Abstract
Background:
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been associated with improved survival in some cancers, but evidence for ovarian cancer is limited.
Methods:
Pooling individual-level data from 12 Ovarian Cancer Association Consortium studies, we evaluated the association between self-reported, pre-diagnosis use of common analgesics and overall/progression-free/disease-specific survival among 7694 women with invasive epithelial ovarian cancer (4273 deaths).
Results:
Regular analgesic use (at least once per week) was not associated with overall survival (pooled hazard ratios, pHRs (95% confidence intervals): aspirin 0.96 (0.88–1.04); non-aspirin NSAIDs 0.97 (0.89–1.05); acetaminophen 1.01 (0.93–1.10)), nor with progression-free/disease-specific survival. There was however a survival advantage for users of any NSAIDs in studies clearly defining non-use as less than once per week (pHR=0.89 (0.82–0.98)).
Conclusions:
Although this study did not show a clear association between analgesic use and ovarian cancer survival, further investigation with clearer definitions of use and information about post-diagnosis use is warranted.
Keywords: ovarian cancer, aspirin, nonsteroidal anti-inflammatory drugs, NSAID, paracetamol, acetaminophen, pooled analysis, survival
Over 238 000 women are diagnosed with ovarian cancer annually (International Agency for Research on Cancer, 2014) and 5-year survival is poor at ∼45% (Howlader et al, 2015). Identifying modifiable factors that could improve survival is therefore important. One possible factor is the use of analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs). Nonsteroidal anti-inflammatory drugs, including aspirin and non-aspirin NSAIDs (NA-NSAIDs), inhibit the pro-inflammatory enzyme cyclooxygenase (COX). COX-2 is over-expressed in many cancers including ovarian (Maccio and Madeddu, 2012), and COX-inhibition can reduce angiogenesis and trigger apoptosis (Xin et al, 2007). While improved survival among NSAID users has been reported for breast (Huang et al, 2015), prostate (Liu et al, 2014), and colorectal (Ye et al, 2014) cancers, two previous observational studies of ovarian cancer found no evidence that pre-diagnosis aspirin, NA-NSAID, or acetaminophen use was associated with survival (Minlikeeva et al, 2015; Nagle et al, 2015). However, both studies were underpowered to detect the likely modest effects. One trial reported no short-term (median follow-up 34 months) survival advantage among women with advanced ovarian cancer when a NA-NSAID was added to standard chemotherapy, but did not examine long-term outcomes (Reyners et al, 2012). Interestingly, a preliminary report (published as a conference abstract) has suggested that NSAID use post-diagnosis may be associated with improved survival (Poole et al, 2016). We used data from a large international consortium to examine the association between pre-diagnosis use of common analgesics and survival after a diagnosis of ovarian cancer. We hypothesised that NSAID users would experience a survival benefit compared to non-users.
Materials and methods
Study population
We pooled data from 12 case–control studies, which included 7694 women with invasive epithelial ovarian tumours, aged <85 years at diagnosis (Supplementary Table 1). Cancers of unknown behaviour (n=93 high grade; n=25 low grade) were assumed to be invasive (Trabert et al, 2014). Most women (59%) had serous cancers; 5%, 15%, and 8% had mucinous, endometrioid, and clear cell cancers, respectively. Most cancers (63%) had distant spread.
Exposure, outcome, and covariates
Studies provided data on self-reported pre-diagnosis analgesic use (Supplementary Table 2; harmonisation described previously (Trabert et al, 2014)). Two studies (UCI/UKO) did not report NA-NSAIDs data, so were only included in ‘aspirin' and ‘any NSAIDs' (aspirin plus non-aspirin) analyses. Regular use was defined as at least once per week vs less often. Frequency, dose, and duration information was available for seven, three, and nine studies, respectively (Table 1). Studies provided data on vital status and time from diagnosis to death or end of follow-up. Disease recurrence/progression (from 3 studies) and cause of death (two studies) was known for 28% and 12% of women, respectively. Ethnicity, smoking status, education, body mass index (BMI), tumour stage, and residual disease was known for 99.8%, 89%, 87%, 92%, 99%, and 24% of women, respectively.
Table 1. The association between regular pre-diagnosis use of common analgesic medications and overall survival following a diagnosis of invasive ovarian cancer.
|
Aspirin |
Non-aspirin NSAIDs |
Any NSAIDs |
Acetaminophen |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure categorisation | n | pHRa | 95% CI | I2 | n | pHRa | 95% CI | I2 | n | pHRa | 95% CI | I2 | n | pHRa | 95% CI | I2 |
|
Regular useb | ||||||||||||||||
| No | 6190 | Ref | 5196 | Ref | 4891 | Ref | 5908 | Ref | ||||||||
| Yes | 1286 | 0.96 | 0.88–1.04 | 5.5 | 1570 | 0.97 | 0.89–1.05 | 0.0 | 2563 | 0.94 | 0.86–1.03 | 32.6 | 1264 | 1.01 | 0.93–1.10 | 0.0 |
|
Frequency (7 studies)c | ||||||||||||||||
| No regular use | 4268 | Ref | 3993 | Ref | 3297 | Ref | 4335 | Ref | ||||||||
| <30 days per month | 234 | 0.92 | 0.72–1.18 | 31.0 | 498 | 0.93 | 0.79–1.09 | 15.2 | 572 | 0.92 | 0.81–1.06 | 0.0 | 532 | 1.05 | 0.85–1.30 | 56.6d |
| Daily | 593 | 1.01 | 0.90–1.14 | 0.0 | 768 | 0.99 | 0.89–1.10 | 0.0 | 1226 | 0.98 | 0.89–1.07 | 2.4 | 419 | 0.99 | 0.86–1.13 | 0.0 |
|
Daily dose (3 studies)e | ||||||||||||||||
| No regular use | 1330 | Ref | 1244 | Ref | 899 | Ref | 1484 | Ref | ||||||||
| Low | 125 | 0.90 | 0.58–1.39 | 51.0 | 253 | 0.98 | 0.81–1.18 | 0.0 | 299 | 0.95 | 0.79–1.13 | 0.0 | 65 | 0.90 | 0.63–1.27 | 0.0 |
| High | 205 | 0.92 | 0.69–1.22 | 37.2 | 230 | 1.10 | 0.91–1.33 | 0.0 | 396 | 0.92 | 0.69–1.24 | 64.2 | 289 | 1.09 | 0.89–1.34 | 18.5 |
|
Duration (9 studies)f | ||||||||||||||||
| No regular use | 3919 | Ref | 3523 | Ref | 2889 | Ref | 4201 | Ref | ||||||||
| <60 months | 426 | 0.96 | 0.82–1.13 | 22.1 | 483 | 0.99 | 0.87–1.13 | 0.0 | 689 | 0.94 | 0.80–1.11 | 40.9 | 253 | 1.04 | 0.87–1.24 | 0.0 |
| 60+ months | 559 | 1.01 | 0.89–1.14 | 0.0 | 519 | 0.90 | 0.78–1.04 | 0.0 | 930 | 0.98 | 0.86–1.13 | 29.8 | 460 | 1.00 | 0.83–1.21 | 34.4 |
Abbreviations: CI=confidence interval; NSAID=nonsteroidal anti-inflammatory drug; pHR=pooled hazard ratio.
Adjusted for age (in years), ethnicity (if <95% of participants were of the same ethnicity) (White/Hispanic/Black/Asian/Other), and education (Less than high-school/Completed high-school including some college/College graduate/Education status unknown).
‘Regular' use defined as at least once per week (depending on the question used by each study to collect information on medication use); includes all 12 studies for aspirin, 10 studies for non-aspirin NSAIDs (excluding UCI/UKO, which did not report these data), 12 studies for any NSAIDs (aspirin or non-aspirin), and 11 studies (excluding UKO) for acetaminophen.
Frequency analyses were conducted in 7 studies with available data (AUS, DOV, HAW, HOP, MAL, NCO, and USC). Frequency of daily use of any NSAID may be slightly underestimated (while less than daily use may be overestimated), because if aspirin and non-aspirin NSAIDs were each taken <7 days per week, this is categorised as less than daily use of any NSAID although it is possible that at least one type of NSAID was taken 7 days per week (this cannot be determined from the data available).
Statistically significant heterogeneity in the pHR across studies.
Low/High defined as </≥ 100 mg/day for aspirin, and </≥500 mg/day for non-aspirin NSAIDs and acetaminophen; includes 3 studies with available data (HAW, HOP, and NCO).
Duration analyses were conducted in 9 studies with available data (CON, DOV, HAW, HOP, MAL, NEC, NJO, UCI, and USC) for aspirin and acetaminophen, and in 8 studies (excluding UCI) for non-aspirin NSAIDs and any NSAIDs.
Statistical analysis
Using Cox proportional hazards regression, we obtained hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between analgesic use and overall survival (OS) in each study. Potential confounders were selected a priori. We did not adjust for treatment type because treatment cannot influence pre-diagnosis analgesic use (as treatment occurs later) and these data were only available for 25% of the cohort. Main models were adjusted for age, education, and ethnicity. Survival time was left-truncated at recruitment to minimise potential bias from eligible women dying before they could be enroled. Following proportional hazards assumption checking (inspecting covariate associations with survival over time), we re-ran models including covariate*time interactions where these interactions were statistically significant (two studies). As the resulting estimates were virtually unchanged, final models did not include these interactions. Site-specific HRs for OS were combined using random-effects meta-analysis. I2 and P-values for heterogeneity (from chi-square tests) were inspected to assess inter-study heterogeneity. Associations between analgesic use and progression-free (PFS) and disease-specific survival (DSS) were estimated from single models, stratified by study, to maximise power. In the same manner we conducted analyses stratified by characteristics likely to modify the association (age, BMI, and disease stage).
This analysis and each contributing study received approval from the appropriate institutional review board/ethics committee. All participants provided written informed consent.
Results
Population characteristics
Mean age at diagnosis was 58 years, 88% of women were non-Hispanic white, and 27% were tertiary-educated. Of the 7694 women, 17%, 23%, and 18% had regularly used aspirin, NA-NSAIDs, and acetaminophen, respectively (Supplementary Table 1). Median follow-up (using reverse Kaplan-Meier (Schemper and Smith, 1996)) was 8.0 years. Over half the women (n=4273, 56%) died and 5-year survival was 55% (Supplementary Table 1), yielding 90% power to detect a HR=0.90 for NSAID users. Among studies with progression/cause-of-death information, 73% of women experienced progression and 95% of deaths were from ovarian cancer.
Primary results
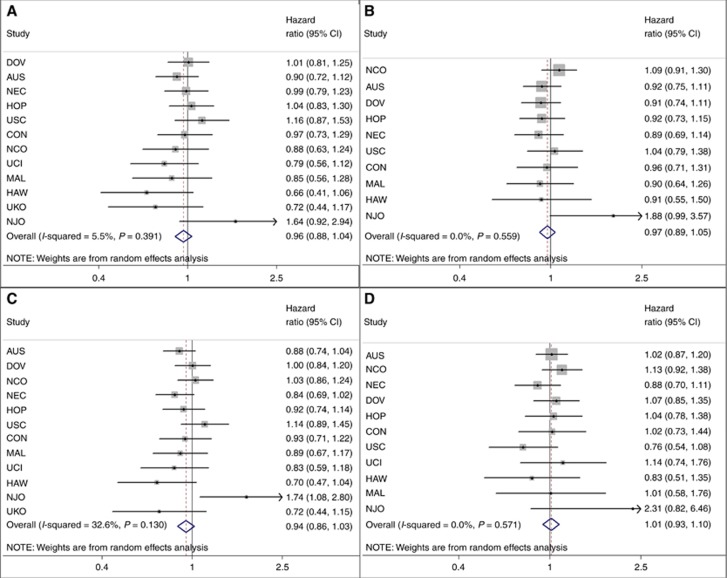
Regular use of analgesics was not associated with OS (pHRs (95% CI): aspirin 0.96 (0.88–1.04); NA-NSAIDs 0.97 (0.89–1.05); any NSAIDs 0.94 (0.86–1.03); acetaminophen 1.01 (0.93–1.10)), nor were frequency, dose, or duration of use (Table 1; Figure 1). There was no significant inter-study heterogeneity (Figure 1). Additional adjustment for tumour stage and grade, residual disease, BMI and smoking status did not appreciably alter effect estimates. Cross-classifying frequency by dosage (among five studies with data) did not demonstrate consistent associations, and long-term (≥5 years) daily use was not associated with survival. Truncating follow-up at five years (when most deaths would be cancer-related) did not affect results. No significant associations were observed with PFS (any NSAIDs, HR=0.96; 95% CI 0.80–1.14) or DSS (HR=0.98; 95% CI 0.82–1.17). There was no significant variation by tumour histology (Table 2; P-interaction=0.3–0.7). Excluding the two studies which had previously examined this association (Minlikeeva et al, 2015; Nagle et al, 2015) (whose participants comprised 25% of this analysis), two studies with high survival rates (these studies recruited a number of prevalent cases), or two studies who asked only about recent use (past 5 years), did not substantially alter effect estimates.
Figure 1.
The association between regular pre-diagnosis use of common analgesic medications and overall survival following a diagnosis of invasive ovarian cancer, adjusted for age, ethnicity (if <95% of participants are of the same ethnicity), and education.(A) Aspirin, (B) non-aspirin NSAIDs, (C) any NSAIDs, (D) acetaminophen.
Table 2. The association between regular pre-diagnosis use of common analgesic medications and overall survival following a diagnosis of invasive ovarian cancer, by histologic subtype.
|
Aspirin (Regulara
vs
no regular use) (12 studies) |
Non-aspirin NSAIDs (Regulara
vs
no regular use) (10 studies) |
Any NSAIDs (Regulara
vs
no regular use) (12 studies) |
Acetaminophen (Regulara
vs
no regular use) (11 studies) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histologic Subtype | nb | pHRc | 95% CI | I2 | nb | pHRc | 95% CI | I2 | nb | pHRc | 95% CI | I2 | nb | pHRc | 95% CI | I2 |
| Serousd | 4386 | 1.04 | 0.92–1.17 | 24.0 | 4034 | 1.01 | 0.90–1.14 | 28.9 | 4394 | 1.01 | 0.93–1.09 | 2.7 | 4255 | 1.02 | 0.93–1.13 | 1.3 |
| High-grade | 4065 | 1.02 | 0.91–1.13 | 11.0 | 3728 | 1.00 | 0.88–1.13 | 30.6 | 4067 | 0.99 | 0.91–1.09 | 9.5 | 3933 | 1.01 | 0.91–1.12 | 1.8 |
| Low-grade | 265 | 1.71 | 0.76–3.87 | 31.2 | 252 | 1.28 | 0.63–2.59 | 46.9 | 277 | 0.99 | 0.56–1.75 | 25.6 | 246 | 1.61 | 0.87–2.99 | 34.6 |
| Mucinous | 248 | 0.92 | 0.42–2.01 | 0.0 | 287 | 1.50 | 0.80–2.80 | 0.0 | 353 | 0.86 | 0.49–1.48 | 0.0 | 209 | 2.17 | 0.82–5.74 | 34.2 |
| Endometrioid | 1126 | 0.87 | 0.63–1.21 | 0.0 | 1000 | 0.93 | 0.69–1.27 | 0.0 | 1118 | 0.94 | 0.66–1.33 | 37.5 | 982 | 0.96 | 0.60–1.53 | 29.2 |
| Clear cell | 474 | 1.22 | 0.74–2.02 | 14.2 | 452 | 0.87 | 0.50–1.53 | 36.2 | 548 | 1.01 | 0.61–1.67 | 43.6 | 392 | 0.93 | 0.37–2.33 | 61.6e |
Abbreviations: CI=confidence interval; NSAID=nonsteroidal anti-inflammatory drug; pHR=pooled hazard ratio.
‘Regular' use defined as at least once per week (depending on the question used by each study to collect information on medication use).
Participants in model (regular users plus participants with no regular use).
Adjusted for age (in years), ethnicity (if <95% of participants were of the same ethnicity) (White/Hispanic/Black/Asian/Other), and education (Less than high-school/Completed high-school including some college/College graduate/Education status unknown).
The number of cases included in high and low-grade serous analyses do not add to the total number of cases included in serous analyses, because some participants (particularly with low-grade serous cancers) could not be included in analyses if there were insufficient cases from their study to estimate a site-specific hazard ratio. A number of serous cancers of unknown grade (8.5% of high-grade serous cancers) were assumed to be high-grade for these analyses.
Statistically significant heterogeneity in the pHR across studies.
To minimise exposure misclassification due to heterogeneous questions between studies, we repeated analyses restricted to six studies clearly defining non-use as less than once per week (Supplementary Table 3). This showed a significant survival advantage among regular users of any NSAIDs (pHR=0.89; 95% CI 0.82–0.98). When we excluded studies with low exposure prevalence (<10%), a similar association was seen among the eight remaining studies (including the six above; any NSAIDs HR=0.92; 95% CI 0.85–0.99).
In stratified analyses, an inverse association between any NSAID use and survival was seen among women aged ≥60 at diagnosis (pHR=0.90; 95% CI 0.82–0.99) or with BMI <25 kg m−2 (0.86; 0.77–0.95). A non-significant inverse association was seen among women with early-stage (localised/regional) tumours (pHR=0.87; 95% CI 0.74–1.04; Supplementary Table 4).
Discussion
Overall, we did not find convincing evidence to support an association between use of aspirin, NA-NSAIDs, or acetaminophen prior to diagnosis and ovarian cancer survival. Although most HRs for aspirin and NA-NSAIDs were <1.0, none was statistically significant. Our results are consistent with the two previous observational studies (Minlikeeva et al, 2015; Nagle et al, 2015) examining pre-diagnosis use of NSAIDs. The relationship did not vary by histologic subtype.
Questions used to define regular use differed between studies. When we restricted analyses to a subset of studies clearly defining non-use as less than once per week, use of any NSAIDs was associated with significantly improved survival. No notable characteristics differentiated these studies from others in the main analyses. This may suggest that exposure misclassification attenuated our primary results, and that analyses using a more consistent definition of use might have more power to detect modest associations with survival. However the results of these post hoc analyses require validation and should be interpreted with caution. The apparent lack of association for long-term daily use of any NSAIDs among the five studies with this information (this included studies with potential misclassification of non-users) could reflect the fact that daily aspirin users are more likely to use a low-dose preparation, which may be insufficient to confer a survival benefit.
We did not have information on medication use after diagnosis, which may be a more relevant time-window, especially if women change their use after diagnosis. A recent meta-analysis of NSAID use and breast cancer survival found post- but not pre-diagnosis use was associated with improved survival (Huang et al, 2015), a pattern also suggested by the preliminary report (conference abstract) examining post-diagnosis use among a small number (N=602) of ovarian cancer patients (Poole et al, 2016). Unless change in use is associated with survival, the likely effect of using pre-diagnosis data to estimate post-diagnosis use would be random misclassification, attenuating any real association. We had insufficient data to stratify by post-diagnosis prognostic factors such as treatment received, but additional adjustment for stage/grade of disease (which predict treatment type) and amount of residual disease after surgery did not appreciably alter our estimates.
In conclusion, we did not find convincing evidence of an association between pre-diagnosis analgesic use and ovarian cancer survival. However, the modest associations in subgroup analyses suggest we cannot exclude the possibility that NSAID use is associated with survival (but we could not detect this due to exposure misclassification and/or a sub-optimal exposure window). Further investigation with more consistent definitions of analgesic use/non-use (including by selective/non-selective COX-2 inhibition) and information about post-diagnosis use is warranted.
Acknowledgments
This work was supported by: the National Cancer Institute at the U.S. National Institutes of Health (K07-CA095666, K07-CA80668, K22-CA138563, N01-CN025403, N01-CN55424, N01-PC67001, N01-PC67010, P01-CA17054, P30-CA072720, P30-CA14089, P50-CA105009, P50-CA159981, R01-CA058860, R01-CA074850, R01-CA080742, R01-CA092044, R01-CA095023, R01-CA54419, R01-CA58598, R01-CA61107, R01-CA61132, R01-CA76016, R01-CA87538, R01-CA112523, R01-CA126841, R03-CA113148, and R03-CA115195); the U.S. Army Medical Research and Materiel Command (DAMD17–01–1–0729, DAMD17-02-1-0666, DAMD17-02-1-0669, and W81XWH-10-1-0280); the National Health and Medical Research Council of Australia (199600, 400281, 1073898 and P.M.W. fellowship); Cancer Councils of Queensland, Victoria, New South Wales, South Australia and Tasmania and the Cancer Foundation of Western Australia (Multi-State Grant Applications 191, 211, and 182); the Danish Cancer Society (94-222-52); the Mermaid I project; the Cancer Institute of New Jersey; the U.K. National Institute for Health Research University College London Hospital Biomedical Research Centre; the U.S. Public Health Service (PSA-042205); the Lon V. Smith Foundation (LVS-39420); The Eve Appeal (The Oak Foundation); and the California Cancer Research Program (00-01389V-20170 and 2II0200). We thank all the individuals who took part in this study and all the researchers, clinicians, and technical and administrative staff who have made possible the many studies contributing to this work. In particular, for their contribution to the design and conduct of the individual studies that contributed to the analysis, we thank: D. Bowtell, G. Chenevix-Trench, D. Gertig, A. Green, P. Parsons, N. Hayward and D. Whiteman (AUS); I. Orlow and L. Rodriguez-Rodriguez (NJO); and I. Jacobs, M.Widschwendter, E. Wozniak, A. Ryan, J. Ford, N. Balogun and C. Karpinskyj (UKO). We also thank the intramural research program of the US National Cancer Institute for harmonisation of the analgesic variables.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
The authors declare no conflict of interest.
Supplementary Material
References
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2015) SEER Cancer Statistics Review, 1975-2012. National Cancer Institute: Bethesda, MD, USA. [Google Scholar]
- Huang X-z, Gao P, Sun J-x, Song Y-x, Tsai C-c, Liu J, Chen X-w, Chen P, Xu H-m, Wang Z-n (2015) Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta-analysis. Cancer Causes Control 26: 589–600. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2014) GLOBOCAN 2012. Available at http://globocan.iarc.fr/.
- Liu Y, Chen J-Q, Xie L, Wang J, Li T, He Y, Gao Y, Qin X, Li S (2014) Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med 12: 55–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccio A, Madeddu C (2012) Inflammation and ovarian cancer. Cytokine 58: 133–147. [DOI] [PubMed] [Google Scholar]
- Minlikeeva AN, Freudenheim JL, Lo-Ciganic W-H, Eng KH, Friel G, Diergaarde B, Modugno F, Cannioto R, Gower E, Szender JB, Grzankowski K, Odunsi K, Ness RB, Moysich KB (2015) Use of common analgesics is not associated with ovarian cancer survival. Cancer Epidemiol Biomarkers Prev 24: 1291–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle CM, Ibiebele TI, DeFazio A, Protani MM, Webb PM (2015) Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, acetaminophen and ovarian cancer survival. Cancer Epidemiol 39: 196–199. [DOI] [PubMed] [Google Scholar]
- Poole EM, Rice MS, Tworoger SS (2016) Abstract B29: Pre- and postdiagnosis analgesic use and ovarian cancer survival. Clin Cancer Res 22: B29. [Google Scholar]
- Reyners AKL, de Munck L, Erdkamp FLG, Smit WM, Hoekman K, Lalisang RI, de Graaf H, Wymenga ANM, Polee M, Hollema H, van Vugt M, Schaapveld M, Willemse PHB DoCaCel Study G (2012) A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, Fallopian tube or primary peritoneal carcinomas: the DoCaCel study. Ann Oncol 23: 2896–2902. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343–346. [DOI] [PubMed] [Google Scholar]
- Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, Brinton LA, Webb PM, Nagle CM, Jordan SJ, Risch HA, Rossing MA, Doherty JA, Goodman MT, Lurie G, Kjaer SK, Hogdall E, Jensen A, Cramer DW, Terry KL, Vitonis A, Bandera EV, Olson S, King MG, Chandran U, Anton-Culver H, Ziogas A, Menon U, Gayther SA, Ramus SJ, Gentry-Maharaj A, Wu AH, Pearce CL, Pike MC, Berchuck A, Schildkraut JM, Wentzensen N (2014) Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst 106: djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin B, Yokoyama Y, Shigeto T, Mizunuma H (2007) Anti-tumor effect of non-steroidal anti-inflammatory drugs on human ovarian cancers. Pathol Oncol Res 13: 365–369. [DOI] [PubMed] [Google Scholar]
- Ye XF, Wang J, Shi WT, He J (2014) Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br J Cancer 111: 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.