Abstract
The transition of the physical phase of lipids in membrane fragments of a blue-green alga Anacystis nidulans was studied by a spin labeling technique. The maximum hyperfine splitting of the electron spin resonance spectrum of the N-oxyl-4′, 4′-dimethyloxazolidine derivative of 5-ketostearic acid plotted against the reciprocal of the absolute temperature gave a discontinuity point that was characteristic of a transition of the physical phase of the hydrocarbon region of membrane lipids. The phase transition appeared at approximately 13 or 24 C in the organisms grown at 28 or 38 C, respectively.
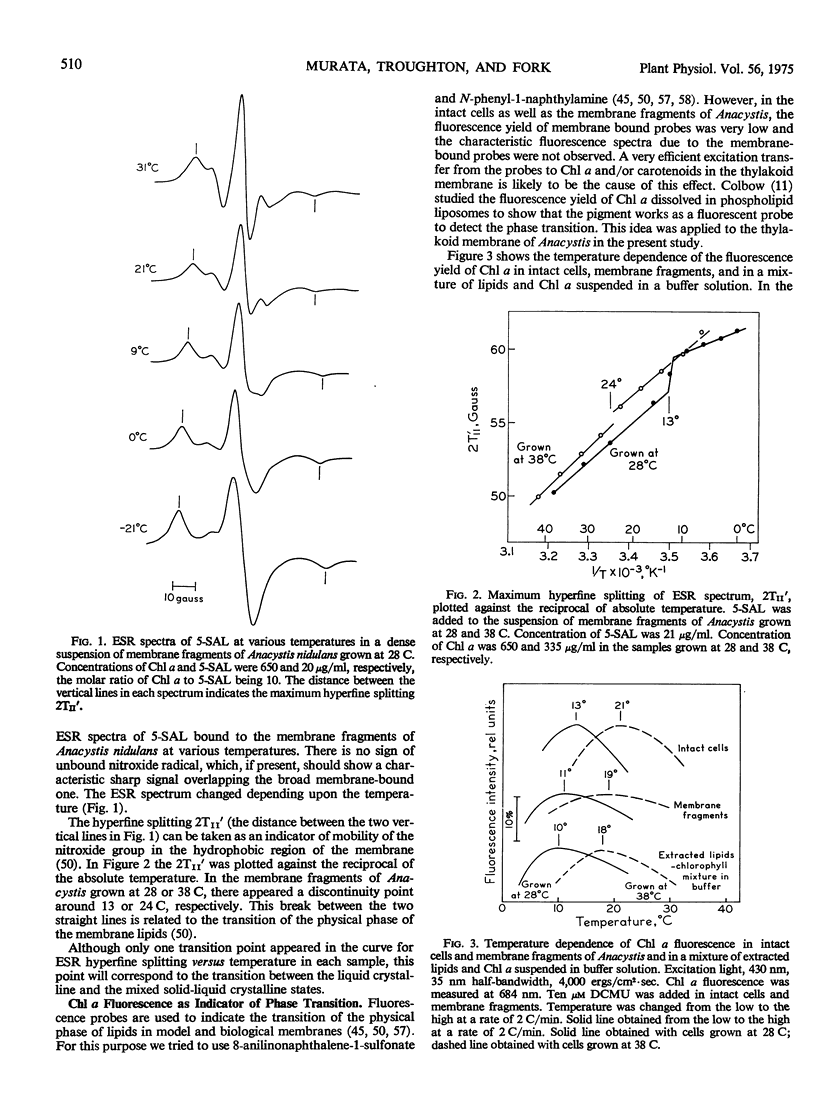
The temperature dependence curve of chlorophyll a fluorescence in intact cells, membrane fragments, and extracted lipids of Anacystis cells suspended in a buffer solution showed that the fluorescence yield became maximum near the phase transition temperatures. These findings suggest that chlorophyll a in the thylakoid membrane works as a native fluorescence probe for the detection of phase transition.
The temperature dependence of photosynthetic electron transport reactions was studied by measuring the oxidoreductive reactions of P700 and by measuring O2 evolution. Each of the Arrhenius plots of the reaction rates was composed of two straight lines with a break near the phase transition temperatures. The activation energy was always lower above than below the transition temperatures. It is proposed to explain these phenomena that a reaction involving plastoquinone is influenced by the physical state of membrane lipids.
The shift between the pigment state 1 and state 2 measured by fluorescence transients also showed a characteristic break in the Arrhenius plots near the phase transition temperatures; below the transition temperatures the shift almost disappeared. This suggests that the configurational change of the thylakoid membrane related to the state 1 and state 2 shift is dependent on the physical state of membrane lipids. In the chloroplasts of lettuce and spinach, on the other hand, there was no break in the Arrhenius plot of the electron transport reactions or of Mg2+-induced changes of chlorophyll a fluorescence.
It is suggested that the transitions of the hyperfine splitting of the ESR signal, electron transport, and the configurational change, as well as the appearance of the maximum of chlorophyll a fluorescence, in the thylakoid membranes of Anacystis nidulans are all related to the transition of the physical phase of membrane lipids between the liquid crystalline state and the mixed liquid crystal-solid state.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMESZ J., DUYSENS L. N. Action spectrum, kinetics and quantum requirement of phosphopyridine nucleotide reduction and cytochrome oxidation in the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1962 Oct 22;64:261–278. doi: 10.1016/0006-3002(62)90736-9. [DOI] [PubMed] [Google Scholar]
- AMESZ J. SPECTROPHOTOMETRIC EVIDENCE FOR THE PARTICIPATION OF A QUINONE IN PHOTOSYNTHESIS OF INTACT BLUE-GREEN ALGAE. Biochim Biophys Acta. 1964 Mar 30;79:257–265. [PubMed] [Google Scholar]
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Allen C. F., Franke H., Hirayama O. Identification of a plastoquinone and two naphthoquinones in Anacystis nidulans by NMR and mass spectroscopy. Biochem Biophys Res Commun. 1967 Mar 9;26(5):562–568. doi: 10.1016/0006-291x(67)90102-7. [DOI] [PubMed] [Google Scholar]
- Amesz J., Visser J. W., van den Engh G. J., Dirks M. P. Reaction kinetics of intermediates of the photosynthetic chain between the two photosystems. Biochim Biophys Acta. 1972 Feb 28;256(2):370–380. doi: 10.1016/0005-2728(72)90067-9. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., McAfee A. G., Ho C. A spin label study of E. coli membrane vesicles. Biochem Biophys Res Commun. 1973 Jul 17;53(2):617–623. doi: 10.1016/0006-291x(73)90706-7. [DOI] [PubMed] [Google Scholar]
- Barratt M. D., Green D. K., Chapman D. E.S.R. studies of nitroxide probes in lecithin-water systems. Chem Phys Lipids. 1969 Apr;3(2):140–144. doi: 10.1016/0009-3084(69)90004-8. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Chapman D., Urbina J. Biomembrane phase transitions. Studies of lipid-water systems using differential scanning calorimetry. J Biol Chem. 1974 Apr 25;249(8):2512–2521. [PubMed] [Google Scholar]
- Cullen J., Phillips M. C., Shipley G. G. The effects of temperature on the composition and physical properties of the lipids of Pseudomonas fluorescens. Biochem J. 1971 Dec;125(3):733–742. doi: 10.1042/bj1250733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletr S., Keith A. D. Spin-label studies of dynamics of lipid alkyl chains in biological membranes: role of unsaturated sites. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1353–1357. doi: 10.1073/pnas.69.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Crowfoot P. D., Wakil S. J. Molecular organization of lipids in Escherichia coli membranes. II. Effect of phospholipids on succinic-ubiquinone reductase activity. J Biol Chem. 1972 Nov 25;247(22):7251–7256. [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- HENNINGER M. D., BHAGAVAN H. N., CRANE F. L. COMPARATIVE STUDIES ON PLASTOQUINONES. I. EVIDENCE FOR THREE QUINONES IN THE BLUE-GREEN ALGA, ANACYSTIS NIDULANS. Arch Biochem Biophys. 1965 Apr;110:69–74. doi: 10.1016/0003-9861(65)90155-4. [DOI] [PubMed] [Google Scholar]
- Haehnel W. Electron transport between plastoquinone and chlorophyll a-I in chloroplasts. Biochim Biophys Acta. 1973 Jun 28;305(3):618–631. doi: 10.1016/0005-2728(73)90081-9. [DOI] [PubMed] [Google Scholar]
- Hirayama O. Lipids and lipoprotein complex in photosynthetic tissues. II. Pigments and lipids in blue-green alga, Anacystis nidulans. J Biochem. 1967 Feb;61(2):179–185. doi: 10.1093/oxfordjournals.jbchem.a128529. [DOI] [PubMed] [Google Scholar]
- Homann P. H. Cation effects on the fluorescence of isolated chloroplasts. Plant Physiol. 1969 Jun;44(6):932–936. doi: 10.1104/pp.44.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Ogawa T., Shibata K. Light-induced spectral changes of P700 in the 800-nm region in Anacystis and spinach lamellae. Biochim Biophys Acta. 1973 May 30;305(2):483–487. doi: 10.1016/0005-2728(73)90194-1. [DOI] [PubMed] [Google Scholar]
- Kemp A., Jr, Groot G. S., Reitsma H. J. Oxidative phosphorylation as a function of temperature. Biochim Biophys Acta. 1969 May;180(1):28–34. doi: 10.1016/0005-2728(69)90190-x. [DOI] [PubMed] [Google Scholar]
- Kok B., Malkin S., Owens O., Forbush B. Observations on the reducing side of the O2-evolving photoact. Brookhaven Symp Biol. 1966;19:446–459. [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Lien S., Bannister T. T. Multiple sites of DCIP reduction by sonicated oat chloroplasts: role of plastocyanin. Biochim Biophys Acta. 1971 Sep 7;245(2):465–481. doi: 10.1016/0005-2728(71)90163-0. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974 Mar 25;84(1):145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J., Sturtevant J. M., Tsong T. Y. Characterization of the plasma membrane of Mycoplasma laidlawii. VII. Phase transitions of membrane lipids. Biochim Biophys Acta. 1970;219(1):114–122. doi: 10.1016/0005-2736(70)90066-0. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. IV. Kinetics of chlorophyll a fluorescence in Porphyra yezoensis. Biochim Biophys Acta. 1970 Jun 30;205(3):379–389. doi: 10.1016/0005-2728(70)90104-0. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. V. Correlation of membrane structure to regulation of excitation transfer between two pigment systems in isolated spinach chloroplasts. Biochim Biophys Acta. 1971 Sep 7;245(2):365–372. doi: 10.1016/0005-2728(71)90155-1. [DOI] [PubMed] [Google Scholar]
- Murata N., Tashiro H., Takamiya A. Effects of divalent metal ions on chlorophyll a fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1970 Mar 3;197(2):250–256. doi: 10.1016/0005-2728(70)90035-6. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- Nozawa Y., Iida H., Fukushima H., Oki K., Onishi S. Studies on Tetrahymena membranes: temperature-induced alterations in fatty acid composition of various membrane fractions in Tetrahymena pyriformis and its effect on membrane fluidity as inferred by spin-label study. Biochim Biophys Acta. 1974 Oct 29;367(2):134–147. doi: 10.1016/0005-2736(74)90038-8. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., McMurchie E. J. Two temperature-induced changes in mitochondrial membranes detected by spin labelling and enzyme kinetics. Biochim Biophys Acta. 1974 Sep 6;363(2):135–140. doi: 10.1016/0005-2736(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Träuble H., Galla H. J., Overath P. Lateral diffusion, protein mobility, and phase transitions in Escherichia coli membranes. A spin label study. Biochemistry. 1973 Dec 18;12(26):5360–5369. doi: 10.1021/bi00750a020. [DOI] [PubMed] [Google Scholar]
- Seelig J. On the flexibility of hydrocarbon chains in lipid bilayers. J Am Chem Soc. 1971 Oct 6;93(20):5017–5022. doi: 10.1021/ja00749a006. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Green J. P., Nichols B. W. The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta. 1973 Jul 18;311(4):531–544. doi: 10.1016/0005-2736(73)90128-4. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]


