Abstract
This review examines both recent and historical literature related to the biophysical chemistry of the proteins in the ageing eye, with a particular focus on cataract development. The lens is a vital component of the eye, acting as an optical focusing device to form clear images on the retina. The lens maintains the necessary high transparency and refractive index by expressing crystallin proteins in high concentration and eliminating all large cellular structures that may cause light scattering. This has the consequence of eliminating lens fibre cell metabolism and results in mature lens fibre cells having no mechanism for protein expression and a complete absence of protein recycling or turnover. As a result, the crystallins are some of the oldest proteins in the human body. Lack of protein repair or recycling means the lens tends to accumulate damage with age in the form of protein post-translational modifications. The crystallins can be subject to a wide range of age-related changes, including isomerisation, deamidation and racemisation. Many of these modification are highly correlated with cataract formation and represent a biochemical mechanism for age-related blindness.
Keywords: Cataract, Crystallin, Eye lens, Post-tanslational modification, Ageing
Introduction
Vision is one of the most important of the human senses, one that is relied upon constantly in daily life. The loss of sight is typically considered a major disability, but there are unfortunately many circumstances that can lead to eye damage and loss of vision. This organ is highly fragile and prone to damage from injury; however, blindness is also associated with genetic mutations, various diseases and, perhaps most commonly, ageing (Pascolini and Mariotti 2012). Cataract is by far the most common cause of blindness in humans, responsible for 51 % of cases of visual impalement worldwide (Rao et al. 2011). Cataract is characterised by a clouding of the eye lens that reduces the quantity of light reaching the retina. Lens opacification from cataract often results in a gradual loss of perception of contrast and colour intensity and an increased sensitivity to glare from bright lights. This progression eventually causes a total loss of sight if allowed to develop untreated (Allen and Vasavada 2006).
Cataract is the result of protein misfolding (Bloemendal et al. 2004). Over time, proteins are subject to a wide range of post-translational modifications that can reduce their stability and promote aggregation (Hains and Truscott 2007; Lampi et al. 1998; Yanshole et al. 2013). The crystallin proteins of the lens are particularly long-lived and any age-related damage they suffer typically accumulates. Cataract occurs when these proteins aggregate into large bodies which cause light scattering and loss of visual acuity (Moreau and King 2012). While the lens has systems to prevent protein aggregation (Ecroyd and Carver 2009; Horwitz 2003), the build-up of damaging modifications over time will eventually overwhelm them and aggregate formation results. Cataract has become an inevitable reality of ageing, especially as the lifetimes of individuals increases (Michael and Bron 2011). Currently, the only effective treatment for cataract-related blindness is surgical removal of the clouded lens and replacement with a prosthesis (Rao et al. 2011). This is naturally an invasive procedure that requires a highly skilled surgeon to perform, and is thus not always readily available in poorer regions of the world (Batlle et al. 2014). It is possible that by studying the eye lens specific cataract-inducing modifications could be identified and therapeutic treatments developed to target them.
The following review provides an overview of the structure and function of the human eye lens and the proteins it contains. Particular attention will be paid to the unique structures of the eye that make it vulnerable to cataract formation and recent discoveries regarding age-related post-translational modifications in the lens crystallin proteins.
Vision and the eye
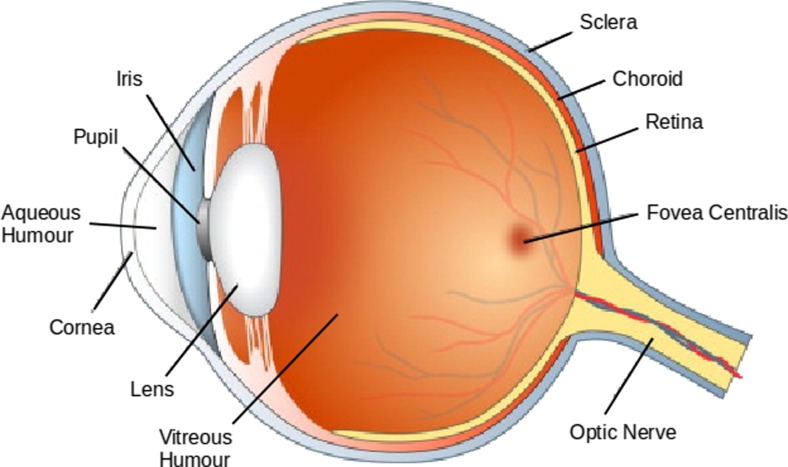
The eye is the organ of vision. It is a complex arrangement of parts that directs light from the external environment to the light-sensitive cells of the retina, converting it into nerve signals (Grossniklaus et al. 2013; Tortora and Derrickson 2008). To be fully functional, the human eye relies upon an interconnected series of components (Fig. 1), all of which must function in harmony to provide vision (Frost et al. 2014). The outermost section of the eye is the cornea, a layer of transparent tissue that covers the anterior chamber. The cornea acts as the eye’s first focusing component, using its shape and refractive index to direct light through the iris into the pupil (Quantock et al. 2015). The cornea also acts as a protective layer for the eye, triggering a blinking response when touched. The anterior chamber is the region between the cornea and iris. It is filled with the aqueous humour, a plasma-like fluid that defines the shape of the region (Goebel et al. 2011; Tortora and Derrickson 2008).
Fig. 1.
Diagram of the human eye with major components labelled (Frost et al. 2014)
The pupil is an aperture defined by the iris that allows light to enter the eye (Wilhelm 2011). Thus, the size of the pupil is determined by contractions of the iris in response to the intensity of light hitting the retina, giving the eye control over light level and preventing damage to the light-sensitive cells via over-stimulation (Neuhuber and Schroedl 2011; Winn et al. 1994). The iris itself is a pigmented circular structure that acts as diaphragm. It consists of fibrillar stroma that are connected to a sphincter muscle that contracts the pupil and a dilator muscle that radially enlarges it (Tortora and Derrickson 2008). The iris consists of two layers, an inner layer of highly pigmented epithelial cells and an outer non-pigmented layer that projects the dilator muscles. The colour of the lens is determined by the concentration of melanin in the epithelium and the scattering of light by the stroma (Mackey et al. 2011).
The primary instrument for focusing light in the eye is the lens (Augusteyn 2010; Bloemendal et al. 2004). The lens sits immediately behind the iris where it can focus incoming light to form a clear image on the retina. The lens exhibits a high transparency and a high refractive index and has flexibility which allows the attached ciliary muscles to alter its curvature (Banh et al. 2006; Bassnett et al. 2011). As a result, the eye can adjust the focal length of the lens to form a real image of objects at various distances with high quality and minimal light scattering (Purnyn 2013; Trokel 1962). The bulk of the eye ball is composed of the vitreous humour, a transparent gel that takes up the space between the lens and the retina (Petrash 2013). This fluid is similar in composition to the aqueous humour but has a lower water content and contains a network of collagen type II fibres that causes a high viscosity (Bishop 2000; Quantock et al. 2015). The vitreous humour has a higher refractive index than water but is primarily considered to be structural in nature rather than acting as a final focusing component. The retina itself consists of light-sensitive tissue that coats the interior surface of the eyeball (Purnyn 2013). Light striking the retina triggers a series of electrochemical events that result in nerve impulses being sent to the visual centres of the brain via the optic nerve. The two layers of the retina consist of a pigmented layer of melanin-rich epithelial cells and a layer of light-sensitive neurons interconnected by synapses (Hoon et al. 2014).
There are many diseases that deleteriously affect vision and many of these are a product of ageing (Grossniklaus et al. 2013). The vitreous humour is one example of a region that is strongly affected by age (Petrash 2013; Sebag 1987). This gel is stagnant and is not replenished over time like the aqueous humour, meaning that material such as blood or cellular debris that invade this region remain there unless removed surgically. Furthermore, the collagen fibres that permeate the gel can aggregate over time causing light scattering which degrades vision quality (Petrash 2013; Quantock et al. 2015). Likewise, the retina is susceptible to age-related macular degeneration which can involve either debris becoming lodged behind the retina or new blood vessels growing behind the retina from the choroid (Chiras et al. 2015; Hoon et al. 2014). In both cases, this causes the retina to become detached and results in loss of vision in the centre of the eye. However, one of the most interesting and well-studied eye components in regards to ageing is the lens, opacification of which leads to impaired vision which characterises cataract disease. (Augusteyn 2010; Banh et al. 2006; Bloemendal et al. 2004; Michael and Bron 2011).
Lens structure and ageing
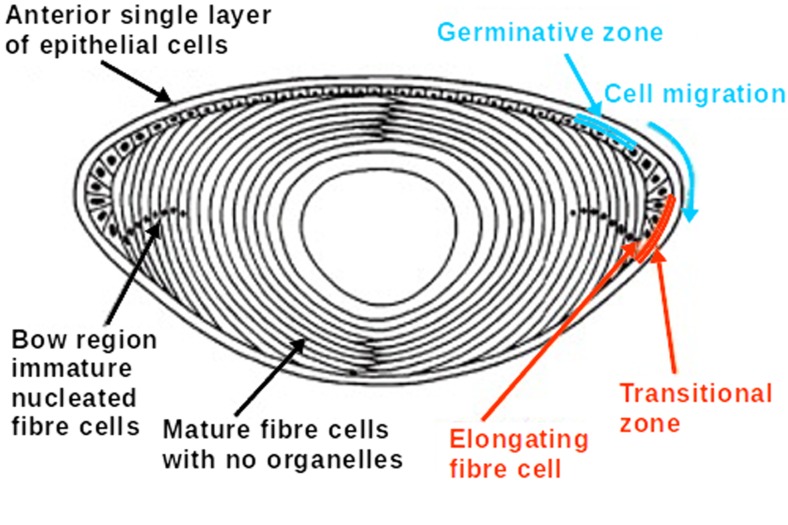
The eye lenses of all mammals are composed of fibre cells arranged in concentric layers around a central nucleus (Moreau and King 2012) (Fig. 2). The outward facing edge of the lens consists of a mono-layer of epithelial cells contacting the iris, from which new fibre cells develop as the lens grows over the lifetime of the individual (Banh et al. 2006; Michael and Bron 2011). The lens epithelial cells are the only part of the lens that has metabolic activity (Dahm et al. 2011). New cells develop from the epithelial layer via mitosis from which they then differentiate into fibre cells (Mochizuki and Masai 2014). As the new fibre cells develop, they express large amounts of crystallin proteins which are responsible for the refractive index and transparency of the lens (Aarts et al. 1989; Zhao et al. 2011). Newly differentiated fibre cells migrate towards the lens equator where they undergo denucleation and lose their internal sub-cellular structures (Bassnett 2002; Mochizuki and Masai 2014). The fibre cells ultimately form multiple layers stacked around the central nucleus of the lens. The newest fibre cells surround the periphery with the oldest cells occurring in the central region of the lens that is formed in utero (Augusteyn 2010).
Fig. 2.
Cross-section of the human eye lens showing layers of lens fibre cells, the epithelial cell layer and the lens nucleus. Fibre cells differentiate form the epithelial layer and migrate to the lens equator. Mature fibre cells elongate and undergo denucleation (Moreau and King 2012)
The long, elongated lens fibre cells form densely packed onion-like layers. The cells can be upwards of 10 mm long in a mature lens and maintain their shape via an extensive cytoskeleton (Perng et al. 2007). The cytoskeletal structure of these cells is vital for transparency, as lenses lacking key filament proteins are opaque despite the fibre cells maintaining typical morphology (Alizadeh et al. 2002). In order to act as an effective optical focusing device, the lens must maintain several unique properties. The lens must display a high transparency in the visual wavelengths, have a high refractive index in order to bend light at as large an angle as possible, and a degree of flexibility that permits changes in focal length (Bloemendal et al. 2004; Trokel 1962). The transparency of the eye lens is limited primarily by the absorption and light scattering of the components of the lens fibre cells (Banh et al. 2006). In healthy eye lenses, absorption and light scattering are negligible. The periphery of the lens exhibits substantial diffraction due to the difference between the refractive index of the fibre cell membrane and the cytoplasm. This is less of an issue along the optical axis of the lens due to angling of the individual fibre cell layers and is absent in the lens nucleus where the refractive index of the membranes is almost equal to that of the cytoplasm (Michael et al. 2003).
The lens achieves a high refractive index due to the very high concentration of proteins expressed in the fibre cells (Aarts et al. 1989). Lens crystallins show high refractive index increments compared to other proteins, due to large quantities of aromatic and sulphurous residues (Mahendiran et al. 2014), and show strong absorbance of UV radiation (Chen et al. 2009; Zhao et al. 2011). In humans, the fibre cells exhibit a protein concentration of ∼320 mg ml−1 uniformly throughout the lens (Bloemendal et al. 2004). The composition and concentration of the proteins found in the eye lens of other species varies greatly, but in all cases these proteins are assigned to a super-family known as the crystallins. All vertebrate species express at least α-, β- and γ-crystallin subtypes in their eye lenses, and in humans these are the only crystallin sub-types (Wistow 2012). It is the structure and stability of the crystallins that define many of the important properties of the eye lens.
The lens maintains its transparency and refractive index due to its unique biophysical characteristics. Light scattering is proportional to the size of objects encountered and their difference compared to their surroundings (Ponce et al. 2006; Trokel 1962), and as such the lens has maximised its transparency by eliminating almost all cellular structures (Bassnett 2002). When a fibre cell differentiates from the epithelial layer, it express crystallin proteins in high concentration and then undergoes degradation of all organelles and large cell structures. The bulk of the eye lens has no blood supply, no cellular structures such as mitochondria, nucleus, or ribosome, and exhibits no metabolic activity (Dahm et al. 2011; Mochizuki and Masai 2014). While this lack of organelle-like sub-structure means the lens fibre cells have exceptional optical properties, it also means that once they have matured they have very little capacity to recycle or repair damaged proteins and no capacity to express new protein (Bloemendal et al. 2004; Wang et al. 2004).
The long life of the fibre cells is one of the defining features of the eye lens (Sharma and Santhoshkumar 2009). Due to the lack of protein turnover in the lens fibre cells, crystallin proteins must last the lifetime of each individual. Thus, post-transnationally modified and damaged proteins build up in the lens over the lifetime of an individual, particularly in the nucleus as it is the oldest lens region and corresponds to the foetal lens (Hains and Truscott 2007; Yanshole et al. 2013). The protein composition of a young lens can be very different to that of an aged lens. The aged eye is prone to diseases that result in loss of vision, the most common of which is cataract in the nucleus of the lens (Hains and Truscott 2007; Moreau and King 2012; Truscott 2005).
Age-related cataract
Cataract is characterised as an opacity in the normally highly transparent lens of the eye (Bloemendal et al. 2004). In general, this opacity can either result from a disruption in the ordered packing of the lens fibre cells or from the formation of large structures within the cells due to a disruption in local ordered protein structure (Michael and Bron 2011; Moreau and King 2012). In the latter case, this disruption is often caused by the formation of high molecular weight structures consisting of aggregated crystallin proteins (Dobson 2004). Such structures exhibit increased light scattering, which reduces lens transparency and eventually causes loss of vision. Age-related nuclear cataract is a common disease of ageing and is highly correlated with fibre cell crystallin protein aggregation (Bloemendal et al. 2004; Harding 2002; Michael and Bron 2011; Petrash 2013).
Lens fibre cells express the crystallin proteins in high concentrations during their development (Aarts et al. 1989). The crystallins form a wide range of oligomeric structures, ranging from monomers in the case of the γ-crystallins (Bloemendal et al. 2004), from dimers to octomers in the case of the β-crystallins (Bateman et al. 2003; Bax et al. 1990; Lampi et al. 1998), and up to 60-mers for the α-crystallins (Horwitz 2003). Larger oligomers are more common towards the centre of the lens and the smaller dimers and monomers make up the bulk of the protein found in the lens cortex. Overall, the polydispersed nature of the lens proteins makes the substance of the lens remarkably glass-like and highly resistant to crystallisation (Augusteyn 2010).
Unfortunately, the high protein concentrations necessary to create the high refractive index material of the lens is also exactly the type of environment that promotes protein aggregation (Ciryam et al. 2015; Moreau and King 2012). While the lens has many systems and special characteristics that are engineered to prevent this aggregation, the effectiveness of these systems tends to diminish as the lens ages (Bloemendal et al. 2004; Ecroyd and Carver 2009; Takemoto et al. 2008). Over time, proteins tend to accumulate post-translational modifications, due to either external damage such as UV radiation exposure or exposure to heat, or by one of several spontaneous chemical modification pathways to which proteins are susceptible (Hains and Truscott 2007; Lampi et al. 1998). Cataract formation is typically the result of such damage building up to a point where individual proteins are no longer able to maintain their structure or solubility (Moreau and King 2012; Morris et al. 2009). Local regions of highly stressed proteins can randomly self-associate into aggregates that grow in size as more proteins unfold and associate. Eventually, damage and stress will cause protein precipitation within the lens fibre cells with these aggregates acting as nucleation sites for further growth. The formation of protein aggregates in the eye lens disrupts the uniformity and transparency of the lens medium leading to light scattering and loss of visual acuity. In general, the larger the size of protein aggregates, the greater the light scattering they cause (Hains and Truscott 2007; Kamei et al. 2001).
Although described by a single term, cataracts can have many underlying causes. Eye lens transparency depends on lens proteins maintaining their ordered structure. Exposing lens proteins to any sources that damages them, or promotes their unfolding, will tend to promote blindness. Cataract has been known to be caused by genetic factors, mutations that produce less stable proteins (Shiels and Hejtmancik 2007), and some diseases such as diabetes (Stevens et al. 1978); however, the most common cause of cataracts is age (Bloemendal et al. 2004; Hains and Truscott 2007; Harding 2002; Michael and Bron 2011).
Lens protein folding and aggregation
The function of globular proteins is defined by their native structure. Under normal circumstances, a protein will fold after it is expressed on the polyribosome, forming a well-defined tertiary structure usually delineated by exposure of hydrophilic surfaces and sequestration of hydrophobic residues into its interior (Dobson 2004).
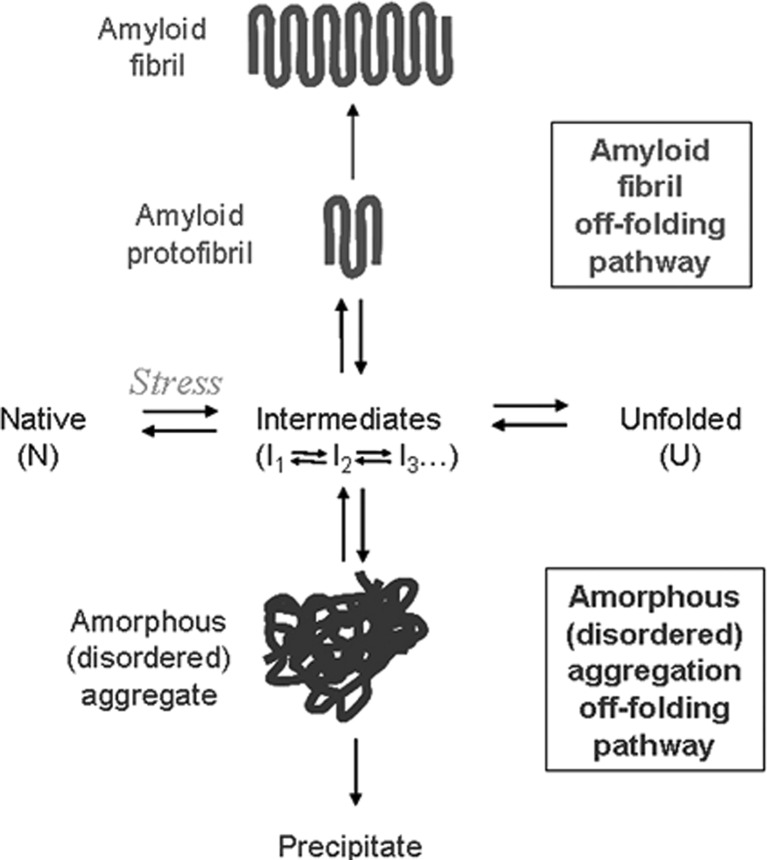
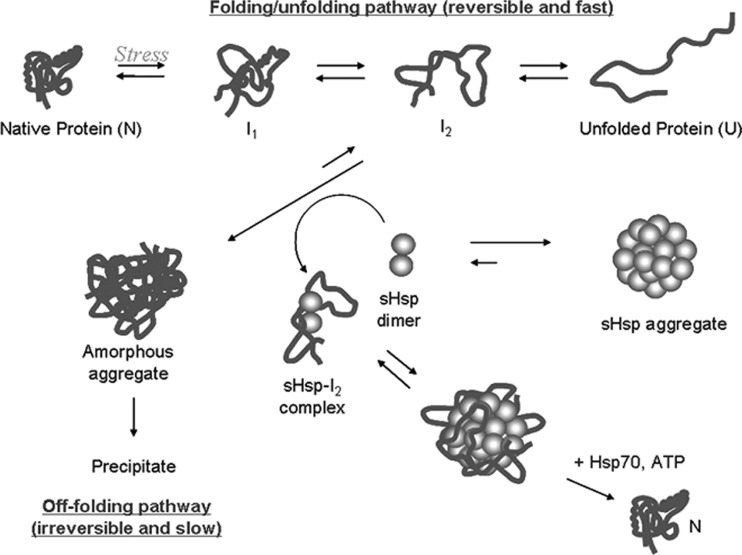
Figure 3 shows a schematic for the typical folding of a polypeptide chain. Following expression, a protein exists in an unfolded state and will fold into its native folded form via a series of partially folded intermediats (Dobson 2004). While in these intermediately folded states, a protein will often have its hydrophobic core exposed to to solution, making it unstable and rendering it prone to aggregation (Dobson 2004). Under normal circumstances, proteins fold rapidly from an unstructured chain to their native states and thus avoid mutual interaction between partially unfolded intermediates. When proteins are exposed to stress conditions, however, the equilibrium between the native and unfolded states is disrupted which can cause the protein to occupy these intermediate states for longer periods of time (Ecroyd and Carver 2009; Haslbeck and Vierling 2015).
Fig. 3.
Protein folding, unfolding and off-folding (aggregation) pathways (Ecroyd and Carver 2009)
Physiological stress is a general term for changes in the environment of a protein that encourages it to unfold from its native state (Kopito 2000). Common causes of stress are elevated temperature, changes in pH or exposure to reducing or oxidising agents. Other stress conditions include chemical alteration of the protein, exposure to metal ions, the disruption of quaternary structure and breaking of inter-residue bonds (Bloemendal et al. 2004; Yanshole et al. 2013). Whatever the root cause of stress, its presence means that a protein will tend to remain in a partially folded intermediate state for longer which increases the possibility of mutual interaction with another partially folded intermediate. This interaction will generally result in protein aggregation (Dobson 2004; Kopito 2000).
Protein aggregation can take on two forms, highly disordered amorphous aggregation and highly ordered amyloid fibrils (Chiti and Dobson 2006; Hall et al. 2015). Amorphous aggregates form when partially unfolded proteins cluster together. When a partially unfolded protein has its hydrophobic core exposed to solution, it will experience an affinity for other hydrophobic regions such as the exposed cores of other proteins. As these protein clusters increase in size, they become less soluble and eventually precipitate (Dobson 2004). Amorphous aggregation and its subsequent precipitation is generally irreversible and protein in this state is entirely inactive; however, amorphous aggregates are believed to be non-toxic to cells and not a significant contributor to protein folding diseases (Kopito 2000).
Amyloid fibrils are a highly ordered form of protein aggregate which are characterised by their β-sheet-rich, long needle-like structure. The amyloid fibril off-folding pathway begins when unstable, as partially folded protein begins to partially take up the highly stable β-sheet conformation (Chiti and Dobson 2006). Other partially folded intermediates that interact with the β-sheet region will also fold into a β-sheet due to the mutual stability of this conformation. As this aggregate gets larger, it begins to act as a nucleus for an amyloid fibril, and any unstable protein it encounters will tend take on a β-sheet structure and attach to the end. This aggregation is highly stable and, unlike an amorphous aggregate, will cause unfolding and aggregation of other proteins present (Norrby 2011; Stefani 2012). Amyloid fibrils are associated with many diseases, particularly age-related neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (Eisenberg and Jucker 2012; Hall and Edskes 2012).
The light scattering that characterises cataract disease is generally believed to be the result of the formation of amorphous aggregates (Bloemendal et al. 2004; Hains and Truscott 2007). When the crystallin proteins are exposed to sufficient stress-inducing modifications, they will unfold and randomly associate to form these large structures. This would not constitute a problem in most tissues as this form of aggregate is readily degradable but in the lens there are no mechanisms for breaking up and recycling these structures. As a result, the lens fibre cells must rely entirely on preventative mechanisms such as high protein stability and large concentrations of molecular chaperone (Bassnett 2002; Takemoto and Sorensen 2008).
Small heat-shock proteins
The small heat-shock proteins (sHsp) are a group of molecular chaperone proteins that are ubiquitous in all organisms (Derham and Harding 1999; Haslbeck and Vierling 2015). The role of the sHsps is to prevent aggregation of other proteins under stress conditions. Proteins that perform the heat shock function are found in all forms of life and have a highly conserved structure across species (De Jong et al. 1993) (see Fig. 6, below). All sHsps are characterised by the presence of a well-structured central region termed the α-crystallin domain (Derham and Harding 1999). This region exhibits a β-sheet-rich immunoglobulin fold, the structure of which is important for the activity of these proteins (Jehle et al. 2010; Kim et al. 1998). This central structured domain is flanked by N- and C-terminal regions, but these are highly variable between species and show differing degrees of structure (Derham and Harding 1999). The expression of sHsps is substantially up-regulated in response to heat stress conditions (Haslbeck and Vierling 2015; Lindquist 1986). These proteins are highly efficient chaperones, which act to prevent protein aggregation by binding to unfolded and partially folded proteins, preventing their association with other unstable proteins (Fu 2014). In this way, they act as a first line of defence against protein unfolding and aggegation (Ecroyd and Carver 2009; Haslbeck and Vierling 2015).
Fig. 6.
General structure of small heat shock proteins. Sequence of αB-crystallin (red) overlaid onto two related proteins, wheat sHSP 16.9 (green) and bacterial sHSP 16.5 (blue) (Ghosh and Clark 2005)
Figure 4 is a diagram of sHsp chaperone action. Under normal physiological conditions, sHsps exist bundled into large stable oligomers containing many subunits. Upon encountering physiological stress, these oligomers become unstable and sHsp dimers dissociate into solution (Delbecq and Klevit 2013; Hochberg and Benesch 2014). The active sHsp dimer interacts with aggregating proteins early in their off-folding pathway (Ecroyd and Carver 2009; Fu 2014). The sHsps bind to proteins that are partially folded, ensuring continued solubility and preventing further unfolding and aggregation. While this binding prevents the formation of both amorphous aggregates and amyloid fibrils, the sHsps have no mechanism for restoring protein to its native fold and rely on other energy-driven processes to recycle any proteins that it binds, such as ATP-driven Hsp70 (Haslbeck et al. 2005). While sHsps are an organism’s first line of defence against protein aggregation, they are also associated with a variety of protein folding diseases. It is common to find sHsp incorporated into the amyloid fibril-rich plaques found in the brains of victims of neurodegenerative disease (Eisenberg and Jucker 2012). The exact causes of sHsps being incorporated into the same structures they are supposed to prevent forming is not well understood (and nor are many of the mechanisms behind their chaperone action) making sHsps an important field of active research (Guzzo 2012; Morrow and Tanguay 2012; van Noort et al. 2012; Treweek et al. 2015).
Fig. 4.
sHSP interaction with unfolding and amorphous aggregation (Ecroyd and Carver 2009)
Eye lens proteins
The lens fibre cells express very high concentrations of highly soluble protein as they develop. In all species, these proteins are classified as the crystallins, of which there are many taxon-specific subtypes (Piatigorsky et al. 1994). All vertebrates express the α-, β- and γ-crystallins in the lens, and in mammals these are the only members of the class present (Slingsby et al. 2013). Crystallin proteins assemble into various oligomers of different sizes and show many weak crystallin–crystallin interactions, resulting in a highly polydisperse fibre cell contents resistant to crystallisation (Ponce et al. 2006; Takemoto and Sorensen 2008). In humans, all crystallins are lens fibre cell-specific with the exception of αB-crystallin which is ubiquitous in all tissues in which it functions as a stress inducible molecular chaperone (Horwitz 2003). All the crystallins are characterised by high solubility and high stability as they must last the lifetime of the lens (Bloemendal et al. 2004; Sharma and Santhoshkumar 2009) (Table 1).
Table 1.
Properties of the human eye lens crystallins (Bloemendal et al. 2004)
| Crystallin | Residues | Mass (Da) | pI | SwissProt | PDB | OMIM |
|---|---|---|---|---|---|---|
| αA | 173 | 19,909 | 5.6 | P02489 | – | 123 580 |
| αB | 175 | 20,159 | 6.8 | P02511 | – | 123 590 |
| βB1 | 251 | 27,892 | 8.6 | P53674 | I0KI | 600 929 |
| βB2 | 204 | 23,249 | 6.5 | P43320 | 1YTQ | 123 620 |
| βB3 | 211 | 24, 230 | 5.9 | P26998 | 3QK3 | 123 630 |
| βA1 | 198 | 23,191 | 6.4 | P05813 | – | 123 610 |
| βA2 | 196 | 21,964 | 5.9 | P53672 | – | 600 836 |
| βA3 | 215 | 25,150 | 5.7 | P05813 | – | 123 610 |
| βA4 | 195 | 22,240 | 5.8 | P53673 | 3LWK | 123 631 |
| γS | 177 | 20,875 | 6.4 | P22914 | 2M3T | 123 730 |
| γA | 173 | 20,761 | 7.8 | P11844 | – | 123 660 |
| γB | 174 | 20,776 | 7.0 | P07316 | 2JDF | 123 670 |
| γC | 173 | 20,747 | 7.0 | P07316 | – | 123 680 |
| γD | 173 | 20,607 | 7.2 | P07320 | 1HK0 | 123 690 |
| γE | Gene inactive in humans | |||||
| γF | Gene inactive in humans | |||||
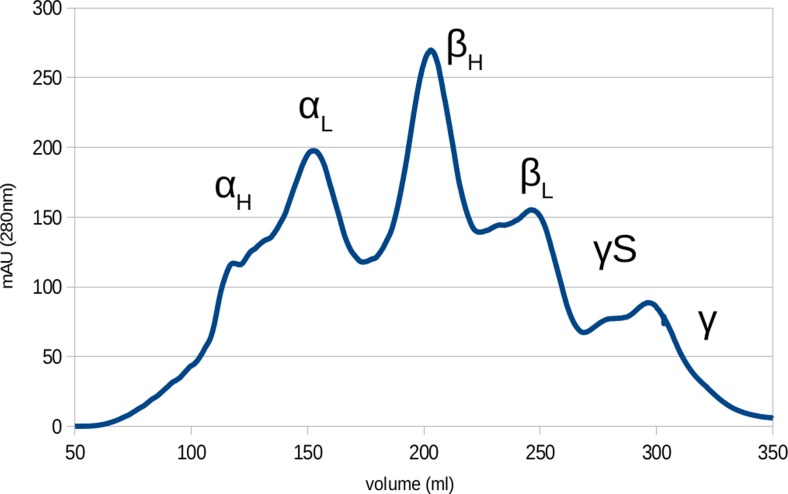
The cytoplasmic protein of the mammalian eye can be separated into its components via size exclusion chromatography (Spector 1964) as shown in Fig. 5. The α-, β- and γ-crystallins separate from one another, forming distinct fractions. The α- and β-crystallins further separate into high- and low-mass species, the separation of which highly buffer-dependent as these species are in a concentration-dependent equilibrium with one another (Bateman et al. 2001). This was ultimately found to be a result of the polydisperse oligomer formation exhibited by the α- and β-crystallins .
Fig. 5.
Chromatogram of lens fibre cells cytoplasm from bovine eye lens separated with gel filtration chromatography. Significant protein groups are marked. α- and β-crystallins separate into high and low mass elution peaks corresponding to different tertiary structures and γS-crystallin elutes separately to the other γ-crystallins
The classification of mammalian eye lens proteins was initially on the basis of net charge and molecular mass (Harding and Dilley 1976). Initially, the α-, β- and γ-crystallins were believed to be individual proteins but now this nomenclature serves as the basis for classifying the three human lens protein families (Wistow 2012). γS-crystallin was originally classified as a β-crystallin as it elutes at a higher apparent mass than the other γ-crystallins but was later found to be much more closely related to the γ-crystallins on the basis of sequence data, and has since been reclassified ( Quax-Jeuken et al. 1985; Smith et al. 1995).
α-crystallin
In humans, the α-crystallins consists of two related proteins, αA and αB (Horwitz 2003). These two proteins share roughly 60 % sequence identity, have high structural similarity and share the same function. Both α-crystallins are sHsps but only αB-crystallin is stress-inducible in other tissues of the body, meaning that αA expression is limited entirely to the eye lens (Bhat and Nagineni 1989; Morrow and Tanguay 2012). Figure 6 shows the general structure of sHsps as a homology model of human αB-crystallin compared with the x-ray crystal structures of wheat sHsp 16.9 and bacterial sHsp 16.5 (Ghosh and Clark 2005).
sHsps are characterised by a central α-crystallin domain flanked an unstructured C-terminal and a variable N-terminal region (Ecroyd and Carver 2009; Hochberg and Benesch 2014; Horwitz 2003). The α-crystallin domain resembles an immunoglobulin fold in structure as seen from X-ray crystallography and NMR-determined structures of truncated αB-crystallin (Jehle et al. 2010; Laganowsky et al. 2010) and from sHsp of other species. The α-crystallin domain consists of a β-sandwich structure of 12 β-strands, of which 2 are shared by an adjacent dimerisation partner (Jehle et al. 2010). The α-crystallins form dimeric species which together assemble into oligomers composed of this base dimer unit (Delbecq and Klevit 2013). These typically consist of 3 dimer pairs arranged in a ring from which the hexamers then further oligomerise to form a grouping of 40–60 units depending on the conditions (Ecroyd and Carver 2009). These large-scale structures are highly polydisperse and dynamic with individual dimers able to freely associate and disassociate from the oligomer. The C-terminal region of sHsps contains a conserved hydrophobic ‘IXI’ motif which binds to a hydrophobic pocket of the α-crystallin domain of adjacent dimers and is proposed to influence subunit dissociation under conditions of stress (Jehle et al. 2010; Laganowsky et al. 2010). α-crystallins are differentiated from other sHsps by the presence of a highly dynamic unstructured C-terminal extension of 10-12 amino acids (Treweek et al. 2010) which contributes to the high solubility shown by these proteins and the loss of this region greatly reduces their stability (Ecroyd and Carver 2009; Hilton et al. 2013; Treweek et al. 2010).
βγ-crystallins
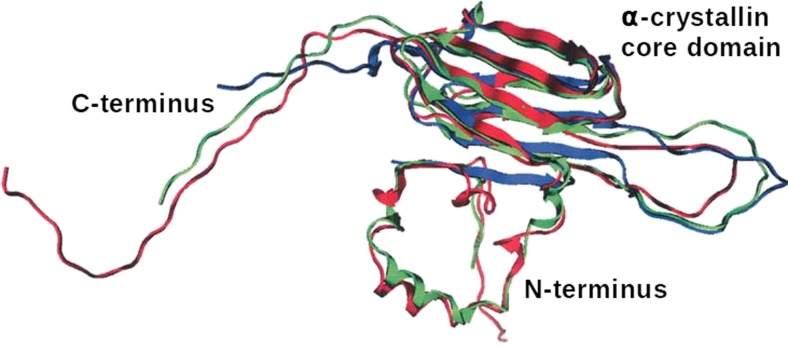
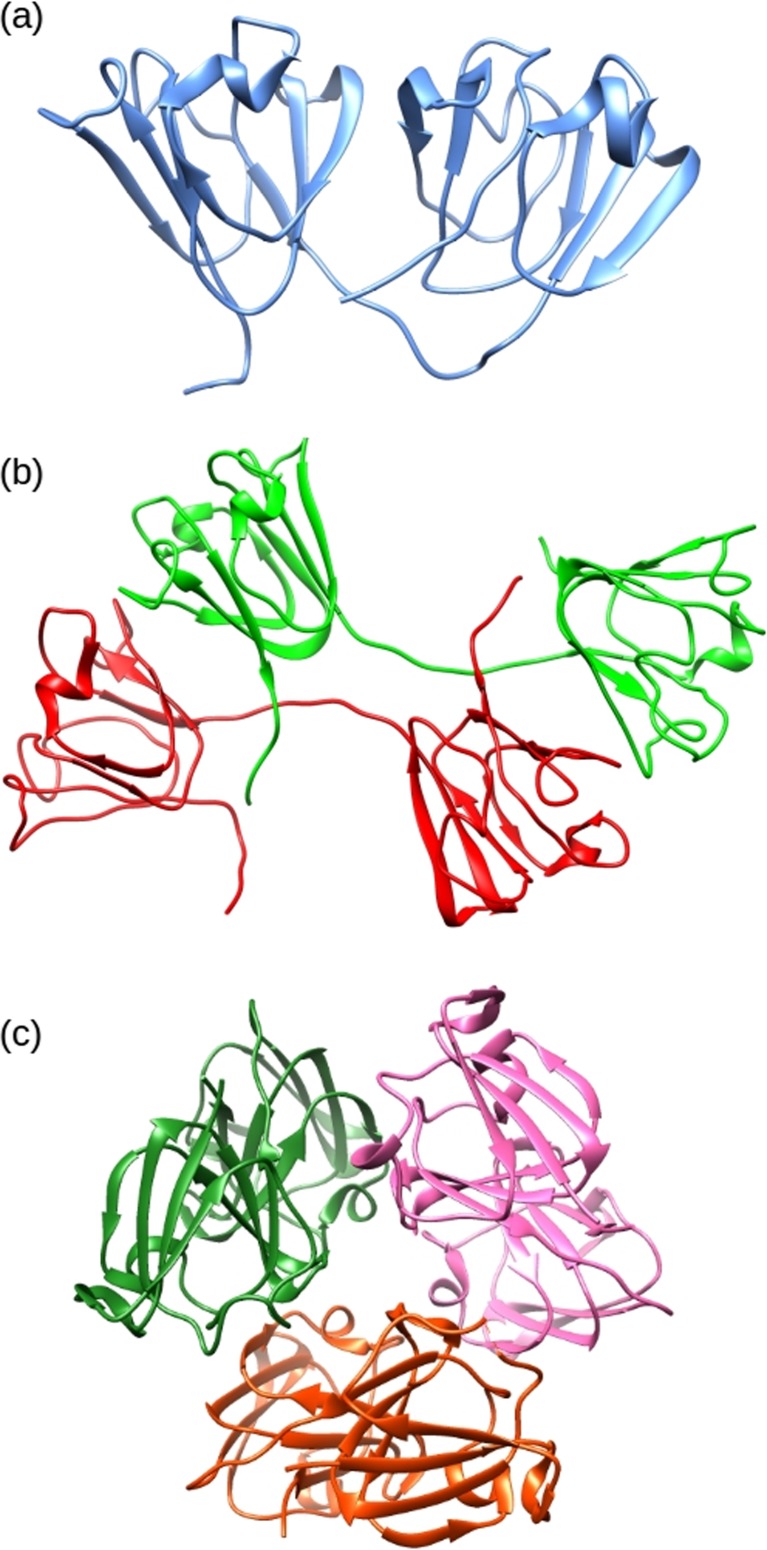
The β- and γ-crystallins are related proteins that act as structural elements in the lens. They have a major role in generating the lens’s high transparency and high refractive index (Mahendiran et al. 2014; Takemoto and Sorensen 2008). This protein family is believed to have originated from primordial Archaeabacteria as single-domain Ca2+ binding proteins (Barnwal et al. 2009; Mishra et al. 2014). The βγ-crystallins are ubiquitous in the eyes of mammals, and in humans they account for ∼50 % of the cytoplasmic proteins by weight (Bloemendal et al. 2004; Slingsby et al. 2013). The β- and γ-crystallins are closely related protein families and they possess a similar structural fold, i.e. a two-domain tertiary structure that roughly divides the peptide chain into halves. Each domain is comprised of two β-sheet rich “Greek-key” motifs with 8 β-strands per domain (Fig. 7).
Fig. 7.
The structures of selected crystallin proteins. a NMR derived structure of human γS-crystallin two domain monomer (PDB 2M3T). b Crystal structure of bovine βB2-crystallin (PDB 1BLB) dimer showing domain sharing. c Crystal structure of human βB3-crystallin trimer (PDB 3QK3) with lattice structure
These proteins pack densely together into the supra-molecular structures via intermolecular cross-domain interactions. The primary distinguishing characteristic between the β- and γ-crystallins is that under physiological conditions the β-crystallins self-assemble into oligomers of between 2 and 8 subunits (Bateman et al. 2003; Bax et al. 1990), whereas the γ-crystallins are largely monomeric (Bloemendal et al. 2004). Both the β-crystallin oligomers and the γ-crystallins monomers are stabilised by hydrophobic interactions between the N- and C-terminal domains. The βγ-crystallins are primarily responsible for the refractive index of the lens and tend to have an above average number of residues with high refractive index increments, i.e. aromatic (tyrosine, tryptophan and phenylalanine) and sulfur-containing (cysteine and methionine) amino acids (Mahendiran et al. 2014; Zhao et al. 2011). The highly soluble, highly stable, poly-dispersed nature of these proteins also minimises aggregation and crystallisation in the lens, ensuring high transparency (Piatigorsky et al. 1994; Slingsby et al. 2013). The structure of the β-crystallins shows distinct differences from the γ-crystallins with the presence of long flexible peptide extensions at the N-terminus. Otherwise, the structures show a close relationship, with similar folds in the C- and N-terminal domains and similar inter-domain interactions. The cause for β-crystallins showing preferential inter-molecular interaction compared to the intra-molecular domain pairing in the γ-crystallins arises from the conformation and length of the domain linker peptides. The inter-domain interface in these proteins is composed of clusters of hydrophobic residues flanked by pairs of polar groups (Flaugh et al. 2005).
β-crystallin
The β-crystallins are divided into acidic βA1, βA2, βA3 and βA4 and basic βB1, βB2 and βB3 proteins (Bloemendal et al. 2004). The βA1 and βA3 crystallins are coded by the same gene and differ only in the length of the N-terminus as the βB3 expression is initiated by an earlier start location (Zigler and Sinha 2015). These two proteins are expressed early in the development of the lens and are found primarily in the nucleus, whereas βB2-crystallin is expressed throughout all stages of eye lens growth from a separate gene (Aarts et al. 1989). All the acidic β-crystallins are expressed throughout the eye lens in humans with the exception of βA2, which appears in only trace quantities when compared to other mammals (Bloemendal et al. 2004). βB1- and βB3-crystallins are highly correlated with the βH fraction of the lens crystallins and form into high mass oligomers such as hexamers and octomers (van Montfort et al. 2003). Conversely, the βL fraction consists of dimers such as βB2 (Aarts et al. 1989). High-mass oligomers of β-crystallin (βH) (hexamers and octomers) are primarily located in the lens nucleus corresponding to the expression of βB1- and βB3-crystallins and thus are generally found in the oldest lens cells (Aarts et al. 1989). The younger fibre cells tend to express more dimeric βB2-crystallin and monomeric γS-crystallin, resulting in somewhat of a gradient, with larger oligomers found in the centre of the eye lens which are replaced by dimers and monomers at the edge of the lens (Augusteyn 2010).
γ-crystallin
There are seven γ-crystallin genes in mammals (Wistow 2012). The first six of these, γA–γF, are closely related and highly similar in sequence. The genes for these proteins appear in a single cluster and show 70–98 % similarity, but in humans the γE and γF genes are inactive (Slingsby et al. 2013). The seventh γ-crystallin, γS, is more distantly related to the others (Smith et al. 1995). It exhibits a unique four-amino-acid-long N-terminus, it lacks the two residue C-terminus of the other six and has a 1- to 2-amino-acid-longer connecting loop between the two domains (Bloemendal et al. 2004). γS-crystallin is induced last during fibre cell development and is not present in the immature cells of the cortex (Aarts et al. 1989; Wang et al. 2004). The γA-D crystallins are similar to the βB1- and βB3-crystallins in that they are expressed early in the lens development, with γC and γD most abundant, and are found primarily in the nucleus of the eye lens (Wang et al. 2004).
γS-crystallin is the primary eye lens crystallin which is expressed post-natally (Wang et al. 2004). It has high structural homology to the other γ-crystallins but is found primarily in the water-rich cortex of the eye lens. Due to its chromatographic properties, γS-crystallin was originally classified as a β-crystallin (βS), but, once the genetic sequence of the crystallins was understood, it was re-classified as an early evolutionary offshoot of the γ-crystallins ( Quax-Jeuken et al. 1985; Smith et al. 1995). The sequence of γS-crystallin is highly conserved throughout all vertebrates and is regarded as one of the more important proteins in the lens structure (Bloemendal et al. 2004; Wenk et al. 2000). The crystallisation of human γS-crystallin has yet to be achieved, but a structure determined by NMR spectroscopy is available (Kingsley et al. 2013).
Post-translational modification
The lack of protein turnover in the lens results in a unique situation not seen in most other bodily tissues. Over time, proteins in any tissue can be subjected to a wide variety of irreversible modifications, due to enzymatic processes or via spontaneous pathways (Bloemendal et al. 2004; Hains and Truscott 2007; Harding 2002; Wilmarth et al. 2006; Yanshole et al. 2013). In the eye lens, the lack of protein expression or turnover means that, instead of these modified proteins being repaired or replaced, they tend to accumulate. Being by far the predominant lens proteins, the modification of the crystallins can be extensive. Crystallins are all highly water soluble upon expression but, over time as post-translational modification occurs, they are altered both structurally and functionally as well as becoming colourised. Loss of solubility occurs and they become prone to aggregation (Sharma and Santhoshkumar 2009). Eventually, the formation of large, poorly-soluble aggregates results in extensive light scattering (Moreau and King 2012).
Cataract formation is highly correlated with age; however, crystallins are often extensively modified in humans even when young (Hains and Truscott 2007; Wilmarth et al. 2006). There are many specific modifications in cataractous lenses that are not present in normal healthy aged lenses (Hooi et al. 2012). It is these modifications in particular that are of interest in order to identify specific causes for cataract formation and therefore identify potential targets for treatment or prevention. Because the proteins in the lens have a significantly longer lifetime than most other proteins in the body (Lynnerup et al. 2008), they have the opportunity to undergo a wide range of modifications and rearrangements that are not normally occur in other tissues. A survey of such modifications follows.
Truncation
Over time, proteins are susceptible to truncation events (Yanshole et al. 2013). Proteases are common throughout tissues where they cleave polypeptides into fragments at specified sites (Page and Di Cera 2008) and represent the basis of natural protein degradation pathways (Goldberg 2003). Crystallins undergo a number of C- and N-terminal truncations which can have a major effect on their stability and solubility (Hains and Truscott 2007; Morris et al. 2008; Treweek et al. 2010). Some of these truncations can be attributed to protease activity, as small amounts of these enzymes are found in the eye of animals (Robertson et al. 2008). However, in the nucleus of the human lens, all enzymes are inactive (Zhu et al. 2010), which means that non-enzymatic pathways are the most likely explanation for the truncation of crystallins from aged eyes, of which there are several (Chaves et al. 2008; Lyons et al. 2014).
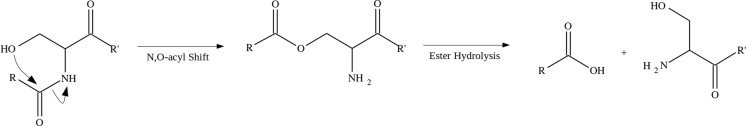
One example of a pathway for spontaneous protein cleavage involves an N,O-acyl shift at serine residues (Lyons et al. 2011), the products of which are encountered frequently in the aged eye lens. The mechanism for this rearrangement, shown in Fig. 8, results from an attack of the serine side-chain hydroxyl group on the preceding backbone amide. The subsequent hydrolysis and peptide chain cleavage is analogous to the intein protein splicing mechanism (Mills and Paulus 2005). This reaction is spontaneous but slow at neutral pH and physiological temperatures; however, the reaction rate increases with elevated temperature (Lyons et al. 2011), exposure to an acidic environment (Tuppy 1951) and the presence of trace metal ions (Yashiro et al. 2003). It is also probable that this truncation occurs for threonine residues as peptides beginning at these residues have been observed in the lens (Su et al. 2011). Protein truncations attributed to this mechanism are common in both aged and cataractous eye lenses. Thus, cleavage of the C-terminus of βA3-crystallin at Ser199 (Su et al. 2011) and γS-crystallin at Ser167 (Friedrich et al. 2012) is common in aged human lenses. Likewise, N-terminal truncation before αB-crystallin ser19 and between Ser59 and Ser75 of βA1-crystallin have been found (Santhoshkumar et al. 2008). It is proposed that these modifications result in a loss of crystallin protein solubility and a disruption of local structure, thereby increasing the probability of protein aggregation and precipitation. The presence of truncated proteins and the resultant peptides in cataractous lenses lends credence to this idea (Friedrich et al. 2012; Santhoshkumar et al. 2008). Additionally, it has been found that some of the small peptides cleaved from the N- or C-termini of lens proteins interact with fibre cell membranes, in some cases binding strongly (Friedrich et al. 2012), potentially weakening the cell walls and leading to the formation of membrane pores. Thus, the peptide products of these spontaneous truncation events may disrupt lens crystallin structure and affect lens fibre cell organisation.
Fig. 8.
Mechanism for spontaneous cleavage of peptide chains at serine residues. The peptide chain undergoes rearrangement via N, O-acyl shift involving the HO-side-chain. Hydrolysis of the resulting ester results in truncation (Lyons et al. 2011)
Deamidation
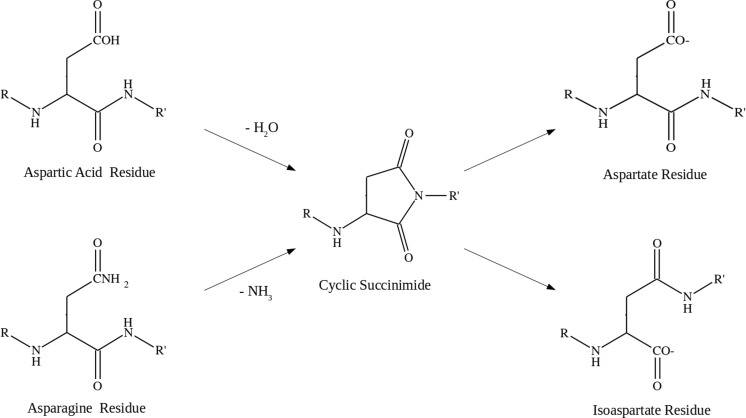
The deamidation post-translational modification is another spontaneous rearrangement commonly found in the older proteins of the body. This modification is so ubiquitous in longer-lived tissues that it is considered one of the factors limiting protein lifetimes (Robinson and Robinson 2004). Deamidation is one of the most common post-translational modification in the eye lens, occurring early in life and building up with age (Hains and Truscott 2010; Lampi et al. 2014; Takemoto et al. 2001). There are some sites of deamidation that occur more frequently in cataract-affected lenses compared to healthy lenses of the same age (Hooi et al. 2012). Deamidation modifies asparagine and glutamine residues by hydrolysing the amide side chain, producing the corresponding carboxylic acid residue (Clarke 1987). The major effect of this modification is the introduction of a negative charge at a formerly neutral location in a protein, and thus it has the potential to destabilise protein structures in the vicinity, including in the lens crystallins (Hains and Truscott 2010; Lampi et al. 2014).
Deamidation is a slow spontaneous rearrangement that occurs via a cyclic succinimide intermediate resulting from the attack by the Asn/Gln side-chain on the backbone amide to form a cyclic succinimide with the loss of ammonia (Reissner and Aswad 2003). The ring then hydrolyses to form the corresponding carboxyl residue, either aspartate or glutamate. Figure 9 shows the deamidation pathway for asparagine. Both asparagine and aspartic acid are capable of ring closing to form a succinimide intermediate, with the intermediate ring opening in both directions to subsequently produce the aspartate and isoaspartate residues (Lampi et al. 2014; Takemoto et al. 2001). The nature of the residue adjacent to the Asn/Gln is an important factor in both the rate of modification and propensity for deamidation. Sequences of Asn-Gly are known to be highly susceptible to deamidation to the extent that it can be difficult to express recombinantly proteins that contain this sequence (Reissner and Aswad 2003). Likewise, deamidation in older proteins is often found adjacent to serine, glycine and histidine residues (Wakankar and Borchardt 2006). Deamidation affects all of the structural proteins in aged eye lenses, but each is modified to a different extent. For example, βB2-crystallin shows deamidation at only one site and only at 3 % abundance even after 60 years of age, whereas γS-crystallin has up to 20 % deamidation at some residues, such as Asn76, by as early as 15 years of age, and up to 60 % deamidation in cataractous lenses (Hooi et al. 2012).
Fig. 9.
Mechanism of deamidation in asparagine and aspartic acid residues via cyclic succinimide intermediate. Subsequent ring opening can produce both aspartic acid (α-amino acid) and isoaspartic acid (β-amino acid) products (Reissner and Aswad 2003)
Racemisation
The conversion of L-amino acids to their D-enantiomer is common in cataractous lenses (Hooi and Truscott 2011). The amino acids that make up proteins are predominantly, with the exception of the non-chiral glycine, expressed as enantiomericly pure L-isomers. Over time, these chiral centres will undergo racemisation as the amino acids convert from pure L-isomers to a mixture of L- and D-isomers (Fujii et al. 1991). Racemisation is typically very slow but depends a great deal on the specific amino acids as well as on external effects such as temperature and radiation exposure (Lyons et al. 2014; Solheim 1993). In normal healthy tissues, the constant recycling of proteins means that significant quantities of D-isomers are unable to build up. However, in dead tissues or among particularly long-lived proteins, the ratio of L- to D-isomers will slowly approach parity (McCudden and Kraus 2006). The rate of conversion of L- to D-isomers in proteins is predictable to the extent that the post-mortem age of biological samples can be estimated by measuring the ratio of isomers using racemisation dating (Meissner and Ritz-Timme 2010; Solheim 1993). This is also true for proteins that do not undergo turnover such as eye lens crystallins, tooth dentin or elastin from ligaments (Ohtani and Yamamoto 1991; Ritz-Timme et al. 2003). Eye lens proteins have been shown to undergo racemisation at many sites, and these locations can be highly sequence-dependent and are often found in unstructured regions (Fujii et al. 2011; Hooi and Truscott 2011). Crystallins in healthy aged eyes exhibit racemisation at 4.5 % of all serine residues and 9 % of asparagine residues, corresponding to an average of two D-amino acids per protein (Hooi and Truscott 2011). Racemisation of threonine, glutamine, glutamic acid and phenylalanine residues has also been observed, but to a smaller degree, which is attributed to incidental exposure to high temperatures of the intact lenses during life. Cataractous lenses show a rate of racemisation in serine, aspartic acid, asparagine and threonine residues which is significantly higher than healthy, age-matched lenses (Hooi and Truscott 2011).
The racemisation of aspariginyl residues, asparigine and aspartic acid, is a special case. These residues show the largest tendency to racemise of any amino acid in the eye due to the existence of a chemical pathway that allows for steric inversion (Lampi et al. 2014; Wakankar and Borchardt 2006). This is the same rearrangement that causes deamidation via a succinimide intermediate ring opening (Fig. 9). This ring opening, already capable of forming structural isomer isaoaspartate, can cause the amino acid to form a D-enantiomer, with one possible product of this interconversion being a D-isoaspartate residue, equivalent to a Dβ-amino acid (Kaji et al. 2007). Both αA-crystallin Asp151 and γS-crystallin Asn76 are reported to undergo gradual racemisation over their lifetime with γS-crystallin Asn76 showing particularly high modification in cataractous lenses (Hooi et al. 2012). Of the 13 aspariginyl residues of αB-crystallin, Asp36 and Asp62 show extensive racemisation in aged lenses compared to young ones, a pattern repeated with βB2-crystallin Asp4 residue (Fujii et al. 1994). In αA-crystallin, Asp151 and 58 are highly modified in older eyes, but the racemisation of these residues can first appear at a young age (Fujii et al. 1997; Hooi et al. 2013b). Other studies suggest that the Asp151 is the primary site of racemisation in αA-crystallin, due to sensitivity to radiation damage from both UV-B (Fujii et al. 1997) and gamma radiation (Fujii et al. 2001). Serine residues are also highly susceptible to racemisation which may be the result of alpha proton abstraction (Lyons et al. 2014). Specific sites of serine racemisation in αA-Crystallin include Ser59 and 62 with 35 % D-amino acid (Hooi et al. 2013a).
Oxidation
Oxidation appears to be a common modification in aggregated protein extracted from cataractous lenses and is especially common in nuclear cataract (Truscott 2005). To protect against oxidation, the lens naturally contains high concentrations of antioxidants, particularly glutathione, and possesses several oxidation defence and repair systems that maintain redox homeostasis (Giblin 2000; Lou 2003). As the lens ages, these systems lose effectiveness and the concentration of antioxidants falls, resulting in an increase in oxidation over time (Xing and Lou 2010). The amino acids tryptophan, cysteine and methionine are commonly subject to oxidation in the lens (Moreau and King 2012; Stadtman 2006). Oxidation of cysteine residues is particularly common, with the modification first observed in the early stages of cataract formation and increasing in prevalence as cataract progresses, to the extent that >90 % of residues are modified in advanced cases (Garner and Spector 1980; Hains and Truscott 2008). Similarly, methionine residues exhibit up to 50 % oxidation upon the appearance of cataract and even non-cataractous lenses can have up to 37 % oxidised methionine (Garner and Spector 1980; Stadtman et al. 2005). Tryptophan residues also show significant oxidation in cataract-affected lenses when compared to healthy lenses, and several particular tryptophan sites have been identified where oxidation is highly correlated with cataract, such as βB1-crystallin Trp192 (Hains and Truscott 2007). Similarly, high levels of oxidation of αB-crystallin Met-68 have been reported in cataractous lenses (Yanshole et al. 2013). The oxidation-mimicking W42Q mutant of γD-crystallin readily shows reduced thermal stability and aggregates in the presence of WT γD-crystallin (Serebryany and King 2015). Likewise, the presence of oxidised βA3-crystallin peptide (152–166) increases aggregation in both α- and γ-crystallin bovine lens fractions (Udupa and Sharma 2005). The oxidation of proteins in the lens promotes the formation of covalent cross-links and the accumulation of mixed disulfide species (Moreau and King 2012). Highly oxidised proteins typically make up a large proportion of insoluble and urea-soluble protein fractions of cataractous lenses (Truscott 2005; Yanshole et al. 2013).
Other chemical modifications
The crystallins are prone to a wide range of chemical modifications such as phosphorylation and glycosylation (Bloemendal et al. 2004). Many proteins also undergo phosphorylation over time, particularly at serine residues. However, this modification is less correlated with cataract than many of the others (Hains and Truscott 2007). Phosphorylation is common to αA- and αB-crystallin at a number of locations and can either increase or reduce its chaperone activity depending on the location (Van Boekel et al. 1996; Ecroyd et al. 2007; Kamei et al. 2001; Li et al. 2012). The other eye lens crystallins are less noted for undergoing phosphorylation. Cysteine residues are also prone to methylation over time and this modification is common in the eye lens. Some prominent locations of crystallin cysteine methylation appear in cataract-affected eye lenses but this modification is also common in both aged healthy lenses and young lenses (Hains and Truscott 2007). Interestingly, the methylation of cysteine residues in γS-crystallin appears to occur early in life and is maximised well before any occurrence of cataract, and therefore appears to play little or no contributory role to lens opacification (Lapko et al. 2002). Glycosylation of the crystallin proteins appears in cataract associated with diabetes (Thorpe and Baynes 2012). The glycosylation at the delta carbon atom of lysine residues with glucose 6-phosphate has been reported and is believed to lead to increased aggregate formation via sulfhydryl oxidation (Stevens et al. 1978). As the aged crystallins become affected by these modifications, their structure can become disrupted, potentially exposing hydrophobic regions of the proteins to the aqueous solvent, encouraging random interactions between the crystallins (Ecroyd and Carver 2009; Moreau and King 2012). The formation of large protein aggregates increases light scattering and reduces vision quality, and leads to opacification (Trokel 1962).
Conclusion
The human eye lens exhibits many biochemical properties that are unique amongst the tissues of the body. The dual requirements of high transparency and high refractive index necessitate a high protein concentration (Bloemendal et al. 2004; Dahm et al. 2011). This makes the lens highly dependent on the stability of the crystallins in maintaining its function and makes the lens very vulnerable to diseases of ageing. The tendency for age-related damage to accumulate in the lens means that cataract is an inevitability for most individuals, especially as lifespans increase (Michael and Bron 2011). The nature of the lens means that repair of such damage is not practical and the only current effective method of treatment for cataract is surgical replacement, a procedure not always available in the developing world (Allen and Vasavada 2006; Batlle et al. 2014; Pascolini and Mariotti 2012). For non-surgical preventative treatment to be developed, the locations of post-translational modification that have a disproportionate effect on lens clarity must be characterised (Hooi and Truscott 2011; Michael and Bron 2011; Moreau and King 2012). The summary of post-translational modifications presented in this review show the wide range of changes that the crystallins are subject to as they age. Many of these modifications lead to protein destabilisation in vitro and many are correlated with cataract formation in vivo. While the scope of this review is necessarily limited, the wide variety of these destabilising modification may suggest that cataract is the result of cumulative damage from many sources rather than that of specific modifications having a disproportionate effect. This state of affairs will inevitably complicate the search for a pharmaceutical treatment for cataract. Nevertheless, the continued identification and characterisation of age-related post-translational modifications in the crystallins is an important and justifiability active field of science.
Acknowledgments
I would like to thank Prof. John Carver and Dr. Damien Hall for their support and supervision. This work was supported in part by an ANU PhD Scholarship and by the National Health and Medical Research Council of Australia (grant #1068087).
Compliance with Ethical Standards
ᅟ
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the author.
References
- Aarts HJ, Lubsen NH, Schoenmakers JG. Crystallin gene expression during rat lens development. Eur J Biochem. 1989;183:31–36. doi: 10.1111/j.1432-1033.1989.tb14892.x. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–3727. [PubMed] [Google Scholar]
- Allen D, Vasavada A. Cataract and surgery for cataract. Br Med J. 2006;333:128–132. doi: 10.1136/bmj.333.7559.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh A, Bantseeva V, Choh V, Moran KL, Sivak JG. The lens of the eye as a focusing device and its response to stress. Prog Retin Eye Res. 2006;25:189–206. doi: 10.1016/j.preteyeres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Barnwal RP, Jobby MK, Devi KM, Sharma Y, Chary KV. Solution structure and calcium-binding properties of M-crystallin, a primordial βγ-crystallin from archaea. J Mol Biol. 2009;386:675–689. doi: 10.1016/j.jmb.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GFJM. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman OA, Lubsen NH, Slingsby C. Association behaviour of human βB1-crystallin and its truncated forms. Exp Eye Res. 2001;73:321–331. doi: 10.1006/exer.2001.1038. [DOI] [PubMed] [Google Scholar]
- Bateman OA, Sarra R, Van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic β-crystallin oligomers and hetero-oligomers. Exp Eye Res. 2003;77:409–422. doi: 10.1016/S0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Batlle JF, Lansingh VC, Silva JC, Eckert KA, Resnikoff S. The cataract situation in Latin America: barriers to cataract surgery. Am J Ophthalmol. 2014;158:242–250. doi: 10.1016/j.ajo.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Bax B, Lapatto R, Nalini V, Driessen H, Lindley P, Mahadevan D, Blundell T, Slingsby C. X-ray-analysis of βB2-crystallin and evolution of oligomeric lens proteins. Nature. 1990;347:776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/S0006-291X(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–344. doi: 10.1016/S1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Chaves JM, Srivastava K, Gupta R, Srivastava OP. Structural and functional roles of deamidation and/or truncation of N-or C-termini in human αA-crystallin. Biochemistry. 2008;47:10069–10083. doi: 10.1021/bi8001902. [DOI] [PubMed] [Google Scholar]
- Chen J, Callis PR, King J. Mechanism of the very efficient quenching of tryptophan fluorescence in human γD- and γS-crystallins: the γ-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry. 2009;48:3708–3716. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiras D, Kitsos G, Petersen MB, Skalidakis I, Kroupis C. Oxidative stress in dry age-related macular degeneration and exfoliation syndrome. Crit Rev Clin Lab Sci. 2015;52:12–27. doi: 10.3109/10408363.2014.968703. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Ciryam P, Kundra R, Morimoto RI, Dobson CM, Vendruscolo M. Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci. 2015;36:72–77. doi: 10.1016/j.tips.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 1987;30:808–821. doi: 10.1111/j.1399-3011.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- Dahm R, van Marle J, Quinlan RA, Prescott AR, Vrensen GFJM. Homeostasis in the vertebrate lens: mechanisms of solute exchange. Philos Trans R Soc Lond B. 2011;366:1265–1277. doi: 10.1098/rstb.2010.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of alpha B crystallin. FEBS Lett. 2013;587:1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derham BK, Harding JJ. α-Crystallin as a molecular chaperone. Prog Retin Eye Res. 1999;18:463–509. doi: 10.1016/S1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15:3–16 [DOI] [PubMed]
- Ecroyd H, Carver JA. Crystallin proteins and amyloid fibrils. Cell Mol Life Sci. 2009;66:62–81. doi: 10.1007/s00018-008-8327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JLB, Robinson C, MacPhee C, Carver J. Mimicking phosphorylation of αB-crystallin affects its chaperone activity. Biochem J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugh SL, Kosinski‐Collins MS, King J. Contributions of hydrophobic domain interface interactions to the folding and stability of human γD‐crystallin. Protein Sci. 2005;14:569–581. doi: 10.1110/ps.041111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Lam J, Truscott RJ. Degradation of an old human protein: age-dependent cleavage of γs-crystallin generates a peptide that binds to cell membranes. J Biol Chem. 2012;287:39012–39020. doi: 10.1074/jbc.M112.391565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Mitchell CH, Boesze-Battaglia K. Autophagy in the eye: implications for ocular cell health. Exp Eye Res. 2014;124:56–66. doi: 10.1016/j.exer.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Chaperone function and mechanism of small heat-shock proteins. Acta Biochim Biophys Sin. 2014;46:347–356. doi: 10.1093/abbs/gmt152. [DOI] [PubMed] [Google Scholar]
- Fujii N, Muraoka S, Satoh K, Hori H, Harada K. Racemization of aspartic acids at specific sites in αA-crystallin from aged human lens. Biomed Res. 1991;12:315–321. doi: 10.2220/biomedres.12.315. [DOI] [Google Scholar]
- Fujii N, Satoh K, Harada K, Ishibashi Y. Simultaneous stereoinversion and isomerization at specific aspartic acid residues in αA-crystallin from human lens. J Biochem. 1994;116:663–669. doi: 10.1093/oxfordjournals.jbchem.a124577. [DOI] [PubMed] [Google Scholar]
- Fujii N, Momose Y, Ishibashi Y, Uemura T, Takita M, Takehana M. Specific racemization and isomerization of the aspartyl residue of αA-crystallin due to UV-B irradiation. Exp Eye Res. 1997;65:99–104. doi: 10.1006/exer.1997.0315. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hiroki K, Matsumoto S, Masuda K, Inoue M, Tanaka Y, Awakura M, Akaboshi M. Correlation between the loss of the chaperone‐like activity and the oxidation, isomerization and racemization of gamma‐irradiated alpha‐crystallin. Photochem Photobiol. 2001;74:477–482. doi: 10.1562/0031-8655(2001)074<0477:CBTLOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fujii N, Kaji Y, Fujii N. D-amino acids in aged proteins: analysis and biological relevance. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:3141–3147. doi: 10.1016/j.jchromb.2011.05.051. [DOI] [PubMed] [Google Scholar]
- Garner M, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Clark JI. Insights into the domains required for dimerization and assembly of human αB crystallin. Protein Sci. 2005;14:684–695. doi: 10.1110/ps.041152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- Goebel K, Ruefer F, Erb C. Physiology of aqueous humor formation, diurnal fluctuation of intraocular pressure and its significance for glaucoma. Klin Monatsbl Augenheilkd. 2011;228:104–108. doi: 10.1055/s-0029-1246040. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Nickerson JM, Edelhauser HF, Bergman LA, Berglin L. Anatomic alterations in aging and age-related diseases of the eye. Invest Ophthalmol Vis Sci. 2013;54:ORSF23. doi: 10.1167/iovs.13-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J. Biotechnical applications of small heat shock proteins from bacteria. Int J Biochem Cell Biol. 2012;44:1698–1705. doi: 10.1016/j.biocel.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim Biophys Acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJ. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Edskes H. Computational modeling of the relationship between amyloid and disease. Biophys Rev. 2012;4:205–222. doi: 10.1007/s12551-012-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Kardos J, Edskes H, Carver JA, Goto Y. A multi-pathway perspective on protein aggregation: implications for control of the rate and extent of amyloid formation. FEBS Lett. 2015;589:672–679. doi: 10.1016/j.febslet.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1:465–479. doi: 10.1016/S1568-1637(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Harding JJ, Dilley KJ. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976;22:1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hilton GR, Hochberg GKA, Laganowsky A, McGinnigle SI, Baldwin AJ, Benesch JLP. C-terminal interactions mediate the quaternary dynamics of alpha B-crystallin. Philos Trans R Soc Lond B. 2013;368:20110405. doi: 10.1098/rstb.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg GKA, Benesch JLP. Dynamical structure of alpha B-crystallin. Prog Biophys Mol Biol. 2014;115:11–20. doi: 10.1016/j.pbiomolbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Hooi MYS, Truscott RJ. Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age. 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MYS, Raftery MJ, Truscott RJW. Racemization of two proteins over our lifespan: deamidation of asparagine 76 in γS crystallin is greater in cataract than in normal lenses across the age range. Invest Ophthalmol Vis Sci. 2012;53:3554–3561. doi: 10.1167/iovs.11-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi M, Raftery MJ, Truscott RJ. Age‐dependent racemization of serine residues in a human chaperone protein. Protein Sci. 2013;22:93–100. doi: 10.1002/pro.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, Truscott RJ. Accelerated aging of Asp 58 in αA crystallin and human cataract formation. Exp Eye Res. 2013;106:34–39. doi: 10.1016/j.exer.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Della Santina L, Wong ROL. Functional architecture of the retina: development and disease. Prog Retin Eye Res. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/S0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Jehle S, Rajagopal P, Bardiaux B, Markovic S, Kühne R, Stout JR, Higman VA, Klevit RE, van Rossum B-J, Oschkinat H. Solid-state NMR and SAXS studies provide a structural basis for the activation of [alpha] B-crystallin oligomers. Nat Struct Mol Biol. 2010;17:1037–1042. doi: 10.1038/nsmb.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji Y, Oshika T, Takazawa Y, Fukayama M, Takata T, Fujii N. Localization of D-β-aspartic acid–containing proteins in human eyes. Invest Ophthalmol Vis Sci. 2007;48:3923–3927. doi: 10.1167/iovs.06-1284. [DOI] [PubMed] [Google Scholar]
- Kamei A, Hamaguchi T, Matsuura N, MASUDA K. Does post-translational modification influence chaperone-like activity of ALPHA.-Crystallin? I. Study on phosphorylation. Biol Pharm Bull. 2001;24:96–99. doi: 10.1248/bpb.24.96. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim S-H. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kingsley CN, Brubaker WD, Markovic S, Diehl A, Brindley AJ, Oschkinat H, Martin RW. Preferential and specific binding of human αB-crystallin to a cataract-related variant of γS-crystallin. Structure. 2013;21:2221–2227. doi: 10.1016/j.str.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/S0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JLP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D (2010) Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci Publ Protein Soc 19:1031–1043 [DOI] [PMC free article] [PubMed]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR, David LL. Lens β-crystallins: the role of deamidation and related modifications in aging and cataract. Prog Biophys Mol Biol. 2014;115:21–31. doi: 10.1016/j.pbiomolbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, Smith JB. S-methylated cysteines in human lens γS-crystallins. Biochemistry. 2002;41:14645–14651. doi: 10.1021/bi0267700. [DOI] [PubMed] [Google Scholar]
- Li R, Zhu Z, Reiser G. Specific phosphorylation of αA-crystallin is required for the αA-crystallin-induced protection of astrocytes against staurosporine and C2-ceramide toxicity. Neurochem Int. 2012;60:652–658. doi: 10.1016/j.neuint.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/S1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B, Jamie J, Truscott RJ. Spontaneous cleavage of proteins at serine residues. Int J Pept Res Ther. 2011;17:131–135. doi: 10.1007/s10989-011-9250-3. [DOI] [Google Scholar]
- Lyons B, Jamie JF, Truscott RJW. Separate mechanisms for age-related truncation and racemisation of peptide-bound serine. Amino Acids. 2014;46:199–207. doi: 10.1007/s00726-013-1619-5. [DOI] [PubMed] [Google Scholar]
- Mackey DA, Wilkinson CH, Kearns LS, Hewitt AW. Classification of iris colour: review and refinement of a classification schema. Clin Exp Ophthalmol. 2011;39:462–471. doi: 10.1111/j.1442-9071.2010.02487.x. [DOI] [PubMed] [Google Scholar]
- Mahendiran K, Elie C, Nebel J-C, Ryan A, Pierscionek BK. Primary sequence contribution to the optical function of the eye lens. Sci Rep. 2014;4:5195. doi: 10.1038/srep05195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Kraus VB. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin Biochem. 2006;39:1112–1130. doi: 10.1016/j.clinbiochem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Meissner C, Ritz-Timme S. Molecular pathology and age estimation. Forensic Sci Int. 2010;203:34–43. doi: 10.1016/j.forsciint.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B. 2011;366:1278–1292. doi: 10.1098/rstb.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R, van Marle J, Vrensen GF, van den Berg TJ. Changes in the refractive index of lens fibre membranes during maturation–impact on lens transparency. Exp Eye Res. 2003;77:93–99. doi: 10.1016/S0014-4835(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Mills KV, Paulus H. Homing endonucleases and inteins. Berlin: Springer; 2005. Biochemical mechanisms of intein-mediated protein splicing; pp. 233–255. [Google Scholar]
- Mishra A, Krishnan B, Srivastava SS, Sharma Y. Microbial βγ-crystallins. Prog Biophys Mol Biol. 2014;115:42–51. doi: 10.1016/j.pbiomolbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Masai I. The lens equator: a platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Develop Growth Differ. 2014;56:387–401. doi: 10.1111/dgd.12128. [DOI] [PubMed] [Google Scholar]
- Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18:273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AM, Treweek TM, Aquilina JA, Carver JA, Walker MJ. Glutamic acid residues in the C‐terminal extension of small heat shock protein 25 are critical for structural and functional integrity. FEBS J. 2008;275:5885–5898. doi: 10.1111/j.1742-4658.2008.06719.x. [DOI] [PubMed] [Google Scholar]
- Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim Biophys Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM. Small heat shock protein expression and functions during development. Int J Biochem Cell Biol. 2012;44:1613–1621. doi: 10.1016/j.biocel.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Neuhuber W, Schroedl F. Autonomic control of the eye and the iris. Auton Neurosci Basic Clin. 2011;165:67–79. doi: 10.1016/j.autneu.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Norrby E. Prions and protein-folding diseases. J Intern Med. 2011;270:1–14. doi: 10.1111/j.1365-2796.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- Ohtani S, Yamamoto K. Age estimation using the racemization of amino acid in human dentin. J Forensic Sci. 1991;36:792–800. doi: 10.1520/JFS13089J. [DOI] [PubMed] [Google Scholar]
- Page MJ, Di Cera E. Serine peptidases: classification, structure and function. Cell Mol Life Sci. 2008;65:1220–1236. doi: 10.1007/s00018-008-7565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Perng M-D, Zhang Q, Quinlan RA. Insights into the beaded filament of the eye lens. Exp Cell Res. 2007;313:2180–2188. doi: 10.1016/j.yexcr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013;54:ORSF54–ORSF59. doi: 10.1167/iovs.13-12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J, Kantorow M, Gopal-Srivastava R, Tomarev SI. Recruitment of enzymes and stress proteins as lens crystallins. EXS. 1994;71:241–250. doi: 10.1007/978-3-0348-7330-7_24. [DOI] [PubMed] [Google Scholar]
- Ponce A, Sorensen C, Takemoto L. Role of short-range protein interactions in lens opacifications. Mol Vis. 2006;12:879–884. [PubMed] [Google Scholar]
- Purnyn H. The mammalian retina: structure and blood supply. Neurophysiology. 2013;45:266–276. doi: 10.1007/s11062-013-9365-6. [DOI] [Google Scholar]
- Quantock AJ, Winkler M, Parfitt GJ, Young RD, Brown DJ, Boote C, Jester JV. From nano to macro: studying the hierarchical structure of the corneal extracellular matrix. Exp Eye Res. 2015;133:81–99. doi: 10.1016/j.exer.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax-Jeuken Y, Driessen H, Leunissen J, Quax W, de Jong W, Bloemendal H. beta s-crystallin: structure and evolution of a distinct member of the beta gamma-superfamily. EMBO J. 1985;4:2597. doi: 10.1002/j.1460-2075.1985.tb03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GN, Khanna R, Payal A. The global burden of cataract. Curr Opin Ophthalmol. 2011;22:4–9. doi: 10.1097/ICU.0b013e3283414fc8. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Aswad DW. Deamidation and isoaspartate formation in proteins: unwanted alterations or surreptitious signals? Cell Mol Life Sci. 2003;60:1281–1295. doi: 10.1007/s00018-003-2287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz-Timme S, Laumeier I, Collins M. Age estimation based on aspartic acid racemization in elastin from the yellow ligaments. Int J Legal Med. 2003;117:96–101. doi: 10.1007/s00414-002-0355-2. [DOI] [PubMed] [Google Scholar]
- Robertson LJ, David LL, Riviere MA, Wilmarth PA, Muir MS, Morton JD. Susceptibility of ovine lens crystallins to proteolytic cleavage during formation of hereditary cataract. Invest Ophthalmol Vis Sci. 2008;49:1016–1022. doi: 10.1167/iovs.07-0792. [DOI] [PubMed] [Google Scholar]
- Robinson NE, Robinson A. Molecular clocks: deamidation of asparaginyl and glutaminyl residues in peptides and proteins. Cave Junction: Althouse; 2004. [Google Scholar]
- Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225:89–93. doi: 10.1007/BF02160337. [DOI] [PubMed] [Google Scholar]
- Serebryany E, King JA. Wild-type human gamma D-crystallin promotes aggregation of its oxidation-mimicking, misfolding-prone W42Q mutant. J Biol Chem. 2015;290:11491–11503. doi: 10.1074/jbc.M114.621581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. Genetic origins of cataract. Arch Ophthalmol. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Yang Z, Lin P, Zaidi Z, Abbasi A, Russell P. The complete sequence of human lens gamma s-crystallin. Biochem J. 1995;307:407–410. doi: 10.1042/bj3070407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim T. A new method for dental age estimation in adults. Forensic Sci Int. 1993;59:137–147. doi: 10.1016/0379-0738(93)90152-Z. [DOI] [PubMed] [Google Scholar]
- Spector A. Methods of isolation of alpha, beta, and gamma crystallins and their subgroups. Invest Ophthalmol Vis Sci. 1964;3:182–193. [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Stefani M. Structural features and cytotoxicity of amyloid oligomers: implications in Alzheimer’s disease and other diseases with amyloid deposits. Prog Neurobiol. 2012;99:226–245. doi: 10.1016/j.pneurobio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Rouzer CA, Monnier VM, Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978;75:2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S-P, McArthur JD, Truscott RJ, Aquilina JA. Truncation, cross-linking and interaction of crystallins and intermediate filament proteins in the aging human lens. Biochim Biophys Acta. 2011;1814:647–656. doi: 10.1016/j.bbapap.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Sorensen CM. Protein-protein interactions and lens transparency. Exp Eye Res. 2008;87:496–501. doi: 10.1016/j.exer.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto L, Fujii N, Boyle D. Mechanism of asparagine deamidation during human senile cataractogenesis. Exp Eye Res. 2001;72:559–563. doi: 10.1006/exer.2001.0983. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Ponce A, Sorensen CM. Age-dependent association of γ-crystallins with aged α-crystallins from old bovine lens. Mol Vis. 2008;14:970–974. [PMC free article] [PubMed] [Google Scholar]
- Thorpe SR, Baynes JW. Nonenzymatic glycosylation of proteins in vitro and in vivo. Glycoconjugates. 2012;2:113–132. [Google Scholar]
- Tortora GJ, Derrickson BH. Principles of anatomy and physiology. New York: Wiley; 2008. [Google Scholar]
- Treweek TM, Rekas A, Walker MJ, Carver JA. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, αA- and αB-crystallin. Exp Eye Res. 2010;91:691–699. doi: 10.1016/j.exer.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Treweek TM, Meehan S, Ecroyd H, Carver JA. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci. 2015;72:429–451. doi: 10.1007/s00018-014-1754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokel S. The physical basis for transparency of the crystalline lens. Invest Ophthalmol Vis Sci. 1962;1:493–501. [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Tuppy H. The amino-acid sequence in the phenylalanyl chain of insulin. Biochem J. 1951;49:481. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udupa PEG, Sharma KK. Effect of oxidized beta B3-crystallin peptide (152–166) on thermal aggregation of bovine lens gamma-crystallins: identification of peptide interacting sites. Exp Eye Res. 2005;80:185–196. doi: 10.1016/j.exer.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Van Boekel MAM, Hoogakker SEA, Harding JJ, De Jong WW. The influence of some post-translational modifications on the chaperone-like activity of α-crystallin. Ophthalmic Res. 1996;28:32–38. doi: 10.1159/000267940. [DOI] [PubMed] [Google Scholar]
- Van Montfort RL, Bateman OA, Lubsen NH, Slingsby C. Crystal structure of truncated human βB1‐crystallin. Protein Sci. 2003;12:2606–2612. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noort JM, Bsibsi M, Nacken P, Gerritsen WH, Amor S. The link between small heat shock proteins and the immune system. Int J Biochem Cell Biol. 2012;44:1670–1679. doi: 10.1016/j.biocel.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Wakankar AA, Borchardt RT. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J Pharm Sci. 2006;95:2321–2336. doi: 10.1002/jps.20740. [DOI] [PubMed] [Google Scholar]
- Wang X, Garcia CM, Shui Y-B, Beebe DC. Expression and regulation of α-, β-, and γ-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–3619. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- Wenk M, Herbst R, Hoeger D, Kretschmar M, Lubsen NH, Jaenicke R. Gamma S-crystallin of bovine and human eye lens: solution structure, stability and folding of the intact two-domain protein and its separate domains. Biophys Chem. 2000;86:95–108. doi: 10.1016/S0301-4622(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Wilhelm H. Disorders of the pupil. Handb Clin Neurol. 2011;102:427–466. doi: 10.1016/B978-0-444-52903-9.00022-4. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. 1994;35:1132–1137. [PubMed] [Google Scholar]
- Wistow G. The human crystallin gene families. Hum Genomics. 2012;6:26. doi: 10.1186/1479-7364-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing K-Y, Lou MF. Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest Ophthalmol Vis Sci. 2010;51:6598–6604. doi: 10.1167/iovs.10-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanshole LV, Cherepanov IV, Snytnikova OA, Yanshole VV, Sagdeev RZ, Tsentalovich YP. Cataract-specific posttranslational modifications and changes in the composition of urea-soluble protein fraction from the rat lens. Mol Vis. 2013;19:2196. [PMC free article] [PubMed] [Google Scholar]
- Yashiro M, Sonobe Y, Yamamura A, Takarada T, Komiyama M, Fujii Y. Metal-ion-assisted hydrolysis of dipeptides involving a serine residue in a neutral aqueous solution. Org Biomol Chem. 2003;1:629–632. doi: 10.1039/b209565c. [DOI] [PubMed] [Google Scholar]
- Zhao H, Brown PH, Magone MT, Schuck P. The molecular refractive function of lens γ-crystallins. J Mol Biol. 2011;411:680–699. doi: 10.1016/j.jmb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Korlimbinis A, Truscott RJ. Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation Res. 2010;13:553–560. doi: 10.1089/rej.2009.1009. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Jr, Sinha D. βA3/A1-crystallin: more than a lens protein. Prog Retin Eye Res. 2015;44:62–85. doi: 10.1016/j.preteyeres.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]