Abstract
Proteins fold and function inside cells which are environments very different from that of dilute buffer solutions most often used in traditional experiments. The crowded milieu results in excluded-volume effects, increased bulk viscosity and amplified chances for inter-molecular interactions. These environmental factors have not been accounted for in most mechanistic studies of protein folding executed during the last decades. The question thus arises as to how these effects—present when polypeptides normally fold in vivo—modulate protein biophysics. To address excluded volume effects, we use synthetic macromolecular crowding agents, which take up significant volume but do not interact with proteins, in combination with strategically selected proteins and a range of equilibrium and time-resolved biophysical (spectroscopic and computational) methods. In this review, we describe key observations on macromolecular crowding effects on protein stability, folding and structure drawn from combined in vitro and in silico studies. As expected based on Minton’s early predictions, many proteins (apoflavodoxin, VlsE, cytochrome c, and S16) became more thermodynamically stable (magnitude depends inversely on protein stability in buffer) and, unexpectedly, for apoflavodoxin and VlsE, the folded states changed both secondary structure content and, for VlsE, overall shape in the presence of macromolecular crowding. For apoflavodoxin and cytochrome c, which have complex kinetic folding mechanisms, excluded volume effects made the folding energy landscapes smoother (i.e., less misfolding and/or kinetic heterogeneity) than in buffer.
Keywords: Protein folding, Macromolecular crowding, Spectroscopy, Protein stability, Excluded volume, Coarse-grained simulation
Introduction
To function, proteins must fold from extended unfolded states to unique compact structures that are biologically active. Through pioneering in vitro and in silico work during the last three decades, using, for example, protein engineering methods (Matouschek et al. 1989) and the energy landscape framework (Bryngelson et al. 1995), significant progress has been made to pinpoint mechanisms and driving forces important for protein folding. However, in reality, proteins fold inside cells where the environment is different from the dilute buffer solutions mostly used in in vitro experiments. The intracellular environment is highly crowded due to the presence of large amounts of macromolecules, including proteins, nucleic acids, ribosomes, and carbohydrates. This means that a significant fraction of the intracellular space is not available to other macromolecular species. It has been estimated that the concentration of macromolecules in the cytoplasm ranges from 80 to 400 mg/ml (Rivas et al. 2004). All macromolecules in physiological fluids collectively occupy between 10 and 40 % of the total volume (Zimmerman and Trach 1991). The crowded environment results in excluded volume effects, increased bulk viscosity and the opportunity for specific, as well as non-specific, inter-molecular interactions. Minton coined the word ‘macromolecular crowding’ in 1981 (Minton 1981) to address the impact of volume exclusion from macromolecules and has provided several key basic studies during the last decades (Sasahara et al. 2003; Minton 2005; Zhou et al. 2008). However, the importance of volume exclusion for biomolecules was already recognized by Ogsteen and Laurent in the 1960s from studies of the connective tissue polysaccharide hyaluronan (Laurent and Ogston 1963).
Due to excluded volume effects, any reaction resulting in a volume change will be affected by macromolecular crowding (Laurent and Ogston 1963; Minton 2005). It has been suggested that macromolecular crowding provides a stabilizing effect to the folded states of proteins indirectly due to destabilization of the more extended and malleable denatured states (Zhou 2004; Cheung et al. 2005). That fluctuations of crowding particles statistically favor compact forms of polymers over extended conformations has been explained by ‘depletion-induced attraction’ (Shaw and Thirumalai 1991). Conceptually, excluded volume effects can simply shift the equilibrium towards the folded state of a protein (F) since the free energy will be raised more for the extended unfolded state than for the folded state in a crowded environment. Alternatively, there may be a direct effect of crowding on the unfolded state (U), forcing it to become more compact at crowded conditions. This will increase the free energy of the unfolded state (since compaction is unfavorable for the unfolded ensemble) and the relative stability of the folded state increases. Such effects on the unfolded ensemble (i.e., conformation and/or energy) may modulate the subsequent folding kinetics; in addition, one may envision that crowded environments can have direct effects on folding mechanisms (Wittung-Stafshede 2011).
Our approach to mimic excluded volume effects in in vitro experiments is to use so-called macromolecular crowding agents. These chemicals are inert, non-charged polymers of certain sizes (i.e., dextrans, Ficoll) that occupy space but do not interact with target proteins (Bohrer et al. 1984; Venturoli and Rippe 2005; Zhou et al. 2008). In addition, for spectroscopic detection of changes, these agents are attractive as they absorb little light in the protein spectral regions. Moreover, they can be dissolved in high concentrations (i.e., up to 400 mg/ml). Ficoll 70 is a 70-kDa sucrose-based polymer that adopts a spherical shape, whereas dextrans are available in various sizes (i.e., 10–70 kDa) and adopt more elongated, rod-like shapes as these glucose-based polymers are less branched than those of Ficoll 70. Our strategy has often been to combine in vitro experiments with in silico simulations on the very same systems (Homouz et al. 2008; Homouz et al. 2009a; Samiotakis et al. 2009; Christiansen et al. 2010). In the computations, we use coarse-grained models of the proteins and impenetrable particles representing the crowding agents. These particles are designed to correspond in size and shape to the agents (dextrans or Ficoll 70) we use in vitro and to exhibit steric repulsion forces. The focus of this review is to report key findings from our unique in vitro/in silico approach on how excluded-volume effects modulate protein stability, unfolded and folded-state conformations and, finally, folding kinetics and mechanisms. We also describe relevant findings by others as appropriate.
Macromolecular crowding effects on protein stability
Many in vitro experiments by us and others have shown that protein stability is increased in the presence of macromolecular crowding agents (Sasahara et al. 2003; Cheung et al. 2005; Stagg et al. 2007; Homouz et al. 2008, 2009b; Pozdnyakova and Wittung-Stafshede 2010). We have probed thermal and chemical stability changes due to the presence of synthetic macromolecular crowding agents using spectroscopic detection methods for several strategic proteins: i.e., α/β-structured Desulfovibrio desulfuricans apoflavodoxin (148 residues), α-helical single-domain Borrelia burgdorferi VlsE (341 residues), α-helical horse-heart cytochrome c (104 residues), the ring-shaped heptamer human mitochondrial co-chaperonin protein 10 (cpn10, 101 residues per monomer), and an α/β thermophilic ribosomal protein S16 (116 residues). In agreement with expectations based on excluded-volume effects (Minton 2000), all proteins are chemically and thermally stabilized by the presence of crowding agents, although to different degrees (Perham et al. 2007; Homouz et al. 2008, 2009b; Samiotakis et al. 2009; Christiansen et al. 2010). In Table 1, we report measured thermal and chemical effects on our studied proteins (Perham et al. 2007; Stagg et al. 2007; Christiansen et al. 2010), along with other such reports taken from the literature. Upon analyzing the available data, no clear trends emerge. The average ∆∆GU with crowding is +3 kJ/mol and the average ∆Tm is +4 °C. There are no reliable trends in ∆∆GU and ∆Tm with respect to protein length for this limited dataset (not shown).
Table 1.
Reported ∆GU and Tm values for protein stability in the presence and absence of crowding agents are listed together with key properties (polypeptide length, fold, folded and unfolded state radius of gyration) of the proteins
| Name of the protein | Protein length (# of residues) | Protein fold (α, β, α/β) | Rg (nm)a | Thermal stability Tm (°C) | Chemical stability ∆GU (kJ/mol) | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Unfolded | Folded | Buffer | Crowdingb | Buffer | Crowdingb | ||||
| D. desulfuricans apoflavodoxin | 148 | α/β | 4.0 | 1.7 | 48 (Hepes) | 64 (Hepes) 400 mg/ml Ficoll70 | 26.3 (phosphate) | 26.5 100 mg/ml Ficoll70 | (Stagg et al. 2007) |
| B. burgdorferi VlsE | 341 | α | 6.6 | 2.3 | 49 | 55 400 mg/ml Ficoll70 | 24 | 29 100 mg/ml Ficoll70 | (Perham et al. 2007) |
| Horse heart cytochrome c | 104 | α | 3.2 | 1.5 | 63 (1 M GuHCl) | 70 (1 M GuHCl) 400 mg/ml Ficoll70 | 45 | 45 200 mg/ml Ficoll70 | (Christiansen et al. 2010) |
| Human co-chaperonin 10 | 102 (heptamer) | β | 3.2c | 1.5c | 67 | 71 300 mg/ml Ficoll70 | 9 c | 12 c 300 mg/ml Ficoll 70 | (Aguilar et al. 2011) |
| phosphoglycerate kinase (PGK) | 415 | α | 7.4 | 2.5 | 39 | 40.5 200 mg/ml Ficoll70 | – | – | (Dhar et al. 2010) |
| A. aeolicus ribosomal protein S16 | 116 | α/β | 3.4 | 1.5 | – | – | 18 | 31 200 mg/ml dextran20 | (Mikaelsson et al. 2012) |
| Hen egg-white lysozyme | 129 | α/β | 3.6 | 1.6 | 56 (pH 2) | 59 (pH 2) 300 mg/ml dextran70 | – | – | (Sasahara et al. 2003) |
| Glutaredoxin 2 | 215 | α/β | 5.0 | 2.0 | – | – | 14.0 | 15.1 100 mg/ml dextran70 | (Kuhnert et al. 2008) |
| Human glutathione transferase A1-1 | 222 (dimer) | α | 5.1c | 2.0c | – | – | 24.0 | 26.2 100 mg/ml dextran70 | (Kuhnert et al. 2008) |
| A. vinelandii apoflavodoxin | 179 | α/β | 4.5 | 1.8 | 48.1 | 52.3 400 mg/ml dextran70 | – | – | (Engel et al. 2008) |
| Cellular retinoic acid-binding protein 1 | 137 | Mostly α | 3.8 | 1.6 | – | – | 35 | 33 150 mg/ml Ficoll70 | (Hong and Gierasch 2010) |
| E coli maltose binding protein | 370 | Mostly α | 6.9 | 2.4 | 62 | 63 300 mg/ml Ficoll70 | – | – | (Kulothungan et al. 2009) |
| Apocytochrome b562 | 106 | α | 3.2 | 1.5 | – | – | 12 | 13 85 mg/ml PEG20 | (Ai et al. 2006) |
| Human FK506-binding protein | 107 | α/β | 3.3 | 1.5 | – | – | 21.0 | 23.1 180 mg/ml Ficoll70 | (Spencer et al. 2005) |
| Deoxyribonuclease I (DNase I) | 282 | α/β | 5.9 | 2.2 | 60 | >80 200 mg/ml PEG4 | – | – | (Sasaki et al. 2007) |
| Skeletal muscle G-actin | 374 | α/β | 7.0 | 2.4 | 45 | 49 100 mg/ml PEG6 | – | – | (Tellam et al. 1983) |
In each case, the amount and kind of crowding agent used in the original study is given. If important, the solution conditions are also given. We note that data for only one crowded condition is listed for each protein; in most cases, several concentrations and types of crowding agents were tested. Rg values were estimated using chain-length correlations in (Millett et al. 2002)
aRadius of gyration (Rg) for unfolded and folded forms of the proteins were estimated using empirical chain length correlations reported in (Millett et al. 2002)
bFicoll70, 70 kDa; dextran70, 70 kDa; dextran20, 20 kDa; PEG4, 4 kDa; PEG6, 6 kDa; PEG20, 20 kDa
cValues for individual monomers
For the cpn10 heptamer, we discovered that the stabilizing effect of crowding arises mostly due to increased stability of each monomer; the energetic effect by crowding on inter-protein interactions is small (Aguilar et al. 2011). For both apoflavodoxin and cytochrome c, we found an inverse correlation between stabilizing effect of added crowder versus protein stability in the buffer: if the stability of apoflavodoxin was decreased by changes of buffer or the cytochrome c stability was decreased by the presence of low amounts of GuHCl, the resulting effect of crowders on thermal stability was larger.
Macromolecular crowding effects on unfolded ensemble
Conformational compaction of the unfolded ensemble as a result of macromolecular crowding has been suggested by SANS experiments on random polymers (Le Coeur et al. 2010) as well as on intrinsically disordered proteins (Johansen et al. 2011). In a study performed in 2008 (Engel et al. 2008), fluorescence experiments indicated that unfolded Azotobacter vinelandii apoflavodoxin was compacted in the presence of crowders, albeit it was noted that this may be due to the coexistence of intermediate states. In 2010, Hong and Gierasch (2010) provided indirect evidence of protein unfolded state compaction in a crowded environment in vitro. For this, they probed changes in Cys and Trp residue accessibility in the cellular retinoic acid-binding protein (CRABP) 1 protein as the means to demonstrate compaction.
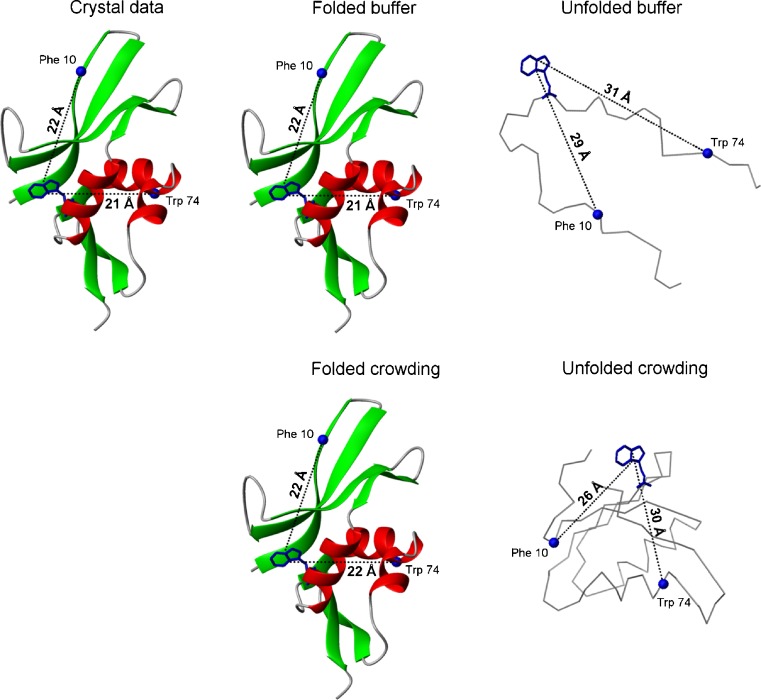
In our work, we used coarse-grained molecular simulations to demonstrate that the radius of gyration values decreased in silico for the unfolded forms of apoflavodoxin, VlsE and cytochrome c in presence of hard sphere crowders (Stagg et al. 2007; Homouz et al. 2008, 2009b). To also study unfolded-state compaction in vitro, we applied intra-molecular pairwise FRET between residues (Trp, donor; BODIPY attached to engineered Cys, acceptor) in the unfolded ensemble of the structural ribosomal protein S16 from Aquifex aeolicus, with and without macromolecular crowding agents. We found that the distances between the pairwise probes in the unfolded ensemble were shorter in the presence of 200 mg/ml dextran 20 as compared to in buffer (Mikaelsson et al. 2012) (Fig. 1). Taken together, it appears that the excluded volume effect alters unfolded-state conformational ensembles (thus, it is not only an energetic effect) such that they are on average more compact.
Fig. 1.
Illustration of how the two distances (between residue-pairs 10–58 and 58–74) probed by FRET in the protein S16 are affected by the presence of crowding agent, in the folded (no urea) and unfolded (at high urea concentrations, i.e., 10 m for 58–74 distances and 12 m for 10–58 distances) states. As discussed in the text, the unfolded polypeptide appears more compact in the presence of crowding agent (Mikaelsson et al. 2012). For comparison, the distances between these residue pairs in the crystal structure is also shown
Macromolecular crowding effects on folded structure
There have been studies indicating that proteins can convert from unfolded to more compact states upon addition of large amounts of crowding agents. For instance, unfolded cytochrome c at pH 2 can adopt a molten globule-like structure in the presence of crowding agents (Sasahara et al. 2003), unfolded RNase A at pH 3 adopts a folded-like structure upon addition of 350 mg/ml PEG 20000 or Ficoll 70 (Tokuriki et al. 2004), and the reduced and carboxyamidated form of RNase T1, that is intrinsically unstructured at pH 7, was found to exhibit some catalytic activity upon the addition of 400 mg/ml dextran 70 (Qu and Bolen 2002). In contrast, for two natively disordered proteins, no induced structuring was observed in crowded conditions (Flaugh and Lumb 2001).
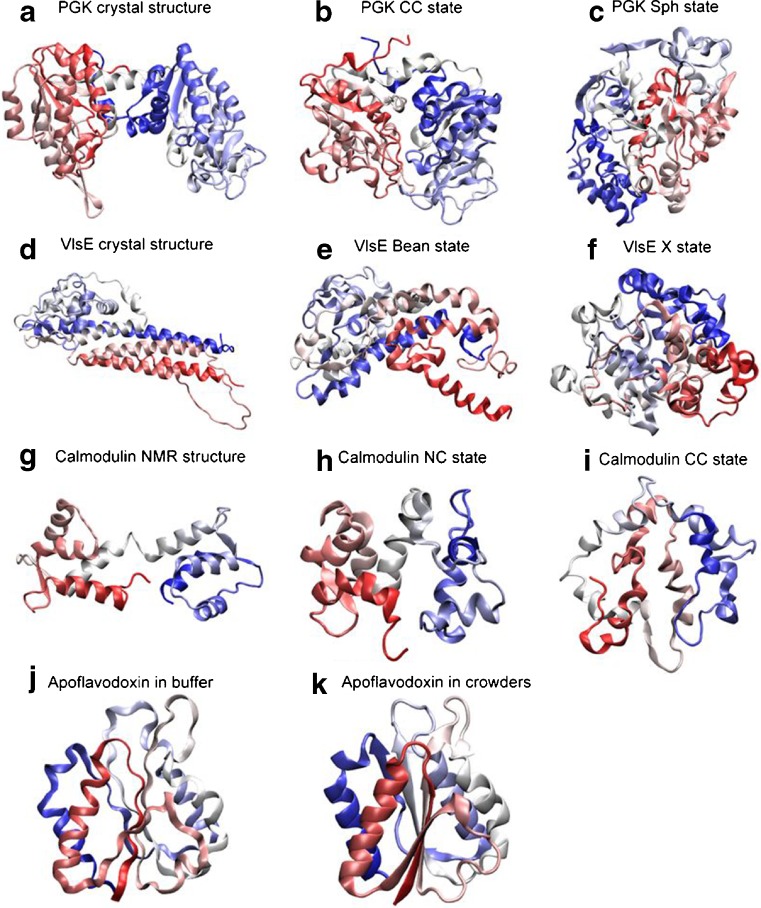
Our contribution to this topic is the discovery that macromolecular crowding affects the folded states of some model proteins at physiological conditions (pH 7). Based on far-UV circular dichroism (CD) data, we concluded that there was increased structural content and, from computations, the overall size and shape of the folded states changed in crowding. For apoflavodoxin, the secondary structure content when in solution in the presence of crowding agents approached that found in the crystal structure. VlsE is aspherical, and for this protein we found two alternative folded-state shapes in crowded conditions (Perham et al. 2007; Stagg et al. 2007; Homouz et al. 2008). With small additions of crowding, the helical content of folded VlsE increased and the elongated shape became bent; addition of more crowders and some perturbation (heat or denaturant), the folded molecules collapsed to more spherical structures and β-like backbone conformations were detected by CD and in the computations. As a result of the collapse, a hidden antigen became surface-exposed. Similarly, using FRET between two fluorescent-proteins (AcGFP1 and mCherry) covalently attached in each end of phosphoglycerate kinase (PGK), PGK folded-state compaction was observed in the presence of Ficoll 70 (Dhar et al. 2010). Of possible biological relevance is that the more compacted PGK states exhibited 15-fold higher enzymatic activity. Also, for the dumbbell-shaped protein calmodulin, the N- and C-lobe distances in the folded state was found to be variable in the presence of crowders, both in silico and in vitro (Homouz et al. 2009a). In Fig. 2, we show computational models of the structural changes found for folded forms of apoflavodoxin, VlsE, calmodulin and PGK.
Fig. 2.
Cartoon representations of folded-state changes (with respect to crystal or NMR structures) found in silico for four proteins (PGK, Dhar et al. 2010; VlsE, Homouz et al. 2008; calmodulin, CaM, Homouz et al. 2009a; apoflavodoxin, Stagg et al. 2007) in the presence of macromolecular crowding. For each row, from left to right, the crowding level is increased. The shapes of the proteins are characterized by the shape parameter S (S ranges from −0.25 to 2: S < 0 is oblate, S = 0 is spherical, and S > 0 is prolate) and the asphericity Δ (Δ ranges from 0 to 1: Δ = 0 is sphere and Δ = 1 is rod). For CaM, due to the fact that the N- and C- lobes have different stability, besides Δ, an overlap function χ is used to characterize structures. χ = 0 indicates that a structure is identical to its NMR structure. χN is the overlap function for the N-lobe of CaM. Cartoon representations are colored from the N-terminus (red) to the C-terminus (blue). a crystal structure of PGK (which is extended; S = 0.26, Δ = 0.26); b collapsed crystal (CC) structure of PGK in crowders (S = 0.05, Δ = 0.1); c spherical compact (Sph) structure of PGK in crowders (S = 0.01, Δ = 0.02); d crystal structure of VlsE (which has an american football shape; S = 0.34, Δ = 0.31); e bean-like structure (Bean) of VlsE in crowders (S = 0.14, Δ = 0.17); f spherical structure (X) of VlsE in crowders (S = 0.02, Δ = 0.02); g NMR structure of CaM (which has a dumbbell-like shape; 0.2 < Δ < 0.4); h ‘native collapsed’ (NC) structure of CaM in crowders (this ensemble has a structurally native-like N-lobe and an unfolded C-lobe that is collapsed over the N-lobe; 0.0 < χN < 0.1 and 0.0 < Δ < 0.2); i completely collapsed (CC) structure of CaM in crowders (here, both N- and C- lobes have collapsed; 0.2 < χN < 0.4 and 0.0 < Δ < 0.2); j structure of apoflavodoxin in buffer (radius of gyration Rg of 15.40 Å) and, k structure of apoflavodoxin in crowders (Rg reduces to 15.35 Å)
That folded state structures may change in crowded conditions is not in conflict with Minton’s theories, although, in most cases, the folded state is modeled as a rigid sphere and there is no room for flexibility. The finding of crowding-induced folded-state plasticity brings up the issue of what are the true functional states of proteins in vivo. Maybe macromolecular crowding effects in vivo modulate local conformations at active sites in proteins in order to tune activity (Homouz et al. 2008). Recent NMR data from the Pielak laboratory suggests that, in addition to entropic origins, enthalpic forces are part of the crowding agent effects (Benton et al. 2012). This finding is compatible with the observed crowding-induced folded-state compaction as this likely involves increased favorable enthalpy.
Macromolecular crowding effects on folding dynamics
While introduction of excluded volume will increase the driving force for folding (since overall stability is increased), the viscosity is increased in high concentrations of macromolecules, which may slow down diffusion-dependent reactions. The microscopic spatial heterogeneity of a solution crowded with macromolecules results in macro- and micro-viscosities that differ in magnitude. Studies have shown that the microviscosity experienced by a protein during short timescale rotation or translation in crowded conditions is less than that measured for the bulk solution (Lavalette et al. 1999, 2006).
There have been a few in vitro crowding studies of peptide dynamics on the nanosecond timescale (Neuweiler et al. 2007; Mukherjee et al. 2009). According to fast fluorescence correlation spectroscopy studies of end-to-end contact formation, nanosecond formation of structural elements was not deterred by hindered diffusion in the presence of macromolecular crowding (Neuweiler et al. 2007). In another study, nanosecond temperature-jump experiments in the presence of crowding agents revealed that helix formation was not affected by crowding although β-hairpins formed slower in crowded conditions (Mukherjee et al. 2009). On the millisecond to second timescales, we have reported folding kinetics for VlsE and apoflavodoxin using stopped-flow mixing (Homouz et al. 2008; Stagg et al. 2010a). We found that the time-resolved folding reaction for VlsE remained two-state, but folding kinetics became faster whereas the unfolding kinetics was not affected, in the presence of low amounts of crowding agents (100 mg/ml Ficoll 70; low enough to not change the structure of the folded state). This result is expected if the presence of macromolecular crowding only affects the unfolded-state ensemble, and not folded- and transition-state ensembles. In contrast to VlsE, apoflavodoxin folds with a complex kinetic mechanism in buffer involving initial misfolding (stopped-flow dead-time, <1 ms) followed by subsequent unwinding and correct folding (seconds) (Stagg et al. 2010b). Our studies of apoflavodoxin variants in the presence of 100 mg/ml Ficoll 70 showed that there was less initial misfolding and the subsequent folding step occurred faster in the presence of crowding than in buffer (Stagg et al. 2010a). Using phi-value analysis, it appeared that the folding-transition state was less ordered with crowding; however, this could also be explained by unfolded-state compaction which would make the transition state appear less structured although it may not be. Another kinetic study by us on apoflavodoxin highlighted that crowder shape matters for the resulting effects on initial misfolding and subsequent folding kinetics (Homouz et al. 2009a).
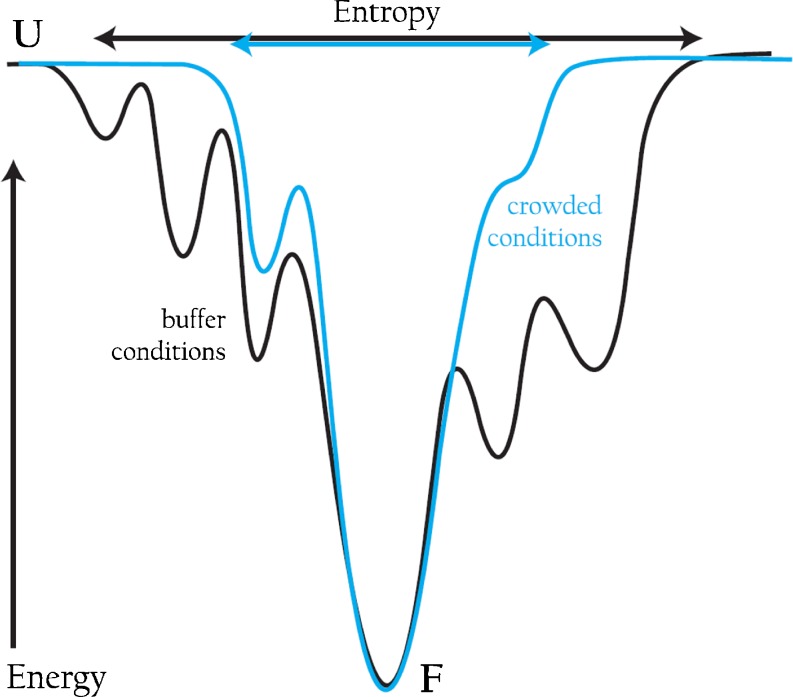
Recently, we bridged the time gap between ns and ms using cytochrome c as our model system in vitro. Cytochrome c folds in a multi-step reaction, involving multiple paths, starting on the μs timescale and completed on the second timescale. To follow early events in folding on the ns to ms timescale we used a ns time-resolved CD (TRCD) approach developed in the Kliger lab (Lewis et al. 1985; Kliger et al. 2012). In this study, electron-transfer (ET)-triggered folding was probed by TRCD and the results demonstrated that there were changes in kinetic partitioning between fast and slow folding phases of cytochrome c in the presence of 220 mg/ml dextran 70. Specifically, fast-folding conformations appeared hindered and most cytochrome c molecules folded on the ms timescale (Chen et al. 2012). Complementary experiments, at the same microviscosity as that in the presence of 220 mg/ml dextran 70, demonstrated that this was not a viscosity effect but indeed due to excluded volume. Our kinetic studies on apoflavodoxin and cytochrome c both suggest that excluded volume effects modulate folding energy landscapes such that they become smoother and there is less misfolding and/or kinetic heterogeneity than in buffer (depicted in Fig. 3).
Fig. 3.
A folding funnel (energy/enthalpy on y-axis vs. entropy on x-axis) for a protein in buffer (black) showing more rugged walls of the funnel (depicting misfolding and/or heterogeneity) and a wider opening (unfolded state entropy which is related to compaction) as compared to the same protein in crowded conditions, where the folding funnel appears to have smoother sides and a narrower top
Relation to macromolecular crowding in vivo
In vivo, crowding agents are not synthetic polymers but proteins and other natural biomolecules. Thus, in vivo, the effects of macromolecular crowding are not solely excluded-volume effects, as is what is probed in in vitro studies using crowding agents. It has become evident that non-specific protein–protein interactions can play a significant role in vivo (Li and Pielak 2009; Wang et al. 2010, 2011). Whereas excluded volume effects will stabilize folded forms, protein–protein nonspecific interactions will counteract this, resulting in reduced (or even reversed) overall effects (Miklos et al. 2011; Schlesinger et al. 2011; Benton et al. 2012). Computer simulations of protein diffusion and stability in a cytoplasmic-like environment had to include interaction energies, on top of excluded volume effects, to match experimental values (McGuffee and Elcock 2010). To avoid deleterious non-specific interactions in vivo due to electrostatic attraction, it appears that organisms have evolved proteins to have like surface charges when occupying the same compartment: for example, the E coli cytoplasm contains mostly negatively-charged proteins.
Protein folding in vivo is often more complicated than what we study in vitro: many proteins interact with chaperones prior to folding; some proteins need metal cofactors to adopt folded active states (Bertini et al. 1994) and these metals are often delivered by metallochaperones (Lutsenko et al. 2007); many polypeptides must be transported to different compartments before folding; there are spatial and temporal differences in local cell conditions, and membranes surround every compartment providing electrostatic gradients in their vicinity (Khramtsov et al. 1992). It is possible that a major role for chaperone proteins is to help newly synthesized proteins coupe with the crowded cellular environment (Burston and Clarke 1995; Frydman 2001).
Technically challenging studies of protein folding inside of living cells on the seconds time scale have been reported by Gruebele et al. (Dhar et al. 2011). Folding experiments with a destabilized variant of PGK were executed in the cytoplasm, the ER and the nucleus of cells. A change from multi-step folding in vitro to two-state folding inside living cells, most pronounced in the ER, was suggested from the data. These results are in agreement with our in vitro data on excluded-volume effects on apoflavodoxin and cytochrome c folding kinetics.
Concluding remarks
In this review, we have mostly focused on results from our combined in vitro/in silico approach. We want to emphasize that there are other crowding-agent studies not reported on here, for example on the role of crowding on enzymatic activity (Vopel and Makhatadze 2012), protein–protein association (Phillip et al. 2012), and mixtures of crowding agents (Batra et al. 2009). Our in vitro/in silico studies, in combination with work by others, underscore that macromolecular crowding results in: (1) more stable proteins, (2) faster and more homogeneous folding kinetics, and (3) population shifts of conformational ensembles. However, in most cases, the absolute effects of crowding agents are small, and in vitro studies in dilute buffers may be good approximations depending on what level of detail one is after. Nonetheless, in vivo reactions are tightly regulated and, therefore, small differences in equilibrium constants (i.e., due to crowding effects) may have dramatic effects on cellular activity and organism fitness.
The goal with in vitro studies of macromolecular crowding effects is often to reveal quantitative changes. It is important to note that there are many pitfalls in experimental setup and data interpretation. Technical problems are for example that some crowding agent stocks contain significant amounts of contaminating metals (e.g., zinc), which is of importance if metalloproteins are studied. Moreover, when crowders are mixed with denaturants, the solvent accessible volume (to the denaturant) is less than the total volume of the sample. Thus, the actual denaturant concentration in the available solution is higher than the value derived based on total volume. Should we correct for this or is this part of the ‘crowding effect’? It is a matter of definition but is crucial when one wants to compare data at ‘similar conditions’.
We would like to end with a question that brings us back to fundamental mechanisms. Can excluded volume effects per se really fold unfolded proteins? In other words, how much free energy can excluded-volume effects provide to polypeptides, and can steric effects alone guide a polypeptide to the ‘right’ folded state? As mentioned, crowding can stabilize folded proteins (i.e., shift equilibria) and change both unfolded- and folded-state structural ensembles. Also, at non-physiological conditions, crowding seems to induce folding of polypeptides towards compact molten globule-like states. However, there are no convincing studies yet that demonstrates that addition of crowding agents to an unfolded polypeptide in buffer at physiological pH triggers folding of that polypeptide to its native state. Pielak reported more than 10 years ago that the unstructured protein FlgM gained structure inside E coli cells and at crowded conditions in vitro, however, the results were based on the disappearance of NMR cross-peaks and no conclusion about the gained structure could be determined (Dedmon et al. 2002).
For a better understanding of protein biophysical properties in living systems, the effects of macromolecular crowding must be taken into account. We now begin to comprehend many aspects of this phenomenon but systematic and quantitative studies of additional protein systems are desired.
Acknowledgements
We would like to thank Allen Minton for his continuous (careful and sometimes critical) feedback on our work on crowding. Minton’s pioneering theoretical contributions to this field has served as the basis for many of our studies and they continue to stimulate new experiments. We thank Eefei Chen, University of California, Santa Cruz for preparing Fig. 3 and Jörgen Åden, Umeå University for preparing Fig. 1; and Magnus Wolf-Watz, Umeå University for helpful comments on the text.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Footnotes
Special Issue: Protein-Protein and Protein-Ligand Interactions in Dilute and Crowded Solution Conditions. In Honor of Allen Minton’s 70th Birthday
References
- Aguilar X, Weise FC, et al. Macromolecular crowding extended to a heptameric system: the Co-chaperonin protein 10. Biochemistry. 2011;50(14):3034–3044. doi: 10.1021/bi2002086. [DOI] [PubMed] [Google Scholar]
- Ai X, Zhou Z, Choy WY. 15N NMR spin relaxation dispersion study of the molecular crowding effects on protein folding under native conditions. J Am Chem Soc. 2006;128:3916–3917. doi: 10.1021/ja057832n. [DOI] [PubMed] [Google Scholar]
- Batra J, Xu K, et al. Nonadditive effects of mixed crowding on protein stability. Proteins. 2009;77(1):133–138. doi: 10.1002/prot.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton LA, Smith AE et al (2012) Unexpected Effects of Macromolecular Crowding on Protein Stability. Biochemistry (in press) [DOI] [PubMed]
- Bertini I, Gary HB, et al. Bioinorganic chemistry. Mill Valley: University Science; 1994. [Google Scholar]
- Bohrer MP, Patterson GD, Carroll PJ. Hindered diffusion of dextran and Ficoll in microporous membranes. Macromolecules. 1984;17:1170–1173. doi: 10.1021/ma00136a011. [DOI] [Google Scholar]
- Bryngelson JD, Onuchic JN, et al. Funnels, pathways, and the energy landscape of protein folding: a synthesis. Proteins. 1995;21(3):167–195. doi: 10.1002/prot.340210302. [DOI] [PubMed] [Google Scholar]
- Burston SG, Clarke AR. Molecular chaperones: physical and mechanistic properties. Essays Biochem. 1995;29:125–136. [PubMed] [Google Scholar]
- Chen E, Christiansen A, et al. The effects of macromolecular crowding on burst phase kinetics of cytochrome c folding. Biochemistry. 2012;51(49):9836–9845. doi: 10.1021/bi301324y. [DOI] [PubMed] [Google Scholar]
- Cheung MS, Klimov D, et al. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci USA. 2005;102(13):4753–4758. doi: 10.1073/pnas.0409630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen A, Wang Q, et al. Factors defining effects of macromolecular crowding on protein stability: an in vitro/in silico case study using cytochrome c. Biochemistry. 2010;49(31):6519–6530. doi: 10.1021/bi100578x. [DOI] [PubMed] [Google Scholar]
- Dedmon MM, Patel CN, et al. FlgM gains structure in living cells. Proc Natl Acad Sci USA. 2002;99(20):12681–12684. doi: 10.1073/pnas.202331299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Samiotakis A, et al. Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding. Proc Natl Acad Sci USA. 2010;107(41):17586–17591. doi: 10.1073/pnas.1006760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Girdhar K, et al. Protein stability and folding kinetics in the nucleus and endoplasmic reticulum of eucaryotic cells. Biophys J. 2011;101(2):421–430. doi: 10.1016/j.bpj.2011.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel R, Westphal AH, et al. Macromolecular crowding compacts unfolded apoflavodoxin and causes severe aggregation of the off-pathway intermediate during apoflavodoxin folding. J Biol Chem. 2008;283(41):27383–27394. doi: 10.1074/jbc.M802393200. [DOI] [PubMed] [Google Scholar]
- Flaugh SL, Lumb KJ. Effects of macromolecular crowding on the intrinsically disordered proteins c-Fos and p27(Kip1) Biomacromolecules. 2001;2(2):538–540. doi: 10.1021/bm015502z. [DOI] [PubMed] [Google Scholar]
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- Homouz D, Perham M, et al. Crowded, cell-like environment induces shape changes in aspherical protein. Proc Natl Acad Sci USA. 2008;105(33):11754–11759. doi: 10.1073/pnas.0803672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homouz D, Sanabria H, et al. Modulation of calmodulin plasticity by the effect of macromolecular crowding. J Mol Biol. 2009;391(5):933–943. doi: 10.1016/j.jmb.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homouz D, Stagg L, et al. Macromolecular crowding modulates folding mechanism of alpha/beta protein apoflavodoxin. Biophys J. 2009;96(2):671–680. doi: 10.1016/j.bpj.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Gierasch LM. Macromolecular crowding remodels the energy landscape of a protein by favoring a more compact unfolded state. J Am Chem Soc. 2010;132(30):10445–10452. doi: 10.1021/ja103166y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen D, Jeffries CM, et al. Effects of macromolecular crowding on an intrinsically disordered protein characterized by small-angle neutron scattering with contrast matching. Biophys J. 2011;100(4):1120–1128. doi: 10.1016/j.bpj.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsov VV, Marsh D, et al. The application of pH-sensitive spin labels to studies of surface potential and polarity of phospholipid membranes and proteins. Biochim Biophys Acta. 1992;1104(2):317–324. doi: 10.1016/0005-2736(92)90046-O. [DOI] [PubMed] [Google Scholar]
- Kliger DS, Chen E, et al. Nanosecond time-resolved natural and magnetic chiroptical spectroscopies. New York: Wiley VCH; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert DC, Gildenhuys S, Dirr H. Effect of macromolecular crowding on the stability of monomeric glutaredoxin 2 and dimeric glutathione transferase A1-1. S Afr J Sci. 2008;104:76–80. [Google Scholar]
- Kulothungan SR, Das M, Johnson M, Ganesh C, Varadarajan R. Effect of crowding agents, signal peptide, and chaperone SecB on the folding and aggregation of E. coli maltose binding protein. Langmuir. 2009;25:6637–6648. doi: 10.1021/la900198h. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Ogston AG. The interaction between polysaccharides and other macromolecules. 4. The osmotic pressure of mixtures of serum albumin and hyaluronic acid. Biochem J. 1963;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalette D, Tetreau C, et al. Microscopic viscosity and rotational diffusion of proteins in a macromolecular environment. Biophys J. 1999;76(5):2744–2751. doi: 10.1016/S0006-3495(99)77427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalette D, Hink MA, et al. Proteins as micro viscosimeters: Brownian motion revisited. Eur Biophys J. 2006;35(6):517–522. doi: 10.1007/s00249-006-0060-z. [DOI] [PubMed] [Google Scholar]
- Le Coeur C, Teixeira J, et al. Compression of random coils due to macromolecular crowding: scaling effects. Phys Rev E. 2010;81(6 Pt 1):061914. doi: 10.1103/PhysRevE.81.061914. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Tilton RF, et al. New technique for measuring circular dichroism changes on a nanosecond time scale—application to (carbonmonoxy)myoglobin and (carbonmonoxy)hemoglobin. J Phys Chem. 1985;89(2):289–294. doi: 10.1021/j100248a023. [DOI] [Google Scholar]
- Li C, Pielak GJ. Using NMR to distinguish viscosity effects from nonspecific protein binding under crowded conditions. J Am Chem Soc. 2009;131(4):1368–1369. doi: 10.1021/ja808428d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S, LeShane ES, et al. Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophys. 2007;463(2):134–148. doi: 10.1016/j.abb.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek A, Kellis JT, Jr, et al. Mapping the transition state and pathway of protein folding by protein engineering [see comments] Nature. 1989;340(6229):122–126. doi: 10.1038/340122a0. [DOI] [PubMed] [Google Scholar]
- McGuffee SR, Elcock AH. Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasm. PLoS Comput Biol. 2010;6(3):e1000694. doi: 10.1371/journal.pcbi.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelsson T, Åden J, et al. Direct observation of protein unfolded state compaction in the presence of macromolecular crowding. Biophys J. 2013;104:694–704. doi: 10.1016/j.bpj.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos AC, Sarkar M, et al. Protein crowding tunes protein stability. J Am Chem Soc. 2011;133(18):7116–7120. doi: 10.1021/ja200067p. [DOI] [PubMed] [Google Scholar]
- Millett IS, Doniach S, et al. Toward a taxonomy of the denatured state: small angle scattering studies of unfolded proteins. Adv Protein Chem. 2002;62:241–262. doi: 10.1016/S0065-3233(02)62009-1. [DOI] [PubMed] [Google Scholar]
- Minton AP. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers. 1981;20:2093–2120. doi: 10.1002/bip.1981.360201006. [DOI] [Google Scholar]
- Minton AP. Effect of a concentrated “inert” macromolecular cosolute on the stability of a globular protein with respect to denaturation by heat and by chaotropes: a statistical-thermodynamic model. Biophys J. 2000;78(1):101–109. doi: 10.1016/S0006-3495(00)76576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton AP. Models for excluded volume interaction between an unfolded protein and rigid macromolecular cosolutes: macromolecular crowding and protein stability revisited. Biophys J. 2005;88(2):971–985. doi: 10.1529/biophysj.104.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Waegele MM, et al. Effect of macromolecular crowding on protein folding dynamics at the secondary structure level. J Mol Biol. 2009;393(1):227–236. doi: 10.1016/j.jmb.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuweiler H, Lollmann M, et al. Dynamics of unfolded polypeptide chains in crowded environment studied by fluorescence correlation spectroscopy. J Mol Biol. 2007;365(3):856–869. doi: 10.1016/j.jmb.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Perham M, Stagg L, et al. Macromolecular crowding increases structural content of folded proteins. FEBS Lett. 2007;581(26):5065–5069. doi: 10.1016/j.febslet.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Phillip Y, Kiss V, et al. Protein-binding dynamics imaged in a living cell. Proc Natl Acad Sci USA. 2012;109(5):1461–1466. doi: 10.1073/pnas.1112171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdnyakova I, Wittung-Stafshede P. Non-linear effects of macromolecular crowding on enzymatic activity of multi-copper oxidase. Biochim Biophys Acta. 2010;1804(4):740–744. doi: 10.1016/j.bbapap.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Qu Y, Bolen DW. Efficacy of macromolecular crowding in forcing proteins to fold. Biophys Chem. 2002;101–102:155–165. doi: 10.1016/S0301-4622(02)00148-5. [DOI] [PubMed] [Google Scholar]
- Rivas G, Ferrone F, et al. Life in a crowded world. EMBO Rep. 2004;5(1):23–27. doi: 10.1038/sj.embor.7400056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiotakis A, Wittung-Stafshede P, et al. Folding, stability and shape of proteins in crowded environments: experimental and computational approaches. Int J Mol Sci. 2009;10(2):572–588. doi: 10.3390/ijms10020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara K, McPhie P, et al. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J Mol Biol. 2003;326(4):1227–1237. doi: 10.1016/S0022-2836(02)01443-2. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Miyoshi D, Sugimoto N. Regulation of DNA nucleases bymolecular crowding. Nucleic Acids Res. 2007;35:4086–4093. doi: 10.1093/nar/gkm445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger AP, Wang Y, et al. Macromolecular crowding fails to fold a globular protein in cells. J Am Chem Soc. 2011;133(21):8082–8085. doi: 10.1021/ja201206t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MR, Thirumalai D. Free polymer in a colloidal solution. Phys Rev A. 1991;44(8):R4797–R4800. doi: 10.1103/PhysRevA.44.R4797. [DOI] [PubMed] [Google Scholar]
- Spencer DS,Xua K,Logan TM,Zhou H-X (2005) Effects of pH, salt, and macromolecular crowding on the stability of FK506-binding protein: an integrated experimental and theoretical study. J Mol Biol 351:219–232 [DOI] [PubMed]
- Stagg L, Zhang SQ, et al. Molecular crowding enhances native structure and stability of alpha/beta protein flavodoxin. Proc Natl Acad Sci USA. 2007;104(48):18976–18981. doi: 10.1073/pnas.0705127104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg L, Christiansen A, et al. Macromolecular crowding tunes folding landscape of parallel alpha/beta protein, apoflavodoxin. J Am Chem Soc. 2010;133(4):646–648. doi: 10.1021/ja107638e. [DOI] [PubMed] [Google Scholar]
- Stagg L, Samiotakis A, et al. Residue-specific analysis of frustration in the folding landscape of repeat beta/alpha protein apoflavodoxin. J Mol Biol. 2010;396(1):75–89. doi: 10.1016/j.jmb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Tellam RL, Sculley MJ, Nichol LW, Wills PR. The influence of poly(ethylene glycol) 6000 on the properties of skeletal-muscle actin. Biochem J. 1983;213:651–659. doi: 10.1042/bj2130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuriki N, Kinjo M, et al. Protein folding by the effects of macromolecular crowding. Protein Sci. 2004;13(1):125–133. doi: 10.1110/ps.03288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol. 2005;288(4):F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- Vopel T, Makhatadze GI. Enzyme activity in the crowded milieu. PLoS One. 2012;7(6):e39418. doi: 10.1371/journal.pone.0039418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li C, et al. Effects of proteins on protein diffusion. J Am Chem Soc. 2010;132(27):9392–9397. doi: 10.1021/ja102296k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhuravleva A, et al. Exploring weak, transient protein-protein interactions in crowded in vivo environments by in-cell nuclear magnetic resonance spectroscopy. Biochemistry. 2011;50(43):9225–9236. doi: 10.1021/bi201287e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittung-Stafshede P. Protein folding inside the cell. Biophys J. 2011;101(2):265–266. doi: 10.1016/j.bpj.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HX. Loops, linkages, rings, catenanes, cages, and crowders: entropy based strategies for stabilizing proteins. Acc Chem Res. 2004;37:123–130. doi: 10.1021/ar0302282. [DOI] [PubMed] [Google Scholar]
- Zhou HX, Rivas G, et al. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SB, Trach SO. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222(3):599–620. doi: 10.1016/0022-2836(91)90499-V. [DOI] [PubMed] [Google Scholar]