroxyl radical (OH•), and hydroperoxyl, also oxidate the biomolecules. This oxidation potential may possibly result in cell death. However, these ROS simultaneously react with surrounding molecules, including the PS. Consequently, the PS cannot be repeatedly activated. The disadvantage of the type I mechanism is the low efficiency of intermolecular reactions, which is attributed to the short lifetime of S1 as well as the destruction of PS (Foote 1991). The type II mechanism for tetrapyrrolic PSs results in reactive 1O2 by energy transfer (kET) from the excited triplet PS to O2 (Fig. 1) (Al-Omari 2010b). An additional deactivation route of the triplet state, one with a lower probability, is electron transfer (kET) to O2 to produce O2 −• (Schweitzer and Schmidt 2003). It should be mentioned that type I and type II mechanisms are dependent on the concentration of O2 in the close vicinity of the PS.
Typical PSs used in PDT should have a high absorbance between 600 and 900 nm as it is in this range where biological tissue shows increased transmission and a high singlet oxygen quantum yield (Allison et al. 2004). Furthermore, it should show a low dark toxicity, which means that PS is nontoxic without illumination.
Dark toxicity
As already mentioned, after the PS is excited by light of the appropriate wavelength, 1O2 and/or other ROS species are formed; these are the direct toxic agents responsible for cell destruction in PDT. However, many researchers have reported on the dark toxicity of PSs in the absence of illumination. These results suggest that 1O2 and other ROS species should not be involved in the process of tumor destruction. In this framework, Coutier (2001) observed the dark toxicity of 5,10,15,20-tetrakis (m-hydroxyphenyl) chlorin (mTHPC) with 500-μm diameter Colo 26 spheroids. The sensitizations with 10 μg mL−1 of mTHPC in the absence of illumination led to about 50 % cell survival. The dark cytotoxicity of m-THPC has also been observed in carcinoma cells of the human breast and in Chinese hamster fibroblasts (Hornung et al. 1999). In another study Ahmed et al. (2004) reported that the insertion of glycosyl units in a quaternized mono-pyridyl-triphenyl-porphyrin derivative resulted in a decrease in dark cytotoxicity, compared with cationic porphyrins with no glycosyl moieties. These authors proposed an increase in targeting because of the inclusion of the sugar residues. Paul et al. (2003) found that pheophorbide a incubated with Jurkat cells was cytotoxic in the dark. Moreover, dark cytotoxicity was found for compounds of pheophorbide, human serum albumin, and pheophorbide (Kuan et al. 2009). Wieder et al. (2006) reported the dark toxicity of phthalocyanines covalently bound to biomolecules. In that study, a dark toxicity for PS compounds was observed when the PSs were present above certain concentrations.
These studies provide examples of the dark toxicity of many PSs in addition to their phototoxicity. The conclusion that can be drawn is that neither 1O2 nor ROS species (which are formed only in the presence of light) are involved in the dark toxicity activities against cancerous cells but that other mechanisms should exist. At the mechanistic level, damage to the enzymes, plasma membrane, and cytoplasmic organelles, as well as to nuclear structures and enzymes, has been observed following the exposure of cells to PDT (Kessel 1986; Moan 1986). The mechanistic action of PDT in experimental cancer therapies is thought to comprise direct damage to tumor vessels and direct killing of tumor cells (Selman et al. 1984; Henderson et al. 1985). A better understanding of the mechanism by which the destruction occurs is needed in order to design the best drugs.
The role of copper in stopping tumor growth
Copper is an essential trace metal for humans and animals. The amount of copper in an organism is strictly regulated (Labbe and Thiele 1999). Angiogenesis, which is the growth of new blood vessels from pre-existing ones, is essential for supplying blood to cancers and therefore for tumor growth, metastasis, and invasion (Eatock et al. 2000). It has been demonstrated that cancers lacking a blood supply do not grow larger than 1–2 mm3 (Ryan and Wilding 2000). The processes involved in angiogenesis that require copper as a necessary cofactor are the stimulation of endothelial growth by tumor cytokine generation, migration of endothelial cells mediated by integrins, and degradation of extracellular matrix proteins by metalloproteinases (Daniel et al. 2004). High levels of copper have been found in many kinds of human cancers of the brain, colon, lung, breast, and prostate (Kuo et al. 2002).
Some anti-copper drugs have been tested in vivo (Brem 1999), of which the copper chelator of tetrathiomolybdate (TM) is the most important. It has been found that TM is effective in deactivating the growth of mammary tumors in lung metastatic carcinoma in C557BL6/J mice and in HER2/neu transgenic mice (Khan et al. 2002; Pan et al. 2002). These findings enhance the notion that copper control can be applied as an anticancer strategy.
Pyrrolidine dithiocarbamate (PDTC) and clioquinol (CQ) have been found to be capable of binding nitrogen with copper, forming new complexes that have apoptosis-inducing activities in tumors and are proteasome inhibitors (Kenyon et al. 2005), but which do not affect normal/non-transformed breast cells. Further, tumor breast cells and premalignant cells which contain high concentrations of copper are sensitive to treatment with CQ or PDTC alone (Kenyon et al. 2005). In order to explain this behavior, these authors suggested that targeting elevated copper should be cancer specific and that the formation of a complex of an active antitumor proteasome inhibitor between PDTC or CQ and tumor cellular copper is a new strategy that has a great potential in breast cancer therapies.
Interaction of PSs with copper
Copper is an essential cofactor in the synthesis of a number of enzymes involved in physiological processes in the human body. In their study on light-emitting molecular devices, Amendola et al. 2006 used fluorescent sensors for monitoring copper concentration in vitro and/or in vivo. There authors observed that upon binding with copper, the several fluorescent sensors being tested were found to undergo fluorescence enhancement or quenching fluorescence.
Porphyrins (tetrapyrrolic PSs) have been intensively used as PSs due to their property of tunable fluorescence emission, high absorption coefficients in the visible region, and high stability against light and chemical reactions (Purrello and Gurrieri 1999). Deviprasad and D’Souza (2000) observed that at the moment of the coupling interaction between copper and a porphyrin compound, the fluorescent sensor 5-[p-N,NO-bis(2-pyridyl)amino]phenyl-10,15,20-tris(p-methoxyphenyl) porphyrin zinc exhibited great selectivity for ionic copper (Cu2+) and showed fluorescence quenching with an “on-off” type switching property. The fluorescence of that PS was retrieved by adding a EDTA disodium solution to the reaction. Fluorescence quenching was also observed when the acetone solution of pheophytin-a was mixed with an aqueous solution of Cu2+ (Hu et al. 2009). The fluorescence quenching in that study was used as a method to quantify the concentration of Cu2+ by measuring the fluorescence intensity of the pheophytin a solution. On the other hand, fluorescence enhancing was observed for the supramolecular self-assembly of the porphyrin–fluorescein hybrid with copper(II) compounds of the 5-(p-amino-phenyl)-10,15,20-triphenylporphyrin system (Lu et al. 2004). Furthermore, in another study, upon adding various metal ions (e.g., Na2+, Cr 3+, Mg2+, Fe2+, Mn2+, Ni2+, Ag+, Zn2+, Co2+, Cd2+, Hg2+, and Cu2+) to zinc porphyrin–dipyridylamino solutions, only copper showed a high selectivity to the PS, causing quenching of the fluorescence signal upon binding with the PS (Weng et al. 2007). Copper plays an essential role in tumor progression and development (Di Vaira et al. 2004). Pan et al. (2002) noticed elevated levels of copper in several types of human tumors and that copper, but not other metals, was the essential cofactor for cancer angiogenesis. Metal chelators have been successfully used to suppress metastasis, angiogenesis, and tumor growth (Ding et al. 2005).
Pyropheophorbide-a derivatives (tetrapyrrolic PSs) were developed as anticancer drugs for applications in PDT, and the role pyropheophorbide-a methyl ester (PPME) has been investigated (Al-Omari et al. 2008; Al-Omari and Al-Noaimi 2010). The tumor selectivity of anticancer drugs is an important therapeutic issue (Brewer 2001). Therefore, it is important to distinguish between the normal and cancerous cells by developing selective anticancer drugs.
Al-Omari and Al-Noaimi (2010) recently reported on the interaction of 19 different metal ions, among which only copper was capable to bind with PPME. This association, which was represented by the fluorescence quenching of PPME, occurred in the presence and absence of the light. One possible conclusion that can be drawn from these findings is that cancer tissue with an elevated concentration of copper can be targeted selectively by PPME following its injection into the body. This indicates that PPME has dark toxicity and phototoxicity due to the binding of copper—in addition to its phototoxicity that results from the formation of ROS species upon illumination (Al-Omari and Ali 2009, 2010).
A new route in PDT for tumor destruction based on PS–copper interaction
There are two types of fluorescence quenching of PSs, namely, static and dynamic quenching (Lakowicz 1999; Al-Omari and Al-Noaimi 2010). The static quenching of fluorescence occurs in the dark in the absence of light, while dynamic quenching requires the PS to be excited by light. In both of these types of fluorescence quenching, the fluorescence, physical, and/or chemical processes have to occur between the PS and other molecules or ions for the quenching to occur (Dolmans et al. 2003; Al-Omari et al. 2004; Allison et al. 2004). The interaction binding between Cu2+ and PPME is an example of such processes (Al-Omari and Al-Noaimi 2010). In this study the static and dynamic quenching of fluorescence were found for the association interaction between Cu2+ and PPME. Actually, since this coupling was assured (Al-Omari and Al-Noaimi 2010) and PPME causes a dark cytotoxicity against tumors (Almeida et al. 2004), the static quenching should take part under the condition of dark cytotoxicity. More general, the mechanism of static quenching between PS and copper probably contributes to the dark toxicity against the cancerous cells. This should be taken into consideration when looking for the explanations for the dark cytotoxicity of PS, especially as neither 1O2 nor ROS species contribute to the dark cytotoxicity. Consequently, in contrast to the usual interpretation of the dark toxicity that is based on guesswork in many cases, it can be suggested that in PDT, dark cytotoxicity is attributed to the binding of copper with PS. Moreover, even in the presence of light, when the phototoxicity of PS is known to result from the generation of 1O2 and/or other ROS species, the coupling of PS with copper should also be considered as contributing to the phototoxicity via the mechanism of dynamic quenching.
What are the implications of these findings?
The recent progress of clinically effective angiogenesis inhibitors has resulted in initiating research on the combination of PS-PDT with such (anti-)angiogenic targeted treatments (Ben-Hur et al. 1988). Clinical trial results have been promising, causing a revival in the field of PS-PDT (Fabbrini et al. 2006). Such combination therapies can help to inhibit cancer recurrence.
Tumor angiogenesis not only provides a target for combination therapy, but it is responsible for the altered tumor vasculature with endothelial cells which have adapted to the increased metabolic requirements of the cancer cells (Ben-Hur et al. 1988; Dellian et al. 1995). The molecular make-up of tumor cells is altered by this activated tumor endothelium (van Beijnum et al. 2006), providing targets for selective delivery of such drugs as PSs (Folli et al. 1994). The selective targeting of the tumor vasculature by the binding of PSs to molecules or antibodies which conjugate to markers of this angiogenic endothelium is a challenge (Folli et al. 1994). The aim of such methods is to increase PDT efficacy while reducing healthy tissue toxicity (Yarmush et al. 1993). The processes of angiogenesis needs copper as an necessary cofactor (Daniel et al. 2004).
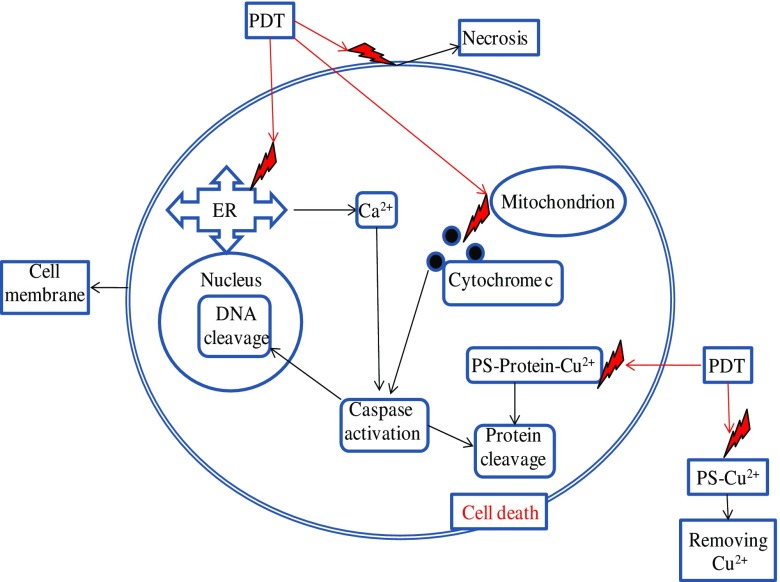
These findings improve our understanding of how PS, through its binding with copper in PDT, contributes to the dark cytotoxicity and phototoxicity of the tumor. The binding of copper with PS can result in the removal of copper from the body (Fig. 2), thereby contributing to the cessation of angiogenesis (Al-Omari and Al-Noaimi 2010). This new concept is important in PDT because PSs can be used to trap vital copper being moved outside of the cell during angiogenesis. Therefore, the PDT process stops the growth of the tumor. Furthermore, the apoptotic mechanism of cell death can start with the coupling with copper in copper-binding proteins.
Fig. 2.
Generalization of the mechanisms of cell death in photodynamic therapy to account for copper–PS binding. ER Endoplasmic reticulum, PDT photodynamic therapy
Based on these implications, we suggest that researchers and clinicians should alter their thinking that PDT 1O2 and ROS species are the only factors directly responsible for tumor destruction, and consider that the binding of PS with copper can also contribute to this destruction.
Where are the binding sites of the copper–PS association?
It has been reported that there is a dark cytotoxicity when the amino group of some PSs binds on the periphery of the PS molecule and that this dark toxicity is absent when the amino group is removed (Poland and Knutson 1982; Rancan et al. 2005). In another study (Weng et al. 2007), the binding site was also defined at the peripheral ring where the nitrogen atom is located. This behavior can be explained by defining the reaction of coordinate bond as follows:
| 1 |
Here, the nitrogen atom is shared covalently by one of its two nonbonding electrons with an empty orbital of Cu2+. As a result, copper is bound to the PS molecule on the periphery where the nitrogen atom is positioned; therefore, PS becomes cationic with charge of 2e (e is the electron charge). Since these PSs were reported to have dark cytotoxicity (Rancan et al. 2005; Weng et al. 2007), it can be concluded that static quenching, which is a physical process, can, at least partially, underlie this cytotoxicity. For many cases of coupling when static quenching occurs in the absence of light, the dynamic quenching of coupling also occurs in the presence of light (Lakowicz 1999; Al-Omari and Al-Noaimi 2010). Hence, the phototoxicity of copper coupling with PS should also be present in addition to that results from the traditional routes of phototoxic agents in PDT.
Alternatively, some PSs do not have nitrogen atoms on their periphery, rather they have oxygen atoms (Al-Omari 2010a; Al-Omari and Ali 2009, 2010; Al-Omari et al. 2011). The oxygen atom can contribute by one of its two pairs of nonbonding electrons with an empty orbital of Cu2+ to form the dative covalent bond as follows:
| 2 |
Thus, copper is coupled to PS by an oxygen atom on its periphery; consequently, the PS becomes cationic with a charge of 2e. The static and dynamic coupling of copper with the PS of peripheral oxygen has been reported (Lakowicz 1999; Al-Omari and Al-Noaimi 2010). The PS molecule of that study was PPME and it was incubated with different types of cancer cells; both dark cytotoxicity and phototoxicity were observed (Almeida et al. 2004). If we correlate the results of the reference study (Al-Omari and Al-Noaimi 2010) with those from another study (Almeida et al. 2004), it might be deduced that in addition to ROS causing the death of cancer cells, the binding of copper to PPME can also cause, at least partially, the death of cancer cells. Generally, whenever the binding between PS and copper is achieved, it should be taken into consideration that this coupling can be responsible, at least partially, for the dark toxicity and phototoxicity.
Generalization of the mechanism of the cell death in PDT to account for copper-PS binding
The well-documented mechanisms of cell death in PDT (Poland and Knutson 1982; Almeida et al. 2004) can be summarized as follows (Fig. 2). Generated free radical attacks on the cellular membrane cause a loss of membrane integrity followed by necrotic death. A similar attack on the endoplasmic reticulum leads to an increase in intracellular Ca2+, which can by the activation of calcineurin induce apoptosis, resulting in the dephosphorylation of the pro-apoptotic bcl-2-associated death promoter (BAD) protein. BAD then migrates to the mitochondria and induces the release of cytochrome c, following which high intracellular Ca2+ and cytochrome c activate the caspase cascade, resulting in DNA cleavage and damage to intracellular proteins.
Based on the above discussion and findings of Al-Omari and Al-Noaimi (2010), the aforementioned mechanisms of cell death can be generalized by adding a new mechanism that includes (Fig. 2) the binding of copper with PS outside of the cell during angiogenesis. This binding of copper stops the process and removes copper from the body. Moreover, the apoptotic mechanism of cell death could start in the cell through the coupling with copper in copper-binding proteins (Fig. 2).
Conclusions
We have shown that in PDT, binding of the PS with copper may contribute to the dark cytotoxicity and phototoxicity of the tumor. Copper may be part of the dark toxicity, but it may also be specific to a certain PS. As the same receptors are on normal tissues/vasculature, dark toxicity may also easily occur in normal tissues. Therefore, PS in PDT is designed to selectively target the diseased tissue. The coupling of copper by PS means that this process could remove the copper from the body thereby stopping tumor angiogenesis. Furthermore, the mechanism of cell death, such as apoptosis and necrosis, can start by the coupling of copper with PS in copper-binding proteins. It is also possible that dark toxicity can also be due to more factors than just copper.
We should change our thinking about 1O2 and ROS species being the only responsible factors in PDT for tumor destruction, but rather that the binding of PS with copper can also contribute this destruction.
Acknowledgments
Conflicts of interest
None.
References
- Ahmed A, Davoust E, Savoie H, Boa AN, Boyle RW. Tetrahedron Lett. 2004;45:6045–6047. doi: 10.1016/j.tetlet.2004.06.021. [DOI] [Google Scholar]
- Allison RR, Downie GH, Cuenca R, Hu XH, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiag Photodyn. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim Biophys Acta. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Al-Omari S. Theoretical and experimental analysis of the molecular system of pyropheophorbide a Me ester in polar solvent. Int Rev Phys. 2010;4:261–269. [Google Scholar]
- Al-Omari S. Energy transfer of pyropheophorbide a methyl ester in dimethylformamide solutions. Rom J Biophys. 2010;20:295–314. [Google Scholar]
- Al-Omari S, Ali A. Photodynamic activity of pyropheophorbide methyl ester and pyropheophorbide a in dime thylformamide solution. Gen Physiol Biophys. 2009;28:70–77. doi: 10.4149/gpb_2009_01_70. [DOI] [PubMed] [Google Scholar]
- Al-Omari S, Ali A. Dose estimation of pyropheophorbide methyl ester and pyropheophorbide a in vitro under oxygenated condition. Int J Biotech Biochem. 2010;6:1093–1100. [Google Scholar]
- Al-Omari S, Al-Noaimi M. Spectroscopic analysis of the binding interaction of cationic Cu2+ with pyropheophorbide-a Me ester: targeting tumor in a copper-dependent manner. Int Rev Phys. 2010;4:152–160. [Google Scholar]
- Al-Omari S, Ermilov E, Helmreich M, Jux N, Hirsch A. Transient absorption spectroscopy of a monofullerene C60-bis-(pyropheophorbide a) molecular system in polar and nonpolar environments. Appl Phys B. 2004;79:617–622. doi: 10.1007/s00340-004-1570-y. [DOI] [Google Scholar]
- Al-Omari S, Ali A, Alsmadi AM, Al-Sugheir M. Photobleaching-electron density for the molecular structures of pyropheophorbide a and pyropheophorbide methyl ester in dimethylformamide solution. Int Rev Phys. 2008;2:419–425. [Google Scholar]
- Al-Omari S, Al-Noaimi M, Raba'eh K, Alna’washi G, Bawa'aneh M, Al-Dweri F, Aqili A. Photophysical properties of sodium zinc(II)-2,9,16,23-phthalocyanine tetracarboxylate in aqueous solution. Int J Pure Appl Phys. 2011;7:57–72. [Google Scholar]
- Amendola V, Fabbrizzi L, Foti F, Licchelli M, Mangano C, Pallavicini P, Poggi A, Sacchi D, Taglietti A. Light-emitting molecular devices based on transition metals. Coord Chem Rev. 2006;250:273–299. doi: 10.1016/j.ccr.2005.04.022. [DOI] [Google Scholar]
- Ben-Hur E, Heldman E, Crane SW, Rosenthal I. Release of clotting factors from photosensitized endothelial cells: a possible trigger for blood vessel occlusion by photodynamic therapy. FEBS Lett. 1988;236:105–108. doi: 10.1016/0014-5793(88)80294-1. [DOI] [PubMed] [Google Scholar]
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter B, Angell-Petersen E, Warloe T, Frandsen N, HØgset A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218:133–147. doi: 10.1111/j.1365-2818.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev. 1995;24:19–33. doi: 10.1039/cs9952400019. [DOI] [Google Scholar]
- Brem S. Angiogenesis and cancer control: from concept to therapeutic trial. Cancer Control. 1999;6:436–458. [PubMed] [Google Scholar]
- Brewer GJ. Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson’s Disease. Exp Biol Med. 2001;226:665–673. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- Coutier St (2001) Unité de Rechereche en Thérapie Photodynamique. PhD thesis. Centre Alexis Vautrin, Vandoeuvre-les-Nancy
- Daniel KG, Harbach RH, Guida WC, Dou QP. Copper storage diseases: Menkes, Wilsons, and cancer. Front Biosci. 2004;9:2652–2662. doi: 10.2741/1424. [DOI] [PubMed] [Google Scholar]
- Dellian M, Abels C, Kuhnle GE, Goetz AE. Effects of photodynamic therapy on leucocyte–endothelium interaction: differences between normal and tumour tissue. Br J Cancer. 1995;72:1125–1130. doi: 10.1038/bjc.1995.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviprasad GR, D’Souza F. Fluorescent chemosensor for selective detection of nicotine and cotinine. Chem Commun. 2000;19:1915–1916. doi: 10.1039/b006055k. [DOI] [Google Scholar]
- Di Vaira M, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P. Clioquinol a drug for Alzheimer’s disease specifically interfering with brain metal metabolism: structural characterization of its zinc(II) and copper(II) complexes. Inorg Chem. 2004;43:3795–3797. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- Ding WQ, Liu B, Vaught J, Yamauchi H, Lind S. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-4385. [DOI] [PubMed] [Google Scholar]
- Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock MM, Schatzlein A, Kaye SB. Tumour vasculature as a target for anticancer therapy. Cancer Treat Rev. 2000;26:191–204. doi: 10.1053/ctrv.1999.0158. [DOI] [PubMed] [Google Scholar]
- Ermilov E, Al-Omari S, Helmreich M, Jux N, Hirsch A. Steady-state and time-resolved studies on the photophysical properties of fullerene-pyropheophorbide a complexes in polar and nonpolar solvents. Opt Commun. 2004;234:245–252. doi: 10.1016/j.optcom.2004.02.024. [DOI] [Google Scholar]
- Ermilov E, Al-Omari S, Helmreich M, Jux N, Hirsch A. Photophysical properties of fullerene-dendron-pyropheophorbide a supramolecules. Chem Phys. 2004;301:27–31. doi: 10.1016/j.chemphys.2004.02.015. [DOI] [Google Scholar]
- Ermilov E, Hackbarth S, Al-Omari S, Helmreich M, Jux N. Trap formation and energy transfer in the hexapyropheophorbide a-fullerene [C60] molecular system. Photochem Photobiol. 2005;250:95–104. [Google Scholar]
- Fabbrini M, Trachsel E, Soldani P, Bindi S, Alessi P, Bracci L, Kosmehl H, Zardi L, Neri D, Neri P. Selective occlusion of tumor blood vessels by targeted delivery of an antibody-photosensitizer conjugate. Int J Cancer. 2006;118:1805–1813. doi: 10.1002/ijc.21412. [DOI] [PubMed] [Google Scholar]
- Folli S, Westermann P, Braichotte D, Pelegrin A, Wagnieres G, van den Bergh H, Mach JP. Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res. 1994;54:2643–2649. [PubMed] [Google Scholar]
- Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659–872. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Gollnick SO, Kabingu E, Kousis PC, Henderson BW. Stimulation of the host immune response by photodynamic therapy (PDT) Proc SPIE. 2004;5319:60–70. doi: 10.1117/12.530437. [DOI] [Google Scholar]
- Henderson BW, Waldow SM, Mang TS, Potter WR, Malone PB, Dougherty TJ. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985;45:572–576. [PubMed] [Google Scholar]
- Hornung R, Jentsch B, Crompton NEA, Haller U, Walt H. In vitro effects and localization of the photosensitizers m-THPC and m-THPC MD on carcinoma cells of the human breast (MCF-7) and Chinese hamster fibroblasts (V-79) Lasers Surg Med. 1999;20:443–450. doi: 10.1002/(SICI)1096-9101(1997)20:4<443::AID-LSM11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hu M, Li H, Chen L, Zhang H, Dong C. Fluorescence quenching of pheophytin-a by copper(II) ions. Chin J Chem. 2009;27:513–517. doi: 10.1002/cjoc.200990084. [DOI] [Google Scholar]
- Kenyon G, Di C, Shirley O, Qiuzhi C, Fred R, Miller Q, Ping D. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Sites of photosensitization by derivatives of hematoporphyrin. Photochem Photobiol. 1986;44:489–494. doi: 10.1111/j.1751-1097.1986.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Khan MK, Miller MW, Taylor J, Gill NK, Dick RD, Van Golen K, Brewer GJ, Merajver SD. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia. 2002;4:164–170. doi: 10.1038/sj.neo.7900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochevar IE, Lambert CR, Lynch MC, Tedesco AC. Comparison of photosensitized plasma membrane damage caused by singlet oxygen and free radicals. Biochim Biophys Acta. 1996;1280:223–230. doi: 10.1016/0005-2736(95)00297-9. [DOI] [PubMed] [Google Scholar]
- Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LAG, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C, Annegret P, Hackbarth S, Wacker M, Langer K. Novel photosensitizer-protein nanoparticles for photodynamic therapy: photophysical characterization and in vitro investigations. J Photochem Photobiol B Biol. 2009;96:66–74. doi: 10.1016/j.jphotobiol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res. 2002;89:1–11. doi: 10.1385/BTER:89:1:1. [DOI] [PubMed] [Google Scholar]
- Labbe S, Thiele DJ. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends Microbiol. 1999;7:500–505. doi: 10.1016/S0966-842X(99)01638-8. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer Academic/Plenum, Dordrecht/New York
- Lu J, Du Y, Wu B, Huang J, Jiang J. Supramolecular self-assembly of porphyrin-fluorescein hybrid with amino-porphyrinatocopper(II) and its fluorescence strengthening character. Inorg Chem Commun. 2004;7:1030–1033. doi: 10.1016/j.inoche.2004.07.008. [DOI] [Google Scholar]
- Moan J. Porphyrin photosensitization and phototherapy. Photochem Photobiol. 1986;43:681–690. doi: 10.1111/j.1751-1097.1986.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- Paul A, Hackbarth S, Mölich A, Luban C, Oelckers S. Comparative study of the photosensitization of Jurkat cells in vitro by pheophorbide-a and a pheophorbide-a diaminobutane poly-propylene-imine dendrimer complex. Laser Phys. 2003;13:22–29. [Google Scholar]
- Poland A, Knutson J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Purrello R, Gurrieri S. Porphyrin assemblies as chemical sensors. Coord Chem Rev. 1999;683:190–192. [Google Scholar]
- Rancan F, Helmreich M, Mölich A, Jux N, Roeder B. Fullerene-pyropheophorbide a complexes as sensitizer for photodynamic therapy: uptake and photo-induced cytotoxicity on Jurkat cells. J Photochem Photobiol B Biol. 2005;80:1–8. doi: 10.1016/j.jphotobiol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Röder B, Hackbarth S, Wiehe A, Rancan F, Jux N, Helmreich M, Hirsch A, Ermilov E, Senge MO, Nöbel M, Al-Omari S, Simonenko K. Influence of tetrapyrroles amphiphilicity on membrane localization and the mechanism of photosensitized cell death. J Porphyrins Phthalocyanines. 2004;8:472–478. [Google Scholar]
- Ryan CJ, Wilding G. Angiogenesis inhibitors. New agents in cancer therapy. Drugs Aging. 2000;17:249–255. doi: 10.2165/00002512-200017040-00001. [DOI] [PubMed] [Google Scholar]
- Schweitzer C, Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- Selman SH, Kriemer-Birnbaum M, Klaunig JE, Goldblatt PJ, Keck RW, Britton SL. Blood flow in transplantable bladder tumors treated with hematoporphyrin derivative and light. Cancer Res. 1984;44:1924–1927. [PubMed] [Google Scholar]
- Solovyov KN, Borisevich EA. Intramolecular heavy-atom effect in the photophysics of organic molecules. Physics-Uspekhi. 2005;48:231–253. doi: 10.1070/PU2005v048n03ABEH001761. [DOI] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- Weng YQ, Teng YL, Yue F, Zhong YR, Ye BH. A new selective fluorescent chemosensor for Cu(II) ion based on zinc porphyrin-dipyridylamino. Inorg Chem Commun. 2007;10:443–444. doi: 10.1016/j.inoche.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a ‘Trojan horse’. Photochem Photobiol Sci. 2006;5:727–734. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- Yarmush ML, Thorpe WP, Strong L, Rakestraw SL, Toner M, Tompkins RG. Antibody targeted photolysis. Crit Rev Ther Drug Carrier Syst. 1993;10:197–252. [PubMed] [Google Scholar]