Abstract
Objectives:
Tenapanor is a first-in-class, small-molecule inhibitor of the gastrointestinal sodium/hydrogen exchanger NHE3. This study assessed the efficacy and safety of tenapanor in patients with constipation-predominant irritable bowel syndrome (IBS-C).
Methods:
In this phase 2, double-blind study, patients with IBS-C (Rome III criteria) were randomized (1:1:1:1) to receive tenapanor 5 mg, 20 mg, or 50 mg b.i.d., or placebo b.i.d. for 12 weeks. The primary end point was the complete spontaneous bowel movement (CSBM) responder rate, defined as the proportion of patients reporting an increase from baseline of ≥1 CSBM/week for ≥6/12 treatment weeks. Secondary end points included abdominal symptom responder rates (≥30% score improvement from baseline for ≥6/12 weeks) and a composite responder rate (CSBM and abdominal pain response in the same week for ≥6/12 weeks).
Results:
Overall, 356 patients were randomized (mean age: 45.7 years; 86.8% women) and 304 completed the study. The CSBM responder rate was significantly higher in the tenapanor 50 mg b.i.d. group than in the placebo group (60.7 vs. 33.7% P<0.001), as was the composite responder rate (50.0 vs. 23.6% P<0.001). Responder rates for abdominal symptoms (pain, discomfort, bloating, cramping, and fullness) were significantly higher in the tenapanor 50 mg b.i.d. group than in the placebo group (all P<0.05). Diarrhea was the most frequent adverse event (tenapanor b.i.d.: 20 mg, 12.4% 50 mg, 11.2%).
Conclusions:
Tenapanor 50 mg b.i.d. significantly increased stool frequency and reduced abdominal symptoms in patients with IBS-C. Further research into tenapanor as a potential treatment for these patients is justified.
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by abdominal pain or discomfort and altered bowel habits (1). IBS affects 7–21% of individuals globally (2, 3), with constipation-predominant IBS (IBS-C) accounting for approximately one-third of cases (4). IBS has both a significant societal impact and a significant personal impact, resulting from impaired quality of life, loss of work productivity, and increased healthcare resource utilization (5).
In addition to typical IBS symptoms such as abdominal pain and bloating, patients with IBS-C commonly report hard and infrequent stools, straining, and a sensation of incomplete evacuation (6). Patients with IBS-C also have a substantially impaired quality of life (4, 5). The negative impacts of IBS-C are often similar to (or worse than) those observed in chronic diseases that are traditionally perceived as being more serious than IBS, such as inflammatory bowel disease, rheumatoid arthritis, and diabetes mellitus (7, 8, 9).
Diagnosis of IBS-C and adequate treatment of individuals with IBS-C present significant challenges, owing to the diverse and dynamic nature of symptoms that accompany a symptom-based diagnosis of diverse pathogenesis (10). Historically, laxatives, dietary fiber, and stool softeners were recommended to patients with IBS-C; however, the evidence supporting these treatments is variable in quantity and quality, and low treatment satisfaction has been reported (10, 11). More recent therapies targeting IBS-C symptoms include the guanylate cyclase-C receptor agonist linaclotide (12, 13) and the selective chloride channel activator lubiprostone (14), both of which are minimally absorbed and act in the gastrointestinal tract. Though linaclotide and lubiprostone have been shown to be more effective than placebo in large randomized controlled trials, fewer than half of patients with IBS-C achieved the primary end points of improvements in stool frequency and abdominal pain (13, 14). Thus, despite the availability of these treatments, there remains an unmet need for further therapeutic options for some patients with IBS-C who continue to experience symptoms (12, 13, 14).
Tenapanor is a first-in-class, small-molecule inhibitor of the gastrointestinal sodium/hydrogen exchanger isoform 3. Tenapanor acts in the gastrointestinal tract to reduce the absorption of sodium and phosphate, with minimal systemic drug exposure (15). Increased sodium retention in the gut in healthy volunteers treated with tenapanor enhances intestinal fluid volume and transit, as demonstrated by softer stools and an increase in the frequency of bowel movements (16). Preclinical studies also suggest that tenapanor may exert antinociceptive effects on visceral sensation (17). Based on these findings, tenapanor was evaluated over 4 weeks at three once-daily doses (10, 30, and 100 mg) in patients with IBS-C in a placebo-controlled study, with tenapanor treatment resulting in improvements in IBS-C symptoms (ClinicalTrials.gov identifier: NCT01340053) (18). A study of tenapanor in healthy volunteers suggested that twice-daily dosing was more effective than once-daily dosing at the equivalent total daily dose at increasing stool sodium (19). We therefore conducted the present phase 2 study to assess the efficacy and safety of three, twice-daily doses of tenapanor (5, 20, and 50 mg) for the treatment of patients with IBS-C.
Methods
Study design
This was a multicenter, phase 2, randomized, double-blind, placebo-controlled study conducted at 79 sites in the USA (ClinicalTrials.gov identifier: NCT01923428) between August 2013 and October 2014 (last patient last visit July 2014). The sites conducting the study were gastroenterology practices (n=26) or primary care and research practices (n=53) specializing in internal medicine and/or gastroenterology. After a 2-week screening period, eligible patients were randomly assigned in a 1:1:1:1 ratio to one of four treatment groups: tenapanor 5 mg, 20 mg, or 50 mg twice daily (b.i.d.; dosed as the hydrochloride salt) or placebo b.i.d. Patients received tenapanor or placebo for 12 consecutive weeks, after which they were followed up for an additional 4 weeks. Patients visited the study site seven times during the study: once during the 2-week screening period (week −2), five times during the active treatment period (weeks 0, 2, 4, 8 and 12), and once more at the end of the 4-week follow-up period (week 16). The study was conducted in accordance with the Declaration of Helsinki and all patients provided written informed consent.
Patients
Men and women aged 18–75 years who met the Rome III criteria for IBS-C (20) were eligible for study enrollment. In addition, patients had to have had a colonoscopy within the past 10 years if more than 50 years of age or in the presence of unexplained warning symptoms (e.g., lower gastrointestinal bleeding, iron-deficiency anemia, clinically significant weight loss, and systemic signs of infection or colitis), and agree to use appropriate methods of contraception, or be surgically sterile or post-menopausal. The main exclusion criteria were: functional diarrhea, IBS with diarrhea, mixed IBS or un-subtyped IBS, as defined by Rome III criteria; any clinically symptomatic biochemical or structural abnormality or active disease of the gastrointestinal tract within 6 months before screening; use of medications that are known to affect stool consistency (except as described below); hepatic dysfunction (defined as alanine aminotransaminase/serum glutamic-pyruvic transaminase or aspartate aminotransaminase/serum glutamic-oxaloacetic transaminase >2.5 × the upper limit of normal) or renal impairment (serum creatinine >2 mg/dl); any surgery on the stomach, small intestine or colon (excluding appendectomy); a major psychiatric disorder requiring hospitalization in the last 3 years, or a history of attempted suicide or uncontrolled bipolar disorder; or clinical evidence of any significant disease that may interfere with the patient successfully completing the trial. Pregnant or lactating women were also excluded.
After the 2-week screening period, in order for patients to be randomly assigned to study treatment, they needed to complete symptom assessments via a touch-tone telephone diary for at least 11 out of 14 days to ensure that they were diary compliant and meet the following study entry criteria: average weekly stool frequency of five or fewer spontaneous bowel movements (SBMs), defined as all non-aided bowel movements, and two or fewer complete spontaneous bowel movements (CSBMs), defined as a bowel movement accompanied by a sensation of complete evacuation; an average weekly stool consistency score of 3 or less using the Bristol Stool Form Scale (BSFS) (21); an average weekly abdominal pain score of at least 3 on a 0–10-point scale; and no liquid stools for any SBM or mushy stools for more than one SBM according to the BSFS.
Rescue medication (bisacodyl 5 mg tablet or 10 mg suppository) was allowed for a maximum of 2 days during the screening period but not during the 48 h before randomization. During the study, rescue medication was allowed to relieve severe constipation (defined as at least 72 h without a bowel movement or when symptoms became intolerable). Bowel movements were not considered to be SBMs and CSBMs if they were reported <24 h after the use of rescue medication.
Patients who were on a stable, continuous regimen of fiber, bulk laxatives, stool softeners, and/or probiotics during the 30 days before the screening visit were included in the study and, provided that they maintained the same treatment and dose throughout the trial, were included in the analysis.
Efficacy variables
The CSBM responder rate was the primary efficacy variable in this study. Secondary efficacy variables included a composite of the CSBM responder rate and the abdominal pain responder rate, and the responder rates for individual abdominal symptoms including abdominal discomfort, abdominal bloating, abdominal fullness, and abdominal cramping. Additional secondary efficacy variables were weekly averages of stool frequency, stool consistency, straining, abdominal pain, constipation severity, and IBS severity. Degree of relief from IBS symptoms, and treatment satisfaction at 12 weeks were additional secondary efficacy variables.
Efficacy assessments
Data for all efficacy variables were recorded by patients using the touch-tone telephone diary. Variables recorded daily included stool frequency (CSBMs and SBMs), stool consistency (BSFS), abdominal symptom scores (pain, bloating, cramping, discomfort, and fullness; each on a 0–10-point scale, 0=absent, 10=very severe), and a straining score (1–5-point scale, 1=not at all, and 5=an extreme amount). Variables scored weekly by patients included constipation severity, IBS severity (each on a 1–5-point scale, 1=none, 5=very severe), degree of relief from IBS symptoms (1–7-point scale, 1=completely relieved, 7=as bad as I can imagine), and treatment satisfaction (1–5-point scale, 1=not at all satisfied, 5=very satisfied).
Responder rates
The CSBM responder rate was defined as the proportion of patients with an increase of at least one CSBM/week compared with baseline for at least 6 of the 12 treatment weeks. The baseline value was defined as the average of the number of CSBMs per week for the 2-week screening period (i.e., weeks −1 and −2).
Responder rates for individual abdominal symptoms (abdominal pain, bloating, cramping, discomfort, and fullness) were defined as the proportion of patients with a decrease of at least 30% from baseline in the average weekly severity score for at least 6 out of 12 treatment weeks. A composite responder rate (22) was calculated as the proportion of patients with an increase of at least one CSBM/week from baseline (primary efficacy variable) and a decrease of at least 30% from baseline in average weekly abdominal pain severity score during the same week, for at least 6 out of 12 treatment weeks.
Safety variables
Adverse events (AEs) were monitored throughout the trial. They were reported by the patient spontaneously and/or in response to an open question from study personnel at each site visit. Safety assessments included: vital signs at each visit (systolic and diastolic blood pressure, heart rate, respiratory rate, body temperature, and weight); clinical laboratory tests (serum electrolytes, hematology, and urinalysis) at weeks −2, 4, and 12; physical examinations at weeks −2, 12, and 16; and 12-lead electrocardiogram monitoring at weeks −2 and 12. Blood samples for pharmacokinetic assessments of tenapanor were collected at site visits at weeks 8 and 12, 1–4 h after morning dosing, from subsets of ~30 randomly selected patients per group.
Statistical methods
A computer-generated randomization scheme was made available to all clinical centers participating in the study via an interactive web response system. The packaging and labeling of the study drug kits were based on a separate drug packaging randomization schedule. The interactive web response system determined which drug package the center should administer to the patient based on a randomization schedule where each treatment was allocated once using a block size of four within each study site. This ensured whole or partial block sizes were allocated, facilitating even distribution of patients amongst each dose group. Data collected from the touch-tone telephone diary system throughout the study were automatically entered into a database; any abnormal values were automatically flagged so the relevant site could follow-up with the patient for clarification. Compliance to study treatment was determined based on the amount of unused study drug returned to study sites.
The intention-to-treat analysis set included all patients who received at least one dose of study drug/placebo and for whom at least one valid week of efficacy assessment data had been collected. A valid week required at least four non-missing days. Scores for stool frequency were standardized to 7 days, with missing days during the week being imputed with the average for the non-missing days. The average weekly scores for other efficacy variables were calculated from the observed number of responses without any standardization. The safety analysis set included all patients who received at least one dose of study drug or placebo.
A sample size of 90 patients in each treatment group was expected to provide 80% power to detect a difference of 20% in the CSBM responder rate between the placebo group and at least one of the tenapanor treatment groups. An assumed responder rate of 20% in patients treated with placebo was based on historical data from a similar trial in patients with IBS-C (23); an assumed responder rate of 40% in patients treated with tenapanor was considered to be clinically meaningful in this patient population.
The primary efficacy variable, the CSBM responder rate, was analyzed using a Cochran–Mantel–Haenszel (CMH) test with pooled investigator sites as a stratification variable. A closed testing procedure was used to control the experiment-wise type I error rate at the 5% significance level. A CMH screening test was conducted first (for the primary efficacy variable only) to look for an association between treatment and responder rate across the placebo, tenapanor 20 mg b.i.d., and tenapanor 50 mg b.i.d. treatment groups. If the result of this test was found to be statistically significant at the 5% level, each of the tenapanor 20 mg b.i.d. and 50 mg b.i.d. treatment groups were compared with the placebo group using a 5% significance level. If either or both of these comparisons were found to have a significant result, either or both of these treatment groups were considered to be individually or simultaneously significantly different from the placebo group. If both the tenapanor 20 mg b.i.d. and tenapanor 50 mg b.i.d. treatment groups were significantly different from the placebo group, the tenapanor 5 mg b.i.d. treatment group was compared with the placebo group using a 5% significance level.
CMH pairwise comparison tests of all tenapanor doses vs. placebo were used to evaluate the abdominal pain responder rate, the composite responder rate, and the responder rates for other abdominal symptoms (abdominal bloating, cramping, discomfort, and fullness). Continuous efficacy variables were assessed using an analysis of covariance model with pooled investigator sites and treatment group as terms and with baseline as the covariate, or an analysis of variance model with pooled investigator sites and treatment as terms.
Results
Patient disposition, demographics, and baseline characteristics
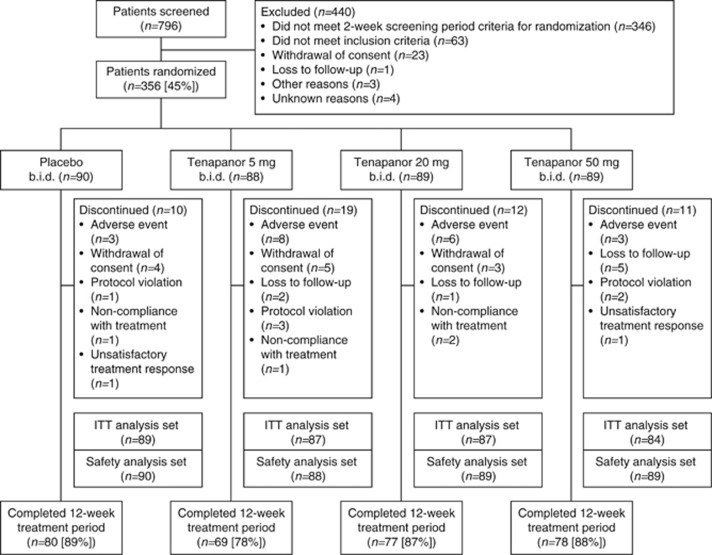
Out of 796 patients who were screened, 356 (45%) met the entry criteria and were randomly assigned to treatment groups (Figure1). Across all treatment groups, the mean age of patients was 45.7 years, with the majority being women (86.8%) and white (75.8%). The proportion of patients completing the study ranged from 78 to 89% across the treatment groups. Patient demographics and baseline disease characteristics were generally comparable across all treatment groups (1, 2).
Figure 1.
Overview of patient flow through the study. The safety analysis set includes all patients who received at least one dose of treatment. The ITT analysis set includes all patients who received at least one dose of treatment and for whom study assessment data had been collected for a minimum of 4 days. b.i.d., twice daily; ITT, intention to treat.
Table 1. Patient demographics and baseline characteristics (safety analysis set).
| Demographic/characteristic | Placebo b.i.d. (n=90) |
Tenapanor b.i.d. |
Overall (n=356) | ||
|---|---|---|---|---|---|
| 5 mg (n=88) | 20 mg (n=89) | 50 mg (n=89) | |||
| Age, years | 46.0 (13.8) | 45.8 (12.7) | 45.3 (14.1) | 45.8 (12.2) | 45.7 (13.2) |
| <65 years, n (%) | 81 (90.0) | 84 (95.5) | 82 (92.1) | 84 (94.4) | 331 (93.0) |
| Sex,n(%) | |||||
| Women | 77 (85.6) | 76 (86.4) | 77 (86.5) | 79 (88.8) | 309 (86.8) |
| Race,n(%) | |||||
| White | 65 (72.2) | 69 (78.4) | 67 (75.3) | 69 (77.5) | 270 (75.8) |
| Black or African American | 22 (24.4) | 18 (20.5) | 19 (21.3) | 17 (19.1) | 76 (21.3) |
| Asian | 1 (1.1) | 0 (0.0) | 3 (3.4) | 2 (2.2) | 6 (1.7) |
| Body mass index, kg/m2 | 28.7 (5.6) | 29.5 (5.7) | 28.5 (5.3) | 28.3 (4.9) | 28.7 (5.4) |
| Duration of IBS-C symptoms before randomization, years | 14.1 (13.6) | 14.7 (13.4) | 12.5 (12.0) | 13.3 (12.7) | 13.7 (12.9) |
b.i.d., twice daily; IBS-C, constipation-predominant irritable bowel syndrome.
Data are shown as mean (s.d.) unless otherwise stated.
Table 2. Baseline IBS-C-related characteristics (intention to treat analysis set).
| Disease characteristic | Placebo b.i.d. (n=89) |
Tenapanor b.i.d. |
||
|---|---|---|---|---|
| 5 mg (n=87) | 20 mg (n=87) | 50 mg (n=84) | ||
| Abdominal paina | 6.1 (1.5) | 6.1 (1.6) | 6.3 (1.5) | 6.0 (1.5) |
| CSBMs/week | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) |
| SBMs/week | 2.0 (1.2) | 1.9 (1.3) | 1.9 (1.1) | 2.0 (1.3) |
| Stool consistencyb | 1.8 (1.0) | 1.8 (1.0) | 1.6 (0.8) | 1.8 (0.9) |
| Strainingc | 3.1 (1.2) | 3.1 (1.1) | 3.1 (1.3) | 3.2 (1.3) |
| IBS severityd | 3.8 (0.7) | 3.9 (0.7) | 3.9 (0.8) | 3.8 (0.7) |
| Constipation severityd | 4.1 (0.7) | 4.2 (0.6) | 4.0 (0.7) | 4.0 (0.8) |
b.i.d., twice daily; BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; IBS, irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; SBM, spontaneous bowel movement.
Assessed daily using a 0–10-point scale: 0=none to 10=very severe; average weekly score was calculated from scores for all days during a valid week.
Assessed using the 7-point BSFS (21); average weekly score calculated from scores for all SBMs during the week.
Assessed for each SBM using a 1–5-point scale: 1=not at all, 5=an extreme amount; average weekly score calculated from scores for all SBMs during the week.
Assessed weekly using a 1–5-point scale: 1=none, 5=very severe.
Data are shown as mean (s.d.) of the average of the weekly scores during the screening period for individual patients.
Efficacy analyses
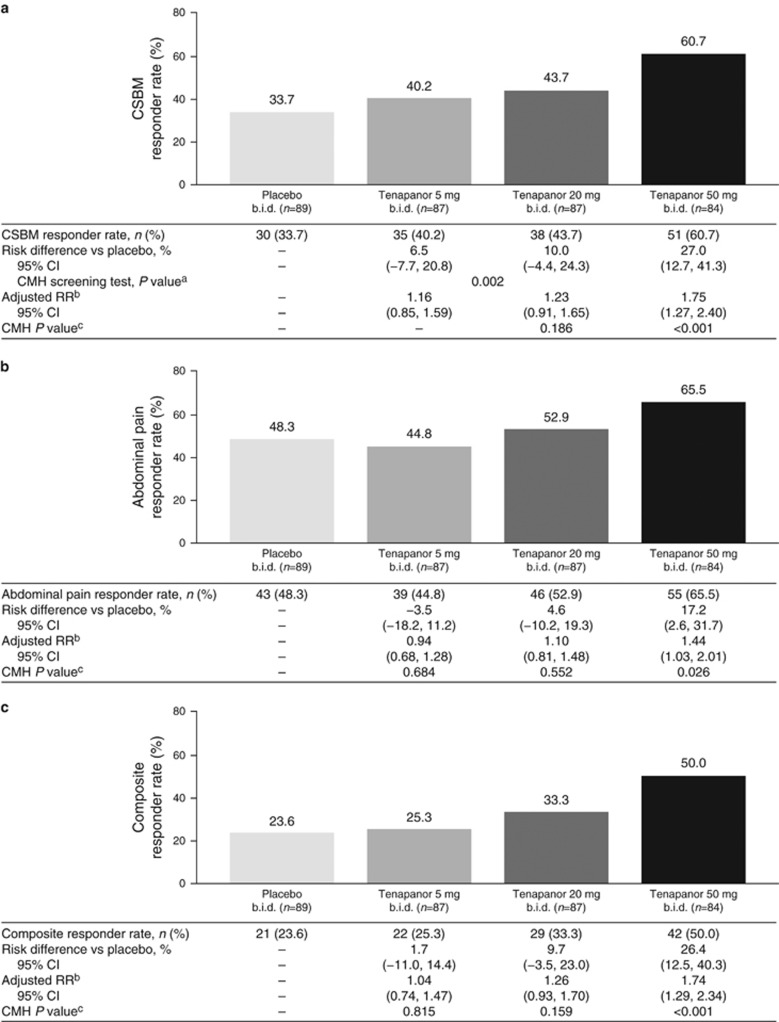
The CSBM responder rate was higher in all tenapanor treatment groups than in the placebo group (Figure2). For the tenapanor 50 mg b.i.d. treatment group, the CSBM responder rate was statistically significantly greater compared with the placebo group (60.7 vs. 33.7% P<0.001). Patients in the tenapanor 20 mg b.i.d. and 50 mg b.i.d. treatment groups both had greater abdominal pain responder rates (52.9 and 65.5%, respectively) than individuals given placebo (48.3%), with those in the tenapanor 50 mg b.i.d. group showing a statistically significant improvement compared with placebo-treated patients (P=0.026; Figure 2). The composite responder rate was greater in all tenapanor treatment groups than in the placebo group (Figure2), with the difference being statistically significant in the tenapanor 50 mg b.i.d. group (50.0 vs. 23.6% P<0.001).
Figure 2.
Responder rates: proportion of patients with: (a) an increase of at least one CSBM/week from baseline for at least 6 of 12 treatment weeks (CSBM responder rate—primary efficacy variable), (b) a decrease in abdominal pain of at least 30% from baseline for at least 6 out of 12 treatment weeks (abdominal pain responder rate), and (c) a decrease in abdominal pain of at least 30% and an increase of at least one CSBM/week (both in the same week) vs. baseline for at least 6 out of 12 treatment weeks (composite responder rate). aCMH screening test P value was based on a two degrees of freedom test for association between treatment (placebo, tenapanor 20 mg b.i.d., or tenapanor 50 mg b.i.d.) and responder rate, stratified by pooled investigator sites. bThe adjusted RR was based on the ratio of responder rates for placebo vs. each tenapanor treatment group, stratified by pooled investigator sites. cThe CMH P value was based on a one degree of freedom test for association between treatment and responder rate (placebo paired with each tenapanor treatment group separately), stratified by pooled investigator sites. b.i.d., twice daily; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; CSBM, complete spontaneous bowel movement; RR, relative risk.
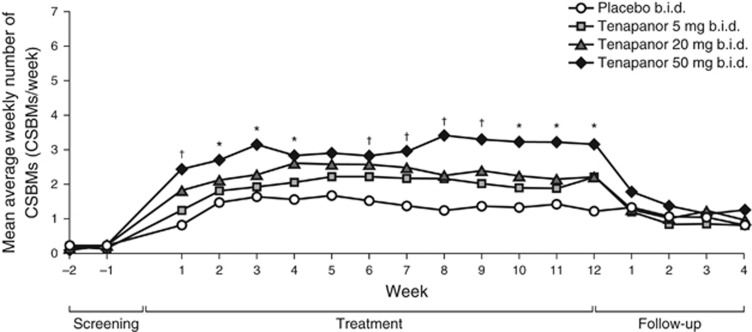
The mean average weekly numbers of CSBMs for all treatment groups are shown in Figure3. During the 12-week treatment period, an increase from baseline in the mean average weekly number of CSBMs was observed in all groups. At several time points over the course of treatment, patients given tenapanor 20 mg or 50 mg b.i.d. had statistically significantly higher mean average weekly numbers of CSBMs than those receiving placebo (P<0.050). Patients in the tenapanor 50 mg b.i.d. group achieved mean average weekly numbers of CSBMs of close to three for the majority of the 12-week treatment period, and over three for half of the 12 weeks, compared with a mean average weekly number of CSBMs of 0.2 at baseline.
Figure 3.
Mean average weekly number of CSBMs over time (intention to treat analysis set). *P<0.050, tenapanor 50 mg b.i.d. vs. placebo. †P<0.050, tenapanor 20 mg b.i.d. and tenapanor 50 mg b.i.d. vs. placebo. P values were based on an analysis of covariance model with treatment and pooled investigator site as factors and baseline value as a covariate. b.i.d., twice daily; CSBM, complete spontaneous bowel movement.
Abdominal symptom responder rates were greater in patients who received tenapanor 20 mg or 50 mg b.i.d. than in those who were given placebo. In the tenapanor 50 mg b.i.d. treatment group, responder rates were statistically significantly greater than those in the placebo group for abdominal discomfort (P=0.003), abdominal bloating (P=0.022), abdominal cramping (P=0.028), and abdominal fullness (P=0.010) (Table3).
Table 3. Abdominal symptom responder rates (intention to treat analysis set).
| Placebo b.i.d. (n=89) |
Tenapanor b.i.d. |
|||
|---|---|---|---|---|
| 5 mg (n=87) | 20 mg (n=87) | 50 mg (n=84) | ||
| Abdominal discomfort responder rate,n(%) | 38 (42.7) | 37 (42.5) | 48 (55.2) | 55 (65.5) |
| Risk difference | – | −0.2 | 12.5 | 22.8 |
| 95% CI | – | (−14.8, 14.4) | (−2.2, 27.1) | (8.3, 37.2) |
| Adjusted RR | – | 1.00 | 1.28 | 1.63 |
| 95% CI | – | (0.73, 1.37) | (0.95, 1.74) | (1.16, 2.28) |
| CMH P value | – | 0.995 | 0.102 | 0.003 |
| Abdominal bloating responder rate,n(%) | 37 (41.6) | 35 (40.2) | 41 (47.1) | 50 (59.5) |
| Risk difference | – | −1.3 | 5.6 | 18.0 |
| 95% CI | – | (−15.9, 13.2) | (−9.1, 20.2) | (3.3, 32.6) |
| Adjusted RR | – | 0.97 | 1.12 | 1.44 |
| 95% CI | – | (0.72, 1.32) | (0.83, 1.50) | (1.05, 1.98) |
| CMH P value | – | 0.864 | 0.467 | 0.022 |
| Abdominal cramping responder rate,n(%) | 41 (46.1) | 40 (46.0) | 47 (54.0) | 53 (63.1) |
| Risk difference | – | −0.1 | 8.0 | 17.0 |
| 95% CI | – | (−14.8, 14.6) | (−6.8, 22.7) | (2.4, 31.6) |
| Adjusted RR | – | 1.01 | 1.18 | 1.43 |
| 95% CI | – | (0.74, 1.37) | (0.87, 1.59) | (1.03, 1.98) |
| CMH P value | – | 0.961 | 0.292 | 0.028 |
| Abdominal fullness responder rate,n(%) | 34 (38.2) | 40 (46.0) | 42 (48.3) | 49 (58.3) |
| Risk difference | – | 7.8 | 10.1 | 20.1 |
| 95% CI | – | (−6.8, 22.3) | (−4.5, 24.6) | (5.5, 34.7) |
| Adjusted RR | – | 1.18 | 1.23 | 1.51 |
| 95% CI | – | (0.87, 1.61) | (0.91, 1.65) | (1.10, 2.08) |
| CMH P value | – | 0.279 | 0.181 | 0.010 |
b.i.d., twice daily; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; RR, relative risk.
Responder rates for abdominal symptoms were defined as the proportion of patients with a decrease of at least 30% from baseline in the average weekly severity score (0–10-point scale: 0=absent, 10=very severe) for at least 6 out of 12 treatment weeks. The adjusted RR was based on the ratio of responder rates for placebo vs. each tenapanor treatment group, stratified by pooled investigator sites. The CMH P value was based on a one degree of freedom test for association between treatment and responder rate (placebo paired with each tenapanor treatment group separately), stratified by pooled investigator sites.
Patients receiving tenapanor 20 mg or 50 mg b.i.d. had greater, statistically significant, improvements over those given placebo for CSBM frequency (P<0.050), stool consistency (P<0.001), straining (P<0.050), and treatment satisfaction (P<0.050; Table 4). Patients receiving tenapanor 50 mg b.i.d. also experienced statistically significant improvements compared with individuals receiving placebo for abdominal pain severity (P=0.014), number of SBMs/week (P=0.006), IBS severity (P=0.024), constipation severity (P<0.001), and degree of relief from IBS (P<0.001; Table 4).
Table 4 Table 4. Other study assessment results (intention to treat analysis set)Continued.
| Placebo b.i.d. (n=89) |
Tenapanor b.i.d. |
|||
| 5 mg (n=87) | 20 mg (n=87) | 5 mg (n=87) | 20 mg (n=87) | |
| Abdominal pain severity score a | ||||
| Change from baseline at week 12 end point, LS mean | −37.0 | −37.6 | −38.9 | −50.4 |
| 95% CI | (−45.1, −28.9) | (−45.6, −29.7) | (−47.0, −30.8) | (−58.8, −42.1) |
| Difference tenapanor vs. placebo, LS mean | – | −0.6 | −1.9 | −13.4 |
| 95% CI | – | (−11.2, 10.0) | (−12.5, 8.7) | (−24.1, −2.8) |
| P value | – | 0.910 | 0.724 | 0.014 |
| CSBMs/week | ||||
| Change from baseline at week 12 end point, LS mean | 0.9 | 1.7 | 2.2 | 2.7 |
| 95% CI | (0.2, 1.7) | (1.0, 2.4) | (1.4, 2.9) | (2.0, 3.5) |
| Difference tenapanor vs. placebo, LS mean | – | 0.8 | 1.2 | 1.8 |
| 95% CI | – | (−0.2, 1.7) | (0.3, 2.2) | (0.8, 2.8) |
| P value | – | 0.115 | 0.012 | <0.001 |
| SBMs/week | ||||
| Change from baseline at week 12 end point, LS mean | 1.6 | 2.4 | 2.6 | 3.4 |
| 95% CI | (0.6, 2.5) | (1.5, 3.4) | (1.7, 3.6) | (2.4, 4.4) |
| Difference tenapanor vs. placebo, LS mean | – | 0.9 | 1.1 | 1.8 |
| 95% CI | – | (−0.4, 2.1) | (−0.2, 2.3) | (0.5, 3.1) |
| P value | – | 0.187 | 0.095 | 0.006 |
| Stool consistencyb | ||||
| Change from baseline at week 12 end point, LS mean | 1.0 | 1.6 | 1.9 | 2.2 |
| 95% CI | (0.6, 1.4) | (1.2, 2.0) | (1.5, 2.3) | (1.8, 2.6) |
| Difference tenapanor vs. placebo, LS mean | – | 0.6 | 0.9 | 1.2 |
| 95% CI | – | (0.1, 1.1) | (0.4, 1.4) | (0.7, 1.7) |
| P value | – | 0.027 | <0.001 | <0.001 |
| Straining c | ||||
| Change from baseline at week 12 end point, LS mean | −0.7 | −0.8 | −1.1 | −1.2 |
| 95% CI | (−0.9, −0.4) | (−1.0, −0.5) | (−1.3, −0.8) | (−1.4, −0.9) |
| Difference tenapanor vs. placebo, LS mean | – | −0.1 | −0.4 | −0.5 |
| 95% CI | – | (−0.4, 0.3) | (−0.8, −0.1) | (−0.8, −0.1) |
| P value | – | 0.584 | 0.020 | 0.006 |
| IBS severity d | ||||
| Change from baseline at week 12 end point, LS mean | −1.1 | −1.0 | −1.1 | −1.4 |
| 95% CI | (−1.3, −0.9) | (−1.3, −0.8) | (−1.3, −0.8) | (−1.7, −1.2) |
| Difference tenapanor vs. placebo, LS mean | – | 0.1 | 0.0 | −0.3 |
| 95% CI | – | (−0.2, 0.4) | (−0.3, 0.3) | (−0.6, −0.0) |
| P value | – | 0.689 | 0.824 | 0.024 |
| Constipation severity d | ||||
| Change from baseline at week 12 end point, LS mean | −1.1 | −1.3 | −1.3 | −1.7 |
| 95% CI | (−1.4, −0.9) | (−1.5, −1.1) | (−1.5, −1.1) | (−1.9, −1.4) |
| Difference tenapanor vs. placebo, LS mean | – | −0.2 | −0.2 | −0.5 |
| 95% CI | – | (−0.5, 0.1) | (−0.5, 0.1) | (−0.8, −0.2) |
| P value | – | 0.233 | 0.299 | <0.001 |
| Degree of relief from IBS e | ||||
| Week 12 end point, LS mean | 3.1 | 2.9 | 3.0 | 2.5 |
| 95% CI | (2.8, 3.4) | (2.7, 3.2) | (2.7, 3.2) | (2.2, 2.8) |
| Difference tenapanor vs. placebo, LS mean | – | −0.2 | −0.1 | −0.6 |
| 95% CI | – | (−0.5, 0.2) | (−0.5, 0.2) | (−0.9, −0.3) |
| P value | – | 0.340 | 0.436 | <0.001 |
| Treatment satisfaction f | ||||
| Week 12 end point, LS mean | 2.9 | 3.1 | 3.3 | 3.7 |
| 95% CI | (2.6, 3.2) | (2.8, 3.4) | (3.0, 3.6) | (3.4, 4.0) |
| Difference tenapanor vs. placebo, LS mean | – | 0.3 | 0.4 | 0.8 |
| 95% CI | – | (−0.1, 0.7) | (0.0, 0.8) | (0.4, 1.2) |
| P value | – | 0.181 | 0.031 | <0.001 |
ANCOVA, analysis of covariance; ANOVA, analysis of variance; b.i.d., twice daily; BSFS, Bristol Stool Form Scale; CI, confidence interval; CSBM, complete spontaneous bowel movement; IBS, irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; LS, least-squares; SBM, spontaneous bowel movement.
Assessed daily using a 0–10-point scale: 0=none, 10=very severe. Average weekly score was calculated from scores for all days during a valid week.
Assessed using the 7-point BSFS (21). Average weekly score calculated from scores for all SBMs during the week.
Assessed for each SBM using a 1–5-point scale: 1=not at all, 5=an extreme amount. Average weekly score calculated from scores for all SBMs during the week.
Assessed weekly using a 1–5-point scale: 1=none, 5=very severe.
Assessed weekly on a 1–7-point scale: 1=complete relief, 7=as bad as I can imagine.
Assessed using a 1–5-point scale: 1=not at all satisfied, 5=very satisfied.
LS means, 95% CIs, and P values were based on an ANCOVA model with treatment and pooled investigator site as factors and baseline as a covariate. Baseline was defined as the average of the respective scores for weeks −1 and −2.
For degree of relief from IBS and treatment satisfaction, LS means, 95% CIs, and P values were based on an ANOVA model with treatment and pooled investigator site as terms.
Compliance and use of rescue medication
Mean compliance to study treatment was >97% in all treatment groups (intention to treat analysis set). In each of the four arms, the majority (≥89%) of patients were more than 80% compliant. The proportion of patients requiring rescue medication during the treatment period was similar across the tenapanor groups (5 mg b.i.d., 28.7% 20 mg b.i.d., 28.7% and 50 mg b.i.d., 26.2%) and slightly higher in patients taking placebo (37.1%).
Safety analyses
Table5 gives an overview of the AEs that occurred during the study. Of those AEs occurring in at least 3% of patients in any tenapanor group and at a higher frequency than in the placebo group, the most frequently reported were diarrhea, headache, nausea, urinary tract infection, and abdominal pain. Treatment-related AEs potentially relating to dehydration were rare in the study, with one patient reporting dry mouth in the placebo group and one patient reporting thirst in the tenapanor 50 mg b.i.d. group. Three patients receiving tenapanor reported serious AEs (laryngeal neoplasm and urinary tract infection (5 mg b.i.d. group) and small intestinal obstruction (20 mg b.i.d. group)), and one patient in the placebo group reported a serious AE of osteomyelitis. None of the serious AEs were considered by the study site investigator to be related to study drug treatment and no serious AEs were reported by patients receiving tenapanor 50 mg b.i.d. Treatment-related diarrhea occurred in 24 (9%) of all tenapanor-treated patients (n=266), leading to study-drug discontinuation in nine patients, three (3%) in each of the tenapanor treatment groups. Three patients who received tenapanor 5 mg b.i.d. reported treatment-related abdominal distension that led to study drug discontinuation. No other AEs led to discontinuation in more than two patients in any treatment group. No deaths occurred over the course of this study and no clinically meaningful changes from baseline were observed in vital signs, clinical laboratory tests, physical examinations, and 12-lead electrocardiograms. Increases from baseline to week 12 in serum phosphorus concentrations from normal to above normal limits were observed in three patients receiving tenapanor (50 mg b.i.d., n=2; 20 mg b.i.d, n=1) and three patients receiving placebo. Decreases in serum phosphorus concentrations from baseline to week 12 from normal to below normal limits were observed in two patients receiving tenapanor (50 mg b.i.d., n=1; 5 mg b.i.d., n=1) and one patient receiving placebo. Decreases in serum sodium concentrations from normal to below normal limits were observed in two patients receiving tenapanor 50 mg b.i.d. These changes in serum phosphorus and sodium were not considered to be clinically meaningful, and none were reported as AEs.
Table 5. Overview of treatment emergent adverse events (safety analysis set).
| AEs, n (%) | Placebo b.i.d. (n=90) |
Tenapanor b.i.d. |
||
|---|---|---|---|---|
| 5 mg (n=88) | 20 mg (n=89) | 50 mg (n=89) | ||
| Any AE | 38 (42.2) | 43 (48.9) | 32 (36.0) | 45 (50.6) |
| Treatment-related AEs | 13 (14.4) | 22 (25.0) | 15 (16.9) | 17 (19.1) |
| Serious AEs | 1 (1.1) | 2 (2.3) | 1 (1.1) | 0 (0.0) |
| Deaths | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AEs leading to study drug discontinuation | 3 (3.3) | 9 (10.2) | 6 (6.7) | 4 (4.5) |
| AEs by preferred term a | ||||
| Diarrhea | 0 (0.0) | 7 (8.0) | 11 (12.4) | 10 (11.2) |
| Nausea | 1 (1.1) | 6 (6.8) | 4 (4.5) | 3 (3.4) |
| Abdominal pain | 2 (2.2) | 7 (8.0) | 0 (0.0) | 4 (4.5) |
| Headache | 5 (5.6) | 6 (6.8) | 1 (1.1) | 3 (3.4) |
| Urinary tract infection | 4 (4.4) | 3 (3.4) | 2 (2.2) | 5 (5.6) |
| Vomiting | 0 (0.0) | 4 (4.5) | 1 (1.1) | 2 (2.2) |
| Gastroesophageal reflux disease | 1 (1.1) | 3 (3.4) | 0 (0.0) | 1 (1.1) |
| Abdominal distension | 0 (0.0) | 3 (3.4) | 1 (1.1) | 0 (0.0) |
| Treatment-related AEs by preferred terma | ||||
| Diarrhea | 0 (0.0) | 7 (8.0) | 9 (10.1) | 8 (9.0) |
| Nausea | 0 (0.0) | 3 (3.4) | 2 (2.2) | 2 (2.2) |
| Abdominal pain | 2 (2.2) | 5 (5.7) | 0 (0.0) | 1 (1.1) |
| Headache | 3 (3.3) | 3 (3.4) | 1 (1.1) | 3 (3.4) |
| Abdominal distension | 0 (0.0) | 3 (3.4) | 1 (1.1) | 0 (0.0) |
AE, adverse event; b.i.d., twice daily.
AEs by preferred tersm occurring in at least 3% of patients in any tenapanor treatment group and at a higher frequency than in the placebo group.
A total of 291 blood samples from patients in the three tenapanor treatment groups were analyzed to determine tenapanor plasma concentrations. Tenapanor levels were above the quantifiable limit of 0.5 ng/ml in only eight samples (range: 0.547–1.03 ng/ml) taken from a total of six individuals in the tenapanor 50 mg b.i.d. group at either week 8 or week 12.
Discussion
In this methodologically rigorous, randomized, placebo-controlled, phase 2 study, tenapanor 50 mg b.i.d. proved to be significantly more effective than placebo at improving symptoms of IBS-C including bowel habits (stool frequency (CSBMs, the primary efficacy variable, and SBMs), stool consistency (BSFS) and straining), abdominal symptoms (pain, discomfort, bloating, cramping, and fullness), as well as a composite end point of increased CSBM frequency and reduced abdominal pain. In addition, tenapanor 50 mg b.i.d. significantly improved global patient-reported treatment measurements compared with placebo including IBS severity, constipation severity, degree of relief from IBS and treatment satisfaction. Benefits were seen during the first week of assessments after therapy had been initiated and were sustained over the 12-week treatment period. Patients receiving tenapanor 50 mg b.i.d. achieved a mean average of ~3 CSBMs/week for the majority of treatment weeks, exceeding 3 CSBMs/week for half of the 12 weeks. Notably, data from a population-based study suggests that a frequency of 3 bowel movements/week to 3 bowel movements/day in adults is ‘normal' (24). Patients in the tenapanor 20 mg b.i.d. and 5 mg b.i.d. dose groups did not achieve statistically significant improvements in CSBM frequency or the composite end point compared with placebo, though trends toward improvements were observed for the 20 mg b.i.d. dose.
Tenapanor is a first-in-class inhibitor of sodium/hydrogen exchanger isoform 3, the predominant intestinal sodium transporter (25), and thereby tenapanor reduces sodium uptake in the gut. This unique mechanism of action leads to increased intestinal fluid volume and shorter transit time (15, 16), which accounts for the softening of stool consistency and increased frequency of bowel movements observed in patients with IBS-C in our study. Tenapanor treatment also led to robust improvements in one of the key symptoms of IBS-C, abdominal pain. It is possible that improvements in constipation with secondary ‘unloading' of the colon may have contributed to the improvements in abdominal pain observed with tenapanor. However, not all drugs that improve constipation also improve abdominal pain. For example, in a 4-week study, polyethylene glycol treatment provided significant increases over placebo in the number of spontaneous bowel movements, without any improvement over placebo in abdominal pain (26). Tenapanor may have direct effects on visceral hypersensitivity, which can be present in patients with IBS-C. Similar to animal studies of linaclotide (27), tenapanor significantly reduced stress-induced colorectal hypersensitivity to mechanical pain stimuli in a rat model (17). The mechanism responsible for this effect remains to be fully elucidated, but these findings support the possibility that tenapanor may benefit pain through effects on visceral hypersensitivity. Further studies to better understand the anti-nociceptive effects of tenapanor are encouraged.
A desire to mitigate the risk of serious systemic drug side effects has fueled a recent trend in developing luminally acting therapeutic agents, and the development of tenapanor extends this trend. Given the minimal systemic availability of tenapanor, as supported by the pharmacokinetic data generated in this study, and its pharmacodynamic effect on sodium/fluid transit in the gastrointestinal tract, it is hardly surprising that the most commonly reported treatment-related AE during the study was diarrhea, occurring in 9% of all tenapanor-treated patients, and causing 3% (n=3) of individuals in each tenapanor group to discontinue treatment. Otherwise, tenapanor appeared to have a generally acceptable safety profile overall. No serious treatment-related AEs or deaths were reported in any of the study groups. There were no clinically meaningful changes from baseline in laboratory parameters, vital signs, physical examinations, and 12-lead electrocardiograms in the study. Given the pharmacodynamic effect of tenapanor in reducing absorption of not only sodium, but also of phosphate from the gut, it is noteworthy that there were no clinically meaningful changes from baseline in serum sodium and phosphorus observed in patients with IBS-C in our study. The study was conducted in an outpatient setting, with no dietary restrictions. Therefore, in this IBS-C patient population, it is possible that dietary intake of sodium and phosphate was adequate to compensate for any impact that reductions in sodium and phosphate absorption with tenapanor may have on serum concentrations of the electrolytes. In addition the body's own complex mechanisms for maintaining sodium and phosphate balance, with factors such as renal handling and body storage, may have been involved.
Given the pharmacodynamic effects of tenapanor on gastrointestinal sodium and phosphate absorption, the agent has also been evaluated in renal-related conditions. In two phase 2 studies, one in patients with chronic kidney disease stage 5D (hemodialysis) and fluid overload (28), and another in patients with type 2 diabetes mellitus and chronic kidney disease stage 3 (29), the pharmacodynamic effects of tenapanor on stool sodium and urinary sodium and phosphorus were confirmed. These observations did not translate into clinically meaningful effects on interdialytic weight gain (28) or albuminuria (a marker of renal function decline) (29). However, results from a 4-week study in patients with hyperphosphatemia in chronic kidney disease stage 5D (hemodialysis), show that the effects of tenapanor on phosphate absorption translate into clinically meaningful reductions in serum phosphate levels vs. placebo (30).
In summary, treatment with tenapanor 50 mg b.i.d. significantly improved key IBS-C symptoms, had positive effects on global IBS-treatment measures, and was generally well-tolerated. The data from this phase 2 study justify the conduct of phase 3 studies (T3MPO-1 and T3MPO-2), which are currently under way (31, 32).
Study Highlights
Acknowledgments
We thank the study participants, the study centers, and the clinical teams. Clinical operations were managed by Susan Edelstein, Lori Marshall, and Jocelyn Tabora from Ardelyx. Andrew Yan from Ardelyx reviewed the manuscript critically for statistical content. Medical writing support was provided by Laura Schmidt MPhil MRes, Michael Molloy-Bland PhD, and Steven Inglis PhD of PharmaGenesis London, London, UK and was funded by Ardelyx.
Footnotes
Guarantor of the article: William D. Chey, MD, AGAF, FACG, FACP, RFF.
Specific author contributions: William D. Chey and Anthony J. Lembo contributed to the planning of the study, interpretation of the data and critical revision of the manuscript for important intellectual content; David P. Rosenbaum contributed to the planning of the study, conduct of the study, interpretation of the data, and critical revision of the manuscript for important intellectual content; all authors approved the final version of the manuscript for submission.
Financial support: Ardelyx was the sponsor of the study. The study was funded by Ardelyx and AstraZeneca.
Potential competing interests: William D. Chey is a consultant for Albireo, Allergan, Ardelyx, IM Health, Ironwood, Nestle, Prometheus, QOL Medical, Valeant, and has received research funding from Ardelyx, Ironwood, Nestle, QOL Medical. Anthony J. Lembo is a consultant for Ardelyx, Ironwood, Allergan, Valeant, and Prometheus, and has received research funding from Prometheus. David P. Rosenbaum is an employee of, and has ownership interest in, Ardelyx, Inc.
References
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–958. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Camilleri M, Mayer EA et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–2131. [DOI] [PubMed] [Google Scholar]
- Pare P, Gray J, Lam S et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 2006;28:1726–1735. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med 2008;358:1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L, Kleinman L, Rentz A et al. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin Ther 2002;24:675–689 discussion 674. [DOI] [PubMed] [Google Scholar]
- Gralnek IM, Hays RD, Kilbourne A et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–660. [DOI] [PubMed] [Google Scholar]
- Pace F, Molteni P, Bollani S et al. Inflammatory bowel disease versus irritable bowel syndrome: a hospital-based, case-control study of disease impact on quality of life. Scand J Gastroenterol 2003;38:1031–1038. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Chey WD, Lembo AJ. New and emerging treatment options for irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2015;11:1–19. [PMC free article] [PubMed] [Google Scholar]
- Lembo A. Irritable bowel syndrome medications side effects survey. J Clin Gastroenterol 2004;38:776–781. [DOI] [PubMed] [Google Scholar]
- Quigley EM, Tack J, Chey WD et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C—a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 2013;37:49–61. [DOI] [PubMed] [Google Scholar]
- Chey WD, Lembo AJ, Lavins BJ et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012;107:1702–1712. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Chey WD, Johanson JF et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome-results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther 2009;29:329–341. [DOI] [PubMed] [Google Scholar]
- Spencer AG, Labonte ED, Rosenbaum DP et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 2014;6:227.ra36. [DOI] [PubMed] [Google Scholar]
- Johansson S, Rosenbaum DP, Knutsson M et al. A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol 2016. doi:10.1007/s10157-016-1302-8. [DOI] [PMC free article] [PubMed]
- Eutamene H, Charmot D, Navre M et al. Visceral antinociceptive effects of RDX5791, a first-in-class minimally systemic NHE3 inhibitor on stress-induced colorectal hypersensitivity to distension in rats. Gastroenterology 2011;140:S-57–58 (abstract). [Google Scholar]
- Ardelyx. A study to evaluate the safety and efficacy of RDX5791 for the treatment of constipation predominant irritable bowel syndrome (IBS-C). In: ClinicalTrials.gov [Internet]. National Library of Medicine (US): Bethesda, MD, USA. 2000. Available at https://clinicaltrials.gov/ct2/show/study/NCT01340053 Accessed on 17 Aug 2016 NLM Identifier: NCT01340053. [Google Scholar]
- Johansson S, Spencer AG, Jacobs JW et alThe safety, tolerability, pharmacodynamics and pharmacokinetics of tenapanor, a minimally absorbed compound inhibiting intestinal sodium uptake in healthy subjects. Poster Presented at the 7th Congress of the International Society for Hemodialysis, 25–27 April 2014, Ginowan City, Japan.
- Rome Foundation. Guidelines-Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointest Liver Dis 2006;15:307–312. [PubMed] [Google Scholar]
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–924. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Guidance for industry: irritable bowel syndrome—clinical evaluation of drugs for treatment (May 2012). Available at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/UCM205269.pdf Accessed on 24 May 2015.
- Center for Drug Evaluation and Research Linzess (Linaclotide). Statistical Review(s). Application number: 202811Orig1s000. NDA/BLA: 202-811. Received on 9 August 2011. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202811Orig1s000StatR.pdf Accessed 30 November 2016.
- Walter SA, Kjellstrom L, Nyhlin H et al. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol 2010;45:556–566. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 1998;19:282–285. [DOI] [PubMed] [Google Scholar]
- Chapman RW, Stanghellini V, Geraint M et al. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol 2013;108:1508–1515. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Bradesi S, Larauche M et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 2010;22:312–e84. [DOI] [PubMed] [Google Scholar]
- Block GA, Rosenbaum DP, Leonsson-Zachrisson M et al. Effect of tenapanor on interdialytic weight gain in patients on hemodialysis. Clin J Am Soc Nephrol 2016;11:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson BV, Rosenbaum DP, Greasley PJ et al. A phase 2 study on the effect of tenapanor on albuminuria in patients with T2DM and CKD. J Am Soc Nephrol 2015;26:239A.25183808 [Google Scholar]
- Block GA, Rosenbaum DP, Leonsson-Zachrisson M et al. Effect of Tenapanor on Serum Phosphate in Patients Receiving Hemodialysis. J Am Soc Nephrol 2017. doi:10.1681/ASN.2016080855. [DOI] [PMC free article] [PubMed]
- Ardelyx. A 12-week study with a 4-week randomized withdrawal period to evaluate the efficacy and safety of tenapanor for the treatment of IBS-C (T3MPO-1). In: ClinicalTrials.gov [Internet]. National Library of Medicine (US): Bethesda, MD, USA. 2000. Available at https://clinicaltrials.gov/ct2/show/NCT02621892 Accessed on 27 Jun 2016 NLM Identifier: NCT02621892. [Google Scholar]
- Ardelyx. A 26-week study to evaluate the efficacy and safety of tenapanor in IBS-C (T3MPO-2). In: ClinicalTrials.gov [Internet]. National Library of Medicine (US): Bethesda, MD, USA. 2000. Available at https://clinicaltrials.gov/ct2/show/NCT02686138 Accessed on 27 June 2016 NLM Identifier: NCT02686138. [Google Scholar]