KEY TEACHING POINTS
|
Introduction
A 54-year-old woman had undergone slow pathway ablation for AV nodal reentrant tachycardia 3 years ago. Before admission she had started to present with recurrent dizziness and syncope due to 2:1 AV block and was referred for pacemaker implantation. She was asymptomatic and had no signs of AV conduction abnormalities until 2 weeks ago. AV conduction abnormality resolved with atropine infusion until stage 2 of a treadmill exercise test. Electrophysiologic study showed suprahisian 2:1 AV block. After cardioneuroablation, the parameters of AV conduction normalized. Holter recordings were normal, and the patient was asymptomatic at the end of 12-month follow-up.
Several clinical conditions comprise autonomic dysfunction by enhanced parasympathetic tone together with decreased sympathetic tone. Enhanced parasympathetic tone may cause sinus bradycardia or pauses, transient or permanent atrioventricular (AV) block, carotid sinus syndrome, or neurally mediated reflex syncope. Although the conduction system is not involved in these patients, they may present with serious symptoms. The parasympathetic postganglionic neuron body cell is located at the cardiac wall in the paracardiac ganglia.1, 2, 3 Cardioneuroablation is a little known technique for management of patients with excessive vagal activation. The technique is based on radiofrequency (RF) catheter ablation of autonomic connections in the 3 main ganglia around the heart.4
Case report
Medical history
A 54-year-old woman was referred to our clinic for implantation of a permanent pacemaker because of symptomatic 2:1 AV block. The patient had undergone successful selective slow pathway ablation for AV nodal reentrant tachycardia 3 years ago.
At EPS, the shortest atrial pacing cycle length that maintained 1:1 anterograde conduction was in the normal range (280 ms before the procedure and 320 ms after the procedure). The A-H interval was unchanged at the end of the procedure. The patient was asymptomatic during 3-year follow-up, and follow-up ECGs revealed no conduction abnormalities.
Admission history
Three weeks before admission, the patient began to present with recurrent dizziness and syncope. Repeated Holter recordings showed 2:1 AV block with a ventricular rate of 45 bpm that was ongoing throughout the day. After atropine sulfate infusion (0.04 mg/kg), 2:1 AV block gradually disappeared (Figure 1). During stage 2 of a treadmill exercise test, her ventricular rate suddenly increased, and stable 1:1 AV conduction with a rate of 120 bpm appeared. There was no chronotropic incompetence. Based on these findings, we considered AV block to be functional; therefore, we decided to perform cardioneuroablation. The patient provided written informed consent for the ablation procedures. The procedure was performed with the patient in a nonsedated state.
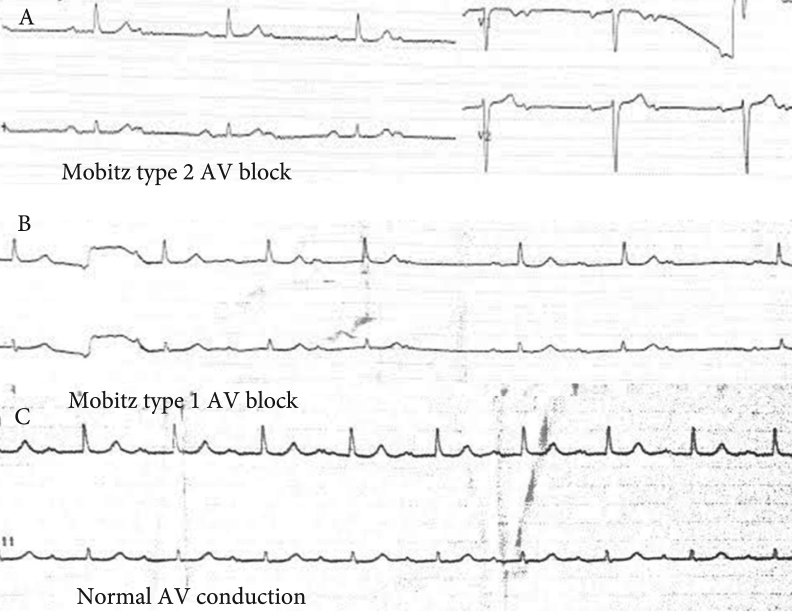
Figure 1.
A: Baseline ECG showing 2:1 AV block. B: After atropine bolus, 2:1 AV block immediately turns to Mobitz type 1 AV block. C: Normal AV conduction is achieved at the late stage of atropine sulfate infusion.
Electrophysiologic study
Three electrophysiologic catheters were placed via venous puncture into the right atrium (RA), right ventricle, and His-bundle region for conventional EPS, which revealed suprahisian 2:1 AV block with normal sinus node function. Using a 4-mm open-tip irrigated RF catheter (Cool Flex, St. Jude Medical, St. Paul, MN), the 3-dimensional geometry of the RA and the left atrium (LA) was created using a 3-dimensional electroanatomic mapping system (EnSite Velocity, St. Jude Medical, Sylmar, CA). Bipolar endocardial electrograms were displayed at filter settings of 30–500 Hz and 300–500 Hz (for EPS and online spectral analysis, respectively) and measured at a sweep speed of 400 mm/s. All recordings were stored on optical disk (EP-Workmate Recording System, St. Jude Medical, St. Paul, MN). Localization of the ganglia was detected by high-frequency stimulation (20 Hz, 0.1–1 mA, 1- to 10-ms pulse width; 3510 multiprogramming stimulator, St. Jude Medical, Sylmar, CA) through the ablation catheter to atrial tissue. This technique has been reported elsewhere.5
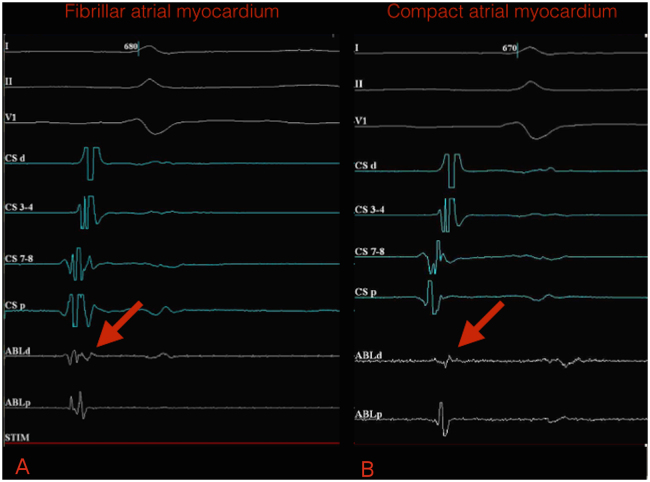
Potentials >300 Hz were shown to have excellent correlation with fibrillar atrial myocardium in the region compatible with paracardiac ganglia, which present a heterogenous and coarse segmented spectrum (Figure 2).
Figure 2.
A: Fibrillar atrial myocardium potentials (heterogeneous and coarse segmented spectrum) (arrow) on intracardiac electrograms. B: Compact atrial myocardium potentials (homogeneous spectrum) on intracardiac electrograms. Bipolar endocardial electrograms are displayed at filter settings of 300–500 Hz at a sweep speed of 400 mm/s. ABLd = distal electrode of ablation catheter ; ABLp = proximal electrode of ablation catheter; CSd = distal electrode of coronary sinus catheter; CSp = proximal electrode of coronary sinus catheter.
Cardioneuroablation
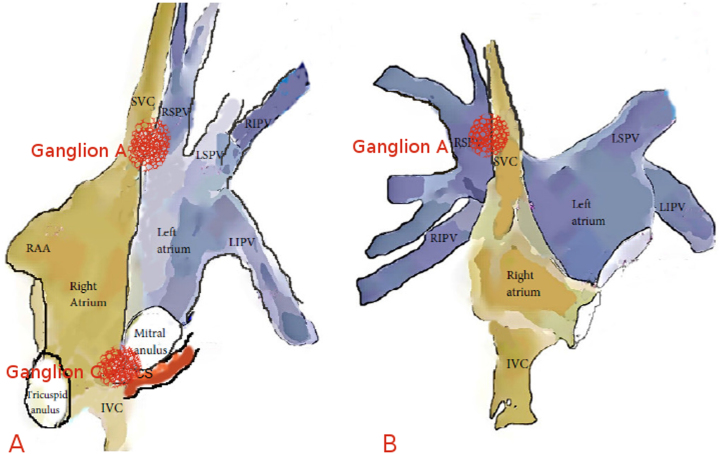
As shown by animal studies, 3 main parasympathetic ganglia located outside the atrial wall in paracardiac fat pads provide innervation to the heart.1, 2, 3 Ganglion A is located between the aorta and the superior vena cava; ganglion B is located between the right pulmonary veins and the RA; and ganglion C is located between the inferior vena cava and the RA/LA (Figure 3). Ganglion A appears to be the “head station” of vagal fibers traveling to both the atria and the sinus and AV nodes.2, 3 Bilateral vagal fibers to the sinus and AV nodes also converge first at ganglion A (a few fibers to the sinus node go directly to ganglion B) and then project to ganglia B and C, which provide vagal innervation to the sinus and AV nodes, respectively.
Figure 3.
Anatomic illustration of ganglia A and C in lateral (A) and anteroposterior (B) views. CS = coronary sinus; IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RAA = right atrial appendage; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
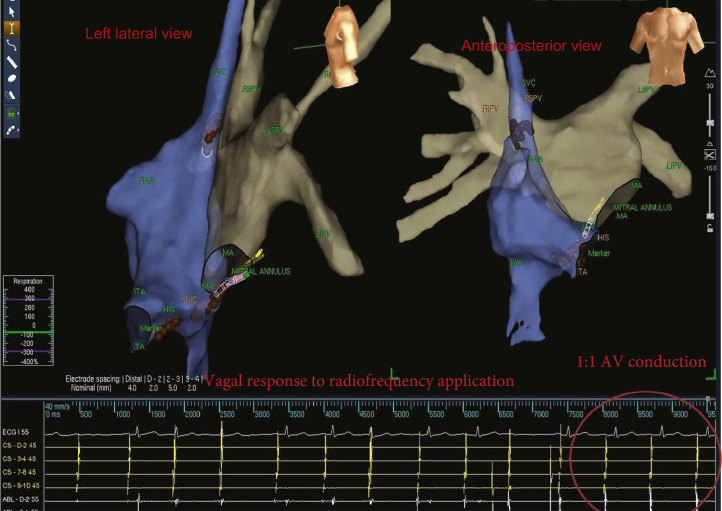
As mentioned earlier, the ablation procedure was performed only via the RA. The RA sites showing a parasympathetic response (bradycardic response with progressive slowing of the sinus rate by 50%) and fibrillar atrial myocardium pattern were assigned as targets and ablated until atrial electrical potential was almost completely eliminated (<0.1 mV; Figure 4 and Online Supplementary Video). A high degree AV block was seen during RF application at targeted sites. After AV node denervation by ablation of ganglia A and C via the RA approach, AV conduction resolved completely.
Figure 4.
Electrogram-guided ablation. Left lateral (left) and anteroposterior (right) views of a 3-dimensional construction of the right atrium (blue) and left atrium (gray). Focal applications of radiofrequency energy were delivered at sites that displayed fibrillar electrograms. Brown tags indicate ablation sites. IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; MA = mitral annulus; RAA = right atrial appendage; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava; TA = tricuspid annulus.
RF current was delivered point by point at a power of 35 W (maximum 40 W) and applied in temperature-controlled mode. The target temperature was 40°C, with a cooling rate of 18 mL/min until elimination (<0.1 mV) of atrial fractionated potentials >300 Hz. Continuous flow during mapping was 2 mL/min.
Postprocedure Wenckebach point was 285 ms. Total procedural time was 75 minutes, and total fluoroscopy time was 17 minutes. A total of 21 endocardial points were treated.
Follow-up
The patient has been completely asymptomatic, experiencing no episodes of dizziness or syncope and taking no medications at the end of 12-month follow-up. All follow-up ECGs and Holter recordings have been normal. No other pause or AV block has yet been recorded. The minimum and mean heart rates changed from 65 and 79 bpm at 1 month to 62 and 77 bpm, 58 and 72 bpm, and 55 and 71 bpm at 3 months, 6 months, and 1 year postprocedure, respectively.
Discussion
Pachon et al4 were the first to report cardiac autonomic modulation through catheter ablation guided by fast Fourier transformation analysis as an alternative treatment of functional intermittent high-degree AV block. In 7 of 21 patients included in their study, the diagnosis was intermittent high-degree AV block (3 occurred during sleep). The procedural end-point was normalization of AV conduction electrophysiologic parameters. In 3 patients, the procedure was performed only via the RA, and 1 of these patients still experienced nocturnal Mobitz type I AV block after the procedure. Long-term follow-up results of the patients were not presented in that study.
The same group reported the long-term outcome of cardioneuroablation in patients with neurally mediated reflex syncope.4 They concluded that endocardial RF catheter ablation of severe neurally meditated reflex syncope via both atria may prevent pacemaker implantation and show excellent long-term results in well-selected patients.
In 2012, Yao et al6 evaluated the feasibility and efficacy of selective endocardial autonomic denervation in the LA as an alternative treatment strategy in patients with highly symptomatic vasovagal syncope. At 30 ± 16 months of follow-up, no patient had any recurrence of syncopal episodes and all patients had significant improvement in symptoms, but 5 of 10 patients reported transient prodromes.
In our case, we aimed to ablate the crux area for “ganglion C” ablation, which provides vagal innervation to the AV node. Only sites that showed a fibrillar atrial myocardium pattern were ablated. In a previous report, Lellouche et al7 aimed to characterize LA sinus rhythm electrogram patterns and their relationship to parasympathetic responses during atrial fibrillation ablation. They revealed that parasympathetic activation during atrial fibrillation ablation is associated with the presence of preablation high-amplitude fractionated electrograms in sinus rhythm. Meyer et al8 showed a similar association between endocardial electrograms and parasympathetic response. The results of our case are consistent with these 2 reports and confirm that paracardiac ganglia can be localized by intracardiac electrograms without using fast Fourier transformation analysis.
The initial response to AV nodal ablation was a change from high-degree AV block to complete AV block during RF application (Figure 4). Then, 1:1 AV conduction was achieved. We also targeted ganglion A by the superior vena cava–RA junction, which provides additional vagal innervation to the AV node. Sinus rate increased markedly after “ganglion A” ablation. The electrophysiologic parameters of AV conduction were completely normal at the end of the RA ablation procedure, so additional ablation via the LA was not performed. To the best of our knowledge, this is the first reported case of permanent functional 2:1 AV block in which normal AV conduction was obtained after neurocardioablation via the RA approach.
A larger patient cohort and randomized controlled trials are needed to confirm the safety and efficacy of this new treatment option in patients with functional AV block. In this case, patient follow-up was only 12 months, and the long-term results are unknown. In addition, the long-term effects of vagal denervation and unrequited sympathetic activity have not yet been compared. An inverse correlation between resting heart rate and life expectancy has been demonstrated in healthy humans.9 Although the postablation mean heart rate of the patients was in an acceptable range, mean heart rate was significantly elevated at the end of study. The long-term results of increased heart rate must be reconstructed in long-term follow-up studies. Pachon et al10 reported the long-term results of a patient with high-degree AV block who underwent the cardioneuroablation procedure guided by spectral endocardial mapping. The electrophysiologic parameters normalized and the patient was asymptomatic, leading a normal life at follow-up 21 months later. However, the authors did not report follow-up heart rate values.
In conclusion, despite these limitations, the results of our case indicate that cardioneuroablation in patients with functional AV block is feasible and may be a valuable adjunctive therapy in patients who cannot be adequately treated by conventional modalities and refuse pacemaker implantation. Further larger, randomized controlled studies are required to demonstrate the efficiency, reliability, and appropriate patient selection criteria of cardioneuroablation.
Footnotes
Supplementary material cited in this article is available online at doi:10.1016/j.hrcr.2014.12.012.
Appendix. Supplementary data
References
- 1.Randall W.C., Milosavljevic M., Wurster R.D., Geis G.S., Ardell J.L. Selective vagal innervation of the heart. Ann Clin Lab Sci. 1986;16:198–208. [PubMed] [Google Scholar]
- 2.Guyton A.C., Hall J.E. Textbook of Medical Physiology. Ninth edition. WB Saunders; Philadelphia: 1996. The autonomic nervous system: the adrenal medulla; pp. 769–781. [Google Scholar]
- 3.Chiou C.W., Eble J.N., Zipes D.P. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes: the third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 4.Pachon J.C., Pachon E.I., Pachon J.C., Lobo T.J., Pachon M.Z., Vargas R.N., Jatene A.D. “Cardioneuroablation”—new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Po S.S., Nakagawa H., Jackman W.M. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 6.Yao Y., Shi R., Wong T., Zheng L., Chen W., Yang L., Huang W., Bao J., Zhang S. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 7.Lellouche N., Buch E., Celigoj A., Siegerman C., Cesario D., De Diego C., Mahajan A., Boyle N.G., Wiener I., Garfinkel A., Shivkumar K. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 2007;50:1324–1331. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 8.Meyer C., Martinek M., Aichinger J., Purerfellner H. Stepwise modulation of the cardiac neural network during ablation at the left superior pulmonary vein-atrial junction. Europace. 2010;12:1025–1028. doi: 10.1093/europace/euq066. [DOI] [PubMed] [Google Scholar]
- 9.Levine H.J. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30:1104–1106. doi: 10.1016/s0735-1097(97)00246-5. [DOI] [PubMed] [Google Scholar]
- 10.Pachon M.J.C., Pachon M.E.I., Lobo T.J., Pachon M.J.C., Pachon M.Z., Vargas R.N., Manrique R.M., Jatene A.D. Syncopal high-degree AV block treated with catheter RF ablation without pacemaker implantation. Pacing Clin Electrophysiol. 2006;29:318–322. doi: 10.1111/j.1540-8159.2006.00340.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.