KEY TEACHING POINTS
|
Introduction
Coronary artery injury or vasospasm can occur as a direct thermal effect of radiofrequency (RF) catheter ablation.1, 2, 3, 4, 5, 6, 7 The right coronary artery (RCA) and left circumflex artery (LCx) are adjacent to the valvular annuli and are likely to suffer from ablation-related injury when RF energy is delivered to accessory pathways1, 2, 3, 4, 5, 6 or the mitral isthmus.7 The right ventricular outflow tract also lies in close proximity to the major coronary arteries.8 However, to the best of our knowledge, injury to the left anterior descending artery (LAD) associated with left ventricular (LV) endocardial ablation has not been reported.
Case report
A 22-year-old man was admitted to our hospital because of palpitations and dyspnea on effort. Twelve-lead electrocardiography showed frequent ventricular premature contractions (VPCs) that were predicted to originate from the LV outflow tract based on QRS morphology (Figure 1A). Echocardiography revealed reduced LV function with an ejection fraction of 35%. A decrease in the number of VPCs and improvement of ejection fraction were observed with oral administration of amiodarone. Hence, VPC-induced cardiomyopathy was assumed, and RF catheter ablation was indicated for treatment of the VPCs.
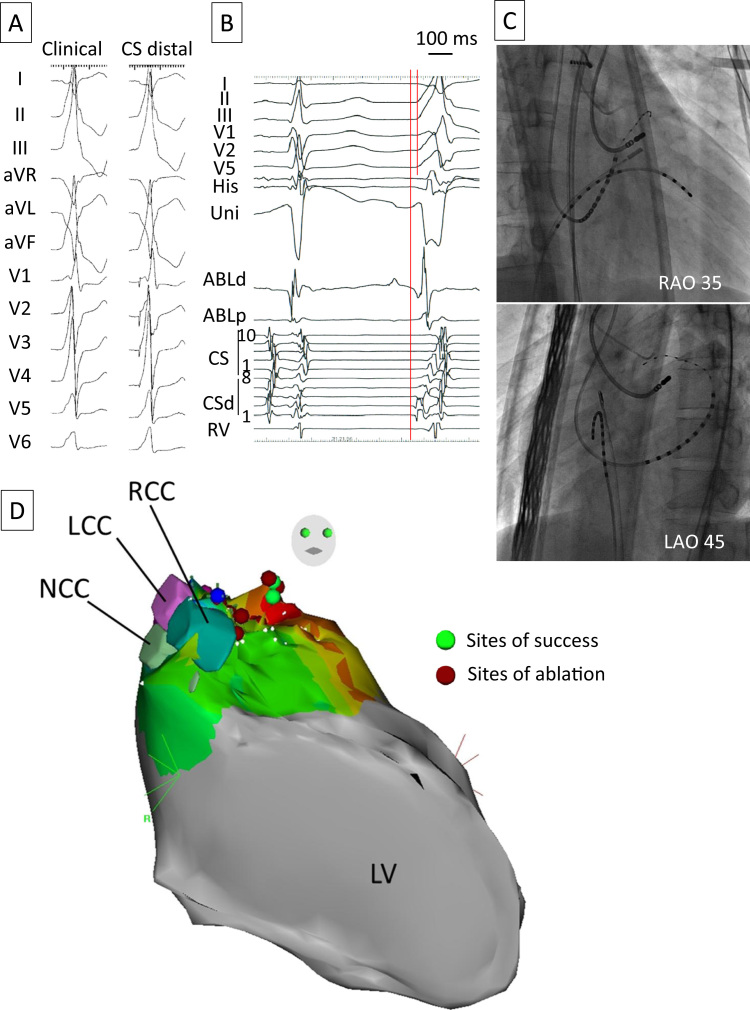
Figure 1.
A: Twelve-lead electrocardiogram showing ventricular premature contractions (left) and the pace-map at the distal CS (right). B: Local electrograms of the distal CS and ablation catheter precede QRS onset by 25 ms. C: Fluoroscopic images showing the CS catheter and the site of successful radiofrequency applications. D: Three-dimensional map depicted by CARTO SOUND showing the anatomic relationship between the coronary sinus cusps and sites of ablation. ABL = ablation catheter; CS = coronary sinus; CSd = distal CS; LAO = left anterior oblique; LCC = left coronary sinus cusp; LV = left ventricle; NCC = noncoronary sinus cusp; RAO = right anterior oblique; RCC = right coronary sinus cusp; RV = right ventricle; Uni = unipolar electrogram.
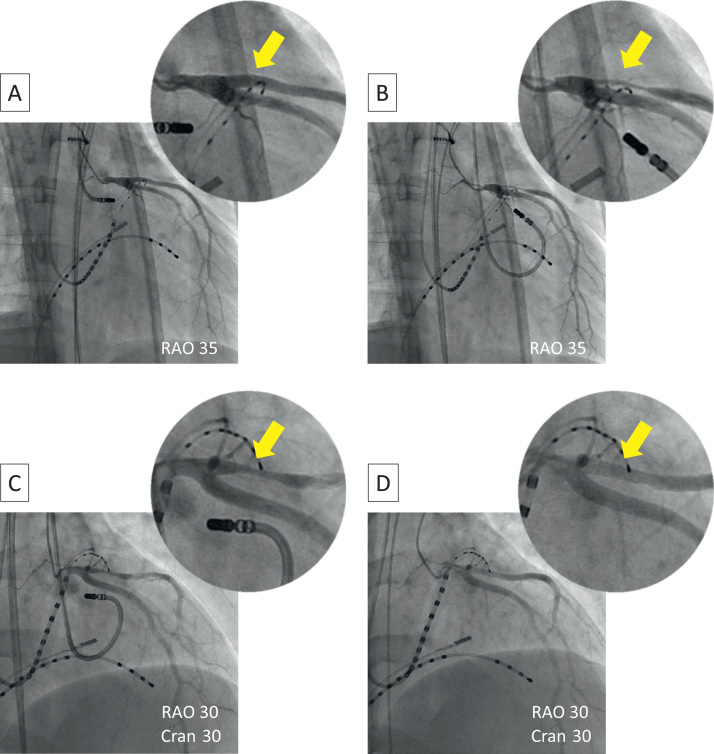
Mapping of the VPCs was performed at the LV endocardial surface, coronary sinus, great cardiac vein (GCV), and aortic left coronary cusp (LCC). Mapping at the GCV with a 2Fr catheter (EPstar Fix, Japan Lifeline, Tokyo, Japan) revealed a good pace-map, and the local electrogram preceded QRS onset by 25 ms (Figures 1A–1C). RF energy was not delivered to this site because of the high impedance and limited accessibility of the ablation catheter9 but was delivered alternatively to the basal anterior portion of the LV endocardium where a good pace-map was also obtained. RF energy was delivered using an irrigated-tip catheter (ThermoCool, Biosense Webster, Diamond Bar, CA) at a power setting of 35 W and maximum temperature of 43°C. VPCs transiently disappeared during RF applications but recurred immediately after applications were stopped. RF energy delivered at the LCC at the same settings also failed to eliminate the VPCs. Coronary angiography performed before and after ablation at the LCC did not reveal any coronary artery injury. Again, we moved to the left ventricle and RF energy was increased to 50 W, which resulted in complete elimination of the VPCs (Figure 1C). The sites of ablation are also shown in the CARTO map (Biosense Webster; Figure 1D). Although the patient did not complain of any symptoms and the 12-lead electrocardiogram did not show any ST-T changes, angiography performed just after the endocardial RF application showed significant stenosis in the proximal portion of the large first diagonal branch of the LAD (Figure 2). This branch also looked like a so-called dual LAD. The distance between the site of successful RF ablation and this branch of the LAD was angiographically measured to be 9 mm. Isosorbide dinitrate (5 mg) was injected immediately, but the coronary stenosis did not improve completely. Several additional vasodilators (nicorandil 2 mg and nitroglycerin 300 μg) were injected, and the coronary stenosis was gradually relieved. Oral administration of aspirin was continued for 1 month. The patient has been free from any arrhythmic and coronary events during a 9-month follow-up period.
Figure 2.
Coronary angiographic images repeated throughout the procedure. A: After ablation at the left coronary sinus cusp, no coronary artery injury was observed. B: Just after radiofrequency ablation at the basal anterior portion of the left ventricular endocardium, severe stenosis of the first branch of the left anterior descending artery was observed. C: Coronary stenosis did not improve completely after immediate intracoronary injection of isosorbide dinitrate. D: Coronary stenosis was gradually relieved after injections of additional vasodilators. Cran = cranial; RAO = right anterior oblique.
Discussion
Coronary arteries can be damaged by RF energy, and the incidence is reported to range from 0.1%1 to 1.4%.2 The majority of reported cases involved RCA injury associated with ablation for right-sided accessory pathways.3, 4 Some cases involving LCx injury by ablation for left-sided accessory pathways also have been reported.5 One reasonable mechanism of coronary artery spasm is direct thermal trauma from RF energy. A previous study in pigs showed that inflammation progresses to the layer of the coronary artery within 48 hours after RF energy delivery to the tricuspid annulus, and such pathophysiologic changes are considered to cause acute coronary spasm.6 Therefore, coronary artery damage is most likely to occur during RF ablation on the atrial side of the valvular annuli and within the coronary sinus because these sites are very close to coronary arteries. Recently, LCx injury associated with mitral isthmus ablation has been widely noted because macroreentrant perimitral atrial flutter is common after ablation of atrial fibrillation. Wong et al7 reported that 15 of 54 patients (28%) undergoing mitral isthmus ablation had acute subclinical LCx injury.
There is only 1 report of LAD occlusion after RF ablation. Dinckal et al10 described a 32-year-old man with proximal LAD occlusion 10 days after RF ablation for a left lateral accessory pathway. Intravenous nitroglycerin was not effective, and a stent was immediately implanted. Their case is consistent with ours in terms of injury to the LAD but different in that the site of obstruction was far from the ablation site (proximal LAD occlusion after ablation of a left lateral accessory pathway), and the time from catheter ablation to LAD injury was much longer.
In our case, the morphology of the bipolar electrogram at the successful ablation site was dull compared with electrograms recorded at the distal coronary sinus, and the unipolar electrogram had a small r wave in its initial portion (Figure 1B), suggesting an intramural or epicardial origin of the VPC. Although direct thermal trauma from RF energy is considered to be the most likely reason for coronary artery injury or vasospasm in general, other possible mechanisms may have been associated with this phenomenon in our patient.
-
1.Direct mechanical trauma by an ablation catheter
- There are a number of reports of left main coronary artery (LMCA) injury.11, 12, 13 For example, Yalin et al13 described a 56-year-old man with LMCA occlusion during RF ablation for a left anterolateral accessory pathway, which was treated by immediate percutaneous coronary angioplasty with a bare-metal stent. All of the cases of LMCA injury were associated with a retrograde transaortic approach. The most probable cause was direct mechanical trauma to the LMCA when the ablation catheter crossed the aortic valve. Although the retrograde transaortic approach was used in our patient, the site of damage was far from the ostium of the LMCA, so direct trauma by the ablation catheter was unlikely.
-
2.Mechanical pressure or high-output pacing by a fine mapping catheter in the GCV
- A recent study9 reported that the close proximity of the GCV to the coronary artery system poses limitations for RF application in the GCV, suggesting the possibility of vasospasm due to mechanical pressure by the mapping catheter in the GCV overlying the LAD. However, this phenomenon is most likely to occur when the catheter is inserted into the GCV at the beginning of the procedure, and in our patient, catheter stability in terms of its location and electrograms was very high throughout the procedure, making this mechanism less likely. Also, high-output pacing stimulation from the mapping catheter in the GCV might capture the vascular smooth muscle of the LAD and induce vasospasm. However, we performed stimulation in the GCV only at the beginning of the procedure, and LAD stenosis occurred during the latter half of the procedure. The same difference in the time course during the procedure in this case makes both mechanisms less likely.
-
3.Angiocatheter-induced coronary spasm
- An angiocatheter tip contacting the vessel wall is a significant factor predisposing patients to catheter-induced coronary spasm.14 In our patient, the LCx appeared hypoplastic. Even if the angiocatheter had been selectively cannulated in the LAD, it is unlikely that the angiocatheter would have advanced deeply to the segment where the vasospasm occurred because we repeatedly performed angiography, and the operator had accurately localized the ostium of the LMCA with the first angiogram.
-
4.Drug-induced vasospasm
- Several drugs, such as catecholamines, acetylcholine, adenosine, and beta-blockers, increase the susceptibility to vasospasm. In our patient, no drugs (including isoproterenol) were used to induce the arrhythmia because the VPCs occurred incessantly during the procedure.
Yokoyama et al15 evaluated the relationship between contact force and tissue temperatures, and lesion size during RF ablation, using a canine thigh muscle preparation. The median value of tissue temperature at a depth of 7 mm was 86°C at settings of 50 W of power and 20g of contact force, and the median value of the maximum lesion depth was 9.4 mm at the same settings. Herein, we may have to take into consideration the cooling effect provided by coronary blood flow. However, these results support our presumption that RF energy delivered from the endocardial side with a high power setting and good catheter–tissue contact can reach not only to the RCA or LCx lying in the atrioventricular groove but also to the LAD on the epicardial side of the ventricular wall, leading to vasospasm or intimal injury.
In conclusion, LV endocardial ablation at high power settings might result in injury to the epicardial coronary arteries due to direct thermal trauma.
Footnotes
Drs. Kimata and Igarashi contributed equally to this work.
References
- 1.Calkins H., Yong P., Miller J.M. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999;99:262–270. doi: 10.1161/01.cir.99.2.262. [DOI] [PubMed] [Google Scholar]
- 2.Solomon A.J., Tracy C.M., Swartz J.F., Reagan K.M., Karasik P.E., Fletcher R.D. Effect on coronary artery anatomy of radiofrequency catheter ablation of atrial insertion sites of accessory pathways. J Am Coll Cardiol. 1993;21:1440–1444. doi: 10.1016/0735-1097(93)90321-q. [DOI] [PubMed] [Google Scholar]
- 3.Hosaka Y., Chinushi M., Takahashi K., Ozaki K., Yanagawa T., Miida T., Oda H., Aizawa Y. Coronary vasospasm triggered ventricular fibrillation delayed after radiofrequency ablation of the right accessory pathway. Europace. 2009;11:1554–1556. doi: 10.1093/europace/eup219. [DOI] [PubMed] [Google Scholar]
- 4.Strobel G.G., Trehan S., Compton S., Judd V.E., Day R.W., Etheridge S.P. Successful pediatric stenting of a nonthrombotic coronary occlusion as a complication of radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2001;24:1026–1028. doi: 10.1046/j.1460-9592.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 5.Spar D.S., Silver E.S., Hordof A.J., Torres A., Liberman L. Coronary artery spasm during radiofrequency ablation of a left lateral accessory pathway. Pediatr Cardiol. 2010;31:724–727. doi: 10.1007/s00246-010-9670-4. [DOI] [PubMed] [Google Scholar]
- 6.Paul T., Bokenkamp R., Mahnert B., Trappe H.J. Coronary artery involvement early and late after radiofrequency current application in young pigs. Am Heart J. 1997;133:436–440. doi: 10.1016/s0002-8703(97)70185-6. [DOI] [PubMed] [Google Scholar]
- 7.Wong K.C., Lim C., Sadarmin P.P., Jones M., Qureshi N., De Bono J., Rajappan K., Bashir Y., Betts T.R. High incidence of acute sub-clinical circumflex artery ׳injury׳ following mitral isthmus ablation. Eur Heart J. 2011;32:1881–1890. doi: 10.1093/eurheartj/ehr117. [DOI] [PubMed] [Google Scholar]
- 8.Vaseghi M., Cesario D.A., Mahajan A., Wiener I., Boyle N.G., Fishbein M.C., Horowitz B.N., Shivkumar K. Catheter ablation of right ventricular outflow tract tachycardia: value of defining coronary anatomy. J Cardiovasc Electrophysiol. 2006;17:632–637. doi: 10.1111/j.1540-8167.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 9.Steven D., Pott C., Bittner A. Idiopathic ventricular outflow tract arrhythmias from the great cardiac vein: challenges and risks of catheter ablation. Int J Cardiol. 2013;169:366–370. doi: 10.1016/j.ijcard.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Dinckal H., Yucel O., Kirilmaz A., Karaca M., Kilicaslan F., Dokumaci B. Left anterior descending coronary artery occlusion after left lateral free wall accessory pathway ablation: what is the possible mechanism? Europace. 2003;5:263–266. doi: 10.1016/s1099-5129(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 11.Hope E.J., Haigney M.C., Calkins H., Resar J.R. Left main coronary thrombosis after radiofrequency ablation: successful treatment with percutaneous transluminal angioplasty. Am Heart J. 1995;129:1217–1219. doi: 10.1016/0002-8703(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 12.Kharrat I., Charfeddine H., Sahnoun M., Rekik S., Krichen S., Hentati M., Kammoun S. Left main coronary thrombosis: unusual complication after radiofrequency ablation of left accessory atrioventricular pathway. J Electrocardiol. 2008;41:683–685. doi: 10.1016/j.jelectrocard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Yalin K., Golcuk E., Bilge A.K., Umman S., Adalet K. Successful stenting of a left main coronary artery occlusion as a complication of RF ablation for Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 2012;35:e43–e46. doi: 10.1111/j.1540-8159.2010.02900.x. [DOI] [PubMed] [Google Scholar]
- 14.Mautner R.K., Cooper M.D., Phillips J.H. Catheter-induced coronary artery spasm: an angiographic manifestation of vasospastic angina? Am Heart J. 1983;106:659–665. doi: 10.1016/0002-8703(83)90083-2. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama K., Nakagawa H., Shah D.C., Lambert H., Leo G., Aeby N., Ikeda A., Pitha J.V., Sharma T., Lazzara R., Jackman W.M. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–362. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]