Abstract
The ovarian follicular reserve has been linked to fertility in cattle. Young adult cattle with low vs. high numbers of antral follicles ≥ 3 mm in diameter in follicular waves also have fewer preantral follicles and decreased fertility. This underscores the importance of understanding the factors that regulate early follicular development and establish the ovarian follicular reserve, but little is known about how the follicular reserve is first established. In ruminants and humans, follicles form during fetal life, but there is a gap (about 50 d in cattle) between the appearance of the first primordial follicles and the first growing, primary follicles. In this review we present evidence that in cattle, fetal ovarian steroids (i.e., estradiol and progesterone) are negative regulators of both follicle formation and of the acquisition by newly formed follicles of the capacity to activate (i.e., initiate growth). The results indicate that capacity to activate is linked to the completion of meiotic prophase I by the oocyte. The inhibitory effects of estradiol on follicle activation were found to be reversible and correlated with inhibition of the progression of meiotic prophase I. Fetal bovine ovaries produce steroid hormones and production varies considerably during gestation and in a pattern consistent with the hypothesis that they inhibit follicle formation and capacity of newly formed follicles to activate in vivo. However, little was known about how steroid production is regulated. In our studies, both LH and FSH stimulated progesterone and estradiol production by ovarian pieces in vitro. The addition of testosterone to the culture medium enhanced estradiol production, especially when FSH was also present, but inhibited progesterone production, even in the presence of gonadotropins. Evidence is also presented for effects of maternal nutrition and health and for potential effects of estrogenic endocrine-disrupting chemicals on the size of the ovarian follicular reserve established during fetal life. In summary, fetal ovarian steroids may be important regulators of the early stages of follicular development in cattle. Therefore, external factors that alter steroid production or action may affect the size of the ovarian follicular reserve.
Keywords: cattle, endocrine-disrupting chemicals, maternal nutrition, ovarian follicle, ovarian follicular reserve, ovary
INTRODUCTION
Differentiation of the bipotential mammalian gonad begins when the primordial germ cells migrate to and enter the genital ridge. In females, the germ cells remain in the outer cortical region of the developing gonad and become oogonia. The oogonia proceed through a series of mitotic divisions and then become primary oocytes as they enter prophase of the first meiotic division. Meiosis arrests at the end of first prophase and primordial follicles form when somatic (pregranulosa) cells surround and flatten against primary oocytes. In the mammalian species that have been examined, mitosis of oogonia and initiation of meiotic prophase I occur before birth, but the timing of meiotic arrest at the diplotene stage of prophase I, follicle formation, and the initiation of follicle growth (i.e., follicle activation) varies from species to species. In rats and mice (reviewed in Pepling (2006)), oocytes proceed in synchrony through the leptotene, zygotene, and pachytene stages of prophase I during the last third of gestation (Fig. 1A). Shortly after birth (the exact time depends on the rodent species and strain), the oocytes arrest at the diplotene stage of first prophase, oocyte nests break down and follicles form fairly synchronously. Soon thereafter some primordial follicles leave the resting pool and activate to become growing, primary follicles. Activation involves a change in the shape of the granulosa cells from flattened (i.e., squamous) to cuboidal and the initiation of oocyte growth (Fig. 2). Some secondary follicles develop around the end of the first postnatal week.
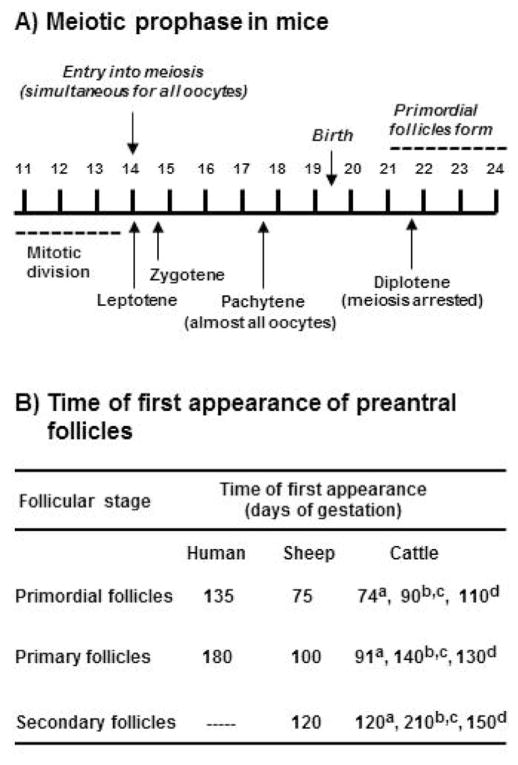
Figure 1.
(A) Timing of meiotic prophase I and formation of primordial follicles during pregnancy and early postnatal life in mice [adapted from Borum (1961)] and (B) reported times of first appearance of primordial, primary, and secondary follicles in fetal ovaries of humans [(van Wagenen and Simpson, 1965), sheep (McNatty et al., 1995), and cattle (aTanaka et al., 2001; bRusse, 1983; cYang and Fortune, 2008; dBurkhart et al., 2010)].
Figure 2.
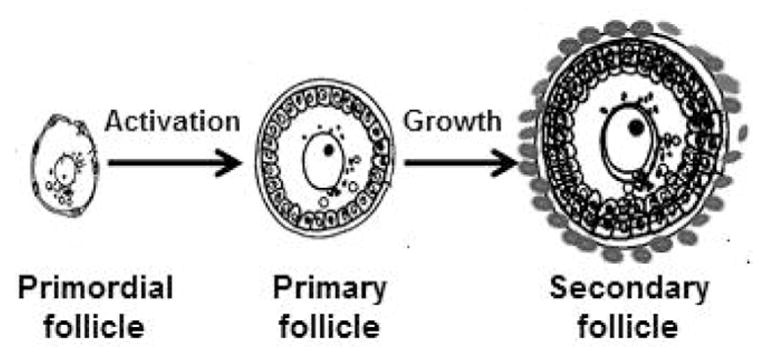
Schematic illustration of early stages of follicular development. Primordial (resting) follicles have a primary oocyte surrounded by flattened granulosa cells. During initiation of follicle growth (activation) granulosa cells become cuboidal in shape and the oocyte begins to grow. Secondary follicles have 2 or more layers of granulosa cells and theca cells progressively accumulate around the basement membrane that surrounds the granulosa cell-oocyte compartment.
The relative synchrony of these events in mice and rats and the fact that follicle formation and activation begin at predictable times after birth have made rodents popular animal models for studying the regulation of follicle formation. In contrast, in species of more practical interest, such as domestic ruminants and humans, meiotic arrest, follicle formation, and the initiation of follicle growth all occur before birth and much less synchronously than in rodents. However, it is of interest to understand the regulation of follicle formation in domestic animals and humans because it is the process that establishes the pool of resting primordial follicles that the female will draw on throughout her reproductive life span. The pool of primordial follicles is sometimes called the “ovarian follicular reserve,” but that term is also used to refer to all healthy ovarian follicles. It has been suggested that the size of the ovarian follicular reserve may determine the length of the reproductive life span.
DOES THE OVARIAN FOLICULAR RESERVE AFFECT FERTILITY IN CATTLE?
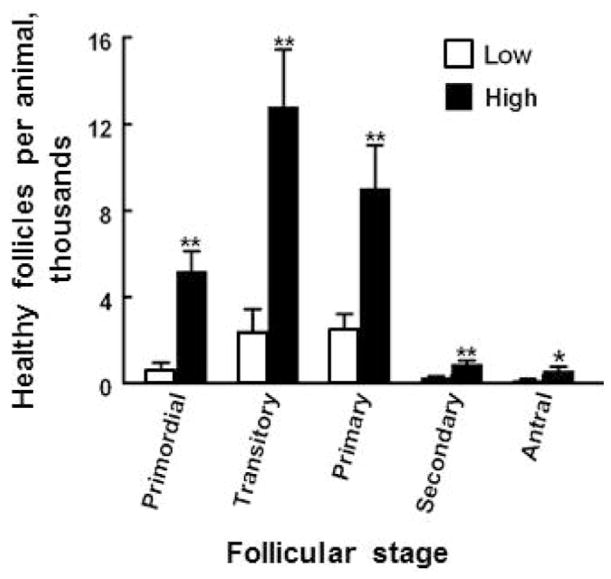
The follicular reserve is considered to be important for women because it may determine the time of menopause (Hansen et al., 2011). Reproductive senescence is not as important in a practical sense for cattle because most are sold for slaughter before it occurs. However, there is evidence that the number of follicles in the ovarian reserve is correlated with the fertility of individual female cattle. Collaborative research by groups at Michigan State University and University College (Dublin, Ireland) has provided interesting evidence of variability among animals in the bovine follicular reserve and of correlation between follicle numbers and fertility [reviewed in Evans et al. (2010, 2012) and Ireland et al. (2011)]. Their research first revealed consistent differences among young, age-matched cattle in numbers of antral follicles ≥ 3 mm in diameter in follicular waves. They further compared the ovaries of animals with high antral follicle count (AFC) to those with low AFC and found that the high or low AFC was reflected in significantly higher or lower numbers of follicles at other follicular stages (Fig. 3). Low AFC is also associated with low circulating concentrations of anti-Mullerian hormone (AMH), a protein hormone made by granulosa cells. Low AFC females had low concentrations of circulating progesterone during the luteal phase and a thinner uterine endometrium. Other experiments indicated that low AFC females respond more poorly to superovulatory protocols and have greater blood concentrations of FSH and LH and oocytes with greater steady state abundance of mRNAs that are markers for poor oocyte quality, compared with cattle with high AFC. More recently these researchers have provided evidence that low AFC is associated with impaired fertility, specifically with reduced conception rates to first service and with a longer interval from calving to conception (Mossa et al., 2012). Taken together, these findings are very provocative because they indicate that events that occur in fetal ovaries, formation of follicles and their survival or demise, may influence ovarian function in adults and, thus, in the reproductive success of a female. Our laboratory is interested in understanding the regulation of the early stages of follicular development and the events that give rise to the ovarian follicular reserve. Some of our findings will be discussed in the sections that follow.
Figure 3.
Numbers of morphologically healthy follicles at various stages of development in ovaries of young adult heifers with a low (≤15 follicles, n = 5) or high (≥25 follicles, n = 5) number of follicles ≥3 mm in diameter (antral follicle count, AFC) during ovarian follicular waves. Data are derived, with permission, from Ireland et al. (2008), where additional experimental details are provided. Asterisks (*P < 0.05; ** P <0.01) indicate difference between means for Low v. High AFC groups.
WHEN DO BOVINE FOLICLES FORM?
As mentioned previously, follicles form in ruminant and human ovaries well before birth. In fetal ovaries of cattle and sheep, oogonia become enclosed in long ovigerous cords that extend from the corticomedullary border to the ovarian surface epithelium (Sawyer et al., 2002; Hummitzsch et al., 2013). Mitosis of oogonia and their transition to primary oocytes occur within the cords, which also contain somatic (pregranulosa) cells. As follicles form, by enclosure of an oocyte and a few pregranulosa cells within a basement membrane, the follicle breaks free of the ovigerous cord. The oocyte nests or cysts that have been described in rodent ovaries do not appear to be a feature of bovine ovaries. Because gestation is relatively long in ruminants and the pace of ovarian development is relatively slow, it can be difficult to pinpoint exactly when specific follicular milestones are reached. This is illustrated by the varying published estimates of when primordial, primary, and secondary follicles first appear in bovine ovaries (Fig. 1B). For example, Russe (1983) first detected these stages at 90, 140, and 210 d of gestation, respectively, whereas the estimates of Tanaka et al. (2001) were quite different (i.e., 74, 91, and 120 d, respectively) and those of Burkhart et al. (2010) were more similar (110, 130, and 150 d) to data of Russe (Fig. 1B). As a prelude to our studies of follicle formation and activation, we reexamined the question of when preantral follicular stages first appear in bovine ovaries, using fetal ovaries from a nearby slaughterhouse and estimating fetal age by crown-rump length (Evans and Sack, 1973). Our observations agreed with those of Russe (1983); the first primordial, primary, and secondary follicles were observed around 90, 140, and 210 d of gestation, respectively (Yang and Fortune, 2008). It is interesting that in humans, sheep, and cattle, there is a considerable lag between the formation of primordial follicles and the appearance of the first activated (primary) follicles (Fig. 1B). This stands in distinct contrast to the almost immediate exit of a subset of newly formed rodent follicles from the resting pool of primordial follicles a few days after birth. Our laboratory previously developed methods for studying follicle activation in vitro, using pieces of ovarian cortex from fetal bovine ovaries obtained during the third trimester, a time when activation is occurring in vivo (Wandji et al., 1996). More recently, we adapted those methods to study the regulation of follicle formation in cattle and to address the question of why newly formed bovine primordial follicles do not activate in vivo.
WHAT REGULATES THE FORMATION OF BOVINE FOLICLES?
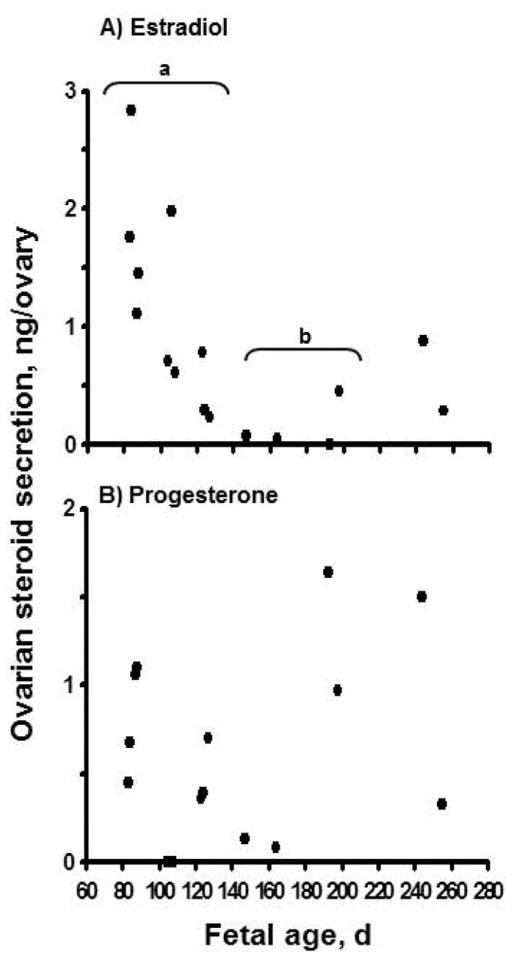
When newborn rat ovaries were treated in vitro with estradiol or progesterone, follicle assembly (i.e., formation) was inhibited (Kezele and Skinner, 2003). Results of studies by M. E. Pepling’s laboratory supported the concept that breakdown of oocyte cysts and follicle formation occur shortly after birth in mice because female pups are no longer exposed to the high concentrations of steroids (i.e., estradiol and progesterone) circulating in the mother (Jefferson et al., 2006; Chen et al., 2007). In cattle and sheep, fetal ovaries synthesize steroids and steroid production varies considerably during gestation (Shemesh et al., 1978; Dominguez et al., 1988; Quirke et al., 2001; Yang and Fortune, 2008; Nilsson and Skinner, 2009). Estradiol and progesterone are increased during early gestation when germ cells (i.e., oogonia) are very active mitotically, but production of these steroids begins to decline around the beginning of the second trimester (Yang and Fortune, 2008; Nilsson and Skinner, 2009), when follicles begin to form and later gain competence to activate (Fig. 4). This indicated that endogenous ovarian steroids may play a role in regulating early follicular development in cattle.
Figure 4.
(A) Estradiol and (B) progesterone secretion (ng/ovary per 24 h) in vitro by ovaries from bovine fetuses at different gestational ages. One ovary from each fetus was cut into pieces (0.5 to 1 mm3). The pieces were cultured for 24 h and steroid concentrations in the culture medium were determined by RIA. Different letters (a, b) indicate that mean estradiol levels differ (P < 0.05). Adapted from Yang and Fortune (2008), with permission.
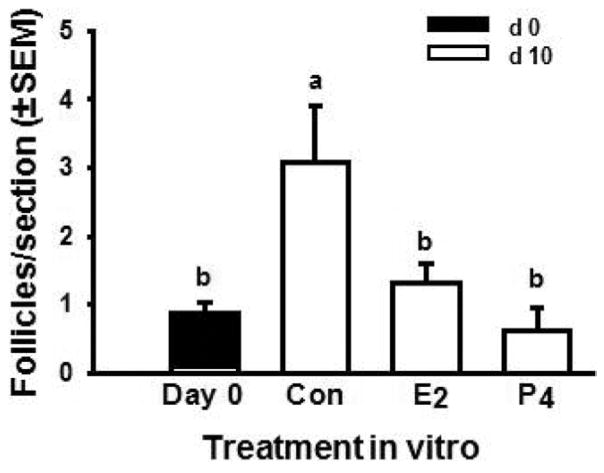
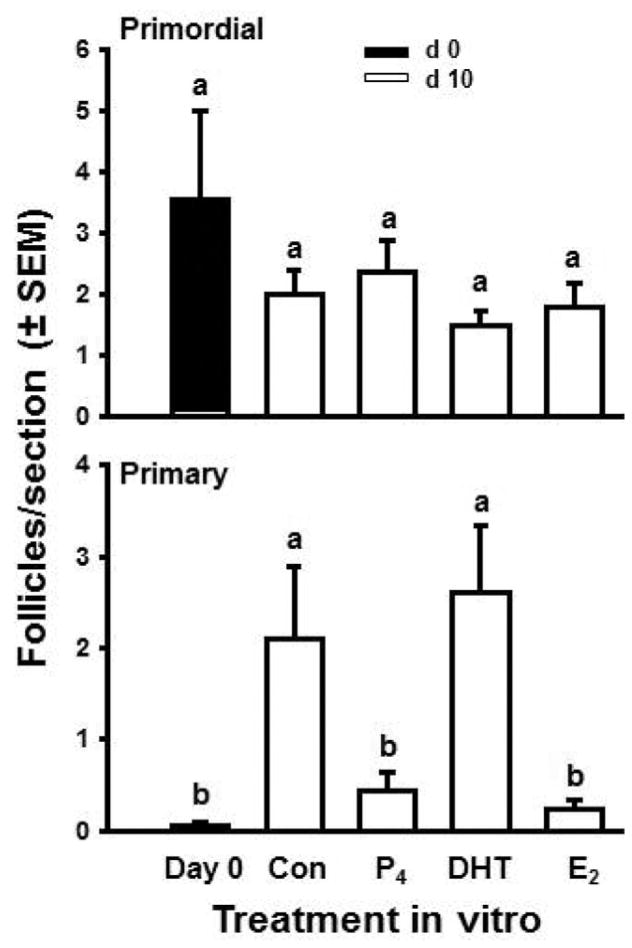
To test that hypothesis we used fetal bovine ovaries at an early stage during follicle formation (approximately 100 d of gestation) and cultured cortical pieces for 10 d with or without 1 μM estradiol or progesterone (Fortune et al., 2010). On d 0 or after 10 d, cortical pieces were fixed, serially sectioned, and analyzed by histological morphometry. Cortical pieces cultured in control medium for 10 d had about three times as many follicles as cortical pieces on d 0 (Fig. 5). In the presence of the steroids, the ovarian tissue and follicles remained healthy, but follicle numbers did not increase during culture. These results show that bovine follicles can form during culture of cortical pieces and that both estradiol and progesterone can inhibit follicle formation in vitro. Consistent with our findings for progesterone, Nilsson and Skinner (2009) reported that 1 μM progesterone significantly reduced the percentage of oocytes that were enclosed within follicles in cultures of fetal bovine cortex obtained during the period of follicle formation. These results, together with the patterns of ovarian steroid production around the time of follicle formation in vivo (Fig. 4), indicate that ovarian steroids may negatively regulate follicle formation in vivo in fetal bovine ovaries. Subsequent experiments showed that lower doses of the steroids can also inhibit follicle formation and that the nonaromatizable androgen, 5a-dihydrotestosterone (DHT), is without effect (M. Y. Yang and J. E. Fortune, unpublished data). Results of the dose–response studies suggested that progesterone is not acting merely as a precursor for estradiol synthesis because cortical pieces were at least as sensitive to progesterone as to estradiol. These findings suggest that in vivo the local concentration of steroids in the micro-environment acts as a regulator of follicle formation. Because there is a 50-d gap between the beginning of follicle formation and the appearance of the first activated, primary follicles in vivo (Fig. 1B), it appeared to us that formation of the initial follicular reserve during fetal life might also include acquisition by newly formed follicles of the ability to activate. This question is addressed in the following section.
Figure 5.
Effects of steroids on bovine follicle formation in vitro. Numbers of follicles (primordial + primary) in ovarian cortical pieces from around d 100 of gestation after 0 (black bar) or 10 (white bar) d in culture with control medium (Con), estradiol (E2), or progesterone (P4; both at 1 μM). Data are means ± SEM; means with different letters (a, b) differ (P < 0.05; n = 4; 2 cultures from each of 2 fetuses). Adapted from Fortune et al. (2010).
HOW DO BOVINE FOLICLES GAIN THE CAPACITY TO ACTIVATE?
Why is there a significant gap between follicle formation and activation? We hypothesized that newly formed follicles might lack the capacity to activate or, alternatively, that they might have that capacity but be prevented from activating by the presence of an inhibitor. Because primordial follicles reside in the ovarian cortex, small pieces of ovarian cortex from 90- to 140-d-old ovaries were cultured for 2 d and then fixed, sectioned, and analyzed histologically for numbers and stages of follicles. Primordial follicles had not activated after 2 d of culture (Yang and Fortune, 2008), although most primordial follicles activate within 1 to 2 d in vitro when cortical pieces are obtained from older, third-trimester ovaries (Wandji et al., 1996). This result indicates that before d 140 of gestation, bovine primordial follicles lack the capacity to activate. To determine if they could gain the capacity to activate in vitro, the experiment was repeated and cortical pieces were cultured for either 2 or 10 d. Again, there was no significant increase in the number of primary follicles after 2 d of culture, compared with time 0, but after 10 d in vitro, activation had occurred (Yang and Fortune, 2008). This showed that primordial follicles gained the capacity to activate at some point during the 10-d culture, earlier than they would have done so in vivo, supporting the hypothesis that an inhibitor of follicular capacity to activate is present in vivo.
When newborn rat ovaries were treated in vitro with progesterone, both follicle assembly and activation were inhibited (Kezele and Skinner, 2003). Treatment of prenatal mouse ovaries with estradiol or progesterone in vitro reduced both primordial and primary follicles (Chen et al., 2007). This indicated that endogenous ovarian steroids might play a role in regulating the acquisition of the capacity to activate by newly formed follicles in cattle. Therefore, we tested the effects of 1 μM estradiol and progesterone, steroids that were effective in blocking follicle formation and activation in mice and rats, on the activation of newly formed bovine follicles in cortical pieces in vitro. After 10 d of culture, both steroids had inhibited follicle activation, maintaining the number of primary follicles at levels similar to control tissue on d 0 (Fig. 6). In contrast, the nonaromatizable androgen, DHT, did not prevent primordial follicles from gaining the capacity to activate and numbers of primary follicles were similar to control cultures on d 10 (Fig. 6; Yang and Fortune, 2008). Subsequent experiments in our laboratory showed that the inhibitory effects of progesterone and estradiol on development of the capacity to activate are dose-dependent (range = 0.01 to 1 μM) and that progesterone is more effective than estradiol at lower doses, suggesting that it does not act simply as a precursor for estradiol synthesis (M. Y. Yang and J. E. Fortune, unpublished data).
Figure 6.
Effects of control medium (Con), progesterone (P4), 5α-dihydrotestosterone (DHT), and estradiol (E2; steroids at 1 μM) on follicle activation in vitro in ovarian cortical pieces from 91- to 140-d-old fetal calves. Mean number of follicles per section (±SEM) at time 0 and after 10 d of culture. Means with different letters (a, b) within a panel differ (P < 0.05; n = 8; 2 cultures from each of 4 fetuses). Adapted from Yang and Fortune (2008), with permission.
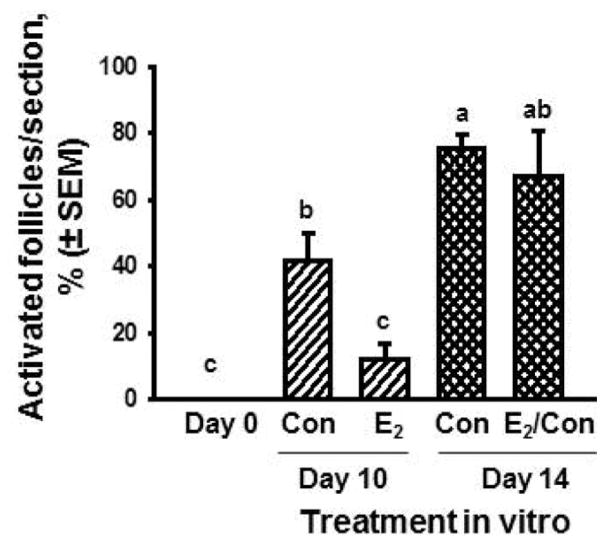
The results of the experiments with exogenous steroids support the hypothesis that the concentration of estradiol and (or) progesterone in the local environment may inhibit (at high concentrations) or allow (at low concentrations) follicular acquisition of the capacity to activate. But why would bovine follicles not be capable of activating when they are first formed and need to be restrained from activating for around 50 d? The older literature is conflicting on this point, but there are suggestions that bovine oocytes may become incorporated into follicles before they have arrested at the diplotene stage at the end of meiotic prophase I (Baker and Franchi, 1967). Therefore, we hypothesized that newly formed bovine follicles cannot activate because they have not achieved meiotic arrest at the diplotene stage of prophase I. Examination of serial paraffin sections stained with hematoxylin and eosin revealed that most oocytes in follicles are at prediplotene stages of prophase I before d 140 of gestation, whereas most are in diplotene after that time (Yang and Fortune, 2008). In addition, steady state levels of mRNA for YBX2, a specific marker for diplotene oocytes, increase in ovarian cortical tissue after d 140 (Yang and Fortune, 2008). More recent experiments showed that the inhibitory effects of estradiol in vitro on acquisition of the capacity to activate are reversible (Fig. 7; Fortune et al., 2010) and are accompanied by inhibition of meiotic progression to diplotene (data not shown). To test the hypothesis that estradiol specifically inhibits activation of newly formed follicles, cortical pieces from fetuses older than 140 d were cultured with estradiol. There was no effect on activation, indicating that estradiol’s inhibitory effects are specific to newly formed follicles that have not achieved meiotic arrest (M. Y. Yang and J. E. Fortune, unpublished data). This finding is consistent with the suggestion by Kezele and Skinner (2003) that the inhibitory effects of steroids on activation of rat follicles are restricted to the initial wave of follicle activation that occurs shortly after follicle formation.
Figure 7.
Percentage of activated follicles in bovine cortical pieces cultured with control medium (Con) or estradiol (E2; 1 μM) for 10 or 14 d (means ± SEM). The E2/Con: d 0 to 10 cultured with E2 and d 10 to 14 with control medium. Cortical pieces were dissected from 100 to 120-d-old fetal calves. Means with no common letters (a, b, c) differ (P < 0.05; n = 4, 2 cultures from each of 2 fetuses). Adapted from Fortune et al. (2010).
Clearly there is much more to be learned about how steroids exert their effects on follicle formation and activation in cattle. However, the results thus far underscore the potential importance of regulation by steroid hormones in the establishment of the bovine follicular reserve and indicate that factors that alter fetal steroid levels or patterns may affect the size of the initial follicular reserve. For example, manipulation of the steroidal environment of the fetus in vivo by injection of androgen or an aromatase inhibitor affected the size of the follicular reserve in adulthood in sheep and baboons, respectively (Steckler et al., 2005; Albrecht and Pepe, 2010).
REGULATION OF FETAL BOVINE STEROIDOGENESIS
The data discussed previously provide evidence that fetal steroids may play an important role in regulating the formation of primordial follicles in cattle and their development of the capacity to initiate growth. Our results and those of other researchers have also shown that fetal bovine ovaries produce steroid hormones and that ovarian steroid production varies considerably over the course of gestation (Shemesh et al., 1978; Dominguez et al., 1988; Quirke et al., 2001; Yang and Fortune, 2008; Nilsson and Skinner, 2009). Garverick et al. (2010) detected mRNA and protein for aromatase and for estrogen receptor (ER) α and β in fetal bovine ovaries during the first trimester of pregnancy. However, little is known about what regulates ovarian steroid production by fetal bovine ovaries. The regulation of steroid production by preovulatory follicles is summarized by the 2-cell, 2-gonadotropin model: theca cells synthesize androgens (androstenedione in cattle) under the influence of LH, but lack the aromatase enzyme necessary to convert them to estrogens, whereas granulosa cells cannot convert progestins to androgens, but respond to FSH by converting exogenous androgen to estradiol [reviewed in Fortune and Quirk (1988)]. However, fetal ovarian steroid production is greatest before any follicles are formed (Fig. 4), so it is not known whether or not the pathway from cholesterol to estradiol is present entirely in one fetal ovarian cell type or if 2 or more cell types participate.
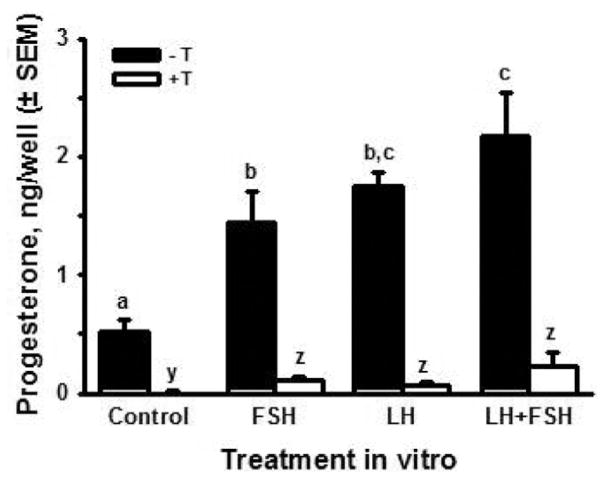
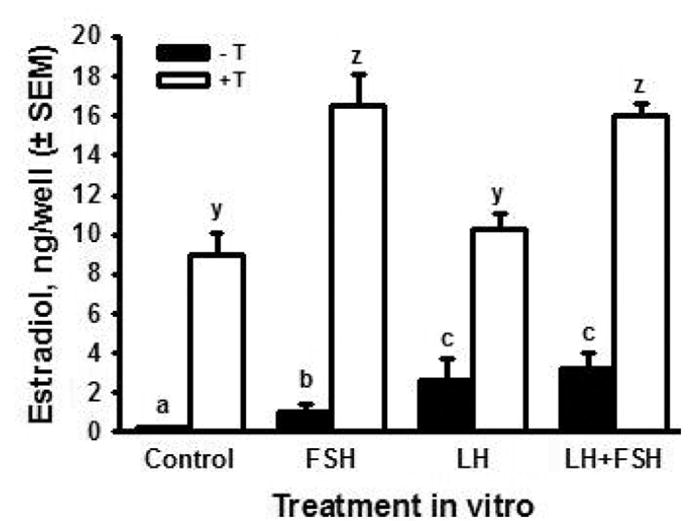
Since gonadotropins have been detected in the fetal bovine circulation (Challis et al., 1974; Tanaka et al., 2001) and fetal ovaries have binding sites for FSH and LH (Wandji et al., 1992), we hypothesized that LH stimulates production of progesterone and androgens and that FSH stimulates the conversion of aromatizable androgens to estradiol. To test these hypotheses, ovaries obtained on d 80 to 89 of gestation (i.e., when steroid production is still fairly robust and just before follicle formation begins) were cut into pieces and the pieces were distributed to culture wells at random (2 pieces per well). Ovarian pieces were cultured in the presence or absence of testosterone (0.5 μM), with or without 100 ng/mL ovine (o) LH (oLH S-26) and (or) FSH (oFSH S-17). Medium was collected and replaced every other day for 10 d. Fig. 8 shows estradiol accumulation in the medium over 10 d of culture. In the presence or absence of gonadotropins, estradiol was much greater in the cultures with exogenous testosterone. This suggests that production of estradiol by the fetal ovary is limited by the availability of androgen precursor, at least during the time period we examined. In the absence of testosterone, estradiol was significantly increased by both LH (about 10-fold) and FSH (about 5-fold) and the effect of their combination was similar to LH alone. In the presence of exogenous testosterone, FSH almost doubled the accumulation of estradiol, compared with testosterone alone, whereas LH had no effect. The combination of FSH plus LH produced the same effect as FSH alone. Taken together, these results support the hypothesis that LH increases the production of androgen by fetal ovarian tissue late in the first trimester, whereas FSH stimulates the conversion of androgen to estradiol. Preliminary results confirmed that LH can increase androgen production in ovarian pieces in vitro, but much more work is needed to provide a fuller understanding of the regulation of fetal estradiol production at this stage of gestation.
Figure 8.
Cumulative production of estradiol (ng/well ± SEM; n = 6, 2 cultures from each of 3 fetuses) by ovarian pieces from fetal bovine ovaries (80 to 89 d of gestation) cultured for 10 d in control medium with or without FSH (100 ng/mL) and (or) LH (100 ng/mL), in the presence (open bars) or absence (black bars) of testosterone (T, 0.5 μM). Means with no common letters (-T: a,b,c and +T: y,z) are different (P < 0.05).
Both FSH and LH increased the accumulation of progesterone in the culture medium, in the absence of testosterone, over 10 d of culture (Fig. 9). In contrast to the positive effect of testosterone on fetal ovarian estradiol production, testosterone dramatically inhibited progesterone accumulation in control cultures and completely abolished the stimulatory effects of the gonadotropins, reducing progesterone to concentrations less than those in the control cultures (Fig. 9). Because the results for estradiol production suggested that androgens are rate-limiting in the production of estradiol by fetal ovarian tissue, it is difficult to know if the inhibitory effect of testosterone on progesterone production by the fetal ovary is meaningful physiologically. Dose–response studies should be helpful in addressing that question.
Figure 9.
Cumulative production of progesterone (ng/well ± SEM; n = 6, 2 cultures from each of 3 fetuses) by ovarian pieces from fetal bovine ovaries (80 to 89 d of gestation) cultured for 10 d in control medium with or without FSH (100 ng/mL) and (or) LH (100 ng/mL), in the presence (open bars) or absence (black bars) of testosterone (T, 0.5 μM). Means with no common letters (-T: a,b,c and +T: y,z) are different (P < 0.05).
These results indicate that gonadotropins may play roles in regulating fetal ovarian steroidogenesis and that steroids themselves may act as regulators of steroid production. It is not clear why the capacity of the fetal ovary to make estradiol decreases dramatically just before the start of follicle formation; the mechanisms of that decline are unknown. Clearly, we are just beginning to scratch the surface of understanding how ovarian fetal steroid production is regulated.
WHAT DETERMINES THE SIZE OF THE OVARIAN FOLICULAR RESERVE?
Maternal Nutrition and Health
Research by the groups at Michigan State University and University College Dublin provided evidence that the size of the follicular reserve is correlated with fertility in dairy cattle (summarized previously). Since the pool of primordial follicles is established primarily during the second trimester of bovine gestation, it seems possible that the size of the follicular pool might be influenced by the environment of the fetus before and during that time. One factor that, logically, might be linked to the number of primordial follicles formed is the nutritional status of the mother. Mossa et al. (2009) fed pregnant cattle either a control diet or a nutritionally restricted diet (60% of maintenance energy requirements) for the first 110 d of gestation and examined the ovaries of their heifer calves at 7, 18, and 35 wk of age. At each time point, the antral follicle count of the females whose dams had been nutritionally deprived was about 60% that of the control heifers (P < 0.01; Table 1). Since the period of nutritional restriction encompassed the differentiation of the gonad as female, mitosis of oogonia, and the first 20 d of follicle formation, and since low AFC has been correlated with low follicle counts in all follicular size classes (Fig. 3), these data suggest that nutrition during early pregnancy is critically important to the size of the follicular reserve. These findings are consistent with the relationship between birth weight and AFC in beef cows reported by Cushman et al. (2009). Similarly, Bernal et al. (2010) reported that in rats maternal undernutrition during pregnancy and lactation, a period that includes follicle formation, resulted in a significant reduction in the number of primordial follicles in female progeny at 150 d of age.
Table 1.
Mean (±SEM) number of follicles ≥3 mm in diameter (antral follicle count) during follicle waves in heifer calves born to mothers fed a control diet (control) or who were nutritionally restricted (restricted, 60% energy requirement) for the first 110 d of gestation (from Mossa et al., 2009)
| Antral follicle count | |||
|---|---|---|---|
|
| |||
| Heifer age, wk | Control (n = 13) | Restricted (n = 10) | P < |
| 7 | 23.9 ± 2.1 | 14.1 ± 0.9 | 0.01 |
| 18 | 26.1 ± 2.9 | 16.2 ± 1.1 | 0.01 |
| 35 | 23.9 ± 2.2 | 16.6 ± 1.2 | 0.01 |
There is also evidence indicating that impairment of maternal health during bovine gestation may negatively affect the size of the follicular reserve. Cows with an elevated somatic cell count in their milk, which is indicative of mammary gland infection, gave birth to heifers with circulating concentrations of AMH that were about one-half those in female calves born to cows with low somatic cell counts (Ireland et al., 2011; Evans et al., 2012). The concentration of AMH in the blood of female cattle has been correlated with the antral follicle count and with the size of the bovine ovarian follicular reserve (Ireland et al., 2011).
Taken together, the results of these experiments indicate that factors like maternal nutrition and health of the dam may affect the number of follicles formed during fetal life. The data provide evidence for fewer antral follicles or lower circulating AMH in the female offspring after birth and these characteristics are correlated with reduced adult fertility.
Endocrine-Disrupting Chemicals
There is increasing concern about the potential threat of endocrine-disrupting chemicals (EDCs) to the health and reproduction of farm animals (Rhind, 2005). Endocrine-disrupting chemicals include phytoestrogens, biologically active natural substances commonly found in forages, and environmental estrogens found in pesticides, plastics, and industrial chemicals. Physiological concentrations of estrogens are essential for female reproduction, but estrogenic EDCs can mimic or disrupt the actions of endogenous estrogens. Cattle are potentially at risk for reproductive effects of both phytoestrogens and environmental estrogens. Adverse effects of phytoestrogens on fetal development have been reported for rodents (Chan, 2009). Brief exposures to environmental estrogens, such as bisphenol A (BPA), during fetal development may adversely affect oocyte development in mice (Susiarjo et al., 2007) and in women, exposure to BPA is correlated with recurrent miscarriage (Sugiura-Ogasawara et al., 2005).
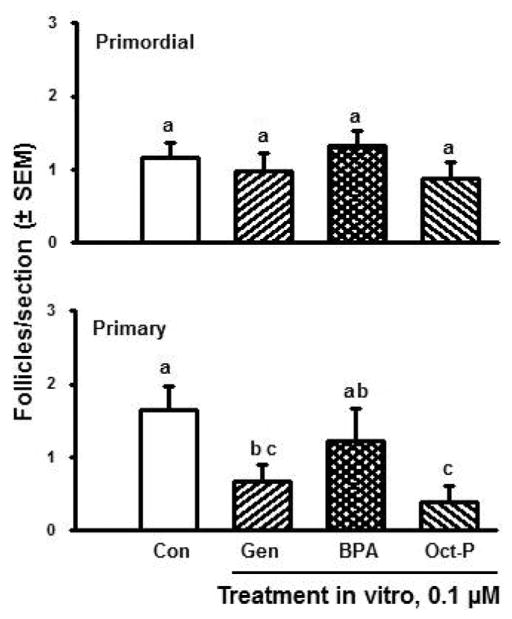
Since estradiol appears to be an important regulator of the formation and capacity to activate of bovine follicles, we have used the in vitro models described previously to begin to assess whether phyto- and environmental estrogens might affect the establishment of the follicular reserve in cattle. Cortical pieces were prepared from fetal ovaries during follicle formation (i.e., d 100 to 120 of gestation), cultured for 10 d, and then processed for morphometric analysis of follicle numbers and types. In control cultures, some follicles had activated after 10 d, as expected (discussed previously). However, the number of primary follicles was significantly reduced in the presence of the phytoestrogen, genistein, and the environmental estrogen, octylphenol, whereas the environmental estrogen BPA was without significant effect (Fig. 10). Further experimentation revealed that genistein and octylphenol both exerted dose-dependent inhibitory effects on follicle numbers; they appeared to inhibit both follicle formation and activation (data not shown). Follicle numbers were not affected by BPA at any of the doses tested (data not shown). However, the results of experiments in progress show that BPA exerts specific effects on steroidogenesis by the fetal bovine ovarian pieces in vitro. These findings indicate that exposure of bovine fetal ovaries to estrogenic EDCs in vivo may negatively affect the processes that lead to the establishment of the initial ovarian follicular reserve, that is, follicle formation and development of the capacity to activate.
Figure 10.
Numbers of primordial and primary follicles (mean number per section ± SEM; n = 6 cultures, 2 from each of 3 fetuses) in ovarian cortical pieces cultured for 10 d in control medium (Con) or with the phytoestrogen genistein (Gen) or the environmental estrogens bisphenol A (BPA) or octylphenol (Oct-P), all at 0.1 μM. Ovarian pieces were isolated from ovaries of bovine fetuses at 100 to 120 d, a time when follicle formation is in progress. Means with no common letters (a,b,c) are different (P < 0.05).
SUMARY AND CONCLUSIONS
In ruminants and humans, the ovarian follicular reserve is established during fetal life when primordial follicles form and develop the capacity to initiate growth. In cattle, results of experiments in vitro suggest that estradiol and progesterone, synthesized by the fetal ovary, play a role in regulating the timing of these critical events, but their mechanisms of action remain to be elucidated. Both LH and FSH stimulate steroid production by fetal ovarian pieces in vitro, and the results suggest that LH increases androgen synthesis, whereas FSH stimulates the aromatization of androgen to estradiol. The finding that the number of antral follicles in follicular waves in cattle is correlated both with the total number of follicles in the ovaries and with fertility indicates that the size of the pool of resting primordial follicles established during fetal life may have an impact on the reproductive performance of adult cattle. This idea is consistent with data showing that nutritional deprivation of the dam during early pregnancy produces heifer calves with fewer follicles in waves compared with daughters of nutritionally-replete cows. In addition, studies in vitro showed that early follicular development can be impaired by exposure of fetal ovarian tissue to estrogenic EDCs. Taken together, these results not only provide evidence of the importance of the ovarian follicular reserve for fertility, but also indicate that the size of the reserve can be affected by the environment of the fetus during development.
Footnotes
Based on a presentation at the Triennial Reproduction Symposium titled “Impediments to Fertility in Domestic Animals” preceding the Joint Annual Meeting, July 15–19, 2012, Phoenix, Arizona. The symposium was sponsored, in part, by the The James Lauderdale Appreciation Club, The ASAS Foundation, Elanco Animal Health (Greenfield, IN), and Pfizer Animal Health (New York, NY), with publication sponsored by the American Society of Animal Science and the Journal of Animal Science.
Research from the authors’ laboratory was supported by the National Research Initiative Competitive Grant 2008-35203-05989 from the USDA National Institute of Food and Agriculture, the NIH (HD060232), and a USDA Hatch award.
LITERATURE CITED
- Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 2010;54:397–407. doi: 10.1387/ijdb.082758ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG, Franchi LL. The fine structure of chromosomes in bovine primordial oocytes. J Reprod Fertil. 1967;14:511–513. doi: 10.1530/jrf.0.0140511. [DOI] [PubMed] [Google Scholar]
- Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res. 1961;24:495–507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- Burkhart MN, Juengel JL, Smith PR, Heath DA, Perry GA, Smith MF, Garverick HA. Morphological development and characterization of aromatase and estrogen receptors alpha and beta in fetal ovaries of cattle from days 110 to 250. Anim Reprod Sci. 2010;117:43–54. doi: 10.1016/j.anireprosci.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Challis JR, Kim CK, Naftolin F, Judd HL, Yen SS, Benirschke K. The concentrations of androgens, oestrogens, progesterone and luteinizing hormone in the serum of foetal calves throughout the course of gestation. J Endocrinol. 1974;60:107–115. doi: 10.1677/joe.0.0600107. [DOI] [PubMed] [Google Scholar]
- Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod Toxicol. 2009;28:52–58. doi: 10.1016/j.reprotox.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Cushman RA, Allan MF, Kuehn LA, Snelling WM, Cupp AS, Freetly HC. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: Investigation of influence of stage of the estrous cycle, age, and birth weight. J Anim Sci. 2009;87:1971–1980. doi: 10.2527/jas.2008-1728. [DOI] [PubMed] [Google Scholar]
- Dominguez MM, Liptrap RM, Basrur PK. Steroidogenesis in fetal bovine gonads. Can J Vet Res. 1988;52:401–406. [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Mossa F, Fair T, Lonergan P, Butler ST, Zielak-Steciwko AE, Smith GW, Jimenez-Krassel F, Folger JK, Ireland JL, Ireland JJ. Causes and consequences of the variation in the number of ovarian follicles in cattle. In: Lucy MC, Pate JL, Smith MF, Spencer TE, editors. Reproduction in Domestic Ruminants VII Soc Reprod Fertil. Vol. 67. Nottingham Univ. Press; Nottingham, UK: 2010. pp. 421–429. [DOI] [PubMed] [Google Scholar]
- Evans ACO, Mossa F, Walsh SW, Scheetz D, Jimenez-Krassel F, Ireland JLH, Smith GW, Ireland JJ. Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod Domest Anim. 2012;47:31–37. doi: 10.1111/j.1439-0531.2012.02052.x. [DOI] [PubMed] [Google Scholar]
- Evans HE, Sack WO. Prenatal development of domestic and laboratory mammals: Growth curves, external features and selected references. Anat Histol Embryol. 1973;2:11–45. doi: 10.1111/j.1439-0264.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Quirk SM. Regulation of steroidogenesis in bovine preovulatory follicles. J Anim Sci. 1988;66:1–8. [Google Scholar]
- Fortune JE, Yang MY, Muruvi W. The earliest stages of follicular development: Follicle formation and activation. In: Lucy MC, Pate JL, Smith MF, Spencer TE, editors. Reproduction in Domestic Ruminants VII Soc Reprod Fertil. Vol. 67. Nottingham Univ. Press; Nottingham, UK: 2010. pp. 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garverick HA, Juengel JL, Smith P, Heath DA, Burkhart MN, Perry GA, Smith MF, McNatty KP. Development of the ovary and ontongeny of mRNA and protein for P450 aromatase (arom) and estrogen receptors (ER) alpha and beta during early fetal life in cattle. Anim Reprod Sci. 2010;117:24–33. doi: 10.1016/j.anireprosci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ. A new model of development of the mammalian ovary and follicles. PLoS One. 2013;8:e55578. doi: 10.1371/journal.pone.0055578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JL, Mossa F, Lonergan P, Evans AC. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev. 2011;23:1–14. doi: 10.1071/RD10226. [DOI] [PubMed] [Google Scholar]
- Ireland JLH, Sheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod. 2008;79:1219–1225. doi: 10.1095/biolreprod.108.071670. [DOI] [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: Inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Hudson NL, Heath DA, Tisdall DJ, WSO, Braw-Tal R. Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. J Reprod Fertil Suppl. 1995;49:123–135. [PubMed] [Google Scholar]
- Mossa F, Kenny D, Jimenez-Krassel F, Smith GW, Berry D, Bulter S, Fair T, Lonergan P, Ireland JJ, Evans ACO. Undernutrition of heifers during the first trimester of pregnancy diminishes size of the ovarian reserve in female offspring. Proc. 42nd Ann. Meet. Soc. Study Reprod; Pittsburg, PA. 2009. p. 77. (Abstr) [Google Scholar]
- Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans AC. Low numbers of ovarian follicles ≥ 3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci. 2012;95:2355–2361. doi: 10.3168/jds.2011-4325. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Progesterone regulation of primordial follicle assembly in bovine fetal ovaries. Mol Cell Endocrinol. 2009;313:9–16. doi: 10.1016/j.mce.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod. 2001;65:216–228. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- Rhind SM. Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod. Domest Anim. 2005;40:282–290. doi: 10.1111/j.1439-0531.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Russe I. Oogenesis in cattle and sheep. Bibl Anat. 1983;24:77–92. [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Shemesh M, Ailenberg M, Mileguir F, Ayalon N, Hansel W. Hormone secretion by cultured bovine pre- and postimplantation gonads. Biol Reprod. 1978;19:761–767. doi: 10.1095/biolreprod19.4.761. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: Prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nakada K, Moriyoshi M, Sawamukai Y. Appearance and number of follicles and change in the concentration of serum FSH in female bovine fetuses. Reproduction. 2001;121:777–782. [PubMed] [Google Scholar]
- van Wagenen G, Simpson ME. Homo sapiens and Macaca mulatta. Yale Univ. Press; New Haven, CT: 1965. Embryology of the ovary and testis. [Google Scholar]
- Wandji SA, Pelletier G, Sirard MA. Ontogeny and cellular localization of 125I-labeled insulin-like growth factor- I, 125I-labeled follicle-stimulating hormone, and 125I-labeled human chorionic gonadotropin binding sites in ovaries from bovine fetuses and neonatal calves. Biol Reprod. 1992;47:814–822. doi: 10.1095/biolreprod47.5.814. [DOI] [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;78:1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]