Abstract
OBJECTIVES:
Non-invasive fibrosis scores are widely used to identify/exclude advanced fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). However, these scores were principally developed and validated in patients aged between 35 and 65 years of age. The objective of this study was to assess the effect of age on the performance of non-invasive fibrosis tests in NAFLD.
METHODS:
Patients were recruited from European specialist hepatology clinics. The cohort was divided into five age-based groups: ≤35 (n=74), 36–45 (n=96), 46–55 (n=197), 56–64 (n=191), and ≥65 years (n=76), and the performance of the aspartate aminotransferase (AST)/alanine transaminase (ALT) ratio, fibrosis 4 (FIB-4), and NAFLD fibrosis score (NFS) for advanced fibrosis (stage F3–F4) for each group was assessed using liver biopsy as the standard.
RESULTS:
Six hundred and thirty-four patients were included. The diagnostic accuracy of the AST/ALT ratio was lower than NFS and FIB-4 in all the age groups. The AST/ALT ratio, NFS, and FIB-4 score performed poorly for a diagnosis of advanced fibrosis in those aged ≤35 years (area under the receiver operating characteristic curves (AUROCs 0.52, 0.52, and 0.60, respectively). For all groups >35 years, AUROCs for advanced fibrosis were similar for the NFS and FIB-4 score (range 0.77–0.84). However, the specificity for advanced fibrosis using the FIB-4 and NFS declined with age, becoming unacceptably low in those aged ≥65 years (35% for FIB-4 and 20% for NFS). New cutoffs were derived (and validated) for those aged ≥65 years, which improved specificity to 70% without adversely affecting sensitivity (FIB-4 2.0, sensitivity 77% NFS 0.12, sensitivity 80%).
CONCLUSIONS:
The NFS and FIB-4 scores have similar accuracy for advanced fibrosis in patients aged >35 years. However, the specificity for advanced fibrosis is unacceptably low in patients aged ≥65 years, resulting in a high false positive rate. New thresholds for use in patients aged ≥65 years are proposed to address this issue.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in many developed countries and now affects 20–30% of the population, although many affected individuals go unrecognized (1, 2, 3, 4). NAFLD has a variable prognosis, with the majority of patients having benign disease without associated liver-related morbidity or mortality (5). However, approximately 40% of patients with NAFLD develop progressive fibrosis that can result in cirrhosis, putting patients at risk of hepatocellular carcinoma, liver failure, and portal hypertension-related complications (6, 7, 8, 9, 10). The development of advanced fibrosis (stage F3–F4) in patients with NAFLD is clinically important as it is associated with a more than threefold increased risk of mortality (all cause and liver related) compared with a reference population (11, 12). It is therefore vital to identify patients with advanced fibrosis so that appropriate lifestyle interventions, treatment, or surveillance for complications can be initiated.
Numerous non-invasive tests for liver fibrosis have been investigated in patients with NAFLD, but to date, none have the accuracy to completely replace liver biopsy (13, 14). Simple non-invasive fibrosis scores derived from routine clinical and biochemical indices, such as the aspartate aminotransferase (AST)/alanine transaminase (ALT) ratio, fibrosis 4 (FIB-4) score and NAFLD fibrosis score (NFS) have shown promise, particularly for excluding advanced fibrosis, and are therefore useful tools to risk stratify patients with NAFLD for further investigation (15, 16, 17, 18, 19, 20). As a result, these non-invasive scores are now widely used “first line” in primary and secondary care to assess fibrosis in patients with abnormal liver enzymes and suspected NAFLD (21). Moreover, a recent Lancet Commission addressing liver disease has recommended that simple non-invasive scores be used to help identify patients with advanced fibrosis in the community (22).
With the increasing use of simple non-invasive fibrosis markers to identify patients with advanced liver disease, it is important to recognize and mitigate against any limitations of these tests. The majority of patients included in the studies on which these non-invasive scores were developed were aged between 35 and 65 years, so there is potential for reduced efficacy of these tests in patients outwith this age distribution. The aim of this study was to assess whether age affects the diagnostic performance of the AST/ALT ratio, FIB-4 score, and NFS for advanced fibrosis in a cohort of patients with biopsy-proven NAFLD. In particular, our aim was to assess the efficacy of these non-invasive scores in younger (≤35 years) and older patients (≥65 years) with NAFLD and if necessary to derive specific cutoffs for use in those patients.
METHODS
This study included consecutive patients with biopsy-proven NAFLD who attended specialist fatty liver clinics at the Freeman Hospital, Newcastle upon Tyne, UK; Addenbrooke's Hospital, Cambridge, UK; Antwerp University Hospital, Edegem, Belgium; and Pitié-Salpêtrière Hospital, Paris, France. These formed the initial ascertainment cohort. Liver biopsies were conducted as per routine clinical care for the investigation of abnormal liver function tests (raised ALT, AST, or gamma-glutamyl transferase) or to stage disease severity in patients with radiological evidence of fatty liver. Clinical and laboratory data were collected prospectively from the time of liver biopsy. Patients with evidence of other liver disease (autoimmune hepatitis, viral hepatitis, drug induced liver injury, hemochromatosis, cholestatic liver disease, or Wilson's disease) were excluded. In addition, subjects consuming excessive amounts of alcohol (alcohol intake >20 g/day for women; >30 g/day for men) at the time of biopsy or in the past were excluded. Patients with incomplete data to calculate all the non-invasive scores were excluded.
Relevant clinical details, including gender, age, weight, and height, were obtained at the time of biopsy. The body mass index was calculated by the formula: weight (kg)/height (m)2. Patients were identified as having diabetes if they had been diagnosed with diabetes according to the 2004 American Diabetes Association criteria or if they were taking an oral hypoglycemic drug or insulin (23).
Percutaneous liver biopsies were performed as per unit protocol at the sites and were assessed by an experienced local hepatopathologist. Patients with liver biopsies specimens <15 mm in length were excluded. Histological scoring was performed according to the non-alcoholic steatohepatitis (NASH) Clinical Research Network criteria (24). The NAFLD activity score was graded from 0 to 8, including scores for steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2). NASH was defined as steatosis with hepatocyte ballooning and inflammation ± fibrosis (25). Fibrosis was staged from F0 to F4 (24). Patients with stage F3 or F4 fibrosis were considered to have advanced fibrosis.
The AST/ALT ratio, FIB-4, and NFS were calculated from blood tests taken at the time of liver biopsy as previously described (16, 26, 27). Details of the formulas and cutoffs for the tests under investigation are shown in Table 1. Previously published cutoffs were used to exclude and diagnose advanced fibrosis for each score (15, 16, 18, 19)
Table 1. An overview of the simple non-invasive fibrosis markers under investigation (15, 16, 18, 19, 26, 27).
| Test | Calculation method | Lower cutoff | Upper cutoff |
|---|---|---|---|
| AST/ALT ratio | AST/ALT | 0.8 | 1 |
| FIB-4 score | Age × AST (IU/l)/platelet count (× 109/l) × √ALT (IU/l) | 1.3 | 2.67 |
| NAFLD fibrosis score (NFS) | −1.675+0.037 × age (years)+0.094 × BMI (kg/m2)+1.13 × impaired fasting glycemia (IFG) or diabetes (yes=1, no=0)+0.99 × AST/ALT ratio −0.013 × platelet (× 109/l)−0.66 × albumin (g/dl) | −1.455 | 0.676 |
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body masss index; FIB-4, fibrosis 4; NAFLD, non-alcoholic fatty liver disease. A score below the lower cutoff is used to exclude advanced fibrosis (stage F3–F4) with reasonable accuracy and a score above the upper cutoff is suggestive of the presence of advanced fibrosis in patients with NAFLD.
To validate new cutoffs for the NFS and FIB-4 score optimized for use in older patients (aged ≥65 years) that were derived in the initial ascertainment cohort, anonymized biochemical, histological, and anthropometric data were collected from a separate group of histologically characterized patients from the EPoS/EASL European NAFLD Registry. The “European NAFLD Registry” was established during the EU FP7 FLIP project (2010-) and is now maintained by the EU H2020 EPoS (Elucidating Pathways of Steatohepatitis) consortium to facilitate collaborative research into NAFLD. It is the largest multi-national registry of patients with histologically characterized NAFLD. These patients had data collected according to the same methodology as the main cohort.
All statistical analyses were performed using the SPSS software version 22.0 (SPSS, Chicago, IL). Continuous normally distributed variables were represented as mean ± s.d. Categorical and non-normal variables were summarized as median and range. Chi squared tests were used to determine the distribution of categorical variables between groups. To compare the means of normally distributed variables between groups, the Student's t-test or analysis of variance test was performed. To determine differences between groups for continuous non-normally distributed variables, medians were compared using the Mann–Whitney U-test. The diagnostic performance of the non-invasive tests was assessed by receiver operating characteristic (ROC) curve analysis. The area under the ROC (AUROC) was used as an index to compare the accuracy of tests. The sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs), positive likelihood ratios (LR +ve), and negative likelihood ratios (LR −ve) for relevant cutoffs were also displayed. In order to assess changes in sensitivity and specificity of the tests with age, plots of sensitivity and specificity in different age groups were displayed graphically. New cutoffs for the FIB-4 and NFS were derived for ≥65-year-old patients by taking the point on the ROC where the combined value of sensitivity and specificity was the highest. As the prevalence of advanced fibrosis can vary in different populations, the PPVs and NPVs for the new cutoffs were displayed at advanced fibrosis prevalence rates of 5, 10, 20, 30, and 40%. A P value of <0.05 was considered significant.
RESULTS
Characteristics of the whole cohort
A total of 634 patients with biopsy-proven NAFLD were included in the study, 369 from Newcastle, 138 from Antwerp, 99 from Paris, and 28 from Cambridge. Overall, 347 (55%) patients were male. Overall, the indication for liver biopsy was the assessment of “raised” transaminases (serum ALT or AST >40 IU/ml) in 464 (73%) patients and for the staging of disease in 170 (27%) patients with imaging evidence of steatosis and “normal” transaminase levels. The mean age was 51±12 years. Forty-three percent of the cohort were diabetic and the mean body mass index was 34.5 ± 6 kg/m2. In total, 461 (73%) patients had histological evidence of NASH and the median NAFLD activity score was 4 (1, 2, 3, 4, 5, 6, 7, 8). Overall, the median fibrosis stage was F1 (F0–F4); 164 patients (25%) had advanced liver fibrosis (stage F3–F4).
Effect of age on clinical factors in patients with NAFLD
In order to assess the effect of age on clinical accuracy of simple non-invasive scores for NAFLD fibrosis, the cohort was stratified by age into five groups: ≤35, 36–45, 46–55, 56–64, and ≥65 years, respectively. Table 2 displays the clinical, biochemical, and histological features of patients in each of these age groups. Overall, increasing age was significantly associated with female gender (P<0.001) and the presence of Type 2 diabetes (P<0.001) and NASH (P=0.001), as well as increased fibrosis stage (P<0.001), AST/ALT ratio (P<0.001), NFS (P<0.001), and FIB-4 score (P<0.001). In addition, there was a significant negative association between age and platelet count (P<0.001), serum ALT (P<0.001), and serum albumin (P<0.001)
Table 2. Effect of age on clinical, biochemical, and histological features in patients with NAFLD.
| Characteristics | ≤35 years, n=74 | 36–45 years, n=96 | 46–55 years, n=197 | 56–64 years, n=191 | ≥65 years, n=76 | P value |
|---|---|---|---|---|---|---|
| Age (years) | 28±5 | 41±3 | 51±3 | 60±3 | 69±3 | |
| Gender (% of male) | 72% | 64% | 57% | 50% | 32% | <0.001a |
| BMI (kg/m2) | 34.9±6.0 | 35.5±6.9 | 34.8±6.2 | 34±5.2 | 33.6±6.3 | 0.17b |
| Diabetes | 38% | 38% | 41% | 53% | 58% | <0.001a |
| ALT (IU/l) | 108±72 | 75±45 | 68±45 | 59±33 | 57±40 | <0.001b |
| AST (IU/l) | 56±33 | 48±33 | 47±32 | 46±24 | 52±42 | 0.1b |
| ALB (g/l) | 46±7 | 45±4 | 45±5 | 44±4 | 45±5 | <0.001b |
| Platelets (× 109/l) | 271±63 | 258±77 | 250±66 | 237±67 | 214±84 | <0.001b |
| AST/ALT ratio | 0.58±0.18 | 0.68±0.29 | 0.74±0.28 | 0.83±0.34 | 1.01±0.45 | <0.001b |
| FIB-4 score | 0.59±0.25 | 0.98±0.62 | 1.24±0.72 | 1.70±1.1 | 2.94±2.98 | <0.001b |
| NAFLD fibrosis score | −3.20±1.14 | −2.03±1.52 | −1.55±1.34 | −0.88±1.36 | 0.08±1.54 | <0.001b |
| Fibrosis stage | 0 (0–3) | 0 (0–4) | 1 (0–4) | 2 (0–4) | 2 (0–4) | <0.001c |
| 0 | 38 (51%) | 50 (52%) | 74 (37%) | 50 (26%) | 16 (21%) | |
| 1 | 20 (27%) | 19 (20%) | 48 (24%) | 44 (23%) | 17 (22%) | |
| 2 | 8 (11%) | 9 (9%) | 33 (17%) | 33 (17%) | 13 (17%) | |
| 3 | 8 (11%) | 15 (16%) | 33 (17%) | 40 (21%) | 15 (20%) | |
| 4 | 0 (0%) | 3 (3%) | 9 (5%) | 24 (13%) | 15 (20%) | |
| Stage F3 or F4 fibrosis | 8 (11%) | 18 (19%) | 42 (22%) | 64 (34%) | 30 (40%) | <0.001a |
| NASH | 45 (61%) | 61 (64%) | 141 (72%) | 154 (81%) | 60 (79%) | 0.002a |
| NAS | 4 (1–7) | 4 (1–7) | 4 (1–8) | 4 (1–8) | 3 (1–7) | 0.24c |
| Raised transaminases | 62 (84%) | 74 (77%) | 142 (72%) | 137 (72%) | 49 (64%) | 0.083a |
ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body masss index; FIB-4, fibrosis 4; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD Activity Score; NASH, non-alcoholic steatohepatitis. Data are expressed as mean±s.d. or median (range). Raised transaminases=serum ALT or AST >40 IU/l.
Chi-square test.
Student's t-test.
Mann–Whitney U-test.
Diagnostic accuracy for advanced fibrosis using simple non-invasive fibrosis scores in patients by age group
A detailed summary of the AUROC, sensitivity, specificity, PPVs, NPVs, LR+ve, and LR-ve for the AST/ALT ratio, the FIB-4 score, and the NFS is shown in Table 3 for patients divided into the five age groups. Overall, the performance of the AST/ALT ratio, NFS, and FIB-4 score was very poor in the ≤35-year-old patients (AUROCs 0.52, 0.52, and 0.60, respectively, all P=NS), suggesting that these scores have insufficient accuracy to have a role in the management of patients with NAFLD in this age group.
Table 3. Performance of the tests for diagnosis/exclusion of advanced fibrosis by age group using established cutoffs (15, 16, 18, 19).
| Test | AUROC (95% CI) | Cutoff | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | LR +ve | LR −ve |
|---|---|---|---|---|---|---|---|---|
| ≤35 years | ||||||||
| AST/ALT ratio | 0.52 (0.29–0.75) | 0.8 | 25 | 89 | 22 | 91 | 2.27 | 0.84 |
| 1 | 0 | 98.5 | 0 | 89 | 0 | 0.13 | ||
| FIB-4 score | 0.60 (0.40–0.81) | 1.30 | 0 | 97 | 0 | 89 | 0 | 1.03 |
| 2.67 | 0 | 100 | 0 | 89 | — | 1 | ||
| 3.25 | 0 | 100 | 0 | 89 | — | 1 | ||
| NFS | 0.52 (0.32–0.73) | −1.455 | 0 | 91 | 0 | 88 | — | 1 |
| 0.676 | 0 | 100 | 0 | 89 | — | 1 | ||
| 36–45 years | ||||||||
| AST/ALT ratio | 0.66 (0.53–0.80) | 0.8 | 39 | 80 | 31 | 85 | 1.95 | 0.76 |
| 1 | 6 | 94 | 19 | 81 | 1 | 1 | ||
| FIB-4 score | 0.79 (0.66–0.91) | 1.30 | 56 | 91 | 59 | 90 | 6.2 | 0.48 |
| 2.67 | 17 | 100 | 100 | 84 | ∞ | 0.83 | ||
| 3.25 | 6 | 100 | 100 | 82 | ∞ | 0.94 | ||
| NFS | 0.86 (0.76–0.96) | −1.455 | 78 | 80 | 48 | 94 | 3.9 | 0.28 |
| 0.676 | 22 | 100 | 100 | 85 | ∞ | 0.78 | ||
| 46–55 years | ||||||||
| AST/ALT ratio | 0.63 (0.53–0.73) | 0.8 | 44 | 74 | 31 | 83 | 1.69 | 0.76 |
| 1 | 20 | 91 | 37 | 81 | 2.22 | 0.88 | ||
| FIB-4 score | 0.77 (0.68–0.86) | 1.30 | 63 | 77 | 42 | 89 | 2.74 | 0.48 |
| 2.67 | 12 | 98.7 | 71 | 81 | 9.23 | 0.89 | ||
| 3.25 | 5 | 99.9 | 93 | 80 | 50 | 0.95 | ||
| NFS | 0.81 (0.73–0.89) | −1.455 | 81 | 65 | 38 | 93 | 2.31 | 0.29 |
| 0.676 | 22 | 97.4 | 69 | 82 | 8.46 | 0.8 | ||
| 56–64 years | ||||||||
| AST/ALT ratio | 0.72 (0.64–0.80) | 0.8 | 64 | 69 | 52 | 79 | 2.06 | 0.52 |
| 1 | 38 | 92 | 71 | 74 | 4.75 | 0.67 | ||
| FIB-4 score | 0.84 (0.78–0.90) | 1.30 | 90 | 61 | 54 | 92 | 2.3 | 0.16 |
| 2.67 | 30 | 97.6 | 86 | 73 | 12.5 | 0.72 | ||
| 3.25 | 20 | 100 | 100 | 71 | ∞ | 0.8 | ||
| NFS | 0.83 (0.64–0.80) | −1.455 | 95 | 44 | 47 | 94 | 1.69 | 0.11 |
| 0.676 | 31 | 99.9 | 99 | 74 | 310 | 0.69 | ||
| ≥65 years | ||||||||
| AST/ALT ratio | 0.73 (0.61–0.85) | 0.8 | 80 | 48 | 50 | 79 | 1.53 | 0.42 |
| 1 | 67 | 76 | 64 | 78 | 2.79 | 0.43 | ||
| FIB-4 score | 0.81 (0.72–0.91) | 1.30 | 93 | 35 | 48 | 89 | 1.43 | 0.2 |
| 2.67 | 53 | 85 | 69 | 74 | 3.53 | 0.55 | ||
| 3.25 | 50 | 91 | 78 | 74 | 5.55 | 0.55 | ||
| NFS | 0.81 (0.71–0.92) | −1.455 | 93 | 20 | 43 | 82 | 1.16 | 0.35 |
| 0.676 | 57 | 85 | 71 | 76 | 3.8 | 0.51 | ||
ALT, alanine transaminase; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; FIB-4, fibrosis 4; LR +ve, positive likelihood ratio, LR −ve, negative likelihood ratio; NFS, non-alcoholic fatty liver disease fibrosis score; NPV, negative predictive value; PPV, positive predictive value; Sens., sensitivity; Spec., specificity.
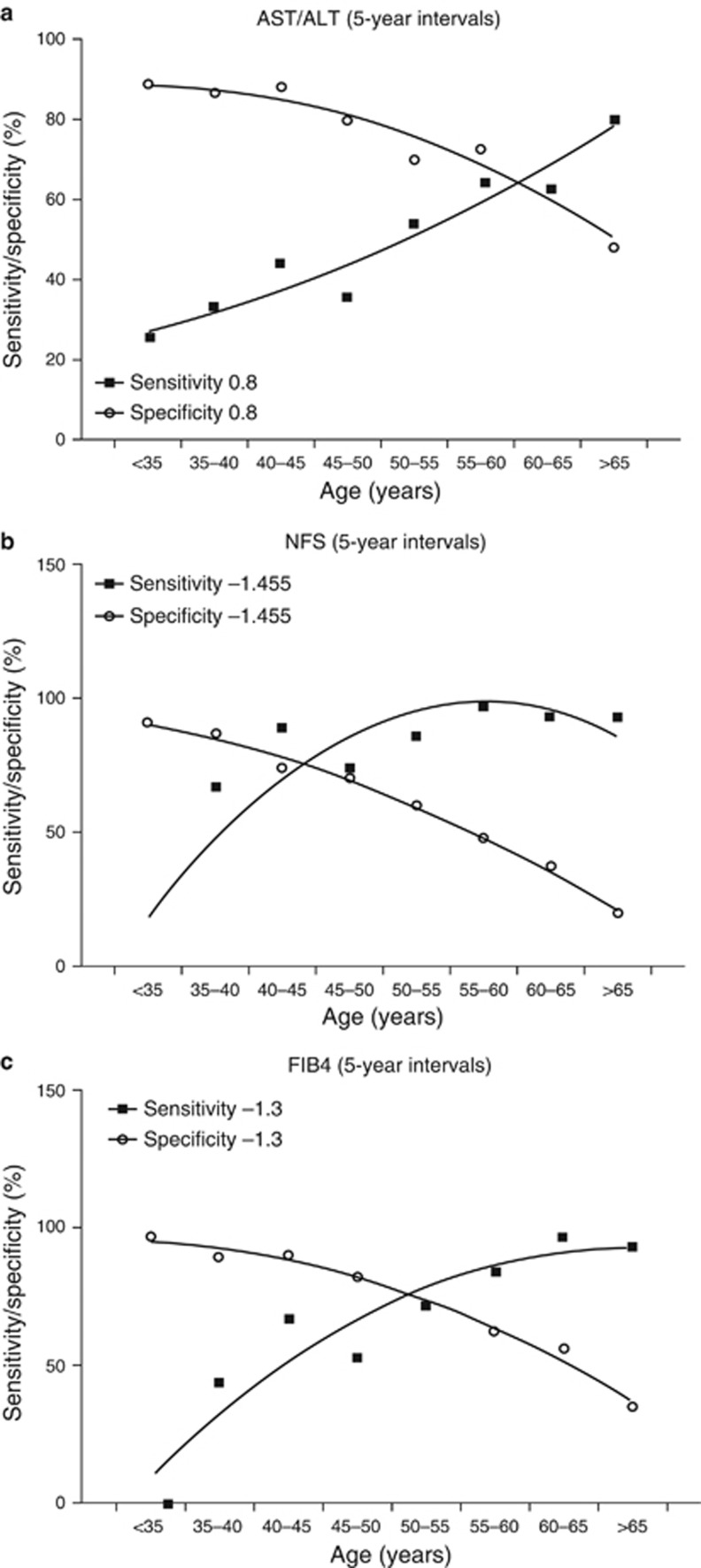
The diagnostic accuracy of the AST/ALT ratio for advanced fibrosis was lower than the NFS and FIB-4 score across all age groups, with modest AUROCs ranging from 0.52 to 0.73. The NFS and FIB-4 score performed similarly well across all the age groups >35 years, with AUROCs for a diagnosis of advanced fibrosis ranging from 0.77 to 0.84. However, despite having similar AUROC for advanced fibrosis across age groups, the specificity of the NFS and FIB-4 for advanced fibrosis fell with increasing age. This is illustrated in Figure 1a–c, which shows plots of the sensitivity and specificity against age for the AST/ALT ratio, NFS, and FIB-4 score. Using the established diagnostic cutoffs for advanced fibrosis, the specificity of the NFS and FIB-4 scores were very low in patients aged ≥65 years (20% at cutoff −1.445 for NFS and 35% at cutoff 1.3 for FIB-4). This equates to an unacceptably high false positive rate for advanced fibrosis in patients aged >65 years. The observed decline in specificity with increasing age is perhaps unsurprising as both the NFS and FIB-4 score incorporate age in the formula, but the fall in specificity for advanced fibrosis with the AST/ALT ratio was unexpected.
Figure 1.
Plots of sensitivity and specificity against age for existing diagnostic cutoffs for advanced fibrosis for: (a) aspartate aminotransferase (AST)/alanine transaminase (ALT) ratio (0.8); (b) non-alcoholic fatty liver disease fibrosis score (NFS) (−1.445); (c) fibrosis 4 (FIB-4) score (1.3). These show a reduction in sensitivity and an increase in specificity with age.
Effects of age on specificity of AST/ALT ratio for advanced fibrosis
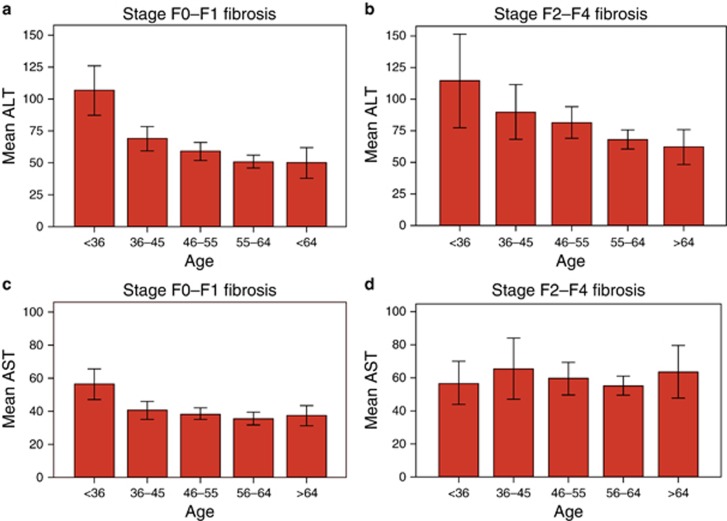
Overall, there was a significant negative association between age and serum ALT (P<0.001), whereas there was no significant relationship between age and AST. When the relationship between age and serum ALT in patients with no/mild fibrosis (stage F0–F1) or moderate-to-advanced fibrosis (stage F2–F4) were analyzed separately to correct for fibrosis, the significant negative relationship between serum ALT and age persisted (P<0.001 for both see Figure 2a, b), suggesting the age-related fall in ALT level was independent of fibrosis stage. This relationship also persisted when males and females were analyzed separately (data not shown). No significant relationship between serum AST level and age was observed (Figure 2c, d).
Figure 2.
Relationship between serum alanine transaminase (ALT) level and age in patients with (a) stage F0–F1 fibrosis and (b) stage F2–F4 fibrosis. Relationship between serum aspartate aminotransferase (AST) level and age in patients with (c) stage F0–F1 fibrosis and (d) stage F2–F4 fibrosis. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
Derivation of new cutoffs to exclude advanced fibrosis for the NFS and FIB-4 score in older patients (≥65 years)
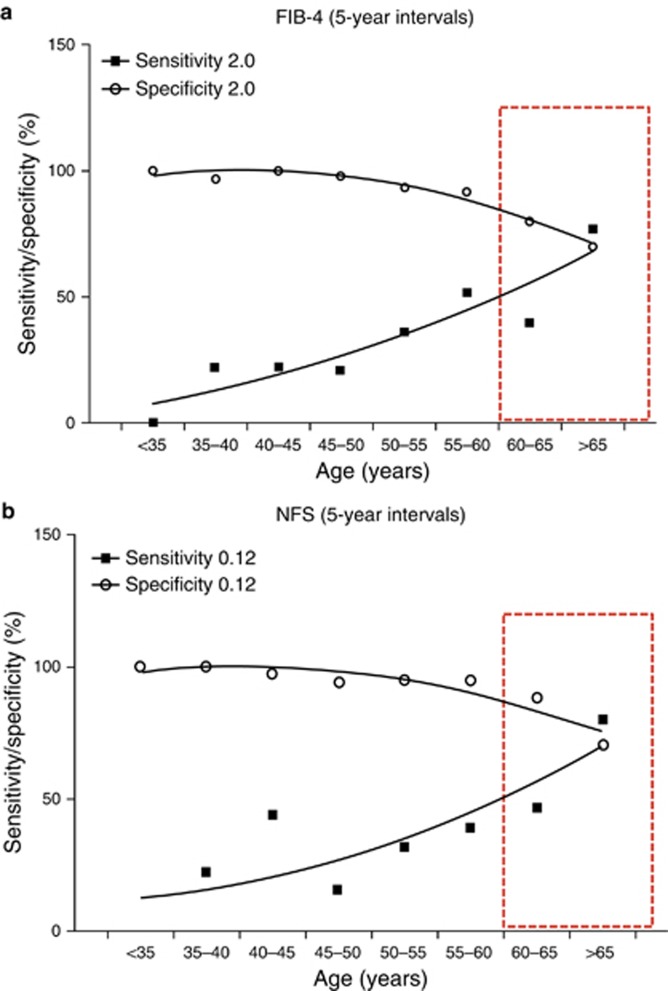
As NAFLD is highly prevalent in older patients, it would be particularly advantageous to have an accurate non-invasive test for fibrosis in this age group. In light of the poor specificity of the NFS and FIB-4 scores for advanced fibrosis when the currently published diagnostic cutoffs are employed in patients aged ≥65 years, our results imply that many patients with mild fibrosis will be wrongly classified as having advanced fibrosis and so would undergo additional unnecessary investigations. To address this problem, optimized cutoffs were derived to improve specificity and thus reduce the false positive rate. Adopting the revised threshold (FIB-4 >2.0) in patients aged ≥65 years, the sensitivity and specificity attained for advanced fibrosis were 77% and 70%, respectively. Similarly, the new age-adjusted NFS threshold (NFS>0.12) yielded 80% sensitivity and 70% specificity for advanced fibrosis. Plots showing the sensitivity and specificity performance of these new thresholds across the age range are shown Figure 3a, b. Application of the new cutoff for the NFS in patients aged >65 years substantially reduced the number of patients with intermediate scores, so that the majority were definitively classified as low risk or high risk and would not necessarily need further investigation (percentage definitively categorized using the new cutoff 80% (61/76) vs. 45% (34/76) for the existing cutoff). This was a substantial improvement over the performance of the existing cutoff with slightly more cases correctly classified (80% vs. 76%, respectively) with respect to their histological stage. For the FIB-4 score, the new cutoff also definitively classified more patients as low risk or high risk than the existing cutoff (82% (62/76) vs. 55% (42/76), respectively) while maintaining the correct classification in 78% of cases. As PPVs and NPVs are a function of disease prevalence in the background population and the tests may be applied to populations where the prevalence of advanced fibrosis is lower than in our study cohort, the PPVs and NPVs for older patients (≥65 years) are displayed across an advanced fibrosis prevalence scale in Table 4, which compares performance of the existing thresholds and our proposed thresholds for the FIB-4 and NFS.
Figure 3.
Plots of sensitivity and specificity against age for new diagnostic cutoffs for advanced fibrosis for: (a) fibrosis 4 (FIB-4) score (2.0); (b) non-alcoholic fatty liver disease fibrosis score (NFS) (0.12). Age range for which proposed thresholds are optimized is highlighted by red box. These show better specificity than current cutoffs without an adverse effect on sensitivity. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
Table 4. Performance of current and proposed new cutoffs to exclude advanced fibrosis (stage F3–F4) in older patients (≥65 years) at different prevalence rates of advanced fibrosis.
| Test | Cutoff | Sens. (%) | Spec. (%) | Prev. (%) | PPV (%) | NPV (%) | LR +ve | LR −ve |
|---|---|---|---|---|---|---|---|---|
| FIB-4 | 1.3 | 93 | 35 | 5 | 7 | 99 | 1.43 | 0.2 |
| 10 | 14 | 98 | ||||||
| 20 | 26 | 95 | ||||||
| 30 | 38 | 92 | ||||||
| 40 | 49 | 88 | ||||||
| 2.0 | 77 | 70 | 5 | 12 | 98 | 2.56 | 0.33 | |
| 10 | 22 | 96 | ||||||
| 20 | 39 | 92 | ||||||
| 30 | 52 | 88 | ||||||
| 40 | 63 | 82 | ||||||
| NFS | −1.455 | 93 | 20 | 5 | 6 | 98 | 1.16 | 0.35 |
| 10 | 11 | 96 | ||||||
| 20 | 23 | 92 | ||||||
| 30 | 33 | 87 | ||||||
| 40 | 44 | 81 | ||||||
| 0.12 | 80 | 70 | 5 | 12 | 99 | 2.67 | 0.29 | |
| 10 | 23 | 97 | ||||||
| 20 | 40 | 93 | ||||||
| 30 | 53 | 89 | ||||||
| 40 | 64 | 84 |
FIB-4, fibrosis 4; LR +ve, positive likelihood ratio, LR −ve, negative likelihood ratio; NFS, non-alcoholic fatty liver disease fibrosis score; NPV, negative predictive value; Prev., prevalence; PPV, positive predictive value; Sens., sensitivity; Spec., specificity.
Independent validation of new cutoffs for the NFS and FIB-4 score in a separate patient cohort
In order to validate the new cutoffs for the NFS and FIB-4 score in older patients, a second cohort of 61 patients with biopsy-proven NAFLD aged ≥65-years but otherwise selected according to the same criteria as the first cohort was assessed. The mean age of these patients was similar to the mean for the age in the ≥65-years group in the ascertainment cohort at 69±3.4 years and 41 (67%) were female. Overall 54 (89%) patients had NASH, 38 (62%) were diabetic, and 24 (39%) had advanced fibrosis. The AUROC for a diagnosis of advanced fibrosis was 0.72 (95% confidence interval 0.59–0.85) for both the NFS and FIB-4 score. The sensitivity and specificity for advanced fibrosis in these patients was similar to the main cohort (NFS: sensitivity 71% and specificity 68% FIB-4 score: sensitivity 75% and specificity 65%), confirming their validity.
DISCUSSION
The use of simple non-invasive fibrosis scores such as the NFS and FIB-4 score to identify or exclude advanced fibrosis as part of a staged approach to diagnosis and risk stratification in patients with NAFLD is now widely adopted and recommended by most guidelines (1, 2, 13, 28). Previous studies have shown that these scores can reliably exclude advanced fibrosis in patients with NAFLD, and as a result, they provide a useful, inexpensive first-line assessment of liver fibrosis for use in primary or secondary care (16, 18, 19). Although fidelity appears lower when applied in bariatric surgery cohorts (29), several studies have now validated their use in large populations of patients with biopsy-proven NAFLD (17, 20, 30, 31, 32). However, the majority of patients included in these studies were in middle age (typical mean age of 45–55 years), with very few younger (≤35 years) or older patients (≥65 years) included. With the widespread adoption of the FIB-4 and NFS, inevitably these tests are increasingly being used in patients outside the age range in which they have been validated. Anecdotally, we have seen quite a number of older patients referred to our fatty liver clinics with intermediate or high NFS and FIB-4 scores who have no evidence of advanced fibrosis when assessed by other methods, such as elastography or biopsy. Therefore, the aim of the present study was to assess the performance of these simple non-invasive fibrosis scores in age-defined cohorts of patients of histologically characterized NAFLD, with a particular focus on cases at the younger (<35 years) and older (≥65 years) ends of the age spectrum.
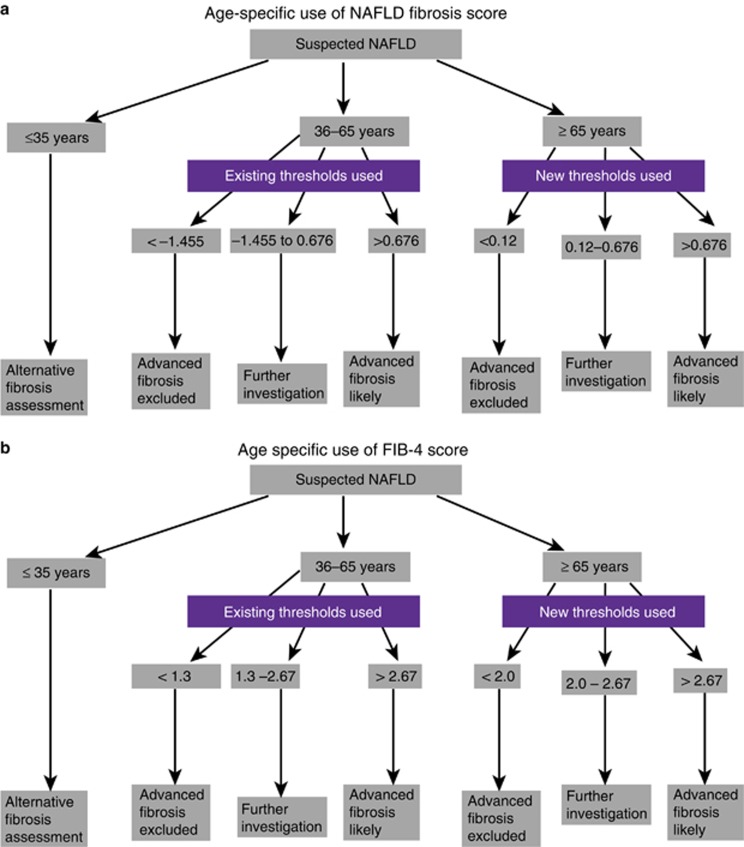
One of the most notable findings of this study was that, despite the FIB-4 and NFS having similar AUROCs in all age groups >35 years old, the specificity for advanced fibrosis using the lower cutoff with these tests fell sharply in the older patients and became unacceptably low in those aged ≥65 years (specificity 35% and 20%, respectively, for FIB-4 score and NFS). In essence, inclusion of age in the FIB-4 and NFS leads to a false inflation of the score in patients aged >65 years, bringing more patients into the intermediate-risk bracket and increasing the high false positive rate. As a result, we have derived new lower cutoffs for the FIB-4 score and NFS for use in patients aged ≥65 years, which reduced the number of patients with an indeterminate score. Use of these thresholds in patients aged ≥65 years increased the specificity for advanced fibrosis using the NFS and FIB-4 score to 70%, effectively controlling the false positive rate without adversely inflating the false negative rate of the test. The upper cutoffs remained the same. The advantage of adopting these cutoffs was confirmed in a separate validation cohort. We believe that introduction of these new cutoffs for older patients will have a direct benefit to the patients by reducing the need for further unnecessary and potentially invasive investigations and will also lead to cost savings by reducing inappropriate onward referral of older patients without advanced liver disease to secondary care. A proposed algorithm for the use of the NFS and FIB-4 score that takes account of patient age is shown in Figure 4a, b. It is important to note that even with these new cutoffs the PPV for advanced fibrosis with the FIB-4 score and NFS was relatively low if used in cohorts with a low prevalence of advanced fibrosis. Therefore, patients with a raised score should undergo a second-line investigation to confirm advanced fibrosis. Previous studies have shown that the major value of these simple non-invasive scores is to exclude rather than diagnose advanced fibrosis (19).
Figure 4.
Proposed algorithm for the use of the (a) non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) and (b) fibrosis 4 (FIB-4) score to help diagnose or exclude advanced fibrosis patients using the age specific cutoffs. The NFS and FIB-4 scores are not suitable for patients aged <35 years. Existing thresholds as previously published should be used for those aged 35–65 years. The new lower thresholds derived and validated in the current paper are recommended for those aged ≥65 years. Existing upper cutoffs remain the same for those aged ≥65 years. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
Both the NFS and the FIB-4 score include age in their models and this might be one explanation for the reduction in specificity for advanced fibrosis with increasing age. However, we also demonstrated that the specificity of the AST/ALT ratio for advanced fibrosis fell with increasing age. This was due to a significant age-related fall in serum ALT levels, which persisted, independent of fibrosis stage and gender, and confirms results of previous studies (33, 34, 35). As both the NFS and FIB-4 score include a ratio of the AST and ALT in their models, this may well also contribute to the overall reduction in specificity with increasing age. Serum ALT levels are well known to fall with increasing fibrosis stage, so one explanation for this observation may be that, with a higher prevalence of advanced fibrosis in older patients, the ALT fall in the older patients was due to increased fibrosis stage. However, re-analysis of the data stratified by fibrosis stage demonstrates that this is not the explanation and so the underlying mechanism for the fall in ALT levels is not known. It is, however, consistent with the observation that many NAFLD patients have serum ALT levels within the normal range, irrespective of disease activity. It once again highlights that clinicians should not rely on a raised serum ALT to support a diagnosis of NAFLD or as a marker of disease severity, particularly in older individuals. It also raises the broader question of whether age-specific normal ranges for serum ALT should be defined. Therefore, the likely explanation for the reduced specificity of the AST/ALT ratio for advanced fibrosis is that, even in the absence of liver disease, there is a natural age-related fall in serum ALT level (while the AST level remains stable). This increases the AST/ALT ratio and, as a result, reduces the specificity of this test for advanced fibrosis. In addition to the effect of age on transaminase levels, we found a negative association between age and platelet count, confirming previous epidemiological studies (36). Both NFS and FIB-4 score include platelet count in their models, likely also contributing to the observed reduction in specificity with increasing age.
Another key finding of this study was that the AST/ALT ratio, NFS, and FIB-4 scores performed very poorly in patients aged <35 years. In this age group, the AUROCs for advanced fibrosis for all three non-invasive scores did not reach statistical significance, suggesting that these scores are inaccurate and therefore should not be used to diagnose or exclude advanced fibrosis. The prevalence of advanced fibrosis was relatively low in this group compared with older patients, with 11% of subjects having stage F3 fibrosis and none being cirrhotic. The low prevalence of advanced fibrosis might be one explanation for the poor performance of these tests in this age group.
In the United Kingdom, a recent Lancet Commission has set out a blueprint that aims to reduce premature mortality rates from liver disease and a key aspect of this is early recognition of patients with progressive and advanced liver disease in the community (22). One of the recommendations of this report is that all liver function test requests in the community should have both ALT and AST measured and the AST/ALT ratio should be displayed on the laboratory report. AST/ALT ratios >1 are to be flagged to clinicians to recommend further liver assessment. Despite having looked very promising in some previous studies, the AST/ALT ratio performed relatively poorly in the present study with an overall AUROC of 0.70 for a diagnosis of advanced fibrosis (15, 19). Results of the present study and others suggest that it might be more beneficial to use the FIB-4 score or NFS as an alternative to the AST/ALT ratio as these appear more accurate (18, 37, 38). Although these are more complex to calculate, the FIB-4 score only requires the addition of the age and platelet count to the AST and ALT and a simple online calculator is available (http://gihep.com/calculators/hepatology/fibrosis-4-score/).
It is salutatory to note that, as with the FIB-4 and NFS, many of the other non-invasive tests for fibrosis that are used in clinical practice have not been validated for use in older-age cohorts, particularly when NASH and advanced fibrosis are seen more commonly on biopsy in older patients (39). Complex fibrosis panels that include markers of matrix turnover, such as the Enhanced Liver fibrosis panel and FibroTest, have shown promise for the assessment of liver fibrosis in NAFLD (40, 41). However, these panels have been primarily developed in young and middle-aged patients with few patients aged ≥65 years studied. The Enhanced Liver fibrosis test may be less susceptible to an age-related bias as it does not include age in its mathematical model; however, there is published evidence that the test exhibits reduced diagnostic accuracy when age is >45 years and so clarification of this point is needed (42). Further evaluation in older patients is clearly warranted before accuracy can be assumed in older patients. Another commonly adopted method for non-invasive assessment of fibrosis is liver stiffness measurement assessed by transient elastography. This technique is generally effective at excluding advanced fibrosis when reliable readings can be obtained and may perform better than simple non-invasive fibrosis tests (43). However, large studies have demonstrated that an insufficient number of valid measurements may be achieved in some patients owing to adiposity and body habitus. Indeed, even when using the Fibroscan XL probe, which is adapted for obese patients, a valid result may not be achieved in up to 26% of subjects (44). In large-scale retrospective studies, older age (>52 years), along with central obesity and Type 2 diabetes mellitus, were the key independent factors predictive of failure to achieve valid liver stiffness measurement (45). There is also recent evidence from an epidemidemiological study that liver stiffness may rise with age (46), suggesting that this test too may have reduced utility in older patients with NAFLD.
Age-related changes in liver morphology may also contribute to the effect of increasing age on the performance of non-invasive fibrosis tests, including elastography. Liver blood flow and liver mass are known to be reduced in the ageing liver (47). It is also recognized that vascular morphology is modified with increasing age with even healthy liver exhibiting defenestration of sinusoidal endothelial cells, sinusoidal fibrosis, increased hepatic arteriolar wall thickness, and decreased arteriolar diameter that may impair oxygen-dependent hepatocyte function (48, 49).
This study does have some limitations. First, the sample size of younger (≤35 years) and older patients (≥65 years) is relatively small despite a large overall cohort size, reflecting that these patients are less likely to have a liver biopsy performed in routine clinical practice. Second, patients are likely to be highly selected, particularly the older patients (≥65 years), as clinicians tend to be reluctant to perform liver biopsy in older patients for fear of complications, unless they believe it is going to significantly change the patient's management. In addition, the prevalence of Type 2 diabetes was high (38%) in the young (<35 years) patients, which suggests selection bias as this rate of diabetes is higher than would be expected in a young cohort of patients with NAFLD. Third, the prevalence of advanced fibrosis was high in this study, again probably reflecting the selection bias, and this will have impacted on the PPVs and NPVs that have been displayed as they are heavily influenced by prevalence. To counter this concern, as prevalence can vary in different populations, we have displayed PPVs and NPVs at different prevalence rates for the new derived cutoffs to aid clinicians using these scores in different clinical settings. Finally, histological assessment of liver fibrosis is an imperfect gold standard that is subject to both the effect of non-uniform distribution of histological lesions throughout the liver parenchyma and ascertainment variation (50, 51, 52). Liver biopsies were analyzed by a local pathologist at each of the centers. Although all the pathologists reading the biopsies are highly experienced hepatopathologists, there is potential for interobserver variability, which may have led to a lowering of the diagnostic accuracy of the tests. Despite these limitations, the overall diagnostic accuracy of the non-invasive scores assessed in the present study are in keeping with other studies and this is the first study to specifically assess these scores in specific age groups (17, 18, 20, 30, 31, 32).
In conclusion, this study shows that age has a significant effect on the performance of simple non-invasive fibrosis scores in diagnosing advanced fibrosis. The AST/ALT ratio, FIB-4 score, and NFS performed poorly in patients aged <35 years, suggesting that clinicians should use alternative means of non-invasive diagnosis of fibrosis in this age group. The overall diagnostic accuracy for advanced fibrosis using the NFS and FIB-4 score was acceptable in patients aged >35 years, but there was a significant fall in specificity for advanced fibrosis in older patients (≥65 years), resulting in a high false positive rate for advanced fibrosis. To rectify this, while the existing thresholds should continue to be used in patients aged 35–65 years, we propose new cutoffs that will significantly improve the accuracy of the NFS and FIB-4 score in patients aged ≥65 years.
Study Highlights
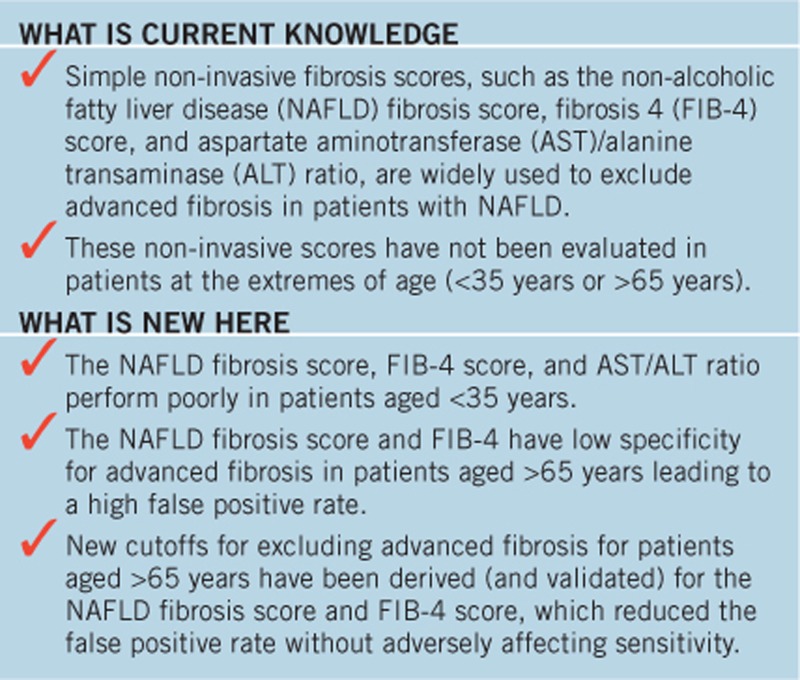
Acknowledgments
The research leading to these results has been supported by the EPoS (Elucidating Pathways of Steatohepatitis) consortium funded by the Horizon 2020 Framework Program of the European Union under Grant Agreement 634413. The authors are contributing members of the EPoS/EASL European NAFLD Registry.
Footnotes
Guarantor of the article: Stuart McPherson, BSc, MBChB, MD, FRCP.
Specific author contributions: Design of study, data collection, analysis, writing manuscript and approval of final manuscript: S.M. and Q.M.A. Data collection, review of manuscript for important intellectual content, and approved final manuscript: T.H., J.-F.D., S.P., M.R.-G., M.A., C.P.O., S.F., L.V.G., J.M.S., D.T., A.B., E.B., V.R., and C.P.D.
Financial support: Q.M.A. is the recipient of a Clinical Senior Lectureship Award from the Higher Education Funding Council for England (HEFCE).
Potential competing interests: None.
References
- Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ 2011;343:d3897. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Nobili V, Tiribelli C. Epidemiology of fatty liver: an update. World J Gastroenterol 2014;20:9050–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–344. [DOI] [PubMed] [Google Scholar]
- Wong VW, Wong GL, Choi PC et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010;59:969–974. [DOI] [PubMed] [Google Scholar]
- Pais R, Charlotte F, Fedchuk L et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol 2013;59:550–556. [DOI] [PubMed] [Google Scholar]
- McPherson S, Hardy T, Henderson E et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Franzen LE, Mathiesen UL et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–873. [DOI] [PubMed] [Google Scholar]
- Bhala N, Angulo P, van der Poorten D et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M, Hagstrom H, Nasr P et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–1554. [DOI] [PubMed] [Google Scholar]
- Angulo P, Kleiner DE, Dam-Larsen S et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol 2013;66:1033–1045. [DOI] [PubMed] [Google Scholar]
- Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013;58:1007–1019. [DOI] [PubMed] [Google Scholar]
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95:734–739. [DOI] [PubMed] [Google Scholar]
- Angulo P, Hui JM, Marchesini G et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- McPherson S, Henderson E, Burt AD et al. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014;60:1055–1062. [DOI] [PubMed] [Google Scholar]
- Shah AG, Lydecker A, Murray K et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson S, Stewart SF, Henderson E et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- McPherson S, Anstee QM, Henderson E et al. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur J Gastroenterol Hepatol 2013;25:652–658. [DOI] [PubMed] [Google Scholar]
- Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014;5:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Aspinall R, Bellis M et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–1997. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 2014;147:754–764. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- Vallet-Pichard A, Mallet V, Nalpas B et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Bellentani S, Cortez-Pinto H et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372–384. [DOI] [PubMed] [Google Scholar]
- Francque SM, Verrijken A, Mertens I et al. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol 2012;10:1162–1168 quiz e1187. [DOI] [PubMed] [Google Scholar]
- Petta S, Vanni E, Bugianesi E et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015;35:1566–1573. [DOI] [PubMed] [Google Scholar]
- Adams LA, George J, Bugianesi E et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536–1543. [DOI] [PubMed] [Google Scholar]
- Demir M, Lang S, Nierhoff D et al. Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol 2013;47:719–726. [DOI] [PubMed] [Google Scholar]
- Dong MH, Bettencourt R, Brenner DA et al. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol 2012;10:285–290 e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong MH, Bettencourt R, Barrett-Connor E et al. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PLoS One 2010;5:e14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh GB, Pagadala MR, Dasarathy J et al. Age impacts ability of aspartate-alanine aminotransferase ratio to predict advanced fibrosis in nonalcoholic fatty liver disease. Dig Dis Sci 2015;60:1825–1831. [DOI] [PubMed] [Google Scholar]
- Balduini CL, Noris P. Platelet count and aging. Haematologica 2014;99:953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gutierrez OZ, Hernandez-Rocha C, Candia-Balboa RA et al. Validation study of systems for noninvasive diagnosis of fibrosis in nonalcoholic fatty liver disease in Latin population. Ann Hepatol 2013;12:416–424. [PubMed] [Google Scholar]
- Xun YH, Fan JG, Zang GQ et al. Suboptimal performance of simple noninvasive tests for advanced fibrosis in Chinese patients with nonalcoholic fatty liver disease. J Dig Dis 2012;13:588–595. [DOI] [PubMed] [Google Scholar]
- Noureddin M, Yates KP, Vaughn IA et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology 2013;58:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Massard J, Tahiri M et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha IN, Parkes J, Roderick P et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008;47:455–460. [DOI] [PubMed] [Google Scholar]
- Fagan KJ, Pretorius CJ, Horsfall LU et al. ELF score >/=9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int 2015;35:1673–1681. [DOI] [PubMed] [Google Scholar]
- Wong VW, Vergniol J, Wong GL et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–462. [DOI] [PubMed] [Google Scholar]
- Myers RP, Pomier-Layrargues G, Kirsch R et al. Discordance in fibrosis staging between liver biopsy and transient elastography using the fibroscan xl probe. J Hepatol 2011;56:564–570. [DOI] [PubMed] [Google Scholar]
- Castera L, Foucher J, Bernard PH et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828–835. [DOI] [PubMed] [Google Scholar]
- Koehler EM, Plompen EP, Schouten JN et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2015;63:138–147. [DOI] [PubMed] [Google Scholar]
- Jansen PL. Liver disease in the elderly. Best Pract Res Clin Gastroenterol 2002;16:149–158. [DOI] [PubMed] [Google Scholar]
- McLean AJ, Cogger VC, Chong GC et al. Age-related pseudocapillarization of the human liver. J Pathol 2003;200:112–117. [DOI] [PubMed] [Google Scholar]
- Fiel MI, Deniz K, Elmali F et al. Increasing hepatic arteriole wall thickness and decreased luminal diameter occur with increasing age in normal livers. J Hepatol 2011;55:582–586. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Bruckert E, Grimaldi A et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–1906. [DOI] [PubMed] [Google Scholar]
- Levene AP, Kudo H, Armstrong MJ et al. Quantifying hepatic steatosis—more than meets the eye. Histopathology 2012;60:971–981. [DOI] [PubMed] [Google Scholar]
- Burt AD, Lackner C, Tiniakos DG. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis 2015;35:207–220. [DOI] [PubMed] [Google Scholar]