Abstract
Background:
Most studies of environmental risk factors and breast cancer are conducted using average risk cohorts.
Methods:
We examined the association between polycyclic aromatic hydrocarbon (PAH)-albumin adducts in bloods from baseline and breast cancer risk in a prospective nested case–control study (New York site of the BCFR, 80 cases and 156 controls). We estimated the 10-year absolute breast cancer risk by a risk model that uses pedigree information (BOADICEA) and evaluated whether the increased risk from PAH differed by absolute risk.
Results:
Women with detectable levels of PAH had a twofold association with breast cancer risk (odds ratio (OR)=2.04; 95% CI=1.06–3.93) relative to women with non-detectable levels. The association increased with higher levels of PAH (⩾median) and by a higher level of absolute breast cancer risk (10-year risk ⩾3.4%: OR=4.09, 95% CI=1.38–12.13).
Conclusions:
These results support that family-based cohorts can be an efficient way to examine gene–environment interactions.
Keywords: breast cancer, PAH, family history, gene–environment interaction, aetiology, prevention
Many epidemiological studies, particularly those using population-based ascertainment, do not include a substantial proportion of subjects with a cancer family history, and are therefore not enriched for underlying genetic susceptibility (Terry et al, 2016). This can have an impact on both the precision and ability to identify associations if the risk gradient depends on underlying genetic susceptibility (i.e., gene–environment interactions). Improving precision can be readily accomplished through sampling more individuals. However, increasing the sample size without ensuring adequate individuals at greater disease susceptibility are included will limit the ability to detect gene–environment interactions. Thus, the role of a given environmental factor may be under-recognised if gene–environment interactions exist. Using a family-based cohort enriched with individuals across the risk spectrum, we illustrate the importance of having sufficient numbers of individuals across the risk spectrum to properly test the role of environmental factors on cancer risk. We illustrate this concept by estimating the association between polycyclic aromatic hydrocarbons (PAHs) and breast cancer risk as a function of a woman's underlying genetic risk inferred from her cancer family history.
Polycyclic aromatic hydrocarbons, a group of compounds with two or more fused benzene rings, are environmental contaminants that play an important carcinogenic role due to widespread population exposure (Phillips, 1983; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2010). Polycyclic aromatic hydrocarbons can be bio-transformed into reactive intermediates that form covalent PAH-DNA adducts that have mutagenic properties to initiate and/or promote tumorigenesis (Phillips, 1983). Polycyclic aromatic hydrocarbons are lipophilic, are stored in the fat tissue of the breast and have shown endocrine-disrupting and obesogenic capabilities to cause mammary cancer in rodents (Morris and Seifter, 1992). The potential carcinogenic mechanisms of metabolised PAHs may be partially due to their structural similarities with oestrogenic compounds that affect hormone signalling pathways or increase the bioaccumulation capacity in adipose tissue and increase breast cancer risk (Macon and Fenton, 2013; Zhang et al, 2016). Therefore, the International Agency for Research on Cancer has classified selected PAHs, such as benzo[a]pyrene, as human carcinogens (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2010); the US Environmental Protection Agency (EPA) also lists PAHs as possible carcinogens (Mumtaz et al, 1996).
Several large studies have examined the role of PAHs on breast cancer risk. The Long Island Breast Cancer Study Project (LIBCSP), a population-based case–control study, found a statistically significant increased risk for women with detectable levels of PAH-DNA adducts in blood (Gammon et al, 2002, 2004), and estimated a 50% greater risk of breast cancer for women in the highest vs lowest quintile of PAH-DNA adducts. Breast cancer was increased by 40% for women reporting ever burning synthetic logs (associated with bulky PAH-DNA adducts) in their homes (White et al, 2014), and 44% for women exposed to the highest level (vs below the median) of vehicular traffic (Mordukhovich et al, 2016b). Polycyclic aromatic hydrocarbon exposures from other sources, including tobacco smoking (Gaudet et al, 2013), dietary intake (White et al, 2016), grilled and smoked meats(Fu et al, 2011; Di Maso et al, 2013), also contribute to increased breast cancer risk. However, two cohort studies have not observed any associations with PAHs and breast cancer risk that may be due to use of the postlabelling assay that is not specific for PAH adducts or measuring non-carcinogenic PAH markers in urine where urinary measures only reflect short-term exposure (Lee et al, 2010; Saieva et al, 2011). A growing number of studies found that exposure to PAHs may further enhance breast cancer risk for women carrying higher susceptibility genetic variants involved in carcinogen metabolism, DNA repair and cell cycle control pathways (Terry et al, 2004; Gaudet et al, 2008; Mordukhovich et al, 2016a). These stronger associations in subgroups defined by genetic variants suggests that women with higher breast cancer risk based on family history would also have higher risk but detecting interactions between environmental carcinogens and underlying risk requires a sufficient number of women at higher risk for cancer. We hypothesised that the women at greater risk of breast cancer from PAH exposure are the women who have higher underlying absolute risk of breast cancer predicted from their cancer family history.
Materials and methods
Study design
We conducted a prospective study within the women unaffected with breast cancer at enrolment in the New York site of the Breast Cancer Family Registry (BCFR), a registry of individuals within families with breast and/or ovarian cancer (for details, see John et al, 2004; Quante et al, 2012). At recruitment, each eligible subject completed a questionnaire that included information on demographics, lifestyle and environmental factors, past surgeries and family history of cancer (John et al, 2004). We actively followed participants for subsequent information on cancer incidence and vital status and attempted to verify cancers through pathology reviews and reports and medical records. In the current nested case–control study, we analysed data for 80 prospectively ascertained breast cancer cases and 156 age- and ethnicity-matched controls. All cohort participants provided written informed consent, and the study was approved by the relevant local ethics committees.
PAH-albumin adducts
We measured plasma PAH-albumin adducts by competitive enzyme linked immunosorbent assay using monoclonal antibody 8E11 that recognises benzo(a)pyrene diolepoxide tetrols and related PAH metabolites. A pooled quality control sample was run within each batch (Santella et al, 1995), and the value was expressed as fmol of PAH per mg albumin.
Absolute risk of breast cancer
We calculated 10-year risk of breast cancer using available family pedigree and vital status data from all family members on cancer diagnoses and age at diagnoses and information on BRCA1 and BRCA2 mutations using the risk model BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) (Antoniou et al, 2004, 2008; Lee et al, 2014). We used this probability as a continuous risk score in the regression analyses.
Statistical analysis
After descriptive analyses, we used conditional logistic regression based on the matched sets (1 : 2 age and race/ethnicity matched) to estimate breast cancer risk for women with detectable PAH-albumin adducts compared with women with non-detectable adducts (referent). We further categorised detectable adducts based on the median for matched control women with detectable adducts to examine high vs low levels of detectable adducts. We considered the potential confounding effects of age at blood draw and body mass index. We further stratified by menopausal status, smoking status, BOADICEA 10-year risk score (⩾3.4 vs <3.4%) and examined interactions across the continuous BOADICEA score by centring PAH at the mean and including an indicator variable for non-detectable levels. We selected 3.4 as a categorical cutoff for absolute 10-year risk because a commonly used clinical cutoff to indicate high risk for 5-year risk is 1.67%. We also compared interactions using a continuous level of PAH-albumin adducts for those with detectable levels and a continuous 10-year BOADICEA score (Figure 1). We formally tested for statistical significance of interactions through a cross-product term for multiplicative interactions and used the relative excess risk due to interaction (RERI) for additive interactions.
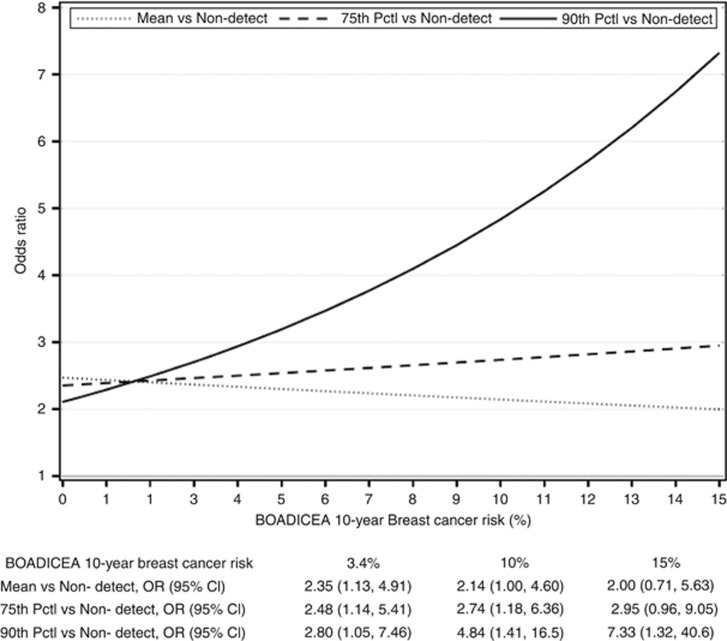
Figure 1.
Increase in breast cancer risk from PAH exposure by absolute risk of breast cancer as estimated by the BOADICEA, New York site of the BCFR. Results from a multivariable conditional logistic regression of breast cancer risk with the following covariates: centred PAH-albumin adducts, indicator variable for women with non-detectable levels, continuous BOADICEA score, interactions between BOADICEA and centred PAH-albumin adducts and indicator variable for women with non-detectable values, and further adjusted for age at blood draw, body mass index and smoking status. P-value for multiplicative interaction=0.09.
Results
Table 1 summarises the overall association with categorised PAH. The highest category of PAHs was associated with breast cancer risk (OR=2.89, 95% CI: 1.25–6.69). The associations were higher for women with 10-year BOADICEA score ⩾3.4 (OR=4.09, 95% CI: 1.38–12.13) compared with women with a 10-year BOADICEA score <3.4. The RERI was positive but not statistically significant (RERI=1.75, 95% CI=−1.90, 5.40). Figure 1 shows the association between PAHs and BOADICEA with BOADICEA considered on a continuous scale and PAH levels on a continuous scale for the mean level, the 75th and the 90th percentiles (test for multiplicative interaction P=0.09). The higher the absolute risk of breast cancer, the stronger the association with PAHs. There was no interaction with BOADICEA scores for women with non-detectable levels of PAHs.
Table 1. Plasma PAH-albumin adducts and breast cancer risk categorised by menopausal status and absolute risk score, New York site of the BCFR.
|
Cases |
Controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAH-albumin adducts (fmol mg−1) | N=80 | % | N=156 | % | ORa | (95% CI) | ORb | (95% CI) | ||
|
All women | ||||||||||
| Non-detectable | 27 | 36.0 | 72 | 49.3 | Reference | Reference | ||||
| Detectable | 48 | 64.0 | 74 | 50.7 | 1.90 | 1.00 | 3.63 | 2.04 | 1.06 | 3.93 |
| <Medianc | 20 | 26.7 | 37 | 25.3 | 1.49 | 0.71 | 3.15 | 1.59 | 0.75 | 3.39 |
| ⩾Median | 28 | 37.3 | 37 | 25.3 | 2.66 | 1.17 | 6.05 | 2.89 | 1.25 | 6.69 |
|
Premenopausal women only | ||||||||||
| Non-detectable | 18 | 36.0 | 52 | 50.0 | Reference | Reference | ||||
| Detectable | 32 | 64.0 | 52 | 50.0 | 2.19 | 0.99 | 4.83 | 2.30 | 1.00 | 5.27 |
| <Medianc | 15 | 30.0 | 27 | 26.0 | 1.81 | 0.73 | 4.49 | 1.93 | 0.73 | 5.06 |
| ⩾Median | 17 | 34.0 | 25 | 24.0 | 2.91 | 1.03 | 8.21 | 2.87 | 1.01 | 8.17 |
|
Interaction with BOADICEA Risk Scored | ||||||||||
| Non-detect or Detect <median, <3.4% | 12 | 16.0 | 38 | 27.0 | Reference | Reference | ||||
| Detect⩾Median, <3.4% | 7 | 9.3 | 15 | 10.6 | 1.75 | 0.54 | 5.68 | 1.81 | 0.55 | 5.94 |
| Non-detect or detect <median, ⩾3.4% | 35 | 46.7 | 68 | 48.2 | 1.65 | 0.72 | 3.77 | 1.54 | 0.66 | 4.04 |
| Detect⩾Median, ⩾3.4% | 21 | 28.0 | 20 | 14.2 | 4.01 | 1.39 | 11.58 | 4.09 | 1.38 | 12.13 |
Conditional logistic regression, unadjusted.
Conditional logistic regression, adjusted for age at blood draw, body mass index and smoking status.
Median=73.0 fmol mg−1 among controls.
Additive interaction tested using RERI (1.75, 95% CI=−1.90, 5.40).
Discussion
Women with higher PAH albumin adducts had 2–3 times greater breast cancer risk in our cohort. The associations were even stronger for women with higher absolute risk of breast cancer (OR=4.09, 95% CI=1.28–12.13). Our results provide further evidence that PAHs are breast carcinogens and support the stronger associations seen in other studies once subgroups were divided based on risk. For example, in the LIBCSP, traffic-related PAH exposures were positively associated with increased breast cancer risk by interactions with specific polymorphisms in DNA repair genes of ERCC2, XRCC1 and OGG1 (ORs from 2.32 to 2.96) (Mordukhovich et al, 2016a). Associations (∼2-fold and higher) were observed for women with greater inherited genetic susceptibility to the effects of PAH due to variants in carcinogen metabolism, DNA repair, response to oxidative stress and cellular apoptosis genes, as well as the tumour suppressor gene p53 (Terry et al, 2004; Gaudet et al, 2008). In addition, constitutional DNA methylation in several breast cancer susceptibility genes (BRCA1, PALB2 and MLH1) has been associated with increased risk of early-onset breast cancers with genetic mutations (both germline and somatic mutations) (Wong et al, 2011; Scott et al, 2016). Changes in DNA methylation in RARβ and APC suggest a stronger aetiologic effect of PAH exposure on breast cancer risk for higher susceptibility women (White et al, 2015). These results are biologically plausible because genetic mutation can disrupt DNA methylation patterns, and hence, impact gene expression and function. Meanwhile, epigenetic changes precede downstream genetic mutation in tumorigenesis that can disable DNA repair functions (You and Jones, 2012).
These findings also illustrate the benefit of using high-risk cohorts to examine gene–environment interactions as we covered the full range of underlying breast cancer risk unlike many cohorts that predominantly have only a small proportion of women at high risk. Thus, family-based cohorts can be useful not only for gene discovery but as a very effective way to examine environmental exposures. If replicated in larger cohorts, these results support the hypothesis that PAH may be a strong breast carcinogen for women with greater underlying breast cancer susceptibility.
Acknowledgments
The New York site of the Breast Cancer Family Registry is supported by grants R01 CA 159868 and UM1 CA164920 from the US National Cancer Institute and ES009089. This work was also supported by the Breast Cancer Research Foundation. John Hopper is a Senior Principal Fellow of the National Health and Medical Research Council of Australia. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products or organisations imply endorsement by the USA Government or the BCFR.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Southey MC, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tryggvadottir L, Syrjakoski K, Kallioniemi OP, Eerola H, Nevanlinna H, Pharoah PD, Easton DF (2008) The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 98(8): 1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou AC, Pharoah PP, Smith P, Easton DF (2004) The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91(8): 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, Negri E, Levi F, La Vecchia C, Franceschi S, Serraino D, Polesel J (2013) Red meat and cancer risk in a network of case-control studies focusing on cooking practices. Ann Oncol 24(12): 3107–3112. [DOI] [PubMed] [Google Scholar]
- Fu Z, Deming SL, Fair AM, Shrubsole MJ, Wujcik DM, Shu XO, Kelley M, Zheng W (2011) Well-done meat intake and meat-derived mutagen exposures in relation to breast cancer risk: the Nashville Breast Health Study. Breast Cancer Res Treat 129(3): 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon MD, Sagiv SK, Eng SM, Shantakumar S, Gaudet MM, Teitelbaum SL, Britton JA, Terry MB, Wang LW, Wang Q, Stellman SD, Beyea J, Hatch M, Kabat GC, Wolff MS, Levin B, Neugut AI, Santella RM (2004) Polycyclic aromatic hydrocarbon-DNA adducts and breast cancer: a pooled analysis. Arch Environ Health 59(12): 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon MD, Santella RM, Neugut AI, Eng SM, Teitelbaum SL, Paykin A, Levin B, Terry MB, Young TL, Wang LW, Wang Q, Britton JA, Wolff MS, Stellman SD, Hatch M, Kabat GC, Senie R, Garbowski G, Maffeo C, Montalvan P, Berkowitz G, Kemeny M, Citron M, Schnabel F, Schuss A, Hajdu S, Vinceguerra V (2002) Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev 11(8): 677–685. [PubMed] [Google Scholar]
- Gaudet MM, Gammon MD, Bensen JT, Sagiv SK, Shantakumar S, Teitelbaum SL, Eng SM, Neugut AI, Santella RM (2008) Genetic variation of TP53, polycyclic aromatic hydrocarbon-related exposures, and breast cancer risk among women on Long Island, New York. Breast Cancer Res Treat 108(1): 93–99. [DOI] [PubMed] [Google Scholar]
- Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ (2013) Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst 105(8): 515–525. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 92: 1–853. [PMC free article] [PubMed] [Google Scholar]
- John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd N, Buys SS, Daly MB, O'Malley FP, Santella RM, Southey MC, Venne VL, Venter DJ, West DW, Whittemore AS, Seminara D (2004) The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 6(4): R375–R389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC (2014) BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 110(2): 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Shu XO, Gao YT, Ji BT, Yang G, Blair A, Rothman N, Zheng W, Chow WH, Kang D (2010) Breast cancer and urinary biomarkers of polycyclic aromatic hydrocarbon and oxidative stress in the Shanghai Women's Health Study. Cancer Epidemiol Biomarkers Prev 19(3): 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon MB, Fenton SE (2013) Endocrine disruptors and the breast: early life effects and later life disease. J Mammary Gland Biol Neoplasia 18(1): 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, Richardson DB, Millikan RC, Engel LS, Shantakumar S, Steck SE, Neugut AI, Rossner P Jr, Santella RM, Gammon MD (2016. a) Polymorphisms in DNA repair genes, traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence. Int J Cancer 139(2): 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, Richardson DB, Millikan RC, Engel LS, Shantakumar S, Steck SE, Neugut AI, Rossner P Jr, Santella RM, Gammon MD (2016. b) Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ Health Perspect 124(1): 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JJ, Seifter E (1992) The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses 38(3): 177–184. [DOI] [PubMed] [Google Scholar]
- Mumtaz MM, George JD, Gold KW, Cibulas W, DeRosa CT (1996) ATSDR evaluation of health effects of chemicals. IV. Polycyclic aromatic hydrocarbons (PAHs): understanding a complex problem. Toxicol Ind Health 12(6): 742–971. [DOI] [PubMed] [Google Scholar]
- Phillips DH (1983) Fifty years of benzo(a)pyrene. Nature 303(5917): 468–472. [DOI] [PubMed] [Google Scholar]
- Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB (2012) Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res 14(6): R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saieva C, Peluso M, Masala G, Munnia A, Ceroti M, Piro S, Sera F, Bendinelli B, Pala V, Sieri S, Tumino R, Giurdanella MC, Panico S, Mattiello A, Vineis P, Polidoro S, Matullo G, Palli D (2011) Bulky DNA adducts and breast cancer risk in the prospective EPIC-Italy study. Breast Cancer Res Treat 129(2): 477–484. [DOI] [PubMed] [Google Scholar]
- Santella RM, Perera FP, Young TL, Zhang YJ, Chiamprasert S, Tang D, Wang LW, Beachman A, Lin JH, DeLeo VA (1995) Polycyclic aromatic hydrocarbon-DNA and protein adducts in coal tar treated patients and controls and their relationship to glutathione S-transferase genotype. Mutat Res 334(2): 117–124. [DOI] [PubMed] [Google Scholar]
- Scott CM, Joo JE, O'Callaghan N, Buchanan DD, Clendenning M, Giles GG, Hopper JL, Wong EM, Southey MC (2016) Methylation of breast cancer predisposition genes in early-onset breast cancer: Australian Breast Cancer Family Registry. PLoS One 11(11): e0165436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Gammon MD, Zhang FF, Eng SM, Sagiv SK, Paykin AB, Wang Q, Hayes S, Teitelbaum SL, Neugut AI, Santella RM (2004) Polymorphism in the DNA repair gene XPD, polycyclic aromatic hydrocarbon-DNA adducts, cigarette smoking, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 13(12): 2053–2058. [PubMed] [Google Scholar]
- Terry MB, Phillips KA, Daly MB, John EM, Andrulis IL, Buys SS, Goldgar DE, Knight JA, Whittemore AS, Chung WK, Apicella C, Hopper JL (2016) Cohort Profile: The Breast Cancer Prospective Family Study Cohort (ProF-SC). Int J Epidemiol 45(3): 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, Steck SE, Mordukhovich I, Eng SM, Engel LS, Conway K, Hatch M, Neugut AI, Santella RM, Gammon MD (2016) Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int 89-90: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Chen J, McCullough LE, Xu X, Cho YH, Teitelbaum SL, Neugut AI, Terry MB, Hibshoosh H, Santella RM, Gammon MD (2015) Polycyclic aromatic hydrocarbon (PAH)-DNA adducts and breast cancer: modification by gene promoter methylation in a population-based study. Cancer Causes Control 26(12): 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Teitelbaum SL, Stellman SD, Beyea J, Steck SE, Mordukhovich I, McCarty KM, Ahn J, Rossner P Jr, Santella RM, Gammon MD (2014) Indoor air pollution exposure from use of indoor stoves and fireplaces in association with breast cancer: a case-control study. Environ Health 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EM, Southey MC, Fox SB, Brown MA, Dowty JG, Jenkins MA, Giles GG, Hopper JL, Dobrovic A (2011) Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. PLoS One 4(1): 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Jones PA (2012) Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22(1): 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong S, Wang H, Tao S, Kiyama R (2016) Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut 213: 809–824. [DOI] [PubMed] [Google Scholar]