Abstract
Background:
Squamous cell scrotal carcinoma (SCSC) is an infrequent skin cancer associated historically with occupational carcinogens. Human papillomavirus (HPV) DNA has been associated with SCSC but there is no definitive proof of its oncogenic role.
Methods:
Human papillomavirus-DNA and –E6*I mRNA were analysed in six invasive histologically typed SCSC. LCM-PCR was used to localise HPV DNA to tumour cells. P16INK4aand p53 expression were studied by immunohistochemistry.
Results:
In three warty or basaloid SCSC HPV16-DNA and E6*I-mRNA were detected. LCM-PCR confirmed HPV16 was in p16INK4a-positive malignant cells. However, of three usual-type SCSC, all were HPV-negative and two expressed p53 protein but not p16INK4a.
Conclusions:
Human papillomavirus 16 was present in tumour cells and oncogenically active in basaloid and warty SCSC, whereas usual SCSC was HPV-negative and showed immunostaining, suggesting p53 mutation. The dual pathways of oncogenesis and relation between histological type of SCSC and HPV are similar to that in penile cancers.
Keywords: human papillomavirus, HPV16, scrotal carcinoma, skin tumours
Squamous cell scrotal cancer (SCSC) is a rare malignancy and was the first cancer associated with occupational carcinogens including soot, lubricating and cutting oils (Melicow, 1975; Sorahan et al, 1989). Since the 1970s, occupational-related SCSC incidence has decreased in the United Kingdom as working conditions have improved (Sorahan et al, 1989). More recent reports, however, indicate a steady incidence in the Netherlands and increasing incidence in the USA (Wright et al, 2008; Verhoeven et al, 2010). Other factors may be involved.
Human papillomaviruses (HPV) play an important aetiological role in many anogenital and oropharyngeal carcinomas (http://www.hpvcentre.net) and have been occasionally linked to skin tumours outside the genital region (Riddel et al, 2011). Human papillomavirus-DNA has been found in tissue sections containing SCSC, precancers and normal scrotum, but biological evidence is missing that directly demonstrates type-specific HPV-DNA in tumour cells, producing oncogenic RNA transcripts (Guran and Pak, 1999; Nielson et al, 2009; Matoso et al, 2014, 2016). We tested 6 cases for 72 mucosal and cutaneous HPV-genotypes. If HPV-DNA was detected, laser-capture microdissection-PCR (LCM-PCR) was performed to assign or not an individual HPV type specifically to cancer cells as we have done for cervical (pre)-cancer and other HPV-related cancers (Quint et al, 2012; Guimera et al, 2013; María José Fernández-Nestosa et al, 2017). Viral transcriptional activity was confirmed by detection of viral E6*I oncogene mRNA. Immunohistochemical (IHC) expression of p16INK4a was also studied as in previous studies, and is a sensitive but not specific marker of oncogenic HPV-E7 gene activity. IHC expression of p53 was analysed as an indicator that p53 mutations may play a causal role in the development of HPV-negative scrotal cancers.
Materials and methods
Case selection
Formalin-fixed paraffin-embedded (FFPE) tissue blocks of six cases of invasive SCSC were collected from Spain (n=2), Australia (n=2) and Nigeria (n=2) (Table 1). Local and Catalan Institute of Oncology ethics committees approved all protocols and an international steering committee oversaw study progress.
Table 1. invasive scrotal cancers by country, year of/age at diagnosis, histological category, HPV DNA detection and genotyping by 4 assays, HPV histological localization by LCM, HPV16 E6*I mRNA detection, p16INK4a and p53 overexpression.
|
Cases studied |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Country of origin | Spain | Spain | Australia | Australia | Nigeria | Nigeria |
| Year of diagnosis | 2003 | 2008 | 2002 | 2007 | 2007 | 2015 |
| Age at diagnosis | 71 | 62 | 59 | 46 | 42 | 66 |
| Histological diagnosis | Usual SCC | Usual SCC | Warty SCC | Usual SCC | Basaloid SCC | Basaloid SCC |
| HPV DNA and RNA analysis | ||||||
| SPF10−DEIA-LiPA25 | HPV negative | HPV negative | HPV16 | HPV negative | HPV 16 | HPV 16 |
| MPTS123-PCR Luminex | HPV negative | HPV negative | HPV 16 | HPV negative | HPV 16 | HPV 16 |
| Beta HPVs assay | HPV negative | HPV negative | HPV negative | HPV negative | HPV negative | HPV negative |
| Cutaneous Wart-Associated HPVs | HPV negative | HPV negative | HPV negative | HPV negative | HPV negative | HPV negative |
| HPV16 E6*I mRNA-Luminex | HPV negative | HPV negative | Positive | HPV negative | Positive | Positive |
| DNA and RNA quality control | Positive | Positive | Positive | Positive | Positive | Positive |
| Laser capture microdissection | ||||||
| Localization of HPV | n.a. | n.a. | SCC | n.a. | SCC | SCC |
| Immunohistochemistry analysis in primary cancer cells | ||||||
| p16INK4a overexpression | <5% | 25% | >75% | <5% | >75% | >75% |
| p53 overexpression | >75% | <25% | <25% | >75% | <25% | <25%a |
Abbreviations: −=negative; SCC=well differentiated squamous cell carcinoma; n.a.=not applicable.
Poor slide quality made difficult the analyses.
Histology and immunohistochemistry
Cases were sectioned using a sandwich technique (de Sanjose et al, 2010; Guimera et al, 2013). At least four pathologists evaluated the haematoxylin-eosin (H&E) slides, reaching a consensus diagnosis of the histological type of SCSC: Warty, basaloid or usual. This classification was based on the criteria defined for penile carcinomas by the WHO (Moch et al, 2016). P16INK4a staining was performed on one FFPE-section (4 μm) (Guimera et al, 2013) and was considered positive if more than 50% of tumour cells showed expression. P53 staining was performed (Halec et al, 2014) and a tumour was considered driven by p53 mutation if >75% of the tumour cells showed p53 expression.
Laser capture microdissection (LCM)
Human papillomavirus detected in whole tissue section was localized to tumour cells by LCM-PCR (Quint et al, 2012). A polyethylene-napthalate (PEN)-membrane slide, stained only with Hematoxylin was used to optimise sensitivity.
DNA isolation and HPV-DNA genotyping
The DNA was isolated using proteinase K solution and HPV-DNA was tested with the SPF10-DEIA-LiPA25 system (SPF10 -HPV-LiPA, version1 (Labo-Bio-Medical Products, Rijswijk, the Netherlands) (Kleter et al, 1998, 1999; Quint et al, 2012). In all samples, the endogenous human gene RNaseP served as a positive amplification control using real time PCR (Luo et al, 2005). On whole tissue sections, the HPV genotype detected was confirmed by sequencing of the SPF10 inter-primer region (Geraets et al, 2012). As SPF10 is based on viral L1 open reading frame (ORF) cases were also analysed with MPTS 123 PCR-Luminex assay (van Alewijk et al, 2013) to detect the HPV E6 ORF from 16 different genotypes. The presence of cutaneous HPV from five genera was evaluated by Beta HPV assay (25 beta HPV genotypes) and Cutaneous Wart-Associated HPV (23 alpha, gamma, mu and nu genotypes) assays (de Koning et al, 2006, 2010). The presence of 72 HPVs was analysed for L1, E1 or E6 HPV ORFs.
RNA isolation and E6*I mRNA detection
RNA extraction, HPV16 E6*I mRNA Reverse Transcription (RT)-PCR and luminex genotyping system were used as previously described (Halec et al, 2013). An endogenous human transcript, ubiquitin C (ubC) served as a positive amplification control (Halec et al, 2014).
Results
All six cases were primary SCSC (Table 1). One primary basaloid SCSC showed metastases at presentation. No cases were immuno-compromised.
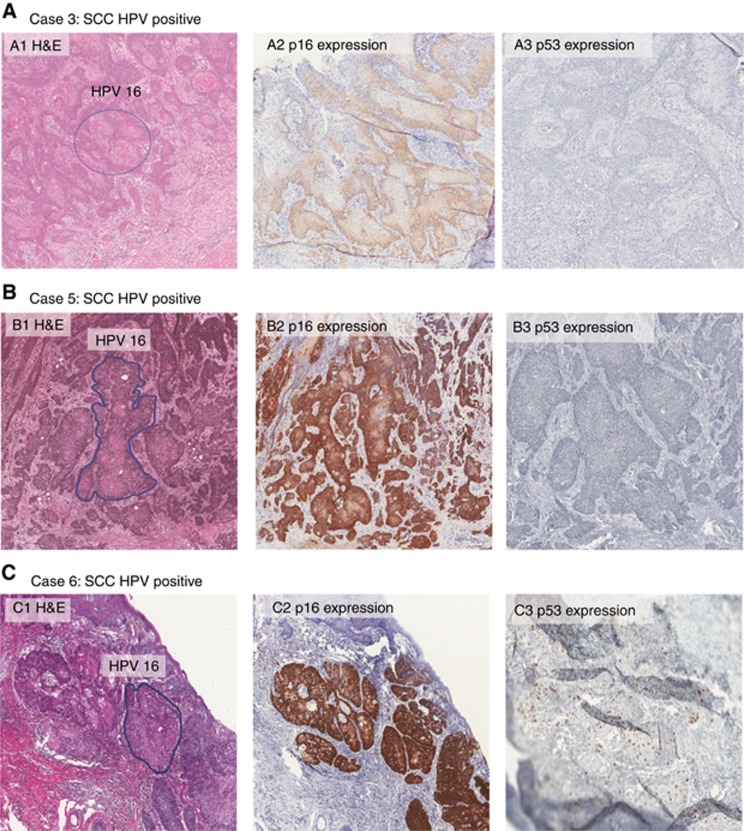
One warty and two basaloid SCSC (case 3, 5 and 6) contained HPV16 DNA as a single infection by both L1 and E6 ORFs assays. E6*I mRNA assay confirmed the presence of HPV16 E6*I transcripts and immunohistochemistry showed diffuse staining for p16INK4a, while p53 was negative. Moreover, LCM-PCR localised HPV16 DNA only in the invasive tumour (Table 1 and Figure 1). From one case with HPV-positive basaloid carcinoma (case 6), HPV16 was confirmed in metastases in three inguinal lymph nodes.
Figure 1.
H&E histological images, p16INK4a and p53 expression pattern of the three HPV16 associated scrotal cancers.The quality of Case C was very poor and the expression of p53 was difficult to analyse.
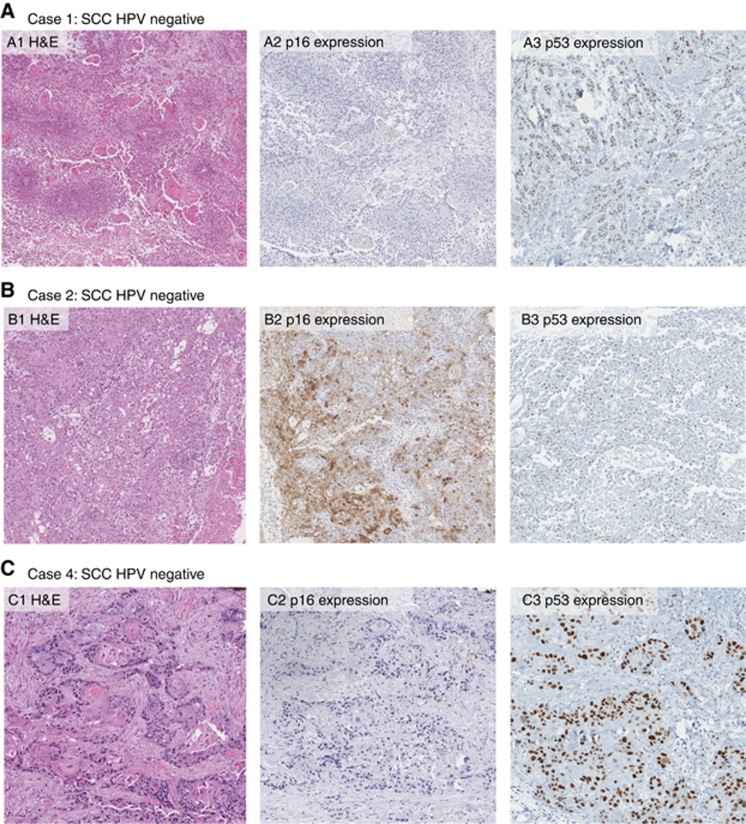
In the three usual-type SCSC specimens (case 1, 2 and 4), no HPV DNA or RNA was detected although quality controls confirmed the presence of human DNA and RNA. In HPV-DNA-negative cases 1 and 4, p53 was expressed in >75% of cancer cells and p16INK4a was completely negative (Table 1 and Figure 2). In case 2, p53 was expressed in around 25% of cancer cells and p16INK4a was expressed only patchily (Table 1 and Figure 2). The median age at diagnosis was 59 years (range: 42–66 years) for HPV-positive cases and 62 years for HPV-negative cases (range: 46–71 years).
Figure 2.
H&E histological images, p16INK4a and p53 expression pattern of the three HPV negative scrotal cancers.
Discussion
Treatments for skin diseases (for example, arsenic and light therapies-UVA/UVB radiation) and HPV have been identified as possible carcinogens to explain the continuing occurrence of SCSC (Stern, 1990; Andrews et al, 1991; Guran and Pak, 1999; Matoso et al, 2016). Previous studies have found HPV-DNA in tissue specimens of SCSC. The current study demonstrates that HPV16 is present specifically in tumour cells of warty and basaloid type SCSC, where it is expressing the HPV-E6 oncogene. In addition, these HPV-positive SCSC show strong expression of p16INK4a. The specific localisation of HPV16 to SCSC tumour cells required the use of LCM-PCR, which excluded potential contamination from adjacent tissue as has been described in studies of cervical cancer and precancer and of penile cancer (Quint et al, 2012; Guimera et al, 2013; María José Fernández-Nestosa et al, 2017). The very sensitive and specific methodology for HPV-DNA detection (Kleter et al, 1998, 1999; Quint et al, 2012), employed in this study together with more specific data on viral transcriptional activity than p16INK4a staining provides new definitive evidence confirming the oncogenic role of HPV16 in some histological types of SCSC (Orihuela et al, 1995; Guran and Pak, 1999; Matoso et al, 2014, 2016).
In two out of three HPV-negative cases of usual type SCSC, p53 was expressed in >75% of the cancer cells indicating the presence of a mutation in this tumour suppressor gene, but p16INK4a was not expressed strongly.
This study clearly demonstrates the two separate aetiological pathways for SCSC suggested previously (Matoso et al, 2014): Human Papillomavirus and non-HPV related, associated with separate histological tumour types. The same pattern has been described by our group using LCM-PCR in penile neoplasia (María José Fernández-Nestosa et al, 2017) and is also seen in vulvar carcinomas, where the HPV prevalence is 33% and 29%, respectively (de Sanjose et al, 2013; Alemany et al, 2016). The association between histology and HPV in SCSC appears the same as that described in penile carcinoma and precancer where neoplasia related to HPV shows a warty/basaloid histology and differentiated PeIN and usual squamous cancer is associated with a HPV-unrelated pathway. In SCSC, HPV16 is the predominant genotype as in all non-cervical sites such as penile, vulval, vaginal, anal or oropharyngeal tumours (de Sanjose et al, 2013; Alemany et al, 2014, 2016; Castellsague et al, 2016). Human papillomavirus16 is also predominant in other cutaneous in situ or invasive SCC outside the genital area such as in periungual and/or subungual skin from immunocompetent patients (Riddel et al, 2011). Scrotal cancer like external vulvar cancer arises in an epithelium that is morphologically genital skin. The embryologic differentiation of scrotal cells resembles that of the vulvar labia majora in women, and may have the same stem-like cells vulnerable to HPV16 infection (Moore et al, 2013).
Although limited by the small number of cases, this study provides biological evidence of an aetiological relationship between HPV16 and SCSC. Larger epidemiological studies are needed to determine if other HPV types apart from HPV16 are involved, the relative role of HPV compared to other environmental and genetic factors and the possible changing role of HPV in scrotal carcinogenesis. Such studies could also determine the potential impact on scrotal cancer of HPV vaccines. Studies of the mechanism and transformation of the scrotal cells by HPV would also contribute to understanding why these events are rare compare to other genital sites, particularly the cervix.
Acknowledgments
Partial support has been obtained from the Stichting Pathologie Ontwikkeling en Onderzoek (SPOO) foundation (the Netherlands). We acknowledge Susana M.Chuva de Sousa Lopes for the fruitful discussion about genital embryologic development.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- Alemany L, Cubilla A, Halec G, Kasamatsu E, Quiros B, Masferrer E, Tous S, Lloveras B, Hernandez-Suarez G, Lonsdale R, Tinoco L, Alejo M, Alvarado-Cabrero I, Laco J, Guimera N, Poblet E, Lombardi LE, Bergeron C, Clavero O, Shin HR, Ferrera A, Felix A, Germar J, Mandys V, Clavel C, Tzardi M, Pons LE, Wain V, Cruz E, Molina C, Mota JD, Jach R, Velasco J, Carrilho C, Lopez-Revilla R, Goodman MT, Quint WG, Castellsague X, Bravo I, Pawlita M, Munoz N, Bosch FX, de Sanjose S group HVs (2016) Role of human papillomavirus in penile carcinomas worldwide. Eur Urol 69(5): 953–961. [DOI] [PubMed] [Google Scholar]
- Alemany L, Saunier M, Tinoco L, Quiros B, Alvarado-Cabrero I, Alejo M, Joura EA, Maldonado P, Klaustermeier J, Salmeron J, Bergeron C, Petry KU, Guimera N, Clavero O, Murillo R, Clavel C, Wain V, Geraets DT, Jach R, Cross P, Carrilho C, Molina C, Shin HR, Mandys V, Nowakowski AM, Vidal A, Lombardi L, Kitchener H, Sica AR, Magana-Leon C, Pawlita M, Quint W, Bravo IG, Munoz N, de Sanjose S, Bosch FX (2014) Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer 50(16): 2846–2854. [DOI] [PubMed] [Google Scholar]
- Andrews PE, Farrow GM, Oesterling JE (1991) Squamous cell carcinoma of the scrotum: long-term followup of 14 patients. J Urol 146(5): 1299–1304. [DOI] [PubMed] [Google Scholar]
- Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, Clavero O, Alos L, Biegner T, Szafarowski T, Alejo M, Holzinger D, Cadena E, Claros E, Hall G, Laco J, Poljak M, Benevolo M, Kasamatsu E, Mehanna H, Ndiaye C, Guimera N, Lloveras B, Leon X, Ruiz-Cabezas JC, Alvarado-Cabrero I, Kang CS, Oh JK, Garcia-Rojo M, Iljazovic E, Ajayi OF, Duarte F, Nessa A, Tinoco L, Duran-Padilla MA, Pirog EC, Viarheichyk H, Morales H, Costes V, Felix A, Germar MJ, Mena M, Ruacan A, Jain A, Mehrotra R, Goodman MT, Lombardi LE, Ferrera A, Malami S, Albanesi EI, Dabed P, Molina C, Lopez-Revilla R, Mandys V, Gonzalez ME, Velasco J, Bravo IG, Quint W, Pawlita M, Munoz N, Sanjose S, Xavier Bosch F Head ICOIHi, Neck Cancer Study G (2016) HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 108(6): djv403. [DOI] [PubMed] [Google Scholar]
- de Koning M, Quint W, Struijk L, Kleter B, Wanningen P, van Doorn LJ, Weissenborn SJ, Feltkamp M, ter Schegget J (2006) Evaluation of a novel highly sensitive, broad-spectrum PCR-reverse hybridization assay for detection and identification of beta-papillomavirus DNA. J Clin Microbiol 44(5): 1792–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning MN, ter Schegget J, Eekhof JA, Kamp M, Kleter B, Gussekloo J, Feltkamp MC, Bouwes Bavinck JN, Purdie KJ, Bunker CB, Proby CM, Meys R, Harwood CA, Quint WG (2010) Evaluation of a novel broad-spectrum PCR-multiplex genotyping assay for identification of cutaneous wart-associated human papillomavirus types. J Clin Microbiol 48(5): 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, Joura EA, Maldonado P, Laco J, Bravo IG, Vidal A, Guimera N, Cross P, Wain GV, Petry KU, Mariani L, Bergeron C, Mandys V, Sica AR, Felix A, Usubutun A, Seoud M, Hernandez-Suarez G, Nowakowski AM, Wilson G, Dalstein V, Hampl M, Kasamatsu ES, Lombardi LE, Tinoco L, Alvarado-Cabrero I, Perrotta M, Bhatla N, Agorastos T, Lynch CF, Goodman MT, Shin HR, Viarheichyk H, Jach R, Cruz MO, Velasco J, Molina C, Bornstein J, Ferrera A, Domingo EJ, Chou CY, Banjo AF, Castellsague X, Pawlita M, Lloveras B, Quint WG, Munoz N, Bosch FX (2013) Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 49(16): 3450–3461. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menendez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andujar M, Castellsague X, Sanchez GI, Nowakowski AM, Bornstein J, Munoz N, Bosch FX Retrospective International S, Group HPVTTS (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11): 1048–1056. [DOI] [PubMed] [Google Scholar]
- Geraets D, Alemany L, Guimera N, de Sanjose S, de Koning M, Molijn A, Jenkins D, Bosch X, Quint W (2012) Detection of rare and possibly carcinogenic human papillomavirus genotypes as single infections in invasive cervical cancer. J Pathol 228(4): 534–543. [DOI] [PubMed] [Google Scholar]
- Guimera N, Lloveras B, Lindeman J, Alemany L, van de Sandt M, Alejo M, Hernandez-Suarez G, Bravo IG, Molijn A, Jenkins D, Cubilla A, Munoz N, de Sanjose S, Bosch FX, Quint W (2013) The occasional role of low-risk human papillomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: results from a global study. Am J Surg Pathol 37(9): 1299–1310. [DOI] [PubMed] [Google Scholar]
- Guran S, Pak I (1999) Cumulation of TP53 mutations and p16INK4A/p15INK4B homozygous deletions in human papilloma virus type 16 positive scrotal cancer. Cancer Genet Cytogenet 109(2): 108–113. [DOI] [PubMed] [Google Scholar]
- Halec G, Schmitt M, Dondog B, Sharkhuu E, Wentzensen N, Gheit T, Tommasino M, Kommoss F, Bosch FX, Franceschi S, Clifford G, Gissmann L, Pawlita M (2013) Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int J Cancer 132(1): 63–71. [DOI] [PubMed] [Google Scholar]
- Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimera N, Grabe N, Lahrmann B, Gissmann L, Quint W, de Sanjose S, Pawlita M (2014) Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol 234(4): 441–451. [DOI] [PubMed] [Google Scholar]
- Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W (1999) Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 37(8): 2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W (1998) Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 153(6): 1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Yang H, Rathbun K, Pau CP, Ou CY (2005) Detection of human immunodeficiency virus type 1 DNA in dried blood spots by a duplex real-time PCR assay. J Clin Microbiol 43(4): 1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- María José Fernández-Nestosa Núria Guimerá, Sanchez DF, Cañete S, Velazquez EF, Jenkins D, Quint W, Cubilla AL (2017) Human Papillomavirus (HPV) genotypes in condylomas, intraepithelial neoplasia and invasive carcinoma of the penis using Laser Capture Microdissection (LCM)-PCR. A study of 191 lesions in 43 patients. Am J Surg Pathol (in press). [DOI] [PubMed]
- Matoso A, Fabre V, Quddus MR, Lepe M, Lombardo KA, Manna P, Epstein JI (2016) Prevalence and distribution of 15 high-risk human papillomavirus types in squamous cell carcinoma of the scrotum. Hum Pathol 53: 130–136. [DOI] [PubMed] [Google Scholar]
- Matoso A, Ross HM, Chen S, Allbritton J, Epstein JI (2014) Squamous neoplasia of the scrotum: a series of 29 cases. Am J Surg Pathol 38(7): 973–981. [DOI] [PubMed] [Google Scholar]
- Melicow MM (1975) Percivall Pott (1713–1788): 200th anniversary of first report of occupation-induced cancer scrotum in chimmey sweepers (1775). Urology 6(6): 745–749. [DOI] [PubMed] [Google Scholar]
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol 70(1): 93–105. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN, Torchia MG (2013) The Developing Human: Clinically Oriented Embryology 9th edn. Saunders/Elsevier: Philadelphia, PA, USA. [Google Scholar]
- Nielson CM, Harris RB, Flores R, Abrahamsen M, Papenfuss MR, Dunne EF, Markowitz LE, Giuliano AR (2009) Multiple-type human papillomavirus infection in male anogenital sites: prevalence and associated factors. Cancer Epidemiol Biomarkers Prev 18(4): 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela E, Tyring SK, Pow-Sang M, Dozier S, Cirelli R, Arany I, Rady P, Sanchez R (1995) Development of human papillomavirus type 16 associated squamous cell carcinoma of the scrotum in a patient with Darier's disease treated with systemic isotretinoin. J Urol 153(6): 1940–1943. [PubMed] [Google Scholar]
- Quint W, Jenkins D, Molijn A, Struijk L, van de Sandt M, Doorbar J, Mols J, Van Hoof C, Hardt K, Struyf F, Colau B (2012) One virus, one lesion—individual components of CIN lesions contain a specific HPV type. J Pathol 227(1): 62–71. [DOI] [PubMed] [Google Scholar]
- Riddel C, Rashid R, Thomas V (2011) Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol 64(6): 1147–1153. [DOI] [PubMed] [Google Scholar]
- Sorahan T, Cooke MA, Wilson S (1989) Incidence of cancer of the scrotum, 1971–84. Br J Ind Med 46(6): 430–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RS (1990) Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. N Engl J Med 322(16): 1093–1097. [DOI] [PubMed] [Google Scholar]
- van Alewijk D, Kleter B, Vent M, Delroisse JM, de Koning M, van Doorn LJ, Quint W, Colau B (2013) A human papilloma virus testing algorithm comprising a combination of the L1 broad-spectrum SPF10 PCR assay and a novel E6 high-risk multiplex type-specific genotyping PCR assay. J Clin Microbiol 51(4): 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven RH, Louwman WJ, Koldewijn EL, Demeyere TB, Coebergh JW (2010) Scrotal cancer: incidence, survival and second primary tumours in the Netherlands since 1989. Br J Cancer 103(9): 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Morgan TM, Lin DW (2008) Primary scrotal cancer: disease characteristics and increasing incidence. Urology 72(5): 1139–1143. [DOI] [PubMed] [Google Scholar]