Abstract
More than a century of research on the Frank–Starling Law has significantly advanced our knowledge about the working heart. The Frank–Starling Law mandates that the heart is able to match cardiac ejection to the dynamic changes occurring in ventricular filling and thereby regulates ventricular contraction and ejection. Significant efforts have been attempted to identify a common fundamental basis for the Frank–Starling heart and, although a unifying idea has still to come forth, there is mounting evidence of a direct relationship between length changes in individual constituents (cardiomyocytes) and their sensitivity to Ca2+ ions. As the Frank–Starling Law is a vital event for the healthy heart, it is of utmost importance to understand its mechanical basis in order to optimize and organize therapeutic strategies to rescue the failing human heart. The present review is a historic perspective on cardiac muscle function. We “revive” a century of scientific research on the heart’s fundamental protein constituents (contractile proteins), to their assemblies in the muscle (the sarcomeres), culminating in a thorough overview of the several synergistically events that compose the Frank–Starling mechanism. It is the authors’ personal beliefs that much can be gained by understanding the Frank–Starling relationship at the cellular and whole organ level, so that we can finally, in this century, tackle the pathophysiologic mechanisms underlying heart failure.
Keywords: Frank–Starling, Heart, Cardiomyocytes, Myofilaments, History
The heart
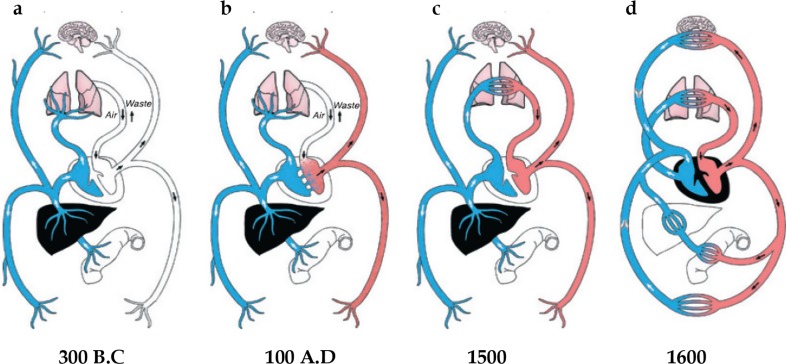
The heart, and its vessels, comprise the cardiovascular system responsible for the motion of blood throughout the body (Harvey 1889). William Harvey’s (1628 publication) “Exercitatio anatomica de motu cordis et sanguinis in animalibus” (On the motion of the heart and blood in animals) showed for the first time: (1) “that the blood moved in a ceaseless stream, as it were in a circle”, and (2) “that the heart is the great propelling power” (Harvey 1889). Although the anatomy of the heart was well known to physicians at the time of Harvey, namely the existence of four cavities divided by an “impermeable” septum and valves that prevented backflow of material, it was however generally accepted that the heart was “a generator of vital spirits, and of heat” and that the propelling of blood was an “act of inspiration, and its flow to any part of the body determined by special excitation” (Fig. 1) (Harvey 1889). William Harvey was the first to correctly define diastolic (relaxation) and systolic (contraction) phases of the heart “Whence the motion which is generally regarded as the diastole of the heart, is in truth its systole. And in like manner the intrinsic motion of the heart is not the diastole but the systole.” (Harvey 1889) It took 300 years before Wiggers (1921a, b) consolidated the meanings of systole and diastole that survive with minor modifications to day (Brutsaert and Sys 1989). Patterson and Starling stated that “[t]he working capacity of a pump is measured by its output.” (Patterson and Starling 1914) In the heart, cardiac output (CO) is the interdependence of blood volume ejected by the ventricles per contraction/heart beat—stroke volume (SV)—and the heart beat frequency—heart rate (HR)—occurring in 1 min (CO = SV × HR). Harvey’s description of the motion of blood greatly advanced the thinking of nineteenth century physiologists (Blasius 1872; Marey 1881; Dreser 1887; Frank 1895) and remains remarkably accurate to day.
Fig. 1.
A schematic overview of the cardiovascular system overtime. a Veins (blue) and arteries (white) are separate. Veins transport blood, in opposition to arteries that transport air. b Arteries (red) transport blood from right side of the heart, after it passes through invisible pores in the septum. c Establishment of the pulmonary circulation that transports blood through the lungs to the left side of the heart, and the liver was the source of veins the propelling power of blood. d Harvey’s view of the cardiovascular system. (Adapted from (2011) with permission)
The Frank–Starling law of the heart
The ability of the heart to adjust the force of its contraction in response to changes in ventricular filling (end-diastolic volume, EDV) forms one of the main pillars of muscle physiology. Ventricular filling sets the relationship between sarcomere length and tension development, and determines the degree of muscle shortening, which thereby regulates ventricular contraction and ejection. The observation that cardiac muscle contraction is the interdependent relationship between tension development, heat production and the extent of muscle shortening, as a function of the initial length of a muscle fiber, was first described for skeletal muscle by Blix (1891) and von Kries (1880, 1892). These were later applied to the heart and advanced by the German physiologist Frank (1895, 1895, 1959), who proposed that the developed pressure was directly proportional to the initial diastolic tension. However, Otto Frank’s experiments were inconclusive to whether an increase in the force of contraction was related to the initial diastolic tension or length of muscle fibers (Patterson et al. 1914). Ernest Starling and colleagues (Patterson and Starling 1914; Patterson et al. 1914; Knowlton and Starling 1912) later showed that “it is length rather than tension which determines the energy of contraction”. Accordingly, the “Law of the Heart” or the “Frank–Starling relationship”, reflect the ability of the heart to adjust the force of its contraction, in response to volume changes in venous return. Thus the Frank–Starling relationship explains beat-by-beat adjustment of cardiac output by both sides of the heart and pathological conditions that directly affect the Frank–Starling response (e.g. diastolic dysfunction), represent life-threatening situations.
Frank–Starling Law of the heart, and its place in history
Several concepts scrutinized this law, including the originality of Frank’s and Starling’s ideas, for instance, Gremels (1936) wrote that “Starling rediscovered [it] ten years later [than Frank]”. Guz (1974) in 1973 suggested: “If we were to give credit in full, we would have to call it the ‘Hales (Stephen 1740)–Haller (1754)–Mϋller (1844)–Ludwig (1856)–Roy (1879; Roy and Adami 1892)–Howell and Donaldson (1884)–Howell and Donaldson (1884)–Frank (1895)–Starling (1918) relationship”. But earlier, Chapman and Mitchell (1965) had argued that: “Starling’s work represents a convergence of various German and British intellectual forces […]. It was his genius that brought these various forces together in meaningful synthesis and it was largely his generalizations that provoked, and still provoke, highly constructive exchanges”. The authors, believe that Ernest Starling stated it as a “Law”, and this view was expressed by Stephen Hawking who stressed the importance of Nature’s Law’s to the success of mankind: “Today most scientists would say a [L]aw of nature is a rule that is based upon an observed regularity and provides predictions that go beyond the immediate situations upon which it is based.” A Law requires the prediction of phenomena or events that goes beyond the mere observation and can be measured and validated by others. The impact of the Frank–Starling relationship gained wide acceptance after Starling presented his concepts in 1915 (Starling 1918, 1920).
Cardiac reserve mechanisms
The normal heart is able to maintain or increase its output by several mechanisms that are mutually related: (1) recruitment of the Frank–Starling reserve; (2) increasing heart rate, which enhances the force of contraction via increases in the force–frequency relationship or Bowditch effect; (3) increases in the peak force generated during a contraction—positive inotropic response or enhanced contractility (e.g., hormones); and (4) elevations of afterload and the Anrep effect. Following an afterload elevation (e.g., vascular resistance), there is a rapid increase of end-diastolic volume that increases contraction (Frank–Starling). This initial rapid response is followed by a progressive and time-dependent (1–2 min) enhancement of contractility, which is independent of length alterations and allows the ventricle to recover towards its normal volume (von Anrep 1912).
Cardiac inotropic reserve
Other factors, such as hormones affect the pump function of the heart. (Starling 1920; von Anrep 1912; Meek and Eyster 1915) In 1891, Erik Johansson observed in dogs that stimulation of the splanchnic nerves raises arterial blood pressure (Johansson 1891). Later Lehndorff (1908) investigated a two-part rise of blood pressure, confirming that the initial rise was due to vasoconstriction of the splanchnic area, and the second due to increased heart rate. Four years later, Elliott (1912) demonstrated that the second rise was caused by secretion of adrenalin. Then, von Anrep (1912) established the link between accelerated heart rate and the secretion of adrenalin found by Elliott (1912). These findings were extended by Starling (1920) who noted that secretion of adrenalin dramatically increases “the energy available at each contraction.”, i.e. the modern positive inotropic effect introduced in 1904 by Engelmann (1904).
The Anrep effect or slow force response
In 1912, Glen von Anrep (1912), working in Ernest Starling’s laboratory, observed that, if arterial resistance was abruptly elevated, the end-diastolic volume first increased, but then after adrenaline administration contractility increased allowing ventricular volume to return to baseline (von Anrep 1912). Two years later, Patterson et al. (1914) observed the same phenomena but attributed it to “improved nourishment of the muscle”, suggesting that myocardial metabolism occurs with increased coronary flow when arterial resistance is increased. Then, Rosenblueth et al. (1959) and Sarnoff et al. (1960; Sarnoff and Mitchell 1961) named this effect “homeometric autoregulation” (Sarnoff et al. 1960) to distinguish it from the length changes in the Frank–Starling relationship, i.e. “heterometric autoregulation”. Parmley and Chuck (1973) used isolated papillary muscle to show that stretching induces the Frank–Starling effect followed by a slow increase in inotropic state if the muscle length remained constant. This slow force response was attributed to the slow increases of [Ca2+] due to stretch-induced activation of sarcolemmal channels, specifically the transient receptor potential canonical 6 (TRPC6) (Seo et al. 2014).
Striated muscle structural unit, the sarcomere
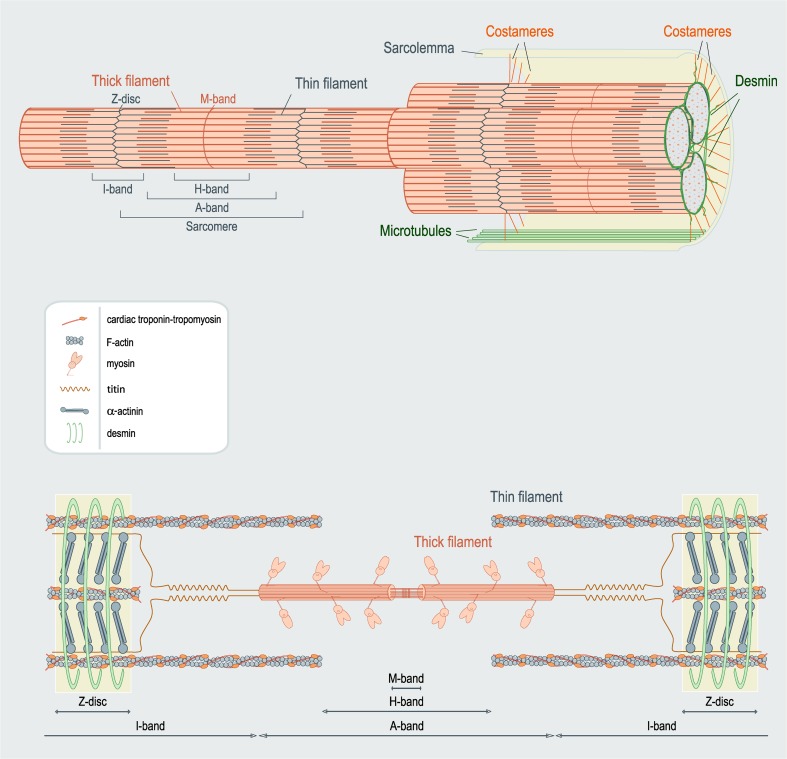
Seventeenth century microscopists, Robert Hooke and Antonie van Leeuwenhoek paved the way for the characterization and description of skeletal and cardiac muscle cells (Bowman 1840; Dobie 1849). Striated muscle fibers are composed of small assemblies, called “myofibrils” which contain the contractile components (Fig. 2, upper). They contain a succession of transverse striations that form the fundamental structural unit - the sarcomere (Fig. 2, lower) (Krause 1869; Schafer 1890). Each sarcomere is delineated by a pair “Z-lines” (from the German word “Zwischenscheibe” meaning “intermediate disc” (Krause 1869)). Sarcomeres are characterized by alternate zones of light I (isotropic) bands and dark A (anisotropic) bands (Engelmann 1873; Hanson and Huxley 1953). Each I-band is bisected by a Z-line and consists of thin filaments, while the central A-band contains both thin and thick filaments (Huxley 1953a). The H-band (from the German “Heller” meaning “brighter”) in the middle of the A-band, solely composed of thick filament structures (Hanson and Huxley 1953; Huxley 1953a). Finally, a dark M-line is the middle of the H-band (so called from the German “Mittelscheibe” meaning “central disc”) (Heidenhain 1913), is critical for the organization of the thick filaments in the sarcomere (Agarkova and Perriard 2005).
Fig. 2.
Anatomy of cardiac muscle. The upper figure illustrates a group of myofibrils connected to the sarcolemma via the costamere network. The lower image shows an individual sarcomere. Note the formation of distinct bands. The components are not drawn to scale. (Adapted from Sequeira et al. (2013a))
Contractile components of muscle
During each heart beat, cardiomyocytes undergo changes in length and load to allow the filling or ejection of blood. The sarcomeres shorten by converting chemical energy into mechanical force to perform work. They contain proteins that govern muscle contraction and relaxation, and structural proteins. Myofilament contraction requires the interaction of the thin (actin-containing) and thick (myosin-containing) filaments (Huxley 1957a). Force production and/or muscle shortening are the collective sum of the tension-generating cross-bridges. Regulation of this interaction depends on Ca2+ and ATP as well as the regulatory troponin–tropomyosin complex bound to the actin thin filaments (McKillop and Geeves 1993; Lehman et al. 2000). The cytoskeleton forms the scaffold that regulates cell shape, provides mechanical integrity and resistance, and stabilizes the sarcomeric proteins. Importantly, this framework mediates biomechanical and biochemical cell signaling that alters gene expression, post-translational modulation and protein synthesis (Kostin et al. 2000; Frank et al. 2006).
The sliding filament hypothesis
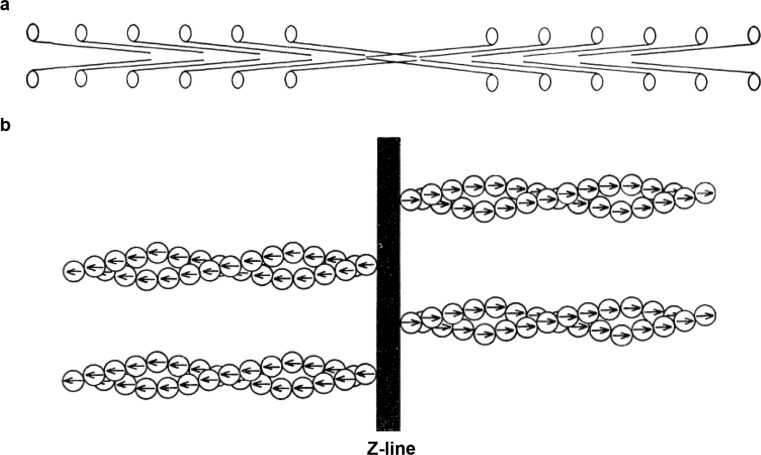
“The official date of the ‘birth’ of the sliding filament theory of muscular contraction is May 22, 1954” (Rall 2014) when Nature published two consecutive papers with the general title ‘Structural changes in muscle during contraction’. The first by Huxley and Niedergerke (1954) and the second by Huxley and Hanson (1954). These papers provided the molecular and mechanical foundations for muscle contraction. Both used high-resolution microscopy to study the structural arrangement of sarcomeres during contraction. They observed that, during contraction, the A-band length remained constant, while the I-band changed length (Huxley and Niedergerke 1954; Huxley and Hanson 1954). These observations signaled the ‘birth’ of the “sliding filament hypothesis”. In 1957, Huxley (1957a) proposed that the two sets of filaments interact and overlap forming several individual structures which H. E. Huxley defined as “cross-bridges” (Huxley 1957b). The resulting force is the collective sum of all “activated” cross-bridges that pull actin filaments towards the center of the sarcomere. Thick filaments are organized in hexagonal arrays, and each thick filament is surrounded by six actin filaments (Huxley 1953b; Stenger and Spiro 1961; Matsubara and Millman 1974a; Page 1974; Robinson and Winegrad 1979). Myosin molecules are packed “tail-to-tail” at the center of the sarcomere in anti-parallel alignment and optimize contact with the actin monomers (Fig. 3a) (Huxley 1961, 1963). Filament movement is directed towards the center of the sarcomere and is entirely determined by the actin filaments on each side of the Z-disc. Relative sliding of the two sets of filaments occurs when actins are “pulled” by myosins (Huxley 1961). The cross-bridges were originally described as “oar-like” (Huxley 1969), but when their structure was revealed at atomic resolution, the cross-bridges are now known to maintain a fixed angle with respect to the thin filaments, and the rotation is due to the converter domain closer to the base of the cross-bridge (Geeves and Holmes 1999).
Fig. 3.
a Diagram of myosin arrangement in the thick filament. b Represents actin molecules polarity pointing away from the Z-line. (adapted from Huxley (1971) with permission)
Muscle ultrastructure: elementary composition
Myosin, the major component of thick filaments
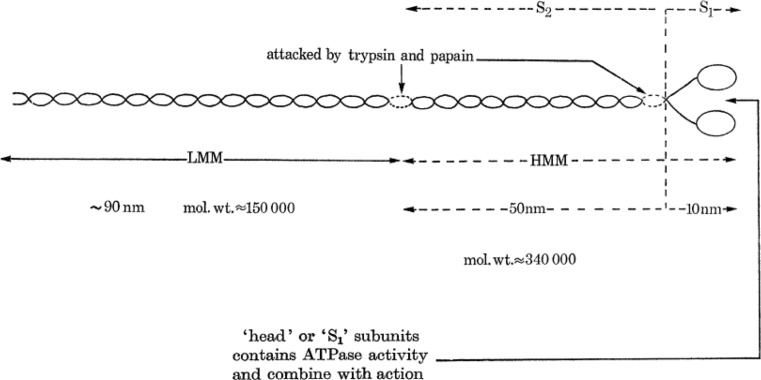
Originally known as “globulin”, myosin was defined in 1895 by von Fürth (1895). It was the first sarcomeric protein to be studied. Major advances in its chemistry and localization were performed by Annemarie Weber who showed that the birefringent properties of the A-bands were the direct result of the birefringence of “myosin threads” (Weber 1934, 1935). In 1939, Engelhardt and Liubimova (1939) reported that myosin is the main enzyme responsible for the hydrolysis of ATP. Structurally and each myosin has a long “rod-like” structure composed of one heavy chain and two light chains (Fig. 4) (Mueller 1965; Slayter and Lowey 1967; Richard Zobel and Carlson 1963; Rice 1961; Weeds and Pope 1971). Each heavy chain has a smaller component, the light meromyosin (LMM) and a larger component, the heavy meromyosin (HMM) (Szent-Györgyi 1953; Woods et al. 1963). They can be cleaved by papain and/or trypsin, and analyzed by gel electrophoresis (Fig. 4) (Gergely 1953; Mihalyi and Szent-Gyorgyi 1953; Mueller and Perry 1961; Kominz et al. 1965). The LMM forms the structure of the thick filament, while the HMM acts as a “hinge”, allowing S1 to move towards actin (Pepe 1966). This hinge property, propelled by the hydrolysis of ATP, governs actin-binding. The HMM is additionally divided into two sub-fragment extractions: sub-fragment 1 (S1) (Mueller 1965; Mueller and Perry 1961; Kominz et al. 1965) and sub-fragment 2 (S2) (Lowey et al. 1967). S1 accounts for 55–60 % of HMM (Mueller 1965) and comprises two globular heads containing an actin-binding component and catalytic ATPase activity. S2 separates the rigid LMM from S1 (Slayter and Lowey 1967).
Fig. 4.
A schematic of myosin (adapted from Huxley (1971)). Here S 1 represents the myosin head, S 1 and S 2 comprise the cross-bridge, and LMM forms the bulk of the thick filament
Actin, the major component of thin filaments
The main constituent of the thin filament is actin (Huxley and Hanson 1954; Perry and Corsi 1958; Ebashi et al. 1969). The actomyosin complex was originally budded “myosin B” in 1941 by Szent-Györgyi (1942) to distinguish it from the myosin (called myosin A) based on their different solubility and viscosity properties. Actin was thought to represent a second form of myosin when extracted from muscle, but Straub (1942) later found that it does not contain myosin B, but instead contains an association of proteins called “actin” which combines with myosin (actin + myosin). Myosin B was later called actomyosin, representing the polymerized form of actin and myosin (Straub 1942). Actin exists in two forms: a continuous monomeric strand of globular “inactive” actin (G-actin) spontaneously polymerizes to form “active” filaments (F-actin) (Huxley and Hanson 1954; Straub 1942, 1943).
Actomyosin/cross-bridges and ATP
An essential property of biological systems is their ability to convert chemical energy into mechanical energy. It was originally believed, and almost unchallenged, that the primary energy-producing reaction of muscle was lactic acid formation via the usage of glycogen. In 1927, Fiske and Subbarow (1927) showed that an “unstable form of phosphorus (which we shall for the present designate as ‘labile phosphorus’)”, i.e. “phosphocreatine” (PCr), decreased during contraction and was restored upon recovery. Evidence for the latter came in the early 1930s from Einar Lundsgaard in a series of papers (Lundsgaard 1930a, 1930b, 1930c) whose concepts overthrew the lactic acid theory of contraction. He showed that muscles poisoned with iodoacetate resulted in muscle spasm and stiffness (rigor) without lactic acid formation. Lundsgaard concluded that PCr was likely the direct source of contraction based on the observation that PCr content fell to zero in the poisoned muscles (Lundsgaard 1930a). Together, these data triggered interest in the study of PCr. Nevertheless, experiments performed in the same decade indicated that no enzyme could use PCr as a direct fuel source in muscle (Lohmann 1934). In 1934 (Lohmann 1934) and 1935 (Lohmann 1935), Lohmann demonstrated that “creatine kinase” (CK) can breakdown PCr, resulting in the conversion of ADP to ATP:
He concluded that ATP breakdown preceded PCr breakdown during contraction, thus providing strong evidence that ATP cleavage is the energy-producing reaction of muscle (Lohmann 1934). Today, we know that ATP hydrolysis (chemical) powers the interaction and sliding of myosin on actin in, i.e., work (mechanical). CK belongs to a group of energy-buffering systems that maintain in vivo levels of ATP. CK catalyzes the transfer of phosphate from PCr to ADP regenerating ATP while preventing the accumulation of cytosolic ADP (Allen and Orchard 1987).
One of the most accepted theories explaining the sliding process is the cross-bridge theory, which suggests that the energy released from ATP hydrolysis is the driving force for myosin extension towards actin (Huxley 1957a; Huxley and Niedergerke 1954; Huxley and Hanson 1954). Here, each bridge performs a number of cycles of attachment to and detachment from actin, which is accompanied by changes in myosin orientation and its initial conformation, changing the affinity of myosin for actin (McKillop and Geeves 1993; Geeves and Conibear 1995; dos Remedios et al. 1972). It has been shown that full ATPase activity of the S1 is greatly stimulated by Mg2+–MgATPase activity (Szent-Györgyi 1942; Kielley and Meyerhof 1948, 1950). When ATP is present, myosin and actin do not interact (step 2, Fig. 5) (Szent-Györgyi 1946). Because myosin-S1 can hydrolyze ATP into ADP and Pi (step 3′, Fig. 5) (Engelhardt 1942; Lymn and Taylor 1971) this process (Wagner and Weeds 1977) is strongly enhanced by binding to actin (step 4, Fig. 5) (Geeves and Holmes 1999). Moreover, formation of weak-binding cross-bridges occurs. A change of actomyosin conformations occurs (steps 5 and 6, Fig. 5) with the release of Pi from the S1 head (step 7, Fig. 5) accompanied by strong-binding cross-bridges (White and Taylor 1976; Chalovich and Eisenberg 1982; Pate and Cooke 1989). Force generation and work accompany muscle shortening. ADP is then released (step 8, Fig. 5) and a new ATP molecule binds to the actomyosin complex to begin a new cross-bridge cycle (step 1, Fig. 5). If ATP is lower than 0.1 mM (Cooke and Bialek 1979; Goldman et al. 1984), cross-bridges become permanently attached (Matsubara and Millman 1974a) (step 8, Fig. 5) and the muscle becomes rigid (“rigor mortis”) (Szent-Györgyi 1946; Huxley and Brown 1967). For a detailed summary of events, see (Gordon et al. 2000).
Fig. 5.
Cross-bridge cycle. (Adapted from Gordon et al. (2000) with permission)
Tropomyosin, a thin filament-associated protein
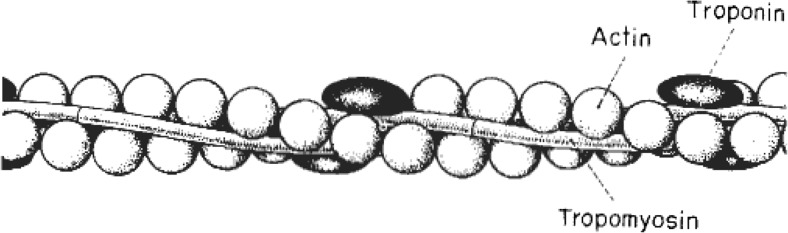
Tropomyosin (Tm) spans each seven actin monomers. It was discovered by Kenneth Bailey in 1946 (Bailey 1946) who proposed that due to its “analytical and structural similarities […] [tropomyosin] is a species of myosin differing mainly in the length of the polypeptide chain” and so “in proposing the present name, we have deemed it desirable to retain the word ‘myosin’ and to add a prefix which suggests this specific relationship.” Today, tropomyosin is far from being a species of myosin. It has two parallel α-helical chains, overlapping head-to-tail along the thin filament.
Setsuro Ebashi was the first to recognize the role of tropomyosin in muscle (Ebashi 1960, 1963). He demonstrated that a new protein with similarities to the tropomyosin described by Kenneth Bailey was required for the ability of the actomyosin complex to be sensitive to Ca2+ (Ebashi 1963). This protein (“native” tropomyosin) was the combination of tropomyosin with a new globular protein, Ebashi, later called “troponin” (Ebashi and Ebashi 1964). Troponin exhibited “cementing” (stabilizing) effects on the interaction between actin and tropomyosin (Ebashi and Kodama 1965; Drabikowski and Nonomura 1968; Pirani et al. 2005), and was essential to regulate tropomyosin’s position on actin, in a Ca2+-dependent manner (Lehman et al. 2000; Huxley 1973a; Haselgrove 1973; Parry and Squire 1973; Vibert et al. 1997).
Troponin complex, thin filament-associated component
Troponin (Tn) was initially termed by Ebashi and colleagues (Ebashi and Kodama 1965, 1966) in 1965 as a “tropomyosin-like protein” due to its similarities to the tropomyosin reported by Bailey (Bailey 1948) “the presence of the [troponin] component makes actomyosin tend to relax, or dissociate, if the concentration of free calcium ions are lowered”. Troponin bound Ca2+ and regulated tropomyosin movement on actin (Parry and Squire 1973). Nevertheless, instead of a single protein exerting multiple roles in muscle contraction, a “flavor” of different troponin subunits was discovered with distinctive structures and functions (Hartshorne and Mueller 1968; Drabikowski et al. 1971a, 1971b; Ebashi et al. 1971; Greaser and Gergely 1971; Hartshorne and Pyun 1971; Sarkar et al. 1971; Schaub et al. 1972; Wilkinson et al. 1972). The troponin complex was separated into 2–4 gel fractions when precipitated under particular conditions, such as the presence or absence of Ca2+, actin, myosin, actomyosin and tropomyosin (Hartshorne and Mueller 1968; Drabikowski et al. 1971a, 1971b; Ebashi et al. 1971; Greaser and Gergely 1971; Hartshorne and Pyun 1971; Sarkar et al. 1971; Schaub et al. 1972; Wilkinson et al. 1972). The identification and clarification of each fraction became a challenge with distinct methodologies producing differing results, including separation of fractions on gels, differing molecular weights and nomenclatures used (troponin A, troponin B, inhibitory factor, Ca2+-sensitizing factor, TN-I, TN-T, TN-C, fraction I, II, III and IV) (Greaser and Gergely 1973). Table 1 (adapted from Perry et al. (1973)) illustrates this dilemma. Several properties of the troponin subunits included components that: (1) were capable of binding Ca2+ (troponin C, troponin A, Ca2+-sensitizing factor, troponin fraction III and troponin 4); (2) inhibited actomyosin interactions (troponin I, troponin B, inhibitory factor, troponin fraction II and troponin 2); and (3) interacted with tropomyosin (troponin T, 37,000 component, troponin fraction T and troponin 3). The fourth fraction was suspected to be a contaminant and is omitted from Table 1 (Drabikowski et al. 1971b; Wilkinson et al. 1972; Schaub 1971).
Table 1.
Components of troponin (adapted from Perry et al. (1973))
| Calcium-binding protein | Inhibitory protein | 37,000 component | References |
|---|---|---|---|
| Troponin A | Troponin B | Hartshorne and Mueller 1968 | |
| Calcium sensitizing factor | Inhibitory factor | 37,000 component | Schaub and Perry 1969; Wilkinson et al. 1971, 1972 |
| Troponin 4 | Troponin 2 | Troponin 3 | Greaser and Gergely 1971 |
| Troponin III | Troponin II | Troponin T | Ebashi et al. 1971 |
| Component III | Component II | Component I | Murray and Kay 1971 |
| Troponin C | Troponin I | Troponin T | Potter and Gergely 1974 |
Based on these data, H. E. Huxley in 1972 (Huxley 1973a) proposed a common nomenclature: “I would therefore like to propose that the following scheme be generally adopted: that the Ca-binding component of troponin (mol wt ~18,000) be called Tp C; that the inhibitory component (mol wt ~23,000) be called Tp I; and that the tropomyosin-binding component (mol wt ~37,000) be called Tp T”.
Today, these are known as TnC, TnI and TnT, and, together with seven actin monomers and one tropomyosin dimer, constitute the thin filament “functional unit” (A7TmTn, Figs. 6 and 7).
Fig. 6.
An early model of the thin filament structure. (Ebashi et al. (1969))
Fig. 7.
Modern schematic model of the thin filament functional unit. Five actin monomers (gray) spanned by one tropomyosin dimer (red) and one troponin complex: cTnC (pink), cTnI (blue) and cTnT (orange). N and C depict N- and C-terminal protein ends, respectively. Dark-blue tropomyosin depicts near-neighbor tropomyosin dimer interaction (Greenfield et al. 2006; Murakami et al. 2008). Myosin-S1 is depicted in solid green (light-green myosin-S1 to better understand its transition states). The orientation of thin filament proteins is: the N-terminal region of cTnT points towards the pointed end (M-band), while the core domain of the troponin complex is oriented to the barbed end (Z-disk) (Paul et al. 2009). Interacting sites and structural regions of actin-tropomyosin-troponin proteins are matched in accordance with available literature (Sequeira et al. 2013b). Cardiac TnI residues 1-34 are arbitrarily positioned. Our figure follows the proposed mechanism for Ca2+-regulation of contraction proposed by Murakami et al. (2005) (Adapted from Sequeira et al. (2013b))
The third filament: titin
The existence of another filament was initially reported by H. E. Huxley and Jean Hanson (1954) in 1954, when through actin–myosin extractions, the authors showed that the sarcomere remained intact. They (Huxley and Hanson 1954) proposed that an elastic component (“S-filaments”) provided continuity between an actin filament and the opposite actin filament in a sarcomere, and would attached to myosin: “The backbone of the muscle fibril is made up of actin filaments which extend from the Z-line up to one side of the H-zone, where they are attached to an elastic component (not the series elastic component) which for convenience we will call the S-filaments”. However, it was difficult at the time to confirm the existence of such an elastic and integrative component. Years later, the existence of a giant elastic protein called “connectin” was reported, consistent with the earlier proposed S-filaments (Maruyama et al. 1977).
Today, it is better known as “titin” (Wang et al. 1979) based on its large proportions (molecular weight ranging from 3 to 3. 8MDa (Maruyama et al. 1984; Labeit and Kolmerer 1995)). Titin is the third-most abundant filament protein by weight. (Labeit et al. 1997) Partially responsible for the generation of resting tension, titin is also the mechano sensor of the sarcomere (Linke and Kruger 2010).
The N-terminal region of titin
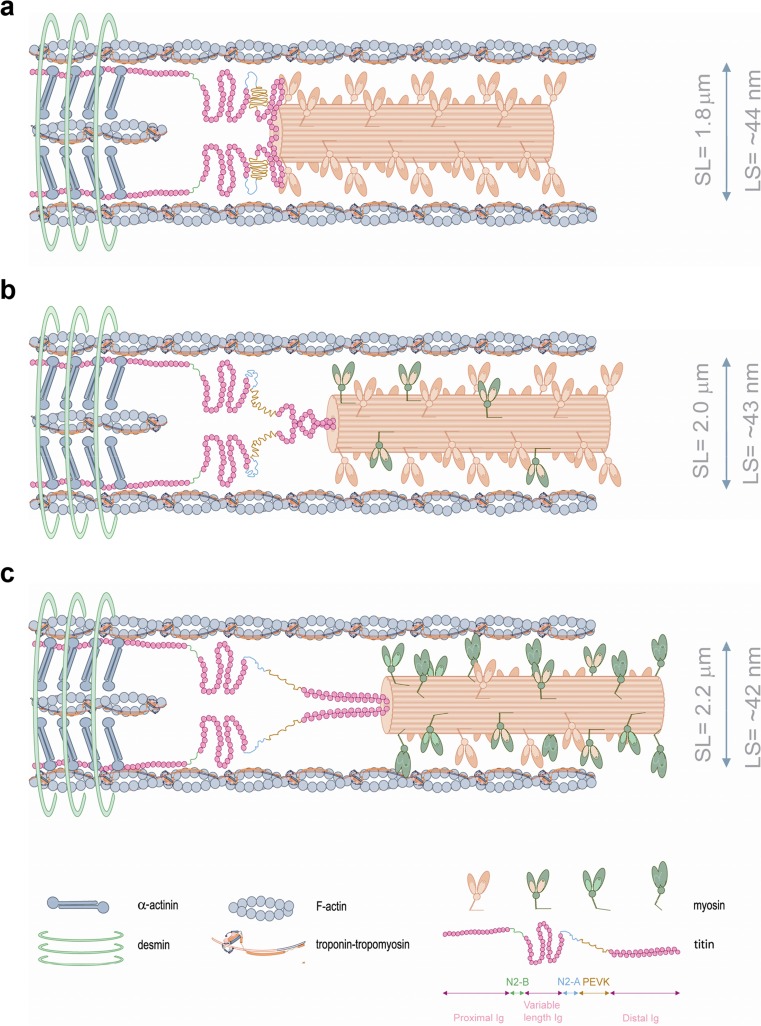
This part of titin resides in the Z-disc and interacts with actin (Trombitás et al. 1997) and possibly with α-actinin (Ohtsuka et al. 1997; Sorimachi et al. 1997) via 45 amino acid repeat regions (Z-repeats) that provide a mechanism of Z-disc assembly resulting from alternative splicing (Gautel et al. 1996). The I-band region of titin is the extensible region and consists of three elastic components that act as a spring element (Fig. 8): (1) tandem immunoglobulin (Ig)-like domains with proximal (near Z-disc) and distal (near I-A regions) segments; (2) the PEVK sequence-region rich in proline (P), glutamic acid (E), valine (V) and lysine (K); and (3) the N2B and N2BA elements (both isoforms contain N2B segments, but only the N2BA has the N2A element) (Labeit and Kolmerer 1995).
Fig. 8.
A schematic overview of titin depicted in half-sarcomere. Note the extension of the elastic components of titin when the sarcomere is stretched. (Adapted from Linke and Kruger (2010))
C-terminal region of titin
The C-terminal A-band region of titin is inextensible. It interacts with thick filament and associated proteins including myosin and cardiac myosin-binding protein C (cMyBP-C) (Zoghbi et al. 2008; Freiburg and Gautel 1996; Maruyama et al. 1985). In the M-band, titins from opposing half-sarcomeres intersect and interconnect with M-band proteins, thereby forming a continuous filament from the M-band towards the Z-disc (Helmes and Granzier 2011; Linke 2008). Titin may be arranged in the thick filament as a dimer (Tskhovrebova et al. 2010) and presumably a bundle of “six titin molecules connect each end of the thick filament to the Z-disk” (Houmeida et al. 2008). Titin is present in both skeletal and cardiac muscle, but differs in its size (Hill and Weber 1986). Cardiac isoforms are smaller, ranging from 3 MDa (N2B) to over 3.2 MDa (N2BA) (Freiburg et al. 2000), as opposed to the even larger skeletal isoforms (N2A) that can reach as much as 3.8 MDa (Labeit and Kolmerer 1995; Linke and Kruger 2010).
Titin and its “elastic” tension of muscle
Under resting conditions, striated muscle resists muscle lengthening by producing passive tension in response to stretch. In the heart, titin accounts for approximately 80 % of total passive tension between physiological operating sarcomere lengths (1.8–2.2 μm). When over-stretched (>2.2 μm), where the contribution of collagen is greater, the titin stretch-based passive tension remains high, indicative of the central role of titin to respond to stretch (Granzier and Irving 1995; Chung and Granzier 2011). Passive (resting) tension results from the extensible I-band spring segment that elongates as sarcomere length increases. The tandem Ig-like segments are the first to extend, followed by the PEVK segment and lastly, the elongation of the N2B segment (Helmes and Granzier 2011; Linke 2008). The flexibility and stretch-based passive tension of the titin spring elements can be regulated by two major mechanisms: a fast “acute” modulation by post-translational modifications and a “chronic” isoform shift due to alternative splicing of the I-band.
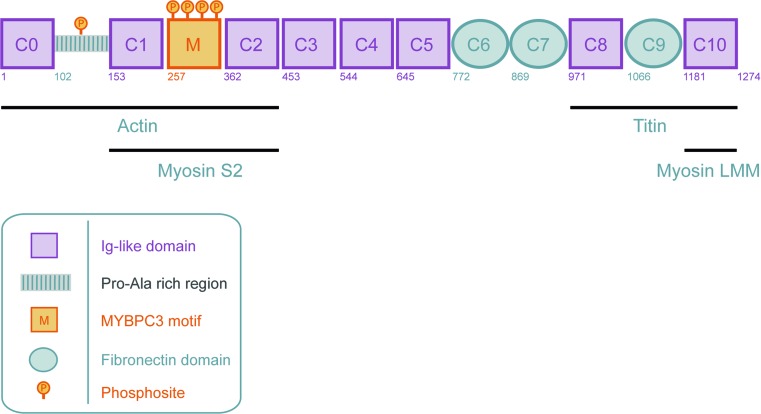
cMyBP-C, thick filament-associated component
The thick filament titin is not solely bound to myosin, since there is another protein in the C-zone of the A-band (Zoghbi et al. 2008; Labeit et al. 1992; Craig and Offer 1976). Called “C-protein” (Offer et al. 1973; Offer 1973) or “cMyBPC” (Vaughan et al. 1993), this protein appears to hold a central role in cross-bridge binding (Herron et al. 2006; Stelzer et al. 2006a) and cycling kinetics (Stelzer et al. 2006a, 2006b). cMyBP-C mainly consists of immunoglobulin-like C2 domains (eight domains) and three/four fibronectin type-III domains. In addition, cMyBP-C has a proline-alanine rich region between domains C0 and C1 and a M-domain between domains C1 and C2 (Fig. 9). Both linker domains have important functional roles (see below). MyBP-C was identified in 1973 by Offer et al. (1973) from skeletal muscle. While MyBP-C’s interaction with myosin (Offer et al. 1973) and actin (Pfuhl and Gautel 2012; Moos et al. 1978; Yamamoto and Moos 1983) were reported soon after its discovery, the exact role of MyBP-C in muscle contraction remained poorly understood. Discovering the individual interaction partners of the N’ and C’ domains was key to understanding the function of MyBP-C in regulating contraction.
Fig. 9.
A schematic domain structure of cMyBP-C. Cardiac MyBP-C consists of eight Ig-like and three fibronectin domains labeled C0 (N-terminus) through C10 (C-terminus). Two additional domains are present in the N-terminal part of the protein, the Proline-Alanine rich region (PA) and the M-domain (M). Four phosphorylation sites (Ser275, Ser284, Ser304 and Ser311) have been described in the M-domain. A recent study (Kuster et al. 2013) revealed a novel phosphorylation site on serine 133 in the PA region. (Adapted from Sequeira et al. (2013c))
C-terminal region of MyBP-C
The C-terminal domains are important for the binding of MyBP-C to thick filaments (Gilbert et al. 1996) that occur via its interaction with the LMM region of myosin heavy chain (MHC) (Miyamoto et al. 1999; Starr and Offer 1978). Although the C10 domain was identified as the myosin LMM binding domain, domains C7-C9 are also needed for proper incorporation into the thick filament (Gilbert et al. 1996). This might be mediated by the interaction of the C’ domains of cMyBP-C with titin, which involves domains C8-C10 (Freiburg and Gautel 1996).
N-terminal region of MyBP-C
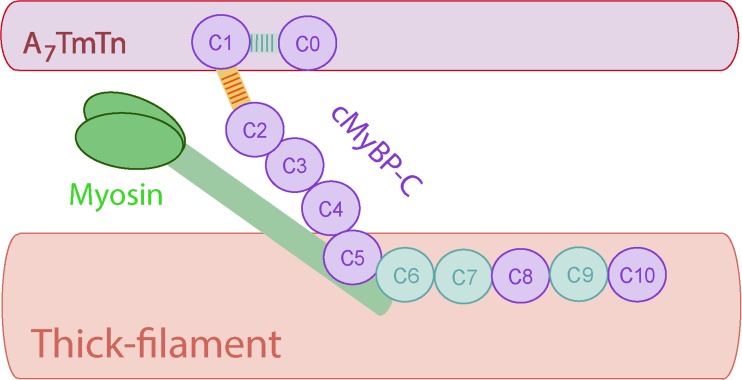
While the C-terminus of cMyBP-C is important for its location and anchoring to thick filaments, the N-terminus is the region through which cMyBP-C exerts its regulatory role on contraction. The best understood function of cMyBP-C’s is its effect on cross-bridge cycling kinetics (Fig. 10). This effect is mediated by the interaction of the cMyBP-C N-terminus with the MHC “neck” region (Gruen and Gautel 1999). This S2 region is the hinge region of MHC and connects the LMM to the myosin head (S1 domain). By binding to this region, cMyBP-C can slow cross-bridge cycling kinetics (Fig. 10). The cMyBP-C/S2 interaction is phosphorylation-dependent and the phosphorylation sites in the M-linker domain are important for modulating this interaction (Gruen et al. 1999). When the cMyBP-C M-domain sites are not phosphorylated, cMyBP-C binds to S2. Upon phosphorylation of cMyBP-C, this interaction is lost. (Gruen et al. 1999) Therefore, the cMyBP-C M-domain, together with the adjacent C1 and C2 domains, are thought to be the sites of interaction with myosin cross-bridges (Gruen and Gautel 1999; Bhuiyan et al. 2012). More controversial has been cMyBP-C’s interaction with actin. Although cMyBP-C was reported to interact with actin in vitro soon after its discovery (Moos et al. 1978; Yamamoto and Moos 1983), the in situ visualization of cMyBP-C‘s direct interaction with actin in intact muscle was lacking. In recent years, accumulating evidence provided by in situ 3D reconstruction approaches (X-ray neutron scattering (Whitten et al. 2008), negative EM staining (Mun et al. 2011; Orlova et al. 2011) and electron tomography (Luther et al. 2011)) demonstrated that cMyBP-C interacts with actin. The N-terminus of cMyBP-C projects towards the thin filament making direct contact with actin. Phosphorylation of sites in the M-domain weakens its interaction with actin (Shaffer et al. 2009). Additionally, recent evidence suggests that the N-terminal extension of cMyBP-C binds the low Ca2+-state (blocked state, B-state; further discussed) position of tropomyosin on actin, indicating that cMyBP-C can interfere with tropomyosin–actin interactions and regulate thin filament transitions (Mun et al. 2011). The functional implications of the putative actin–cMyBP-C interaction are not yet fully understood. Another sarcomeric protein that was recently identified to interact with cMyBP-C’s N-terminus (more specifically the C0 domain) is the regulatory light chain (Ratti et al. 2011). Again, the functional consequences of this interaction are not yet understood.
Fig. 10.
Schematic structure of cMyBP-C. cMyBP-C consists of eight Ig and three fibronectin domains labeled C0 (N-terminal) to C10 (C-terminal), with two additional linker domains the PA (Proline-Alanine; light blue stripes) region between C0 and C1, and the M-domain (M; yellow and orange stripes), between C1 and C2. The C5–C10 domains extend along the thick filament, while the C0-C4 extend to the thin filament. A7TmTn depict a functional unit composed of 7 actin monomers, 1 tropomyosin (Tm) dimer and 1 troponin (Tn) complex
Central role for Ca2+ in muscle contraction
It is well established that cardiac muscle contraction, defined as “excitation–contraction coupling” by Alexander Sandow in 1952 (Sandow 1952), is initiated by electrical activation of cardiomyocytes and results in increase in intracellular [Ca2+]. The first indications for the importance of Ca2+ in the activation of cardiac muscle were demonstrated in the 1880s by Ringer (1882, 1882, 1883), who observed that the ventricle of frog hearts beat faster when he used solutions prepared from tap water supplied by the New River Water Company in England, but not when distilled water was used. The difference was due to Ca2+ in the tap water. Ringer could maintain cardiac contractions if CaCl2 and KCl were added during saline perfusion (0.75 % NaCl) at concentrations of about 0.5 and 1.3 mM, respectively. The great next step for Ca2+ research was reported by Locke and Rosenheim in 1907 (Locke and Rosenheim 1907) using Ringer’s solution (0.75 % NaCl, 0.5 mM CaCl2, 1.3 mM KCl), who showed that omission of Ca2+ and K+ from the Ringer’s solution maintained metabolic activity of the heart, but blocked muscle contraction: “Calcium is necessary for the conversion of the heart’s chemical energy into the mechanical energy of its beat, while potassium is more necessary for the merely chemical processes of cardiac activity”. Similar observations were made in 1913 by George R. Mines, who reported that a Ca2+-free solution generates normal action potentials, but no mechanical response (Mines 1913). Further progress was made in 1940 by Heilbrunn (Heilbrunn 1940), who showed that damaged muscle fibers could generate contractions in solutions containing high [CaCl2] (>20 mM), and in 1942 Bailey (Bailey 1942) observed that mM concentrations of Ca2+ activated the ATPase activity of myosin.
The essential role of Ca2+ in muscle contraction and relaxation remained obscure in the 1960s as noted by Ebashi and Endo (1968): “It is ironic that recognition of the essential role of Ca ion in contraction has resulted mainly from the investigation into the mechanism of relaxation”. In the 1950s, it was well established that ATP was required for contraction in concert with Ca2+. However, muscle physiologists did not understand how muscle relaxation proceeded. Marsh (1951, 1951, 1952) proposed that a “relaxing factor” of small molecular size existed, responsible for the relaxation of muscle. It was believed myokinase was responsible for the “relaxing/Marsh factor” (Bendall 1953, 1954). In 1954 and 1955, Bozler (1954) and Watanabe (1955) reported that in the presence of ATP, a synthetic compound called “EDTA” mimicked the action of this “relaxing factor”, since EDTA by Ca2+-chelation was able to make the muscle relax. Furthermore, Weber recognized in 1959 (Weber 1959) that actomyosin preparations only hydrolyzed ATP if > μM of Ca2+ was present. Final confirmation for the role of Ca2+ in muscle activation came from Ebashi in a series of papers in the 1960s (Ebashi 1960, 1961a, 1961b, 1963; Ebashi and Ebashi 1962; Ebashi and Lipmann 1962) demonstrating that indeed μM Ca2+ was required for the superprecipitation of the actomyosin complex, and that the “relaxing factor” was in fact a vesicular factor capable of removing and storing Ca2+, derived from an organelle with an extensive tubular network, i.e. the sarcoplasmic reticulum (SR). Ford and Podolsky (1970) suggested that Ca2+ release from the SR could be induced by external Ca2+, which was confirmed by Endo et al. (1970) (Ca2+ promotes Ca2+-release from the SR). Despite the strong evidence for the role of Ca2+ in contraction–relaxation at the time, several weaknesses were presented by Weber and Winicur (1961), who observed that some preparations of synthetic actomyosin (a mixture of myosin and actin separately prepared) were less sensitive to Ca2+: “Some actomyosin preparations superprecipitate very little or not at all and hydrolyse adenosine triphosphate at one quarter or one-half of the maximal rate obtained on addition of CaCl2 to give a concentration of 0.1 mM”. As shown previously, Ebashi reported that this was due to a third muscle component, tropomyosin (Ebashi 1963).
Roles of Ca2+ and ATP in muscle contraction
Cardiac muscle contraction is initiated upon electrical activation of cardiomyocytes and the resulting increase in intracellular [Ca2+] and regeneration of ATP. In the 1970s, the idea raised that cross-bridge cycling occurs in two stages. In the absence of Ca2+, tropomyosin physically blocks the myosin-bindings sites on actin (the steric blocking model) and then the concept that, with raised intracellular [Ca2+], myosin binds to actin and induces force development (Haselgrove and Huxley 1973; Huxley 1973b). McKillop and Geeves (1993) in 1993 advanced the later ideas and proposed a three-state model of the thin filament, in which myosin-binding to actin in the presence of Ca2+ does not occur in a single step, but instead in two steps that reflect changes in the affinity of myosin for actin. In concert, Ca2+ and ATP characterize three distinctive states of a muscle fiber: (1) relaxed, (2) activated and (3) rigor. In addition, a three-state model of thin filament regulation comprises three distinct biochemical states of muscle: “blocked-state”, “closed-state” and “open-state”. Electron microscopy reconstructions (Lehman et al. 2000) of thin filament proteins confirmed the solution studies from McKillop and Geeves (1993), and proposed new generic terms to “avoid nomenclature with unintended connotations” (Lehman et al. 2000): the blocked-state corresponds to the “blocked (B-state)”; the closed-state to “Ca2+-induced (C-state)”, and the open-state to “myosin-induced (M-state)” (Fig. 11) (Lehman et al. 2000).
Fig. 11.
A schematic model of thin filament transitions. Seven actin monomers (gray) spanned by one tropomyosin dimer (red) and one troponin complex: cardiac troponin C (pink), cardiac troponin I (blue) and cardiac troponin T (orange). N and C indicate the N- and C-terminal ends of protein. This diagram is based on the structure of actin subdomains (Kabsch et al. 1990; Murakami et al. 2010), the position of tropomyosin on F-actin (Lehman et al. 2000; Pirani et al. 2005; Vibert et al. 1997) and the core domain of human troponin (Takeda et al. 2003; Vinogradova et al. 2005). The tropomyosin overlap region (head-to-tail) depicts interaction with near-neighbor tropomyosin dimer (dark-blue) (Greenfield et al. 2006; Murakami et al. 2008). The orientation of thin filament proteins is as follows: the N-terminal region of cardiac troponin T points towards the pointed end (M-band), while the core domain of the troponin complex is oriented to the barbed end (Z-disk) (Paul et al. 2009). Interacting sites and structural location of actin-tropomyosin-troponin proteins were matched the best as possible in accordance with the available literature (Murakami et al. 2008; Takeda et al. 2003; Pearlstone and Smillie 1982, 1983; Biesiadecki et al. 2007, 2010; Morris and Lehrer 1984; Manning et al. 2011; Tardiff 2011). a B-state (blocked); when ATP is present and cytoplasmic [Ca2+] is low and is not bound to cTnC, tropomyosin is sterically blocking the myosin-binding sites on actin. b C-state (Ca2+-induced); cytoplasmic [Ca2+] rises such that Ca2+ binds to cTnC, inducing conformational changes of the troponin complex, resulting in a ~25° movement of tropomyosin on the thin filament, thereby exposing most of the myosin-binding sites on actin. Note the movement of tropomyosin away from subdomains 1 and 2 of actin. In the C-state, the myofilament is not yet activated as non-tension-generating cross-bridges bind weakly to actin. c M-state (myosin-induced); the strong-binding of tension-generating cross-bridges induces a ~10° movement of tropomyosin on actin, resulting in myofilament activation and contraction. Note the transition of tropomyosin into subdomains 3 and 4 of actin. (Adapted from Sequeira et al. (2013b) with permission)
Relaxed state, B-state
In the relaxed state where Ca2+ levels are low and ATP is present, the muscle does not develop active force and muscle stiffness is low, corresponding to the physiological relaxation (diastole) and determines the amount of passive resting tension. At the molecular level, the decline of Ca2+ levels is associated with uncoupling of Ca2+ from TnC, and relaxation proceeds (Huxley 1957a; Huxley and Niedergerke 1954; Ebashi 1968). In the B-state, tropomyosin sterically blocks myosin-binding sites on the outer domain of actin, thereby promoting inhibition of myosin S1 binding to actin (Fig. 11a). We know that specific regions of TnI bind to the outer domain of actin and thereby “drag” tropomyosin with it, pulling it away from the inner groove of actin, blocking actomyosin interactions in the outer region (Murakami et al. 2005; Eisenberg and Kielley 1970).
Activated state
In an activated state, where Ca2+ levels are high and ATP is hydrolyzed, the muscle generates active force, shortens and becomes very stiff. It corresponds to the physiological contraction phase, systole. As cytosolic free Ca2+ increases, it binds to regulatory sites on TnC, resulting in the structural arrangement of the TnC subunit (Herzberg et al. 1986). Because TnC is structurally attached to TnI (Takeda et al. 2003; Poole et al. 2006), it moves TnI away from actin, shifting tropomyosin back towards the inner domain of actin. The uncovering of the outer domain is propagated from 1 actin to, at least, 14 neighboring actins (2 functional units), leading to cooperative activation of the thin filament (Hill et al. 1980; Nagashima and Asakura 1982; Geeves and Lehrer 1994). Activation takes place in two distinct biochemical steps:
C-state
A movement of ~25° of tropomyosin around actin, corresponding to the C-state, exposes most of the myosin-binding sites (Fig. 11b) (Pirani et al. 2005; Vibert et al. 1997). Nonetheless, the myofilament is not yet activated because non-tension-generating cross-bridges bind weakly to actin (myosin-ADP-Pi). Notably, not only does tropomyosin change its orientation but slight movements of actin subdomains have also been reported (Squire et al. 1993, 1994). McKillop and Geeves (1993) reported that this specific transition represents the state of weak binding of the S1 (acto-S1-ATP or acto-S1-ADP-Pi) to actin (Greene and Eisenberg 1980; Stein and Schwarz 1979). Weak-binding cross-bridges are defined by low affinity to actin, very fast myosin attachment to and detachment from actin (faster attachment/detachment kinetics), and inability to activate thin filament regulatory units (A7TmTn).
M-state
The third and last state, the M-state, involves the release of Pi from the cross-bridge and strong-binding cross-bridge formation (myosin-ADP) that induces an extra ~10° movement of tropomyosin on the actin filament, resulting in myofilament contraction and force development (Fig. 11c) (Lehman et al. 2000; Vibert et al. 1997). The shape complementary of tropomyosin to actin (Gestalt binding (Holmes and Lehman 2008)) might prevent tropomyosin from extensively rolling away from actin (Behrmann et al. 2012). Strong-binding cross-bridges are defined by higher affinity to actin, myosin binds very strongly to actin and detaches very slowly (slower attachment/detachment kinetics), and the ability to activate thin filament regulatory units.
Overall, the troponin complex inhibits a kinetic transition by trapping the position of tropomyosin (Chalovich and Eisenberg 1982). An increase in the free acto-S1-ADP concentration, enhanced by cooperative thin filament activation (Greene and Eisenberg 1980; Bremel and Weber 1972; Regnier et al. 2002), in a Ca2+-dependent manner, is the trigger for S1-strong-binding and consequent cycling and contraction (McKillop and Geeves 1993; Geeves and Lehrer 1994). A new cycle of relaxation ensues, if the Ca2+ levels decline and ATP is readily accessible.
Rigor state
In particular cases, the rigor state can be induced (<0.1 mM ATP) (Cooke and Bialek 1979), and, although it is as stiff as the activated state (Goldman and Simmons 1977), the muscle does not shorten (Szent-Györgyi 1946; Huxley and Brown 1967).
Muscle mechanics: length-dependent changes
Changes in length and load allow the heart to change its ventricular filling and regulate ventricular contraction and ejection. Ventricular filling sets the initial sarcomere length–tension and determines the amount of cardiomyocyte shortening, ultimately regulating ventricular contraction and ejection (de Tombe et al. 2010). The Frank–Starling relationship thus allows the heart to work on a beat-to-beat basis, capable of adjusting the output of both its sides to any alteration affecting venous return, or preload. The sarcomere length–tension relationship in skeletal and cardiac muscle differs, though active tension shows similar dependence on length. The changes in skeletal muscle are discussed below and enable the understanding the Frank–Starling relationship.
Skeletal muscle length dependency
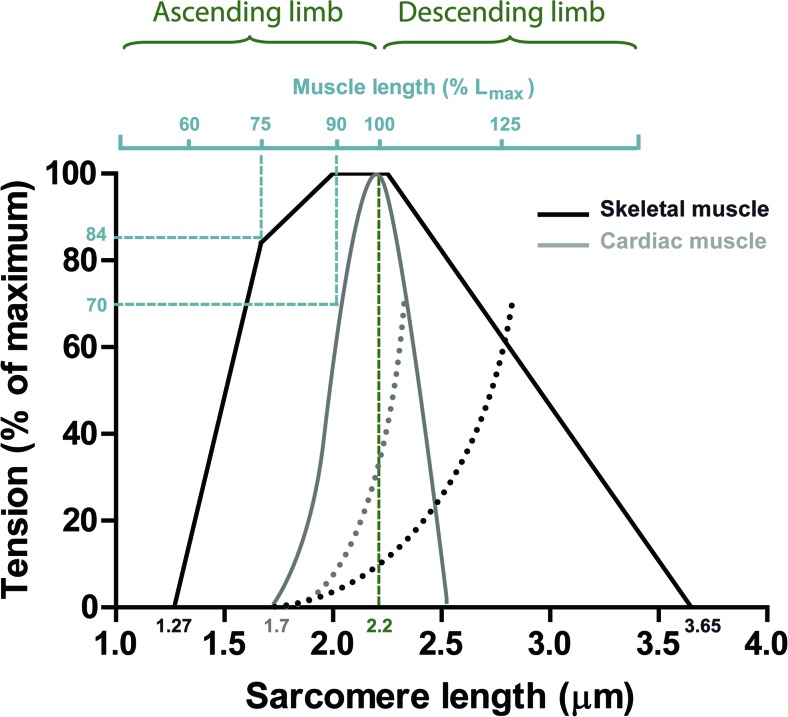
Blix (1891) and von Kries (1880, 1880, 1892) proposed that the energy of a contracting muscle is a function of its length and that muscle shortening depends on its load. Evans and Hill (1914) in the 1910s showed that developed tension grew by increasing fiber length. In addition, a parallel increase of heat production was observed. The complete length–tension relationship for isolated skeletal muscle fibers was provided for the first time by Ramsey and Street (1940) in 1940 (previous experiments rarely exceeded 30 % resting length changes), who demonstrated the biphasic shape alteration of tension development upon length. Almost three decades were needed to combine the results obtained from Ramsey and Street (1940) to the growing advances obtained from the analysis of the ultrastructure of skeletal muscle fibers, the sliding filament theory. In a historic paper published in 1966, Gordon et al. (1966) confirmed the biphasic shape of tension upon length alterations and suggested that such changes resulted directly from the overlap of individual cross-bridges. They demonstrated that active tension in skeletal frog muscles consisted of a maximal tension plateau region between sarcomere lengths of 2.0 and 2.25 μm; a region wherein myofilament overlap of thick and thin filaments was optimal and constant (Fig. 12). Stretching the muscle above 2.25 μm progressively reduced active tension (known as the descending limb of the active tension–length curve) and reached a zero level at 3.65 μm sarcomere length, where myofilament overlap ceased (Gordon et al. 1966). Upon shortening the muscle below 2.0 μm (known as the ascending limb of the active tension–length curve), active tension decreased, which was proposed to be the result of thin filaments colliding at the center of the sarcomere (double overlap) in addition to collision of the thick filaments at the Z-disc. However, a poor correlation between the ascending limb and the number of active cross-bridges was subsequently suggested, so that as the muscle is shortened below slack length the number of cross-bridges remains constant while tension drops (Jewell and Wilkie 1960; Hill 1964; Edman and Kiessling 1971; Rack and Westbury 1969). These observations provided the first indications that factors other than the degree of myofilament overlap of thin and thick filaments existed, and accounted for deactivation upon shortening. In agreement, Taylor and Rüdel (Taylor and Rüdel 1970; Rϋdel and Taylor 1971) demonstrated that the addition of low caffeine concentrations (which causes the release of Ca2+ from the SR) to the bathing solution increased the developed tension at short sarcomere lengths. A similar study was performed by Close (1972) in frog skeletal muscles, showing that inducing twitch or tetanic activation generated different length–tension curves. Close proposed that length-independent changes in activation could also play a role to increase tension (Close 1972): “It may be concluded from this that the principal variations in the length dependence of the twitch are the result of differences in some extrinsic process involved in activation and not differences in the intrinsic strength of the contractile material.”
Fig. 12.
A comparison of length-tension relationships for skeletal and cardiac muscle. Skeletal muscle As proposed by Gordon et al. (1966) using the frog heart, a maximal plateau region over the range of 2.0 and 2.25 μm sarcomere length is expected due to optimal and constant myofilament overlap. When the muscle is stretched above 2.25 μm (descending limb) active tension declines to almost zero at a sarcomere length of 3.65 μm. At shorter sarcomere lengths (below 2.0 μm) (ascending limb) the thin filaments collide in the middle of the sarcomere, and thick filaments collide at the Z-disc and tension ceases. Cardiac muscle The myofilament overlap theory that was the basis for skeletal muscle length-tension relationships cannot account for the cardiac length-tension relationship. Apart from the smaller sarcomere lengths at which the mammalian heart operates (estimated physiological levels range from 1.8 to 2.2 μm), cardiac muscle was demonstrated to present length-dependent changes in activation. Please note that skeletal muscle almost fully activates at 75 % Lmax (length at which force is maximal), which contrasts with cardiac muscle where at the same % of Lmax, active tension is zero. Lengthening cardiac muscle an extra 15 % in length (~90 % Lmax) raises the developed tension from 0 up to 70 %, hence active tension in cardiac muscle is length-dependent. Diagrams adapted from Gordon et al. (1966), Sonnenblick and Skelton (1974), and Allen et al. (1974)
Frank–Starling relationship
In 1895, using the frog heart, Frank (1895) measured isovolumic pressures developed at varying lengths. Extending the findings of Blix and von Kries, Frank (1895, 1895, 1959) observed that the developed pressure was directly proportional to the initial (diastolic) tension. Nonetheless, Frank’s experiments to determine whether an increase in the force of contraction was either related to the initial tension or length of the muscle fibers were inconclusive (Patterson et al. 1914). Starling and coworkers (Patterson and Starling 1914; Patterson et al. 1914; Starling 1918) measured cardiac shortening as a function of cardiac output and its intrinsic relationship to initial EDV, and demonstrated that the initial fiber length, rather than tension, is the main determinant for contraction.
The performance of skeletal muscle length–tension relationships were investigated with cardiac muscle by Abbott and Mommaerts (Abbott and Mommaerts 1959) in the 1960s and later re-examined in more detail by the groups of Sonnenblick (1968), Sonnenblick et al. (1963, 1964) and Grimm (Grimm and Whitehorn 1966, 1968). Both groups demonstrated that active tension exhibited a similar dependence on length to skeletal muscle, namely both change with sarcomere length. However, the shape of cardiac length-tension curves is distinctly different. Skeletal muscle is almost fully active at 75 % of its optimal length (Lmax; muscle length at which tension is maximal) compared with cardiac muscle where active tension is zero at the same % of Lmax (Fig. 12) (Sonnenblick and Skelton 1974; Allen et al. 1974). Stretching cardiac muscle an extra 15 % (~90 % Lmax) increases developed tension from 0 up to 70 % (Fig. 12) (Sonnenblick and Skelton 1974; Allen et al. 1974). Based on this observation, it was suggested that active tension in cardiac muscle is length-dependent. Several other inconsistencies between skeletal and cardiac muscle were reported, including the fact that maximal tension in the heart muscle only presents a peak at maximal lengthening in contrast to the plateau in skeletal muscle, and that the decline in active tension (both ascending and descending limb) is much steeper for cardiac. A simple approach based on myofilament overlap could not account for the observed differences between skeletal and cardiac muscle (Sonnenblick and Skelton 1974; Sonnenblick et al. 1963). With the growing evidence from skeletal muscle for the role of Ca2+ in the “manipulation” of length–tension curves, it was apparent that factors other than the degree of myofilament overlap could explain the striking differences in length–tension curves and the enigmatic length dependence of heart muscle. We noted that later findings from (Edman 2010) showed that the length–tension curve in skeletal muscle exhibits a much smoother shape than proposed by Gordon et al. (1966). Specifically, the length–tension relationship does not have a pronounced plateau region at 2.0–2.2 μm sarcomere length, while the descending limb was also non-linear. Instead, there was a slight sigmoid shape (Edman 2010), where the extrapolated zero tension level was reached at 3.49 μm rather than 3.65 μm sarcomere length.
Length-dependent activation: Ca2+ as a modulator
It was previously demonstrated in membrane-permeabilized skeletal muscle that the shape of the length–tension curve could be varied by adding μM Ca2+ (Hellam and Podolsky 1969; Endo 1972, 1973). To this extent, Fabiato and Fabiato (1975) initially suggested that shortening the cardiac muscle partially inhibits the Ca2+-triggered Ca2+-release from the SR by monitoring free Ca2+ with the Ca2+-sensitive photoprotein aequorin. The authors, however, later dismissed their claims and provided proof that the contractile machinery itself is Ca2+-sensitive to alterations in muscle length at sarcomere lengths greater than 2.35 μm (descending limb) (Fabiato and Fabiato 1978). Later evidence demonstrated that indeed increasing sarcomere length (over the entire sarcomere length range) increases myofilament sensitivity to Ca2+, thus “cementing” the myofilament length-dependent activation hypothesis (Hibberd and Jewell 1982; Kentish et al. 1986).
Current conceptions of the mechanisms underlying length-dependent activation
Over a century of research on the Frank–Starling effect has elucidated our understanding of the fundamental mechanical basis of muscle contraction. The Frank–Starling relationship dictates that an increase in fiber length enhances the maximal force generating capacity and Ca2+-sensitivity of myofilaments, leading to increased force development (de Tombe et al. 2010; Allen and Kentish 1985). Although a unifying concept that explains how myofilaments “sense” length alterations is still to be proven, stretch-induced effects rather than changes in filament spacing dominate the literature. Also, changes in Ca2+ activation upon muscle lengthening have to be accounted for. Overall, myofilament length-dependent activation is the composite of several synergistic mechanisms. Length-dependent activation is associated with: (1) increased Ca2+ affinity of cTnC; (2) alterations in interfilament lattice spacing; (3) titin-induced stretch and formation of strong-binding cross-bridges; (4) cTn complex changes; and (5) cooperative mechanisms.
cTnC-dependent alterations
Ca2+ affinity to cTnC
Two hypotheses were proposed to explain length-dependent activation of the contractile apparatus by Ca2+. The first is the length-dependent regulation of Ca2+ release by the SR suggested by Fabiato and Fabiato (1975), but this has not been confirmed by others. The second hypothesis, that length-dependent modulation of Ca2+ affinity by cTnC is emerging as a solid candidate (Allen and Kurihara 1982; Housmans et al. 1983; Allen and Kentish 1988; Hofmann and Fuchs 1987a, 1988). Allen and Kurihara (1982) microinjected aequorin into isolated papillary and trabeculae muscles, and observed a rise in intracellular [Ca2+] following a quick step release during contraction, which they attributed to dissociation of Ca2+ from the contractile proteins. Housmans et al. (1983) observed similar phenomena. This view was further supported in membrane-permeabilized muscle preparations by Allen and Kentish (1988) who concluded that Ca2+ was released by the contractile apparatus. Additional evidence by Hofmann and Fuchs (1987a, 1987a, 1988) showed that length-dependent changes affect the Ca2+ affinity of cTnC where a decrease in sarcomere length reduced the Ca2+-binding affinity of cTnC.
cTnC is not a length-sensing molecule
It remains to be seen how cTnC “senses” sarcomere length alterations. Initially, cTnC was considered to act as a “length sensor” itself. Babu et al. (1988) reported that length dependence of Ca2+ sensitivity was substantially diminished when cTnC was exchanged by slow skeletal troponin C (ssTnC) in membrane-permeabilized cardiac muscle. They (Babu et al. 1988) attributed the isoform difference of cTnC as the basis for cardiac length-dependent activation compared to skeletal muscle. However, this proposal was dismissed because other groups showed that length-dependent activation is independent of cTnC isoform differences (Moss et al. 1991; McDonald et al. 1995; Wang and Fuchs 1994).
Interfilament lattice spacing versus myocyte lengthening
It has been suggested that the increased Ca2+ sensitivity upon myocyte lengthening results from lattice spacing reduction, which increases the proximity of myosin towards actin. Alterations in interfilament lattice spacing, such as increases in the lattice spacing as a result of shortening, leads to sarcomere thickening and intracellular volume redistribution (Elliott et al. 1963, 1967; Brandt et al. 1967). In turn, the developed active tension decreases via decreased approximation of myosin and actin filaments, and thus less strong binding cross-bridges are formed (Rome 1972; Matsubara and Millman 1974b). Although myocyte lengthening and subsequent lattice reduction are intimately linked, accumulating evidence suggests that lattice reduction itself does not play per se a major role in length-dependent cardiac muscle activation.
In 1977, Godt and Maughan (1977) were able to vary the maximum tension of Ca2+-activated fibers using high-molecular-weight polymers, such as dextran. Because of its inability to diffuse into the lattice spacing, dextran could compress the sarcomere and decrease the interfilament distance (Godt and Maughan 1977). Next, it was found that variations in fiber width (as a function of lattice spacing) could be regulated by concentration of dextran (Magid and Reedy 1980). The groups of Moss et al. (1983) and Stienen et al. (1985) indicated that lattice spacing, rather than length, was responsible for the changes in Ca2+-sensitivity in membrane-permeabilized skeletal muscles. These results were confirmed in skeletal (Godt and Maughan 1981; Wang and Fuchs 1995; Martyn and Gordon 1988) and cardiac (Wang and Fuchs 1995; McDonald and Moss 1995) muscle preparations which demonstrated that osmotic compression (as a function of muscle width) enhances Ca2+ sensitivity. These findings were in agreement with the proposition of Hofmann and Fuchs (1987a, 1987a, 1987b) who demonstrated that Ca2+-binding to cTnC directly regulates cross-bridges interactions (rather than sarcomere length). Fuchs and Wang (1996) observed that both Ca2+ sensitivity and Ca2+ affinity to cTnC were directly correlated with the lattice spacing but not with sarcomere length in cardiac muscle. This hypothesis, however, remains largely disputed, and evidence from the last decade suggests another view. In the previous studies, alterations in lattice spacing as a function of dextran addition were not directly measured, but were indirectly based on alterations in muscle width. To visualize the interfilament lattice spacing as a function of dextran application, the group of de Tombe (Irving et al. 2000; Konhilas et al. 2002a, 2003) used synchrotron X-ray diffraction in membrane-permeabilized and intact cardiac tissue. The authors observed that Ca2+-sensitivity was not in a linear relationship with interfilament spacing as a result of osmotic compression. Rather, compression of the lattice spacing with 4 % dextran to match the decreased lattice spacing as observed when the sarcomere length is increased to the optimal length (≈2.2 μm) did not affect myofilament Ca2+-sensitivity (Konhilas et al. 2002a, 2003). These studies are a major obstacle against the suggestion that interfilament lattice spacing is the major mechanism in the regulation of length-dependent activation.
Titin
Evidence from the last decade revealed that the giant elastic protein, titin, may be involved in the modulation of active tension and serve as a length-dependent sensor. Titin could exert such length-dependent behavior by two possible mechanisms:
Titin-induced reduction of lattice spacing
A titin-based passive tension potentiation of cross-bridge formation could reduce the filament lattice spacing upon stretch (Cazorla et al. 2001; Fukuda et al. 2003; Fukuda and Sasaki 2001). Along these lines, a correlation between enhanced length-dependent activation and higher levels of passive tension has been reported (Cazorla et al. 1999, 2001; Fukuda et al. 2003). A recent study reporting a reduced myofilament force development and impaired length-dependent activation (Mateja et al. 2012) in a rat with a homozygous autosomal mutation expressing a giant titin isoform (N2BA-G, ~3.9 MDa) (Greaser et al. 2008) appears to support this idea.
Stretch potentiates titin-induced strong-binding cross-bridge recruitment
Another view, however, suggests that titin-induced stretch effects a reduction in lattice spacing. Titin is obliquely to the sarcomere axis and, since it is attached to both myosin and cMyBP-C (Zoghbi et al. 2008; Freiburg and Gautel 1996; Maruyama et al. 1985), it may impose a passive strain on the thick filament proteins, thereby reducing lattice spacing and changing the geometry of the cross-bridges (Fukuda and Sasaki 2001; Fukuda et al. 2000). Recruitment of strong-binding cross-bridges via a titin-induced stretch is vital to length-dependent and stretch-activation mechanisms. It has been reported in frog skeletal muscle that sarcomere lengthening increases myosin periodicity (Wakabayashi et al. 1994), such that the transition of the population of rested (order; on the axis plane of the thick filament) cross-bridges to weak binding cross-bridges increases (disorder, Fig. 13) (Xu et al. 1997; Malinchik et al. 1997). A similar finding was recently observed in cardiac muscle where the orientation of myosin heads becomes more perpendicular to the thick filament axis when sarcomere length is increased (Farman et al. 2011). Altogether, these studies suggest that stretch-induced activation by titin-induced radial strain of the thick filament is likely to increase the number of cross-bridges at the thick filament, which would allow more myosins to attach to actin, in strong-binding states (Fig. 13).
Fig. 13.
A schematic model of half-sarcomere at varying sarcomere lengths. Lattice spacing dimensions at each varying length were taken from Konhilas et al. (2002b). As the muscle is stretched from a relatively short sarcomere length (a) to higher sarcomere lengths (b, c), lattice spacing becomes smaller with increased transition of order cross-bridges (a; projection of cross-bridges in X-ray diffraction studies) into disorder (active) states (b, c). The I-band region of titin is the extensible region and consists of three elastic components that act as a spring element: (1) tandem immunoglobulin (Ig)-like domain regions, with proximal (near Z-disc) and distal (near I-A regions) segments; (2) the PEVK sequence-region rich in proline (P), glutamic acid (E), valine (V) and lysine (K); and (3) the N2B and N2BA elements (both isoforms contain N2B segments, but only the N2BA isoform contains an additional N2A element) (Labeit and Kolmerer 1995). Titin-induced stretch imposes a passive strain on the thick filament proteins, reduces lattice spacing and changes the arrangement of cross-bridges. Distinct myosin colors are depicted to better illustrate the transition of ordered to disordered projections. α-actinin and desmin illustrate the Z-disc border. According to detailed calculations from Gordon et al. (2000), ~1 cross-bridge binds each A7TmTn. Note: cardiac myosin-binding protein C (cMyBP-C) was omitted to simplify the drawing and the width and sarcomere length dimensions are not to scale
Cardiac troponin and thin filament transitions: regulator of length-dependent activation
Stabilization of the B-state formation: length-dependent sensitive step
There is evidence to suggest that length-dependent activation is regulated via an “on–off” switch of the thin filament (Smith and Fuchs 1999). The relevance of the transition from the B-state to the C-state for proper length-dependent activation has been shown by Smith and Fuchs (1999), who were the first to provide evidence for a length-sensitive step in the transitions of thin filament activation. A reduction in ionic strength (<0.05 M), known to shift the B-state equilibrium towards a stable C-state (where the disordered population of cross-bridges is increased (Head et al. 1995; Xu et al. 1987); i.e. it mimics the effects of stretch), coincided with impaired length-dependent activation (Smith and Fuchs 1999). Terui et al. (2008) recently demonstrated that length-dependent activation is associated with titin-induced strain on the thick filament, which is highly dependent on the troponin complex. The authors observed that reconstitution of cardiac thin filaments with fast sTn reduced length-dependent activation to a level similar to that of skeletal muscle. In turn, reconstitution with cTn restored length-dependent activation and decreased Ca2+ sensitivity. The authors associated the latter findings as an increased transition of the B-state towards the C-state (Terui et al. 2008).
Recent findings from stretch-activation studies in insect flight muscle support the view that troponin–tropomyosin thin filament transitions are central to the length-dependent response. Flight muscles require stretch-activation mechanisms in addition to Ca2+ to activate the muscle (Pringle 1949, 1978). Because transition of the B-state requires Ca2+ to move tropomyosin to the C-state, insect flight muscles are switched “on" due to Ca2+-induced binding to TnC; however, Ca2+ alone is not sufficient to uncover the myosin-binding sites, stretch is required. Since muscle stretching appears to increase myosin periodicity with orientation of myosin heads on the thick filament (Wakabayashi et al. 1994; Farman et al. 2011), one can speculate that insect flight muscle requires the formation of strong-binding cross-bridges, presumably by stretch, in order to move tropomyosin and uncover myosin-binding sites. In support, Perz-Edwards et al. (2011) recently demonstrated that stretch-activation of insect flight muscle requires the steric blocking-unblocking model (thin filament transitions) for the regulation of the actomyosin complex, and that stretching the muscle causes tropomyosin movement and uncovers myosin-binding sites on actin. The similarities between insect flight and vertebrate muscle thus suggest that passive strain imposed to the thick filament increases the “activation” of cross-bridges which presumably via binding to troponin (“troponin bridges” (Perz-Edwards et al. 2011)) and/or tropomyosin (Behrmann et al. 2012) regulate length-dependent activation. We have recently provided evidence that a reduced length-dependent increase in Ca2+-sensitivity is common in cardiomyopathies with sarcomeric mutations (Sequeira et al. 2013b). This indicates that the latter can potentially impair the equilibrium affinity and thin filament transitions, and support the important role of thin filament alterations for myofilament length-dependent activation.
Post-translational modulation by protein kinase A (PKA) in length-dependent activation
The suggestion that PKA-mediated myofilament protein phosphorylation has a modulatory role in length-dependent activation comes from studies on ferret papillary muscles (Komukai and Kurihara 1997), in which isoprenaline, a stimulator of the β-adrenergic receptor pathway, enhanced the length-dependent change in the force-Ca2+ relationship. Reconstitution of cardiac thin filaments with ssTnI showed higher myofilament Ca2+ sensitivity, but significantly reduced length-dependent activation (Konhilas et al. 2003; Arteaga et al. 2000), indicating a role for cTnI phosphorylation in length-dependent activation. In support, recent data from our group (Wijnker et al. 2014) and others (Hanft et al. 2013; Hanft and McDonald 2010) clearly demonstrate that PKA-induced phosphorylation of cTnI-Ser23/Ser24 is essential for length-dependent activation. One can speculate that, because PKA-induced phosphorylation decreases cTnC–cTnI interactions, this leads to greater cTnI–tropomyosin interactions and, hence, the B-state is favored; this is associated with fewer myosin-binding sites available on actin. We recently provided evidence that indeed the B-state is strengthened upon PKA-induced phosphorylation which is partly the resulting contribution of cTnI but also of cMyBP-C (Sequeira et al. 2015). Finally, a study by Cazorla et al. (2006) using transgenic mice lacking cMyBP-C demonstrated high Ca2+ sensitivity and reduced length-dependent activation compared to wild-type mice, and that this could not be restored by exogenous PKA treatment. This study suggests that cMyBP-C is also required for proper length-dependent sarcomere activation.
Cooperativity
What is cooperativity?
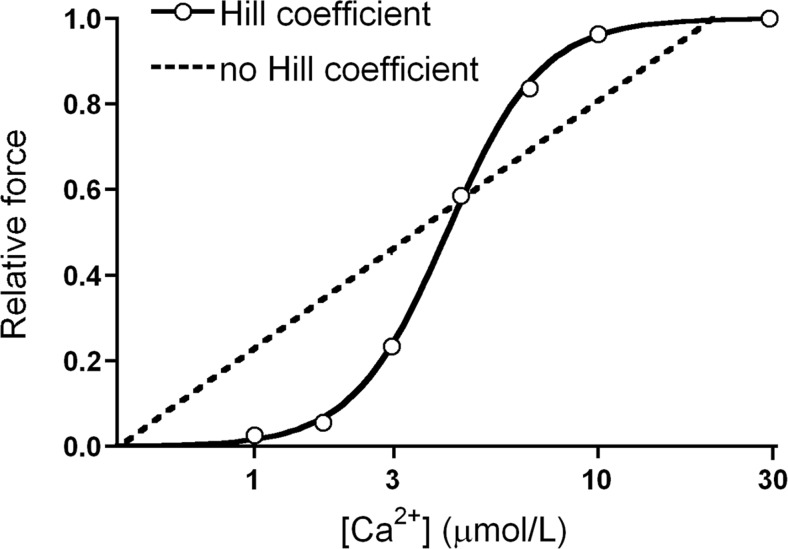
The sigmoidal relationship between [Ca2+] and force and/or ATPase activity is one of the earlier demonstrations that the binding of Ca2+ appeared to activate muscle contraction. Filo et al. in 1965 (Filo et al. 1965) were the first to correlate tension as a function of the free [Ca2+] using glycerinated skeletal and smooth muscle preparations. This observation was confirmed by Hellam and Podolsky (1969) in membrane-permeabilized muscle preparations. They were the first to describe the relationship between free [Ca2+] (defined by its inverse logarithm: pCa, i.e., –log10 of the [Ca2+]) and force as a sigmoidal curve. Six years later, Donaldson and Kerrick (1975) introduced the widely known term, “pCa50”. When studying the effects of Mg2+ on muscle Ca2+ contraction in membrane-permeabilized fibers, the authors observed no difference in maximal tension generated whether or not Mg2+ was present. However, to their surprise, if a submaximal tension comparison was made (at 50 % of the maximum tension), Mg2+ was shown to reduce force generation. In addition, Donaldson and Kerrick (1975) also assumed the existence of a cooperative system: “The tension in the curves […] rises from 10 to 90 % of maximum in less than 2 pCa […] units which is indicative of interacting sites. Because of the evidence for interacting sites these data were analyzed using the Hill equation (Hill 1913) which accounts for cooperative forces in the binding of a ligand to a macromolecule”. The coefficient of Hill (or nH) is thus an indication of the relative number of interacting sites and represents a measure of the cooperativity of Ca2+ activation of the contractile machinery. A hypothetical system with an nH value of 1 describes a one-to-one relationship, where 1 mole of Ca2+ activates 1 functional unit (A7TmTn). The nH for cardiac muscle contraction exceeds 1 both in humans (van der Velden et al. 2000, 2006) and in animals (Konhilas et al. 2003; Boontje et al. 2011; van der Velden et al. 2004), indicative of a highly cooperative system (Fig. 14).
Fig. 14.
Cooperativity in cardiac muscle. In this plot of Ca2+ versus relative force the solid line depicts a unique cooperative relationship between [Ca2+] and force. The dashed line depicts a hypothetical system where cooperative activation is non-existent (x-axis here is non-logarithmic)
Cooperativity of length-dependent activation
The thin filament functional unit comprises seven actin monomers spanned by one tropomyosin dimer and one cTn complex (A7TmTn) (Huxley 1973a). Ca2+-binding to cTnC promotes cTnI detachment from actin and potentiates tropomyosin movement to expose myosin-binding sites on the surface of F-actin. This tropomyosin movement allows Ca2+ cooperative activation of the thin filament with additional recruitment of strong-binding myosin and actin monomers. Structural data suggest that individual strongly-bound cross-bridges bind to the regulatory unit (A7TmTn spanning ~38.5 nm) and regulate tropomyosin movement up to ~3 units (covering ~115 nm) along the thin filament, in the presence of Ca2+ (Vibert et al. 1997). This was also validated in biochemical studies (Geeves and Lehrer 1994; Maytum et al. 1998). Mounting evidence defines the role of tropomyosin in thin filament Ca2+ activation as three biochemical transitions. Tropomyosin increases “communication” between neighboring regulatory units, a property that is governed by the head-to-tail interaction (i.e., overlap region) (Hill et al. 1980; Nagashima and Asakura 1982; Geeves and Lehrer 1994; Pan et al. 1989; Heeley et al. 1989). Removal of this overlap reduces cooperative binding of myosin (Pan et al. 1989; Heeley et al. 1989; Johnson and Smillie 1977).
Two recent studies support the idea that Ca2+ cooperative effects are independent of myosin-binding and are strongly associated with the thin filaments (Sun et al. 2009; Farman et al. 2010). Sun et al. (2009) reconstituted cardiac thin filaments with fluorescent-labeled TnC and analyzed changes in the orientation of the structure of troponin. They observed that blebbistatin (which prevents strong-binding formation) had no effect on the Hill coefficient (Sun et al. 2009). Farman et al. (2010) reached similar conclusions, because, in their experiments, blebbistatin decreased Ca2+ sensitivity and force, but did not affect the Hill coefficient. Importantly, the authors reconstituted rat cardiac thin filaments with a cTnC mutant incapable of binding Ca2+ and observed that both Ca2+ sensitivity and nH were decreased (Farman et al. 2010). In addition, they observed that the effects of the cTnC mutant were greater at short (2.0 μm) than at longer (2.2 μm) sarcomere lengths. The authors attributed this result to the ability of tropomyosin to recruit more regulatory units (A7TmTn) upon stretching; tropomyosin stiffness increases at longer sarcomere length and affects up to three to four A7TmTn units, whereas at shorter sarcomere length, tropomyosin is less stiff and would only affect one or two A7TmTn units.
Taken together, these data imply that cooperative activation via thin filaments is partly responsible for the length-dependent behavior. This is consistent with the observation of impaired Ca2+ cooperative activation in muscle carrying cardiomyopathy-causing mutations in genes encoding cTnT (Manning et al. 2011, 2012). Because tropomyosin overlap regions are required for proper formation of a ternary complex with the N-terminal tail of cTnT (Palm et al. 2003) (which in turn is essential to maintain the thin filament in the B-state (Tobacman et al. 2002; Gollapudi et al. 2012)), this suggests that troponin mutations disrupt the structure of the troponin–tropomyosin complex (Sequeira et al. 2015). Also, impaired tropomyosin–tropomyosin interactions could decrease near-neighbor interactions and decrease length-dependent activation.
Discussion
Over a century of research on the Frank–Starling Law has greatly advanced our knowledge of the fundamental basis of muscle. It mandates that, at the single cardiomyocyte level, there is a direct relationship between sarcomere length and myofilament sensitivity to Ca2+ ions, such that more force is generated at a given concentration of Ca2+ as sarcomere length is increased. Although a unifying idea to explain how the myofilament “senses” length alterations remains in dispute, evidence supports the stretch-induced effects are central key to length-dependent force changes. Also, changes in Ca2+ activation upon muscle lengthening must be considered. This review has provided substantial evidence that myofilament length-dependent activation is a composite of several synergistically mechanokinetic processes. Length-dependent activation is the sum of increased Ca2+-affinity of cTnC, alterations in interfilament lattice spacing, titin-induced stretch, and the formation of strong-binding cross-bridges, cTn complex changes, and Ca2+ cooperative mechanisms.
Acknowledgments
We acknowledge support from the Netherlands organization for scientific research (NWO; VIDI grant 91711344).
Compliance with ethical standards
Conflict of interest
Vasco Sequeira declares that he has no conflict of interest.
Jolanda van der Velden declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the authors.
References
- Abbott BC, Mommaerts WFHM. A study of inotropic mechanisms in the papillary muscle preparation. J Gen Physiol. 1959;42:533–561. doi: 10.1085/jgp.42.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarkova I, Perriard J-C. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Aird W. Discovery of the cardiovascular system: from Galen to William Harvey. J Thromb Haemost. 2011;9:118–129. doi: 10.1111/j.1538-7836.2011.04312.x. [DOI] [PubMed] [Google Scholar]
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17:821–840. doi: 10.1016/S0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D, Kentish J. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J Physiol. 1988;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987;60:153–168. doi: 10.1161/01.RES.60.2.153. [DOI] [PubMed] [Google Scholar]
- Allen DG, Jewell BR, Murray JW. The contribution of activation processes to the length-tension relation of cardiac muscle. Nature. 1974;248:606–607. doi: 10.1038/248606a0. [DOI] [PubMed] [Google Scholar]
- Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A, Sonnenblick E, Gulati J. Molecular basis for the influence of muscle length on myocardial performance. Science. 1988;240:74–76. doi: 10.1126/science.3353709. [DOI] [PubMed] [Google Scholar]
- Bailey K. Myosin and adenosinetriphosphatase. Biochem J. 1942;36:121–139. doi: 10.1042/bj0360121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of muscle. Nature. 1946;157:368–369. doi: 10.1038/157368b0. [DOI] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43:271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann E, et al. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150:327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall JR. Further observations on a factor (the ‘Marsh’ factor) effecting relaxation of ATP-shortened muscle-fibre models, and the effect of Ca and Mg ions upon it. J Physiol. 1953;121:232–254. doi: 10.1113/jphysiol.1953.sp004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall JR. The relaxing effect of myokinase on muscle fibres; Its identity with the ‘Marsh’ factor. Proc R Soc Lond B Biol Sci. 1954;142:409–426. doi: 10.1098/rspb.1954.0033. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MS, Gulick J, Osinska H, Gupta M, Robbins J. Determination of the critical residues responsible for cardiac myosin binding protein C’s interactions. J Mol Cell Cardiol. 2012;53:838–847. doi: 10.1016/j.yjmcc.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiadecki BJ, Chong SM, Nosek TM, Jin J-P. Troponin T core structure and the regulatory NH2-terminal variable region. Biochemistry. 2007;46:1368–1379. doi: 10.1021/bi061949m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiadecki BJ, et al. Removal of the cardiac troponin I N-terminal extension improves cardiac function in aged mice. J Biol Chem. 2010;285:19688–19698. doi: 10.1074/jbc.M109.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius W. Am Froschherzen angestellte versuche uber die Herz-Arbeit unter verschiedenen innerhalb des Kreislaufes herrschenden Druck-Verhaltnissen. Verhandl Phys Med Ges. 1872;2:49. [Google Scholar]
- Blix M. Die Lange und Spannung des Muskels. Skandinavisches Archiv Physiol. 1891;5:173–206. doi: 10.1111/j.1748-1716.1894.tb00199.x. [DOI] [Google Scholar]
- Boontje NM, et al. Enhanced myofilament responsiveness upon b-adrenergic stimulation in post-infarct remodeled myocardium. J Mol Cell Biol. 2011;50:487–499. doi: 10.1016/j.yjmcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Bowman W. On the minute structure and movements of voluntary muscle. Phil Trans Royal Soc Lond. 1840;130:457–501. doi: 10.1098/rstl.1840.0022. [DOI] [Google Scholar]
- Bozler E. Relaxation in extracted muscle fibers. J Gen Physiol. 1954;38:149–159. doi: 10.1085/jgp.38.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P, Lopez E, Reuben J, Grundfest H. The relationship between myofilament packing density and sarcomere length in frog striated muscle. J Cell Biol. 1967;33:255–263. doi: 10.1083/jcb.33.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Vassort G, Garnier D, Le Guennec J-Y. Length modulation of active force in tat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol. 1999;31:1215–1227. doi: 10.1006/jmcc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- Cazorla O, et al. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res. 2006;69:370–380. doi: 10.1016/j.cardiores.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chalovich JM, Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982;257:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chapman CB, Mitchell JH (1965) Starling on the heart. Facsimile reprints including the Linacre Lecture on the Law of the Heart. Dawsons of Pall Mall
- Chung CS, Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol. 2011;50:731–739. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close RI. The relations between sarcomere length and characteristics of isometric twitch contractions of frog sartorius muscle. J Physiol. 1972;220:745–762. doi: 10.1113/jphysiol.1972.sp009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979;28:241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976;192:451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, et al. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie WM. XII - ”Observations on the minute structure and mode of contraction of voluntary muscular fibre"; being the abstract of a paper read before the Royal Medical Society, Edinburgh, December 15th, 1848. J Nat Hist. 1849;3:109–119. doi: 10.1080/03745485909494605. [DOI] [Google Scholar]
- Donaldson SK, Kerrick WG. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975;66:427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Remedios CG, Millikan RGC, Morales MF. Polarization of tryptophan fluorescence from single striated muscle fibers. A molecular probe of contractile state. J Gen Physiol. 1972;59:103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabikowski W, Nonomura Y. The interaction of troponin with F-actin and its abolition by tropomyosin. Biochim Biophys Acta. 1968;160:129–131. doi: 10.1016/0005-2795(68)90075-5. [DOI] [PubMed] [Google Scholar]
- Drabikowski W, Dabrowska R, Barylko B. Separation and characterization of the constituents of troponin. FEBS Lett. 1971;12:148–152. doi: 10.1016/0014-5793(71)80055-8. [DOI] [PubMed] [Google Scholar]
- Drabikowski W, Rafalowska U, Dabrowska R, Szpacenko A, Barylko B. The effect of proteolytic enzymes on the troponin complex. FEBS Lett. 1971;19:259–263. doi: 10.1016/0014-5793(71)80528-8. [DOI] [PubMed] [Google Scholar]
- Dreser H. Ueber Herzarbeit und Herzgifte. Naunyn-Schmiedeberg’s Archiv Pharmacol. 1887;24:221–240. doi: 10.1007/BF01918403. [DOI] [Google Scholar]
- Ebashi S. Calcium binding and relaxation in the actomyosin system. Biochem J. 1960;48:150–151. [Google Scholar]
- Ebashi S. Calcium binding activity of vesicular relaxing factor. J Biochem. 1961;50:236–244. doi: 10.1093/oxfordjournals.jbchem.a127439. [DOI] [PubMed] [Google Scholar]
- Ebashi S. The "role" of the relaxing factor and the contraction-relaxation cycle of skeletal muscle. Prog Theor Phys. 1961;17:35–40. doi: 10.1143/PTPS.17.35. [DOI] [Google Scholar]
- Ebashi S. Third component participating in the super precipitation of ‘natural actomyosin’. Nature. 1963;200:1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S. E.M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi F, Ebashi S. Removal of calcium and relaxation in actomyosin systems. Nature. 1962;194:378–379. doi: 10.1038/194378a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Ebashi F. A new protein component participating in the superprecipitation of myosin B. J Biochem. 1964;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:121–139. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Kodama A. A new protein factor promoting aggregation of tropomyosin. J BIochem. 1965;58:107–108. doi: 10.1093/oxfordjournals.jbchem.a128157. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Kodama A. Native tropomyosin-like action of troponin on trypsin-treated myosin B. J Biochem. 1966;60:733–734. doi: 10.1093/oxfordjournals.jbchem.a128504. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Lipmann F. Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J Cell Biol. 1962;14:389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S, Endo M, Ohtsuki I. Control of muscle contraction. Quatert Rev Biophys. 1969;2:351–384. doi: 10.1017/S0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Wakabayashi T, Ebashi F. Troponin and its components. J Biochem. 1971;69:441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Edman KAP. Contractile performance of striated Muscle. Muscle Biophys. 2010;682:7–40. doi: 10.1007/978-1-4419-6366-6_2. [DOI] [PubMed] [Google Scholar]
- Edman K, Kiessling A. The time course of the active state in relation to sarcomere length and movement studied in single skeletal muscle fibres of the frog. Acta Physiol Scand. 1971;81:182–196. doi: 10.1111/j.1748-1716.1971.tb04891.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Kielley WW. Native tropomyosin: Effect on the interaction of actin with heavy meromyosin and subfragment-1. Biochem Biophy Res Comm. 1970;40:50–56. doi: 10.1016/0006-291X(70)91044-2. [DOI] [PubMed] [Google Scholar]
- Elliott T. The control of the suprarenal glands by the splanchnic nerves. J Physiol. 1912;44:374–409. doi: 10.1113/jphysiol.1912.sp001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott GF, Lowy J, Worthington CR. An X-ray and light-diffraction study of the filament lattice of striated muscle in the living state and in rigor. J Mol Biol. 1963;6:295–305. doi: 10.1016/S0022-2836(63)80090-X. [DOI] [Google Scholar]
- Elliott GF, Lowy J, Millman BM. Low-angle X-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967;25:31–45. doi: 10.1016/0022-2836(67)90277-X. [DOI] [PubMed] [Google Scholar]
- Endo M. Stretch-induced increase in activation of skinned muscle fibres by calcium. Nat New Biol. 1972;237:211–213. doi: 10.1038/newbio237211a0. [DOI] [PubMed] [Google Scholar]
- Endo M. Length dependence of activation of skinned muscle fibers by calcium. Cold Spring Harb Symp Quant Biol. 1973;37:505–510. doi: 10.1101/SQB.1973.037.01.061. [DOI] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y. Calcium Induced Release of Calcium from the Sarcoplasmic Reticulum of Skinned Skeletal Muscle Fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Engelhardt W. Enzymatic and mechanical properties of muscle proteins. Yale J Biol Med. 1942;15:21–38. [PMC free article] [PubMed] [Google Scholar]
- Engelhardt WA, Liubimova MN. Myosine and adenosinetriphosphatase. Nature. 1939;144:668–669. doi: 10.1038/144668b0. [DOI] [Google Scholar]
- Engelmann TW. Mikroskopische Untersuchungen über die quergestreifte Muskelsubstanz. Pflügers Archiv Eur J Physiol. 1873;7:33–71. doi: 10.1007/BF01613312. [DOI] [Google Scholar]
- Engelmann TW. Das herz und seine tätigkeit im lichte neuerer forschung. Th Wilhelm Engelmann. 1904;1:44. [Google Scholar]
- Evans CL, Hill AV. The relation of length to tension development and heat production on contraction in muscle. J Physiol. 1914;49:10–16. doi: 10.1113/jphysiol.1914.sp001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature. 1975;256:54–56. doi: 10.1038/256054a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978;72:667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe PP. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J. 2010;99:2978–2986. doi: 10.1016/j.bpj.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farman GP, et al. Myosin head orientation: a structural determinant for the Frank-Starling relationship. Am J Physiol. 2011;300:2155–2160. doi: 10.1152/ajpheart.01221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filo RS, Bohr DF, Ruegg JC. Glycerinated skeletal and smooth muscle: calcium and magnesium dependence. Science. 1965;147:1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The nature of the "inorganic phosphate" in voluntary muscle. Science. 1927;65:401–403. doi: 10.1126/science.65.1686.401. [DOI] [PubMed] [Google Scholar]
- Ford LE, Podolsky RJ. Regenerative calcium release within muscle cells. Science. 1970;167:58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Frank O. Zur Dynamik des Herzmuskels. Ztschr Biol. 1895;32:370. [Google Scholar]
- Frank O. On the dynamics of cardiac muscle (Translation) Am Heart J. 1959;58:282–317. doi: 10.1016/0002-8703(59)90345-X. [DOI] [Google Scholar]
- Frank D, Kuhn C, Katus H, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med. 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem. 1996;235:317–323. doi: 10.1111/j.1432-1033.1996.00317.x. [DOI] [PubMed] [Google Scholar]
- Freiburg A, et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86:1114–1121. doi: 10.1161/01.RES.86.11.1114. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Wang Y-P. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Sasaki D. Ishiwata, S.i. & Kurihara, S. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001;104:1639–1645. doi: 10.1161/hc3901.095898. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Kajiwara H, Ishiwata SI, Kurihara S. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ Res. 2000;86:e1–e6. doi: 10.1161/01.RES.86.1.e1. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol. 2003;553:147–154. doi: 10.1113/jphysiol.2003.049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürth O. Ueber die Eiweisskörper des Muskelplasmas. Naunyn-Schmiedeberg’s Archiv Pharmacol. 1895;36:231–274. doi: 10.1007/BF01930759. [DOI] [Google Scholar]
- Gautel M, Goulding D, Bullard B, Weber K, Furst DO. The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J Cell Sci. 1996;109:2747–2754. doi: 10.1242/jcs.109.11.2747. [DOI] [PubMed] [Google Scholar]
- Geeves M, Conibear P. The role of three-state docking of myosin S1 with actin in force generation. Biophys J. 1995;68:199S–201S. [PMC free article] [PubMed] [Google Scholar]
- Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J. 1994;67:273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely J. Studies on myosin-adenosinetriphosphatase. J Biol Chem. 1953;200:543–550. [PubMed] [Google Scholar]
- Gilbert R, Kelly MG, Mikawa T, Fischman DA. The carboxyl terminus of myosin binding protein C (MyBP-C, C-protein) specifies incorporation into the A-band of striated muscle. J Cell Sci. 1996;109:101–111. doi: 10.1242/jcs.109.1.101. [DOI] [PubMed] [Google Scholar]
- Godt R, Maughan D. Swelling of skinned muscle fibers of the frog. Biophys J. 1977;19:103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Maughan DW. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981;391:334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Goldman Y, Simmons R. Active and rigor muscle stiffness. J Physiol. 1977;269:55P–57P. [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, Trentham DR. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5′-triphosphate. J Physiol. 1984;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi, Sampath K, Mamidi R, Mallampalli, Sri L, Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys J. 2012;103:940–948. doi: 10.1016/j.bpj.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Huxley A, Julian F. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser ML, Gergely J. Reconstitution of troponin Activity from three protein components. J Biol Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- Greaser ML, Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973;248:2125–2133. [PubMed] [Google Scholar]
- Greaser ML, et al. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J Mol Cell Cardiol. 2008;44:983–991. doi: 10.1016/j.yjmcc.2008.02.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LE, Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980;77:2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield NJ, et al. Solution NMR structure of the junction between tropomyosin molecules: implications for actin binding and regulation. J Mol Biol. 2006;364:80–96. doi: 10.1016/j.jmb.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Gremels H. Über die Steuerung der energetischen Vorgänge am Säugetierherzen. Naunyn Schmiedeberg’s Arch Pharmacol. 1936;182:1–54. doi: 10.1007/BF01858695. [DOI] [Google Scholar]
- Grimm AF, Whitehorn WV. Characteristics of resting tension of myocardium and localization of its elements. Am J Physiol. 1966;210:1362–1368. doi: 10.1152/ajplegacy.1966.210.6.1362. [DOI] [PubMed] [Google Scholar]
- Grimm A, Whitehorn W. Myocardial length-tension sarcomere relationships. Am J Physiol. 1968;214:1378–1387. doi: 10.1152/ajplegacy.1968.214.6.1378. [DOI] [PubMed] [Google Scholar]
- Gruen M, Gautel M. Mutations in β-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286:933–949. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- Gruen M, Prinz H, Gautel M. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett. 1999;453:254–259. doi: 10.1016/S0014-5793(99)00727-9. [DOI] [PubMed] [Google Scholar]
- Guz A (1974) Chairman’s Introduction. In: Ciba Foundation Symposium 24 - Physiological Basis of Starling’s Law of the Heart 1–5
- Haller AV (1754) Physiology: being a course of lectures upon the visceral anatomy & vital economy of human bodies. Translated by Samuel Mihles. Innys and Richardson, London
- Hanft LM, McDonald KS. Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol. 2010;588:2891–2903. doi: 10.1113/jphysiol.2010.190504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft LM, Biesiadecki BJ, McDonald KS. Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24. J Physiol. 2013;591(Pt 18):4535–4547. doi: 10.1113/jphysiol.2013.258400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Huxley HE. Structural basis of the cross-striations in muscle. Nature. 1953;172:530–532. doi: 10.1038/172530b0. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Mueller H. Fractionation of troponin into two distinct proteins. Biochim Biophys Acta Res Comm. 1968;31:647–653. doi: 10.1016/0006-291X(68)90610-4. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Pyun HY. Calcium binding by the troponin complex, and the purification and properties of troponin A. Biochim Biophys Acta. 1971;229:698–711. doi: 10.1016/0005-2795(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Harvey W (1889) On the motion of the heart and blood in animals. G. Bell and Sons
- Haselgrove JC. X-Ray evidence for a conformational change in the actin-containing filaments of vertebrate striated muscle. Cold Spring Harb Symp Quant Biol. 1973;37:341–352. doi: 10.1101/SQB.1973.037.01.044. [DOI] [Google Scholar]
- Haselgrove JC, Huxley HE. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973;77:549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Head JG, Ritchie MD, Geeves MA. Characterization of the equilibrium between blocked and closed states of muscle thin filaments. Eur J Biochem. 1995;227:694–699. doi: 10.1111/j.1432-1033.1995.tb20190.x. [DOI] [PubMed] [Google Scholar]
- Heeley DH, Smillie LB, Lohmeier-Vogel EM. Effects of deletion of tropomyosin overlap on regulated actomyosin subfragment 1 ATPase. Biochem J. 1989;258:831–836. doi: 10.1042/bj2580831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenhain M. Über die Entstehung der quergestreiften Muskelsubstanz bei der Forelle. Archiv für Mikros Anat. 1913;83:A427–A447. doi: 10.1007/BF02980507. [DOI] [Google Scholar]
- Heilbrunn LV. The action of calcium on muscle protoplasm. Physiol Zool. 1940;13:88–94. doi: 10.1086/physzool.13.1.30151530. [DOI] [Google Scholar]
- Hellam DC, Podolsky RJ. Force measurements in skinned muscle fibres. J Physiol. 1969;200:807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmes M, Granzier H. Cardiomyocyte stretch-sensing. Oxford: OUP; 2011. [Google Scholar]
- Herron TJ, et al. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ Res. 2006;98:1290–1298. doi: 10.1161/01.RES.0000222059.54917.ef. [DOI] [PubMed] [Google Scholar]
- Herzberg O, Moult J, James MN. A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J Biol Chem. 1986;261:2638–2644. [PubMed] [Google Scholar]
- Hibberd M, Jewell B. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The combinations of haemoglobin with oxygen and with carbon monoxide. I. Biochem J. 1913;7:471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The effect of tension in prolonging the active state in a twitch. Proc R Soc Lond B Biol Sci. 1964;159:589–595. doi: 10.1098/rspb.1964.0021. [DOI] [PubMed] [Google Scholar]
- Hill C, Weber K. Monoclonal antibodies distinguish titins from heart and skeletal muscle. J Cell Biol. 1986;102:1099–1108. doi: 10.1083/jcb.102.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TL, Eisenberg E, Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980;77:3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann PA, Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987;253:C541–C546. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol. 1987;253:C90–C96. doi: 10.1152/ajpcell.1987.253.1.C90. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Fuchs F. Bound calcium and force development in skinned cardiac muscle bundles: Effect of sarcomere length. J Mol Cell Cardiol. 1988;20:667–677. doi: 10.1016/S0022-2828(88)80012-9. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Lehman W. Gestalt-binding of tropomyosin to actin filaments. J Muscle Res Cell Motil. 2008;29:213–219. doi: 10.1007/s10974-008-9157-6. [DOI] [PubMed] [Google Scholar]
- Houmeida A, et al. Evidence for the oligomeric state of ‘elastic’ titin in muscle sarcomeres. J Mol Biol. 2008;384:299–312. doi: 10.1016/j.jmb.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Housmans PR, Lee NK, Blinks Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science. 1983;221:159–161. doi: 10.1126/science.6857274. [DOI] [PubMed] [Google Scholar]
- Howell WH, Donaldson F. Experiments upon the heart of the dog with reference to the maximum volume of blood sent out by the left ventricle in a single beat, and the influence of variations in venous pressure, arterial pressure, and pulse-rate upon the work done by the heart. Phil Trans Royal Soc Lond. 1884;175:139–160. doi: 10.1098/rstl.1884.0007. [DOI] [Google Scholar]
- Huxley HE. Electron microscope studies of the organisation of the filaments in striated muscle. Biochim Biophys Acta. 1953;12:387–394. doi: 10.1016/0006-3002(53)90156-5. [DOI] [PubMed] [Google Scholar]
- Huxley HE. X-Ray analysis and the problem of muscle. Proc R Soc Lond. 1953;141:59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Huxley A. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley HE. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957;3:631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley HE. The contractile structure of cardiac and skeletal muscle. Circulation. 1961;24:328–335. doi: 10.1161/01.CIR.24.2.328. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J Mol Biol. 1963;7:281–308. doi: 10.1016/S0022-2836(63)80008-X. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;20:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The Croonian Lecture, 1970: The structural basis of muscular contraction. Proc R Soc Lond. 1971;178:131–149. doi: 10.1098/rspb.1971.0057. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Muscle 1972: progress and problems. Cold Spring Harb Symp Quant Biol. 1973;37:689–693. doi: 10.1101/SQB.1973.037.01.084. [DOI] [Google Scholar]
- Huxley AF. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973;183:83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Brown W. The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967;30:383–434. doi: 10.1016/S0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley A, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173:917–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol. 2000;279:H2568–H2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- Jewell B, Wilkie D. The mechanical properties of relaxing muscle. J Physiol. 1960;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JE (1891) Die reizung der vasomotoren nach der lähmung der cerebrospinalen herznerven. Archiv für Physiol, 103–156
- Johnson P, Smillie LB. Polymerizability of rabbit skeletal tropomyosin: effects of enzymic and chemical modifications. Biogeosciences. 1977;16:2264–2269. doi: 10.1021/bi00629a035. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin: DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986;58:755–768. doi: 10.1161/01.RES.58.6.755. [DOI] [PubMed] [Google Scholar]
- Kielley WW, Meyerhof O. A new magnesium-activated adenosinetriphosphatase from muscle. J Biol Chem. 1948;174:387–388. [PubMed] [Google Scholar]
- Kielley WW, Meyerhof O. Studies on adenosinetriphosphatase of muscle; a new magnesium-activated a denosinetriphosphatase. J Biol Chem. 1950;176:591–601. [PubMed] [Google Scholar]
- Knowlton FP, Starling EH. The influence of variations in temperature and blood-pressure on the performance of the isolated mammalian heart. J Physiol. 1912;44:206–219. doi: 10.1113/jphysiol.1912.sp001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominz DR, Mitchell ER, Nihei T, Kay CM. The papain digestion of skeletal myosin A. Biochemistry. 1965;4:2373–2382. doi: 10.1021/bi00887a017. [DOI] [Google Scholar]
- Komukai K, Kurihara S. Length dependence of Ca2+-tension relationship in aequorin-injected ferret papillary muscles. Am J Physiol. 1997;273:1068–1074. doi: 10.1152/ajpheart.1997.273.3.H1068. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res. 2002;90:59–65. doi: 10.1161/hh0102.102269. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Physiol. 2002;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas JP, et al. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev. 2000;5:271–280. doi: 10.1023/A:1009813621103. [DOI] [PubMed] [Google Scholar]
- Krause W. Die motorischen Endplatten der quergestreiften Muskelfasern. Hannover: Hahn; 1869. [Google Scholar]
- Kuster DWD, et al. GSK3β phosphorylates newly identified site in the Pro-Ala rich region of cardiac myosin binding protein C and alters cross-Bridge cycling kinetics in human. Circ Res. 2013;112:633–639. doi: 10.1161/CIRCRESAHA.112.275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Labeit SGM, Lakey A, Trinick J. Towards a molecular understanding of titin. EMBO J. 1992;11:1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res. 1997;80:290–294. doi: 10.1161/01.RES.80.2.290. [DOI] [PubMed] [Google Scholar]
- Lehman W, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Lehndorff A (1908) Über die ursachen der typischen schwankungen des allgemeinen blutdruckes bei reizung der vasomotoren. Archiv für Physiol, 362–391
- Linke WA. Sense and stretchability: The role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Linke WA, Kruger M. The giant protein titin as an integrator of myocyte signaling pathways. Physiology. 2010;25:186–198. doi: 10.1152/physiol.00005.2010. [DOI] [PubMed] [Google Scholar]
- Locke FS, Rosenheim O. Contributions to the physiology of the isolated heart. The consumption of dextrose by mammalian cardiac muscle. J Physiol. 1907;36:205–220. doi: 10.1113/jphysiol.1907.sp001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann K. Uber die enzymatische aufspaltung der kreatinphosphorsaure; zugleich ein beitrag zum chemismus der muskelkontraktion. Biochem Z. 1934;271:264–277. [Google Scholar]
- Lohmann K. Konstitution der adenylpyrophosphorsaure and adeninediphosphorsaure. Biochem Z. 1935;282:120–123. [Google Scholar]
- Lowey S, Goldstein L, Cohen C, Luck SM. Proteolytic degradation of myosin and the meromyosins by a water-insoluble polyanionic derivative of trypsin: Properties of a helical subunit isolated from heavy meromyosin. J Mol Biol. 1967;23:287–304. doi: 10.1016/S0022-2836(67)80106-2. [DOI] [PubMed] [Google Scholar]
- Ludwig C (1856) Lehrbuch Der Physiologie Des Menschen. C.F. Winter, Heidelberg 1852-1856 2
- Lundsgaard E. Untersuchungen fiber muskelkontraktion ohne milchsaure. Biochem Z. 1930;217:162–177. [Google Scholar]
- Lundsgaard E. Weitere untersuchungen uber muskelkontraktionen ohne milchsaurebildung. Biochem Z. 1930;227:51–83. [Google Scholar]
- Lundsgaard E. Uber die einwirkung der monoiodoessigsaure auf den spaltungs- und oxydationsstoffwechsel. Biochem Z. 1930;220:8–18. [Google Scholar]
- Luther PK, et al. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc Natl Acad Sci U S A. 2011;108:11423–11428. doi: 10.1073/pnas.1103216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Magid A, Reedy M. X-ray diffraction observations of chemically skinned frog skeletal muscle processed by an improved method. Biophys J. 1980;30:270–340. doi: 10.1016/S0006-3495(80)85074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinchik S, Xu S, Yu LC. Temperature-induced structural changes in the myosin thick filament of skinned rabbit psoas muscle. Biophys J. 1997;73:2304–2312. doi: 10.1016/S0006-3495(97)78262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EP, Tardiff JC, Schwartz SD. A model of calcium activation of the cardiac thin filament. Biochemistry. 2011;50:7405–7413. doi: 10.1021/bi200506k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EP, Tardiff JC, Schwartz SD. Molecular effects of familial hypertrophic cardiomyopathy-related mutations in the TNT1 domain of cTnT. J Mol Biol. 2012;421:54–66. doi: 10.1016/j.jmb.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marey EJ. La circulation du sang à l’ état physiologique et dans les maladies. Paris: Libraire de L’Academie de Medecine; 1881. [Google Scholar]
- Marsh BB. A factor modifying muscle fibre syneresis. Nature. 1951;167:1065–1066. doi: 10.1038/1671065a0. [DOI] [PubMed] [Google Scholar]
- Marsh BB. The effects of adenosine triphosphate on the fibre volume of a muscle homogenate. Biochim Biophys Acta. 1952;9:247–260. doi: 10.1016/0006-3002(52)90159-5. [DOI] [PubMed] [Google Scholar]
- Martyn DA, Gordon AM. Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil. 1988;9:428–445. doi: 10.1007/BF01774069. [DOI] [PubMed] [Google Scholar]
- Maruyama K, et al. Connectin, an elastic protein of muscle. Characterization and function. J Biochem. 1977;82:317–337. [PubMed] [Google Scholar]
- Maruyama K, Kimura S, Yoshidomi H, Sawada H, Kikuchi M. Molecular size and shape of beta-connectin, an elastic protein of striated muscle. J Biochem. 1984;95:1423–1433. doi: 10.1093/oxfordjournals.jbchem.a134750. [DOI] [PubMed] [Google Scholar]
- Maruyama K, et al. Connectin filaments link thick filaments and Z lines in frog skeletal muscle as revealed by immunoelectron microscopy. J Cell Biol. 1985;101:2167–2172. doi: 10.1083/jcb.101.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateja RD, Greaser ML, de Tombe PP. Impact of titin isoform on length dependent activation and cross-bridge cycling kinetics in rat skeletal muscle. Biochim Biophys Acta. 2012;1833:804–811. doi: 10.1016/j.bbamcr.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I, Millman BM (1974) X-Ray diffraction studies on cardiac muscle. In: Ciba Foundation Symposium 24 - Physiological Basis of Starling’s Law of the Heart 31-41. John Wiley & Sons, Ltd
- Matsubara I, Millman BM. X-ray diffraction patterns from mammalian heart muscle. J Mol Biol. 1974;82:527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Maytum R, Lehrer SS, Geeves MA. Cooperativity and Switching within the Three-State Model of Muscle Regulation. Biochemistry. 1998;38:1102–1110. doi: 10.1021/bi981603e. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res. 1995;77:199–205. doi: 10.1161/01.RES.77.1.199. [DOI] [PubMed] [Google Scholar]
- McDonald KS, et al. Length dependence of Ca2+ sensitivity of tension in mouse cardiac myocytes expressing skeletal troponin C. J Physiol. 1995;483:131–139. doi: 10.1113/jphysiol.1995.sp020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek WJ, Eyster JAE. The effect of adrenalin on the heart-rate. Am J Physiol. 1915;38:62–66. [Google Scholar]
- Mihalyi E, Szent-Gyorgyi AG. Trypsin digestion of muscle proteins. I. Ultracentrifugal analysis of the process. J Biol Chem. 1953;201:189–196. [PubMed] [Google Scholar]
- Mines GR. On functional analysis by the action of electrolytes. J Physiol. 1913;46:188–235. doi: 10.1113/jphysiol.1913.sp001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto CA, Fischman DA, Reinach FC. The interface between MyBP-C and myosin: site-directed mutagenesis of the CX myosin-binding domain of MyBP-C. J Muscle Res Cell Motil. 1999;20:703–716. doi: 10.1023/A:1005513312939. [DOI] [PubMed] [Google Scholar]
- Moos C, Mason CM, Besterman JM, Feng INM, Dubin JH. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978;124:571–586. doi: 10.1016/0022-2836(78)90172-9. [DOI] [PubMed] [Google Scholar]
- Morris EP, Lehrer SS. Troponin-tropomyosin interactions. Fluorescence studies of the binding of troponin, troponin T and chymotryptic troponin T fragments to specifically labeled tropomyosin. Biogeosciences. 1984;23:2214–2220. doi: 10.1021/bi00305a018. [DOI] [PubMed] [Google Scholar]
- Moss L, Swinford A, Greaser M. Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys J. 1983;43:115–119. doi: 10.1016/S0006-3495(83)84329-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, Nwoye LO, Greaser ML. Substitution of cardiac troponin C into rabbit muscle does not alter the length dependence of Ca2+ sensitivity of tension. J Physiol. 1991;440:273–289. doi: 10.1113/jphysiol.1991.sp018708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965;240:3816–3828. [PubMed] [Google Scholar]
- Mueller H, Perry SV. Studies on the tryptic digestion of heavy meromyosin. Biochim Biophys Acta. 1961;50:599–601. doi: 10.1016/0006-3002(61)90030-0. [DOI] [PubMed] [Google Scholar]
- Müller J (1844) Handbuch der Physiologie des Menschen für Vorlesungen. Vierte Auflage, Coblenz. J Hölscher 1837 1
- Mun JY, et al. Electron microscopy and 3D reconstruction of F-actin decorated with cardiac myosin-binding protein C. J Mol Biol. 2011;410:214–225. doi: 10.1016/j.jmb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, et al. Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol. 2005;352:178–201. doi: 10.1016/j.jmb.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Murakami K, et al. Structural basis for tropomyosin overlap in thin (actin) filaments and the generation of a molecular swivel by troponin-T. Proc Natl Acad Sci U S A. 2008;105:7200–7205. doi: 10.1073/pnas.0801950105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, et al. Structural basis for actin assembly, activation of ATP hydrolysis, and delayed phosphate release. Cell. 2010;143:275–287. doi: 10.1016/j.cell.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Murray AC, Kay CM. Separation and characterization of the inhibitory factor of the troponin system. Biochem Biophys Res Comm. 1971;44:237–244. doi: 10.1016/S0006-291X(71)80184-5. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Asakura S. Studies on co-operative properties of tropomyosin-actin and tropomyosin-troponin-actin complexes by the use of N-ethylmaleimide-treated and untreated species of myosin subfragment 1. J Mol Biol. 1982;155:409–428. doi: 10.1016/0022-2836(82)90479-X. [DOI] [PubMed] [Google Scholar]
- Offer G. C-protein and the periodicity in the thick filaments of vertebrate skeletal muscle. Cold Spring Harb Symp Quant Biol. 1973;37:87–93. doi: 10.1101/SQB.1973.037.01.013. [DOI] [Google Scholar]
- Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils: Extraction, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Ohtsuka H, Yajima H, Maruyama K, Kimura S. Binding of the N-terminal 63 kDa portion of connectin/titin to α-actinin as revealed by the yeast two-hybrid system. FEBS Lett. 1997;401:65–67. doi: 10.1016/S0014-5793(96)01432-9. [DOI] [PubMed] [Google Scholar]
- Orlova A, Galkin VE, Jeffries CMJ, Egelman EH, Trewhella J. The N-terminal domains of myosin binding protein C can bind polymorphically to F-actin. J Mol Biol. 2011;412:379–386. doi: 10.1016/j.jmb.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SG (1974) Measurements of structural parameters in cardiac muscle. In: Ciba Foundation Symposium 24 - Physiological Basis of Starling’s Law of the Heart 13-30. John Wiley & Sons, Ltd
- Palm T, Greenfield NJ, Hitchcock-DeGregori SE. Tropomyosin ends determine the stability and functionality of overlap and troponin T complexes. Biophys J. 2003;84:3181–3189. doi: 10.1016/S0006-3495(03)70042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BS, Gordon AM, Luo ZX. Removal of tropomyosin overlap modifies cooperative binding of myosin S-1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem. 1989;264:8495–8498. [PubMed] [Google Scholar]
- Parmley W, Chuck L. Length-dependent changes in myocardial contractile state. Am J Physiol. 1973;224:1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- Parry DAD, Squire JM. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973;75:33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Pate E, Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 1989;414:73–81. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48:357–379. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SW, Piper H, Starling E. The regulation of the heart beat. J Physiol. 1914;48:465–513. doi: 10.1113/jphysiol.1914.sp001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DM, Morris EP, Kensler RW, Squire JM. Structure and orientation of troponin in the thin filament. J Biol Chem. 2009;284:15007–15015. doi: 10.1074/jbc.M808615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstone JR, Smillie LB. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem. 1982;257:10587–10592. [PubMed] [Google Scholar]
- Pearlstone JR, Smillie LB. Effects of troponin-I plus -C on the binding of troponin-T and its fragments to alpha-tropomyosin. J Biol Chem. 1983;254:2534–2542. [PubMed] [Google Scholar]
- Pepe FA. Some aspects of the structural organization of the myofibril as revealed by antibody-staining methods. J Cell Biol. 1966;28:505–525. doi: 10.1083/jcb.28.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SV, Corsi A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958;68:5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SV, Cole HA, Head JF, Wilson FJ. Localization and mode of action of the inhibitory protein component of the troponin complex. Cold Spring Harb Symp Quant Biol. 1973;37:251–262. doi: 10.1101/SQB.1973.037.01.035. [DOI] [Google Scholar]
- Perz-Edwards RJ, et al. X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc Natl Acad Sci U S A. 2011;108:120–125. doi: 10.1073/pnas.1014599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfuhl M, Gautel M. Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J Muscle Res Cell Motil. 2012;33:83–94. doi: 10.1007/s10974-012-9291-z. [DOI] [PubMed] [Google Scholar]
- Pirani A, et al. Single particle analysis of relaxed and activated muscle thin filaments. J Mol Biol. 2005;346:761–772. doi: 10.1016/j.jmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Poole KJV, et al. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol. 2006;155:273–284. doi: 10.1016/j.jsb.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Potter JD, Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ ion regulation of muscle contraction. Biochemistry. 1974;13:2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Pringle JWS. The excitation and contraction of the flight muscles of insects. J Physiol. 1949;108:226–232. doi: 10.1113/jphysiol.1949.sp004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JWS. The Croonian Lecture, 1977: stretch activation of muscle: function and mechanism. Proc Royal Soc Lond. 1978;201:107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Rack PMH, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA. Mechanism of muscular contraction. New York: Springer; 2014. Birth of the sliding filament model of muscular contraction: proposal; pp. 29–57. [Google Scholar]
- Ramsey RW, Street SF. The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. J Cell Comp Biol. 1940;15:11–34. [Google Scholar]
- Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem. 2011;286:12650–12658. doi: 10.1074/jbc.M110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M, et al. Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca2+ relations. J Physiol. 2002;540:485–497. doi: 10.1113/jphysiol.2001.013179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RV. Conformation of individual macromolecular particles from myosin solutions. Biochim Biophys Acta. 1961;52:602–604. doi: 10.1016/0006-3002(61)90427-9. [DOI] [PubMed] [Google Scholar]
- Richard Zobel C, Carlson FD. An electron microscopic investigation of myosin and some of its aggregates. J Mol Biol. 1963;7:78–89. doi: 10.1016/S0022-2836(63)80020-0. [DOI] [PubMed] [Google Scholar]
- Ringer S. Concerning the influence exerted by each of the constituents of the blood on the contraction of the ventricle. J Physiol. 1882;3:380–393. doi: 10.1113/jphysiol.1882.sp000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the Heart. J Physiol. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TF, Winegrad S. The measurement and dynamic implications of thin filament lengths in heart muscle. J Physiol. 1979;286:607–619. doi: 10.1113/jphysiol.1979.sp012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. Relaxation of glycerinated muscle: Low-angle X-ray diffraction studies. J Mol Biol. 1972;65:331–345. doi: 10.1016/0022-2836(72)90285-9. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Alanís J, López E, Rubio R. The adaptation of ventricular muscle to different circulatory conditions. Arch Int Physiol Biochim. 1959;67:358–373. doi: 10.3109/13813455909072295. [DOI] [PubMed] [Google Scholar]
- Roy CS. On the Influences which modify the work of the heart. J Physiol. 1879;1:452–496. doi: 10.1113/jphysiol.1879.sp000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CSA, Adami JG. Contributions to the physiology and pathology of the mammalian heart. Br Med J. 1892;1:428–430. doi: 10.1136/bmj.1.1626.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rϋdel R, Taylor SR. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science. 1971;172:387–388. doi: 10.1126/science.172.3981.387. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;25:176–201. [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Sreter FA, Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971;68:946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnoff SJ, Mitchell JH. The regulation of the performance of the heart. Am J Med. 1961;30:747–771. doi: 10.1016/0002-9343(61)90211-X. [DOI] [PubMed] [Google Scholar]
- Sarnoff SJ, Mitchell JH, Gilmore JP, Remensnyder JP. Homeometric autoregulation in the heart. Circ Res. 1960;8:1077–1091. doi: 10.1161/01.RES.8.5.1077. [DOI] [PubMed] [Google Scholar]
- Schafer EA. On the minute structure of the muscle-columns or sarcostyles which form the wing-muscles of insects. Proc R Soc Lond. 1890;49:280–286. doi: 10.1098/rspl.1890.0092. [DOI] [Google Scholar]
- Schaub MP. SV. The regulatory proteins of the myofibril. Characterization and properties of the inhibitory factor (troponin B) Biochem J. 1971;123:367–377. doi: 10.1042/bj1230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub MC, Perry SV. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969;115:993–1005. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Perry S, Häcker W. The regulatory proteins of the myofibril. Characterization and biological activity of the calcium-sensitizing factor (troponin A) Biochem J. 1972;126:237–249. doi: 10.1042/bj1260237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, et al. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circ Res. 2014;114:823–832. doi: 10.1161/CIRCRESAHA.114.302614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira V, Nijenkamp LLAM, Regan JA, van der Velden J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim Biophys Acta. 2013;1838:700–722. doi: 10.1016/j.bbamem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Sequeira V, et al. Perturbed length-sependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013;112:1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira V, Witjas-Paalberends ER, Kuster DD, Velden J. Cardiac myosin-binding protein C: hypertrophic cardiomyopathy mutations and structure-function relationships. Pflugers Arch. 2013;2:201–206. doi: 10.1007/s00424-013-1400-3. [DOI] [PubMed] [Google Scholar]
- Sequeira V, Najafi A, Wijnker PJM, dos Remedios C, Michels M, Kuster DWD, van der Velden J (2015) ADP-stimulated contraction: a predictor of thin-filament activation in cardiac disease. Proc Natl Acad Sci. (in press) [DOI] [PMC free article] [PubMed]
- Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009;284:12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H, Lowey S. Substructure of the myosin molecule as visualized by electron microscopy. Proc Natl Acad Sci U S A. 1967;58:1611–1618. doi: 10.1073/pnas.58.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SH, Fuchs F. Effect of ionic strength on length-dependent Ca2+ activation in skinned cardiac muscle. J Mol Cell Cardiol. 1999;31:2115–2125. doi: 10.1006/jmcc.1999.1043. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH. Correlation of myocardial ultrastructure and function. Circulation. 1968;38:29–44. doi: 10.1161/01.CIR.38.1.29. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH, Skelton CL. Reconsideration of the ultrastructural basis of cardiac length-tension relations. Circ Res. 1974;35:517–526. doi: 10.1161/01.RES.35.4.517. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH, Spiro D, Cottrell TS. Fine structural changes in heart muscle in relation of the lengthtension curve. Proc Natl Acad Sci U S A. 1963;49:193–200. doi: 10.1073/pnas.49.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenblick EH, Spotnitz H, Spiro D. Role of the sarcomere in ventricular function and the mechanism of heart failure. Circ Res. 1964;15:70–81. doi: 10.1161/01.res.15.3.270. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, et al. Tissue-specific expression and α-actinin binding properties of the Z-disc titin: implications for the nature of vertebrate Z-discs. J Mol Biol. 1997;270:688–695. doi: 10.1006/jmbi.1997.1145. [DOI] [PubMed] [Google Scholar]
- Squire JM, Al-Khayat HA, Yagi N. Muscle thin-filament structure and regulation. Actin sub-domain movements and the tropomyosin shift modelled from low-angle X-ray diffraction. J Chem Soci. 1993;89:2717–2726. [Google Scholar]
- Squire JM, Harford JJ, Al-Khayat HA. Molecular movements in contracting muscle: towards "muscle - the movie". Biophys Chem. 1994;50:87–96. doi: 10.1016/0301-4622(94)85022-4. [DOI] [PubMed] [Google Scholar]
- Starling EH. The linacre lecture on the law of the heart given at Cambridge, 1915. Nature. 1918;101:18. doi: 10.1038/101018a0. [DOI] [Google Scholar]
- Starling EH. On the circulatory changes associated with exercise. J Royal Army Med Corps. 1920;34:258. [Google Scholar]
- Starr R, Offer G. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem J. 1978;171:813–816. doi: 10.1042/bj1710813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LA, Schwarz RP. Chock, P.B. & Eisenberg, E. Mechanism of the actomyosin adenosine triphosphatase. Evidence that adenosine 5′-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979;18:3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res. 2006;98:1212–1218. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- Stenger R, Spiro D. The ultrastructure of mammalian cardiac muscle. J Biophys Biochem Cytol. 1961;9:325–351. doi: 10.1083/jcb.9.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen H. Foundations of anesthesiology. An account of some hydraulic and hydrostatical experiments made on the blood and blood-vessels of animals. J Clin Monit Comput. 1740;16:45–47. doi: 10.1023/a:1009980631686. [DOI] [PubMed] [Google Scholar]
- Stienen GJM, Blangé T, Treijtel BW. Tension development and calcium sensitivity in skinned muscle fibres of the frog. Pflugers Arch. 1985;405:19–23. doi: 10.1007/BF00591092. [DOI] [PubMed] [Google Scholar]
- Straub FB. Actin studies. Int Med Chem Univ Szeged. 1942;2:3–15. [Google Scholar]
- Straub FB. Actin II studies. Int Med Chem Univ Szeged. 1943;3:23–37. [Google Scholar]
- Sun Y-B, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587:155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi A. Myosin and muscular contraction. Inst Med Chem Univ Szeged. 1942;1:1–71. [Google Scholar]
- Szent-Györgyi A. Contraction and the chemical structure of the muscle fibril. J Colloid Sci. 1946;1:1–19. doi: 10.1016/0095-8522(46)90002-5. [DOI] [Google Scholar]
- Szent-Györgyi AG. Meromyosins, the subunits of myosin. Arch Biochem Biophys. 1953;42:305–320. doi: 10.1016/0003-9861(53)90360-9. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- Tardiff J. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108:765–782. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Rϋdel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970;167:882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Terui T, et al. Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle. J Gen Physiol. 2008;131:275–283. doi: 10.1085/jgp.200709895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman LS, et al. The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem. 2002;277:27636–27642. doi: 10.1074/jbc.M201768200. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Greaser ML, Pollack GH. Interaction between titin and thin filaments in intact cardiac muscle. J Muscle Res Cell Motil. 1997;18:345–351. doi: 10.1023/A:1018626210300. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, et al. Shape and flexibility in the titin 11-domain super-repeat. J Mol Biol. 2010;397:1092–1105. doi: 10.1016/j.jmb.2010.01.073. [DOI] [PubMed] [Google Scholar]
- van der Velden J, de Jong JW, Owen VJ, Burton PBJ, Stienen GJM. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res. 2000;46:487–495. doi: 10.1016/S0008-6363(00)00050-X. [DOI] [PubMed] [Google Scholar]
- van der Velden J, et al. Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ Res. 2004;95:e85–e95. doi: 10.1161/01.RES.0000149531.02904.09. [DOI] [PubMed] [Google Scholar]
- van der Velden J, et al. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006;69:876–887. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Weber FE, Einheber S, Fischman DA. Molecular cloning of chicken myosin-binding protein (MyBP) H (86-kDa protein) reveals extensive homology with MyBP-C (C-protein) with conserved immunoglobulin C2 and fibronectin type III motifs. J Biol Chem. 1993;268:3670–3676. [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Vinogradova MV, et al. Ca2 + -regulated structural changes in troponin. PNAS. 2005;102:5038–5043. doi: 10.1073/pnas.0408882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. 1912;45:307–317. doi: 10.1113/jphysiol.1912.sp001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kries J (1880) Untersuchungen zur mechanik des quergestreiften muskels. Archiv für Physiol, 348–374
- von Kries J (1892) Untersuchungen zur mechanik des quergestreiften muskeln. Archiv für Physiol, 1–21
- Wagner D, Weeds A. Studies on the role of myosin alkali light chains. J Mol Biol. 1977;109:455–470. doi: 10.1016/S0022-2836(77)80023-5. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, et al. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994;67:2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YP, Fuchs F. Length, force, and Ca(2+)-troponin C affinity in cardiac and slow skeletal muscle. Am J Physiol. 1994;266:1077–1082. doi: 10.1152/ajpcell.1994.266.4.C1077. [DOI] [PubMed] [Google Scholar]
- Wang Y-P, Fuchs F. Osmotic compression of skinned cardiac and skeletal muscle bundles: Effects on force generation, Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1995;27:1235–1244. doi: 10.1016/S0022-2828(05)82385-5. [DOI] [PubMed] [Google Scholar]
- Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979;76:3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. Relaxing effects of EDTA on glycerol-treated muscle fibers. Arch Biochem Biophys. 1955;54:559–562. doi: 10.1016/0003-9861(55)90071-0. [DOI] [PubMed] [Google Scholar]
- Weber H. Die Muskeleiweisskörper und der Feinbau des Skeletmuskels. Ergebn Physiol Biol Chem Exp Pharmakol. 1934;36:109–150. doi: 10.1007/BF02322523. [DOI] [Google Scholar]
- Weber HHD. Feinbau und die mechanischen Eigenschaften des Myosinfadens. Pflügers Archiv Eur J Physiol. 1935;235:205–233. doi: 10.1007/BF01764179. [DOI] [Google Scholar]
- Weber A. On the role of calcium in the activity of adenosine 5′-triphosphate hydrolysis by actomyosin. J Biol Chem. 1959;234:2764–2769. [PubMed] [Google Scholar]
- Weber A, Winicur S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961;236:3198–3202. [PubMed] [Google Scholar]
- Weeds AG, Pope B. Chemical studies on light chains from cardiac and skeletal muscle myosins. Nature. 1971;234:85–88. doi: 10.1038/234085a0. [DOI] [PubMed] [Google Scholar]
- White HD, Taylor EW. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976;15:5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: Implications for cardiac function. Proc Natl Acad Sci U S A. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggers CJ. Studies on the consecutive phases of the cardiac cycle. Am J Physiol. 1921;56:415–438. [Google Scholar]
- Wiggers CJ. The present status of cardiodynamic studies on normal and pathologic hearts. JAMA. 1921;27:475–502. [Google Scholar]
- Wijnker PJM, et al. Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol. 2014;306:H1171–H1181. doi: 10.1152/ajpheart.00580.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JM, Perry SV, Cole H, Trayer IP. Characterization of components of inhibitory-factor (troponin B) preparations of the myofibril. Biochem J. 1971;124:55P–56P. doi: 10.1042/bj1240055P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JM, Perry S, Cole H, Trayer I. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972;127:215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods EF, Himmelfarb S, Harrington WF. Studies on the structure of myosin in solution. J Biol Chem. 1963;238:2374–2385. [PubMed] [Google Scholar]
- Xu S, Kress M, Huxley HE. X-ray diffraction studies of the structural state of crossbridges in skinned frog sartorius muscle at low ionic strength. J Muscle Res Cell Motil. 1987;8:39–54. doi: 10.1007/BF01767263. [DOI] [PubMed] [Google Scholar]
- Xu S, et al. X-ray diffraction studies of cross-bridges weakly bound to actin in relaxed skinned fibers of rabbit psoas muscle. Biophys J. 1997;73:2292–2303. doi: 10.1016/S0006-3495(97)78261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Moos C. The C-proteins of rabbit red, white, and cardiac muscles. J Biol Chem. 1983;258:8395–8401. [PubMed] [Google Scholar]
- Zoghbi ME, Woodhead JL, Moss RL, Craig RW. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci U S A. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]