Abstract
Background:
Associations of stomach cancer risk with histamine type-2 receptor antagonists (H2RA) and proton-pump inhibitors (PPI) are controversial. We hypothesised that proximal extension of Helicobacter pylori infection from acid suppression would disproportionately increase cancers at proximal subsites.
Methods:
A total of 1 563 860 individuals in the Danish Prescription Drug Registry first prescribed acid-suppressive drugs 1995–2011 were matched to unexposed population-based controls. Hazard ratios (HR) were calculated by Cox proportional hazard regression for stomach cancers diagnosed more than one year after first prescription.
Results:
There were 703 stomach cancers among H2RA-exposed individuals and 1347 among PPI-exposed. Restricted to individuals with five or more prescriptions, subsite-specific HRs for H2RA and PPI were 4.1 and 6.4 for proximal subsites vs 8.0 and 10.3 for distal subsites, respectively.
Conclusions:
Moderate exposures to acid-suppressive drugs did not favour proximal tumour localisation. Given confounding by indication, these findings do not resolve potential contribution to gastric carcinogenesis overall.
Keywords: acid-suppressing therapy, anatomic subsite, gastric cancer, histamine type-2 receptor antagonists, national registry, proton-pump inhibitors
Even though stomach cancer burden has declined over recent decades worldwide, elevated incidence in the middle portion of the stomach has recently been observed in the United States data (Camargo et al, 2011; Torre et al, 2015). Previous studies suggested that acid-inhibitor therapies alter the distribution within the stomach of H. pylori infection, the primary risk factor for noncardia stomach cancer (Hodgson et al, 2005; Garcia Rodriguez et al, 2006). We therefore hypothesised that the proximal extension of H. pylori infection resulting from acid suppression would disproportionately increase cancers of the proximal stomach.
Materials and methods
Our study combined information from the Danish Prescription Drug Registry (DPDR), the Danish Civil Registration System (CRS) and the Danish Cancer Registry (DCR). All individuals who filled a prescription for histamine-2 receptor antagonist (H2RA; ATC-code: A02BA) and proton pump inhibitors (PPI; ATC-code: A02BC) from 1 January 1994 were identified. Individuals with a prescription during 1994 were excluded from the main analysis. For each exposed individual in the DPDR, up to 10 unexposed control persons matched on sex, age (+/−180 days) and municipality were randomly selected from the CRS using the first filled prescription as the index date. Both cohorts were linked to the DCR for cancers reported between 1943 and 2011. Incident stomach cancers were anatomically classified according to the International Classification of Diseases (ICD)-10 into cardia (C16.0), noncardia (C16.1–C16.6), proximal (C16.1 and C16.2), distal (C16.3 and C16.4), and unspecified (C16.5, C16.6, C16.8 and C16.9) subsites (Gjerstorff, 2011). Participants were followed starting one year after the index date until an event of stomach cancer, emigration, death or end of follow-up (31 December 2011), and controls were also censored at the event of a filled acid-suppressing drug prescription.
We estimated stomach cancer subsite-specific hazard ratios (HR) with 95% confidence intervals (CI) for individuals with a filled prescription for H2RA or PPI compared to those without, using Cox proportional hazard regression models stratified on matched sets. Time since index date was the underlying time, with the periods 1–2.9 and 3+ years considered separately and combined in the main analyses. In addition, sensitivity analyses exploring 0–1 year after initial exposure were included. Models were further stratified for number of filled prescriptions as 1–4, 5–14 and ⩾15. The effect of exposure was estimated with restricted cubic spline plots with knots specified at 0.25, 0.5, 0.75, 1, 3, 5 and 10 years since first recorded prescription. We also performed a sensitivity analysis to study the effect of combined exposure to both types of acid-suppressing drugs.
To assess the influence of H. pylori infection, we performed a sensitivity analysis where individuals were censored at time of H. pylori eradication treatment. We defined H. pylori treatment as filled prescriptions of antibiotics and an acid-suppressing drug (H2RA or PPI) within 35 days, as either triple, sequential or quadruple (with Bismuth subcitrate (ATC-code: A02BX05)) therapy (Malfertheiner et al, 2017). The antibiotics considered were as follows: clarithromycin (ATC-code: J01FA09), amoxicillin (ATC-code: J01CA04), metronidazole (ATC-code: P01AB01) and tetracycline (ATC-code: J01A). For another sensitivity analysis, time-dependent propensity scores based on the DNPR data (Supplementary Table S1; (Lynge et al, 2011)) were used to select an alternative control group with similar chance of exposure to acid-suppressing drugs (maximum difference 0.2 s.d. within matched sets). A priori, if the overall HRs did not differ >10%, further analyses only considered the cohort without propensity score matching.
All analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC, USA). We used only the anonymised data without contact or active participation of research subjects and our study complied with regulations of the Danish Data Protection Agency (Danish Protection Board approval no. 2015-57-0102).
Results
Our study population consisted of 1 563 860 individuals prescribed acid-suppressing drugs between 1 January 1995, and 31 December 2011, excluding 201 917 individuals with prescriptions during 1994, Table 1.
Table 1. Baseline characteristics of study cohort, years 1995–2011.
|
First prescription |
||
|---|---|---|
| Characteristics | H2RA (N) | PPI (N) |
|
Sex | ||
| Female | 220 565 | 646 607 |
| Male | 164 093 | 532 595 |
|
Age (years) at first acid-suppressing drug prescription | ||
| <40 | 148 923 | 349 109 |
| 40–59 | 123 327 | 375 182 |
| 60–69 | 48 149 | 183 904 |
| 70–79 | 40 546 | 151 600 |
| 70+ | 23 713 | 119 407 |
|
Year of first acid-suppressing drug prescription | ||
| 1995–1998 | 206 806 | 170 494 |
| 1999–2002 | 107 574 | 223 918 |
| 2003–2006 | 59 060 | 294 881 |
| 2007–2011 | 11 218 | 489 909 |
|
Number of filled H2RA prescriptions at end of follow-up | ||
| 0 | 0 | 161 574 |
| 1 | 202 435 | 51 821 |
| 2–4 | 113 265 | 61 762 |
| 5–14 | 43 601 | 52 579 |
| 15+ | 25 357 | 56 922 |
|
Number of filled PPI prescriptions at end of follow-up | ||
| 0 | 1 110 146 | 0 |
| 1 | 35 056 | 439 244 |
| 2–4 | 19 212 | 351 153 |
| 5–14 | 9 283 | 215 361 |
| 15+ | 5 505 | 173 444 |
|
Type of H2RA (ATC; A02BAXX) at first filled prescription | ||
| Cimetidine (01) | 204 933 | NA |
| Ranitidine (02) | 87 362 | NA |
| Famotidine (03) | 175 | NA |
| Nizatidine (04) | 91 648 | NA |
| Ranitidine bismuth citrate (07) | 540 | NA |
| Total H2RA | 384 658 | NA |
|
Type of PPI (ATC; A02BCXX) at first filled prescription | ||
| Omeprazole (01) | NA | 364 161 |
| Pantoprazole (02) | NA | 253 216 |
| Lansoprazole (03) | NA | 360 439 |
| Rabeprazole (04) | NA | 9 694 |
| Esomeprazole (05) | NA | 191 692 |
| Total PPI | NA | 1 179 202 |
Abbreviations: H2RA=histamine type-2 receptor antagonist; NA=not applicable; PPI=proton-pump inhibitor.
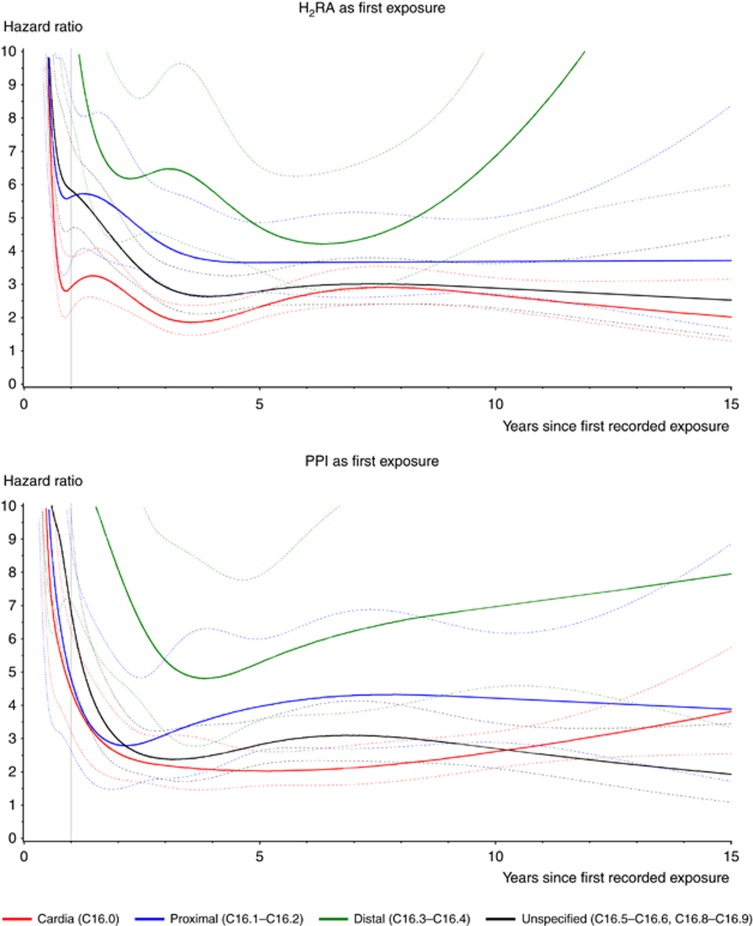
We observed 703 cases of stomach cancer among individuals with a filled H2RA prescription (34.4% cardia, 12.4% proximal, 17.1% distal, and 36.1% unspecified anatomic subsite) and 1 347 cases among individuals with a filled PPI prescription (35.6% cardia, 15.1% proximal, 14.2% distal and 35.2% unspecified anatomic subsite), and at least 90% were adenocarcinomas. Stratum-specific HRs for the various numbers of filled prescriptions, time periods since first exposure and anatomic subsites were nearly all significantly greater than 1.0, Figure 1. For individuals with 5+ prescriptions, HRs decreased over time from initial exposure for both H2RAs and PPIs, and were consistently greater for distal subsites than for proximal subsites at all intervals, Table 2. Considering the highest exposure category of 15+ prescriptions, HRs were greater for distal than proximal cancer for both H2RAs (4.6 (95% CI, 1.8–12.1) vs 3.3 (95% CI, 1.3–8.9), respectively) and PPIs (6.5 (95% CI, 4.1–10.5) vs 3.7(95% CI, 2.5–5.6), respectively).
Figure 1.
Subsite-specific hazard ratios (HR), solid lines, with 95% confidence intervals (CI), dashed lines, of cardia (C16.0), proximal (C16.1–C16.2), distal (C16.3–C16.4) and unspecified (C16.5–C16.6, C16.8–C16.9) site of stomach cancer with 1+ prescriptions of histamine type-2 receptor antagonists (H2RA) and proton-pump inhibitors (PPI), time since first exposure, years 1995–2011.
Table 2. Hazard ratios (HR) with 95% confidence intervals (CI) of stomach cancer for individuals with filled prescription of H2RA and PPI, years 1995–2011.
| Drug | Number of filled prescriptions | Time after initial exposure, years | Cardia C16.0 HR (95% CI) | Noncardia C16.1-C16.6 HR (95% CI) | Proximal C16.1-C16.2 HR (95% CI) | Distal C16.3-C16.4 HR (95% CI) | Unspecified C16.5-C16.6, C16.8-C16.9 HR (95% CI) |
|---|---|---|---|---|---|---|---|
| H2RA | Total 1+ | <1 | 21.7 (16.7–28.1) | 23.7 (17.3–32.4) | 19.0 (11.5–31.3) | 25.5 (16.2–40.0) | 19.6 (16.0–24.1) |
| 1–2.9 | 2.66 (1.91–3.72) | 4.95 (3.58–6.85) | 2.94 (1.66–5.20) | 8.22 (5.21–13.0) | 3.52 (2.73–4.55) | ||
| 3+ | 2.53 (2.14–2.99) | 4.63 (3.77–5.68) | 3.99 (2.94–5.41) | 6.20 (4.53–8.49) | 2.69 (2.24–3.24) | ||
| Totala | 2.55 (2.20–2.97) | 4.72 (3.97–5.61) | 3.72 (2.85–4.86) | 6.78 (5.23–8.80) | 2.94 (2.53–3.42) | ||
| Total 5+ | <1 | 19.5 (5.86–64.7) | 39.5 (8.52–183) | 71.0 (8.86–568) | 8.37 (0.52–135) | 27.9 (12.5–62.1) | |
| 1–2.9 | 2.70 (1.21–6.04) | 11.1 (5.81–21.4) | 11.8 (3.74–37.3) | 11.8 (4.74–29.4) | 5.48 (3.43–8.75) | ||
| 3+ | 3.08 (2.23–4.26) | 4.93 (3.32–7.32) | 2.83 (1.42–5.61) | 6.86 (3.89–12.1) | 3.28 (2.36–4.54) | ||
| Totala | 3.02 (2.24–4.08) | 6.14 (4.39–8.60) | 4.06 (2.28–7.22) | 8.01 (4.94–13.0) | 3.85 (2.94–5.03) | ||
| 1–4 | <1 | 21.8 (16.7–28.5) | 23.1 (16.7–31.8) | 16.5 (9.74–28.0) | 26.2 (16.5–41.4) | 19.1 (15.4–23.7) | |
| 1–2.9 | 2.66 (1.84–3.83) | 3.76 (2.55–5.55) | 1.85 (0.90–3.82) | 7.26 (4.26–12.4) | 2.97 (2.19–4.04) | ||
| 3+ | 2.36 (1.93–2.87) | 4.52 (3.56–5.74) | 4.37 (3.11–6.14) | 5.92 (4.06–8.64) | 2.46 (1.97–3.08) | ||
| Totala | 2.42 (2.03–2.88) | 4.30 (3.51–5.26) | 3.63 (2.69–4.91) | 6.34 (4.65–8.63) | 2.62 (2.19–3.15) | ||
| 5–14 | <1 | 19.5 (5.86–64.7) | 39.5 (8.52–183) | 70.9 (8.86–568) | 8.37 (0.52–135) | 35.9 (14.6–88.2) | |
| 1–2.9 | 1.89 (0.71–5.01) | 14.7 (6.73–32.2) | 44.9 (5.24–385) | 12.3 (4.67–32.3) | 5.65 (3.37–9.49) | ||
| 3+ | 2.15 (1.35–3.43) | 5.24 (3.24–8.48) | 2.71 (1.17–6.26) | 8.55 (4.27–17.1) | 3.51 (2.31–5.32) | ||
| Totala | 2.10 (1.38–3.19) | 7.03 (4.68–10.5) | 4.52 (2.20–9.30) | 9.70 (5.50–17.1) | 4.20 (3.04–5.81) | ||
| 15+ | <1 | NA | NA | NA | NA | 4.32 (0.39–47.7) | |
| 1–2.9 | 10.7 (1.76–64.2) | 5.37 (1.51–19.0) | 3.97 (0.73–21.7) | 8.49 (0.53–136) | 4.78 (1.60–14.3) | ||
| 3+ | 4.59 (2.89–7.31) | 4.34 (2.16–8.72) | 3.09 (0.94–10.2) | 4.27 (1.54–11.9) | 2.94 (1.74–4.99) | ||
| Totala | 4.85 (3.10–7.59) | 4.55 (2.47–8.40) | 3.34 (1.26–8.87) | 4.63 (1.77–12.1) | 3.21 (1.99–5.17) | ||
| PPI | Total 1+ | <1 | 31.4 (27.6–35.7) | 32.1 (27.3–37.7) | 27.0 (21.2–34.4) | 42.1 (32.7–54.2) | 27.9 (24.4–31.9) |
| 1–2.9 | 2.72 (2.26–3.27) | 5.42 (4.45–6.60) | 4.91 (3.64–6.64) | 6.89 (5.11–9.27) | 4.37 (3.71–5.16) | ||
| 3+ | 2.42 (2.13–2.75) | 4.28 (3.66–5.00) | 3.84 (3.09–4.77) | 5.82 (4.47–7.58) | 2.73 (2.36–3.17) | ||
| Totala | 2.51 (2.26–2.79) | 4.68 (4.14–5.29) | 4.17 (3.50–4.98) | 6.26 (5.14–7.64) | 3.34 (2.99–3.73) | ||
| Total 5+ | <1 | 16.6 (10.6–25.8) | 38.6 (22.7–65.6) | 44.6 (18.4–108) | 73.8 (29.1–187) | 23.3 (15.9–34.1) | |
| 1–2.9 | 4.71 (3.55–6.25) | 9.44 (6.95–12.8) | 7.58 (4.75–12.1) | 14.6 (9.13–23.3) | 8.65 (6.71–11.7) | ||
| 3+ | 3.04 (2.55–3.63) | 6.47 (5.20–8.05) | 5.92 (4.37–8.00) | 8.15 (5.62–11.8) | 4.48 (3.66–5.47) | ||
| Totala | 3.42 (2.94–3.97) | 7.35 (6.15–8.78) | 6.36 (4.93–8.20) | 10.3 (7.68–13.7) | 5.73 (4.90–6.71) | ||
| 1–4 | <1 | 33.1 (28.9–37.9) | 31.4 (26.5–37.3) | 25.7 (20.0–33.1) | 39.8 (30.6–51.8) | 28.6 (24.7–33.0) | |
| 1–2.9 | 1.92 (1.49–2.46) | 3.66 (2.80–4.78) | 3.65 (2.44–5.47) | 3.80 (2.48–5.80) | 2.66 (2.11–3.36) | ||
| 3+ | 1.91 (1.58–2.31) | 2.74 (2.16–3.47) | 2.41 (1.73–3.36) | 4.05 (2.75–5.98) | 1.57 (1.24–1.99) | ||
| Totala | 1.91 (1.64–2.22) | 3.09 (2.59–3.70) | 2.82 (2.19–3.65) | 3.93 (2.95–5.24) | 2.01 (1.70–2.37) | ||
| 5–14 | <1 | 16.3 (10.4–25.4) | 40.5 (23.5–69.8) | 43.0 (17.8–104) | 73.8 (29.1–187) | 24.7 (16.7–36.4) | |
| 1–2.9 | 4.84 (3.55–6.59) | 11.9 (8.34–16.9) | 9.59 (5.68–16.2) | 18.3 (10.6–31.7) | 9.38 (7.05–12.5) | ||
| 3+ | 2.60 (2.00–3.38) | 8.30 (6.12–11.3) | 8.88 (5.78–13.6) | 10.0 (6.00–16.7) | 5.23 (3.94–6.94) | ||
| Totala | 3.32 (2.72–4.05) | 9.69 (7.69–12.2) | 9.16 (6.57–12.8) | 13.4 (9.25–19.5) | 6.97 (5.70–8.52) | ||
| 15+ | <1 | NA | 8.49 (0.53–136) | NA | NA | NA | |
| 1–2.9 | 4.12 (2.05–8.26) | 4.41 (2.28–8.53) | 2.88 (0.91–9.10) | 6.95 (2.66–18.2) | 6.36 (3.63–11.1) | ||
| 3+ | 3.50 (2.75–4.44) | 4.94 (3.60–6.79) | 3.89 (2.50–6.04) | 6.41 (3.72–11.1) | 3.85 (2.90–5.11) | ||
| Totala | 3.56 (2.83–4.46) | 4.84 (3.64–6.43) | 3.73 (2.48–5.63) | 6.54 (4.07–10.5) | 4.25 (3.30–5.47) |
Abbreviations: CI=confidence interval; H2RA=histamine type-2 receptor antagonist; HR=hazard ratio; NA=not applicable; PPI=proton-pump inhibitors.
Does not include first year of exposure. NA- not applicable due to low numbers.
In a sensitivity analysis including all 1 765 777 individuals with prescriptions from 1 January 1994, the results did not differ substantially, Supplementary Table S2. In addition, considering the various exposure intensities combined, propensity score-matched HRs were within 10% of the unmatched HRs in all time periods since first exposure for all anatomic subsites, Supplementary Table S3. Change of drug type during follow-up, either from H2RA to PPI prescription or from PPI to H2RA prescription, was associated with the highest risks of stomach cancer across the various subsite categories, Supplementary Table S4. However, the increases were largely confined to the first year after changing therapy, after which HRs became similar to exposures of the respective individual drugs. Censoring at time of H. pylori eradication treatment changed our results only marginally, but numbers were sparse.
Discussion
Acid-suppression alters the distribution of H. pylori infection within the stomach, secondarily leading to development of a proximal-predominant atrophic gastritis a precursor lesion for cancer. This physiologic alteration provides a potential explanation for the recent observation of a relative increase in incidence of tumours localised to the corpus (Camargo et al, 2011). Approximately 20% of the Danish population is colonised with H. pylori (Wildner-Christensen et al, 2002), with lower prevalence in young age groups and higher prevalence among elderly individuals (Tindberg et al, 2001). Given their frequent prescription for premonitory symptoms of stomach cancer and the consequent confounding by indication, our analysis focused upon comparing associations with these drugs across anatomic subsites. A priori, distal cancers would be equally likely to be preceded by indications for treatment, whereas any additional increase in proximal cancers would support an interpretation that the association is causative. Our findings in general did not support this hypothesis, but we cannot preclude that a proximal shift may emerge in the future with longer durations of exposure.
Previous studies, including one in a county of Denmark (Poulsen et al, 2009), have reported significant associations of acid-suppressive drugs with increased risks of stomach cancer overall, even after exclusion of cancers within the first few years of exposure to address confounding by indication (Ahn et al, 2013). Our findings are similar, with the largest numbers of exposed individuals and the longest durations after exposure reported to date. HRs were significantly elevated in most strata of number of filled prescriptions and time after initial exposure for H2RA, PPI and their combinations. Inconclusively, even with restriction to 10 or more years after documented exposure, we found that both drug classes were associated with increases for both the H. pylori-driven noncardia cancers as well as the H. pylori-unrelated cardia cancers.
Our study used the data on acid-suppressing drug prescriptions and stomach cancer diagnoses derived from the entire population of Denmark, thus minimising any potential for methodologic biases. In Denmark, all healthcare is free of charge and encouraged irrespective of income, diminishing incompleteness of health registries and/or confounding by socioeconomic factors. There are limitations worth mentioning: the exposure definition implies that a filled prescription was equivalent to drug use, even though some individuals may not have used the prescribed drugs, thereby biasing the results towards null. Another key issue in observational studies is the possibility of unmeasured confounding.
We performed separate sensitivity analyses either matched on propensity score (based on health characteristics collected from the DNPR (Lynge et al, 2011)) or assessing modification by H. pylori infection.
In the present study, acid-suppressing drugs can only be attributed to H. pylori eradication therapy in a few individuals, in agreement with a recent Danish study (Pottegard et al, 2016). Nevertheless, we confirmed our findings with a sensitivity analysis censoring on filled prescription for eradication drugs since successfully treated patients would no longer be at risk of the hypothesised shift in bacterial distribution. This suggests that neither general nor specific chronic disease strongly influenced our findings.
As a limitation, one-third of cancers were classified with unspecified subsite, reducing statistical power but not a source of bias. Cancers of unspecified anatomic subsite represent a mixture among the specified subsites. Importantly, the probability for a given cancer subsite to be registered as ‘unspecified' should not depend on whether the patient was exposed.
To ensure an unexposed control group, we censored the controls at the time of prescription if they received an acid-suppressing drug. Since these medications may be prescribed for premonitory symptoms, such censoring could have artificially decreased gastric cancer incidence in this group overall. However, any such effect should have been similar for proximal and distal cancers. In conclusion, moderate duration exposure to acid-suppressive drugs did not favour proximal localisation of stomach cancer. These findings do not resolve a potential contribution to stomach carcinogenesis overall. Future studies should employ longer intervals after initiation of these therapies, consistent with the extended natural history of this malignancy.
Acknowledgments
The Intramural Research Program of the National Cancer Institute, National Institutes of Health, USA and the OAK foundation supported this work.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Disclaimer
The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Ahn JS, Eom CS, Jeon CY, Park SM (2013) Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol 19(16): 2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, Eheman CR, Rabkin CS (2011) Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 60(12): 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Lagergren J, Lindblad M (2006) Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut 55(11): 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstorff ML (2011) The Danish Cancer Registry. Scand J Public Health 39(7 Suppl): 42–45. [DOI] [PubMed] [Google Scholar]
- Hodgson N, Koniaris LG, Livingstone AS, Franceschi D (2005) Gastric carcinoids: a temporal increase with proton pump introduction. Surg Endosc 19(12): 1610–1612. [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M (2011) The Danish National Patient Register. Scand J Public Health 39(7 Suppl): 30–33. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66(1): 6–30. [DOI] [PubMed] [Google Scholar]
- Pottegard A, Broe A, Hallas J, de Muckadell OB, Lassen AT, Lodrup AB (2016) Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 9(5): 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen AH, Christensen S, McLaughlin JK, Thomsen RW, Sorensen HT, Olsen JH, Friis S (2009) Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer 100(9): 1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindberg Y, Bengtsson C, Bergstrom M, Granstrom M (2001) The accuracy of serologic diagnosis of Helicobacter pylori infection in school-aged children of mixed ethnicity. Helicobacter 6(1): 24–30. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- Wildner-Christensen M, Touborg LA, Lindebjerg J, Schaffalitzky de Muckadell OB (2002) Diagnosis of Helicobacter pylori in bleeding peptic ulcer patients, evaluation of urea-based tests. Digestion 66(1): 9–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.