Introduction
Cardiac resynchronization therapy (CRT) has proved to be beneficial for patients with moderate-to-severe heart failure with prolonged QRS duration and reduced left ventricular ejection fraction. Placement of a left ventricular (LV) lead in an adequate location has been shown to increase response to CRT1, 2 but can become a complex procedure because of challenging coronary vein anatomies. We report the case of a patient in whom a novel LV lead was successfully implanted in a very-large-diameter coronary vein.
Case report
KEY TEACHING POINTS
|
A 75-year-old man was diagnosed with heart failure symptoms (New York Heart Association [NYHA] class III) due to postmyocardial infarction dilated cardiomyopathy. Based on ejection fraction of 30%, left bundle branch block, QRS duration of 160 ms, and permanent atrial fibrillation, CRT implantation was recommended.
Echocardiography with velocity vector imaging, speckle tracking, and strain rate measurements was performed to identify the region of latest mechanical activation and the optimal location for LV lead placement. Echocardiographic measurements revealed significant interventricular and intraventricular delay, with the mid to basal lateral wall being most delayed. Therefore, this location was chosen as the target for the LV lead.
A pocket was made in the left pectoral region. The coronary sinus was cannulated using a diagnostic catheter Medtronic Torqr 4F6F JSN inside together with an Attain Command+ Surevalve 6250VI-MB2 delivery system.
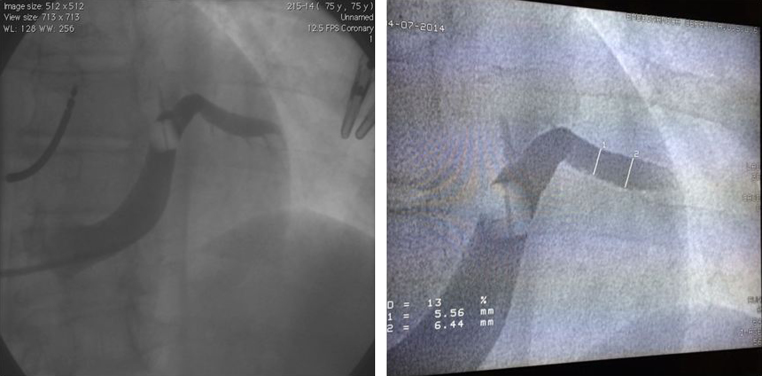
Venography of the coronary venous anatomy was performed using a Medtronic Attain Balloon 6215-80 cm to determine whether a suitable target vein for LV lead placement was available. The venogram revealed only 1 very-large-diameter coronary vein that did not have any side branches (Figure 1). The large-diameter vein was measured, and the vein size in the targeted location ranged from 6.44 mm (19.3Fr) to 5.56 mm (16.7Fr).
Figure 1.
Cardiac venography showing only a very-large-diameter coronary vein.
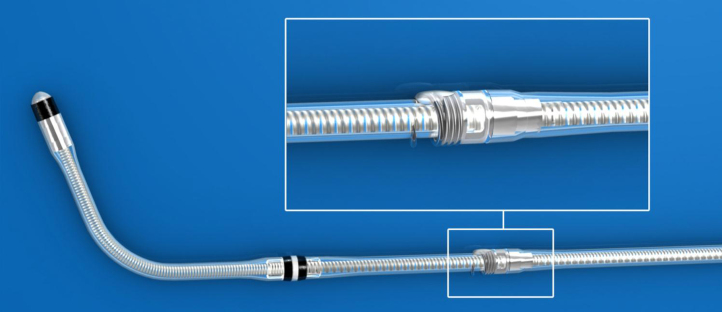
We discussed all the potential options and decided to place the Attain Stability LV lead (model 20066, Medtronic, Maastricht, the Netherlands) into the targeted location. This new LV lead, CE-marked and recently commercially available (outside the United States), is the first for use in the coronary sinus branches and has an active fixation mechanism based on a screw (Figure 2). The lead is a 4Fr bipolar, over-the-wire, steroid-eluting, active fixation lead that is a derivative of the market-released Attain Ability model 4196 lead. Like all other market-released LV leads, it can be delivered using a guidewire or stylet, and its size is compatible with subselection catheters for anatomies that have acute-angle side branches. The model 20066 LV lead has a small exposed side helix, located 36 mm proximal to the tip, that enables the implanter to securely fixate the lead to the vein wall by rotating the lead body. A key design feature is a mechanical stop at the base of the helix that prevents overtorquing and entrapment of the venous wall tissue. The helix is designed to avoid becoming caught on any component of the delivery sheath or the vein wall during insertion or retraction. It straightens when traction force must be applied during lead extraction. In a multicenter clinical study in which this lead was investigated for its safety and performance, 1 patient had a lead extraction at 12 months, with no issues during the study.3
Figure 2.
Model 20066 left ventricular lead with a close-up of the helix fixation mechanism.
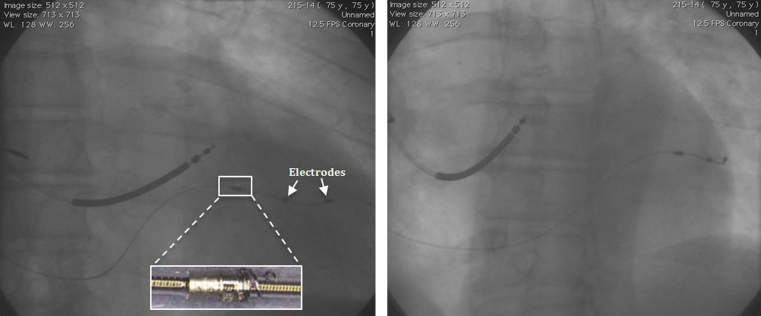
The lead was delivered to the target location using an Attain hybrid guidewire. We advanced the guidewire as distal as possible and were able to bow the helix toward the vessel wall. Despite the extreme diameter of the vein, we were able to easily rotate the lead body and screw the helix into the endothelial wall on our first attempt. The electrodes were placed in the mid lateral wall as intended (Figure 3).
Figure 3.
Final left ventricular lead position fixated to the main coronary sinus (left: right anterior oblique projection; right: left anterior oblique projection). Inset, left: Close-up of the helix.
Electrical measurements revealed a normal capture threshold of 0.7 V at 0.5-ms pulse width in a bipolar configuration, impedance of 800 Ω, and R wave of 9 mV. At 2-month follow-up, the electrical parameters were normal (threshold 0.75 V at 0.5 ms and impedance 850 Ω), and electrode position had remained stable (Table 1). After 4 months (November 2014), the patient had clinically improved from NYHA class III to class I–II despite a suboptimal percentage of pacing. Ejection fraction improved from 30% to 38%, with a consensual reduction of LV volumes from 180 to 160 mL. QRS duration decreased from 160 to 130 ms. Permanent atrial fibrillation was still present, as was moderate mitral regurgitation. Considering the patient’s clinical and echocardiographic improvement, atrioventricular nodal ablation was not planned per our usual protocol. The dose of beta-blocker was increased.
Table 1.
Electrical parameters at implant and at 2-month follow-up
| At implant | At 2-month follow-up | |
|---|---|---|
| R-wave amplitude | 9 mV | NA (9 mV manual) |
| Pacing threshold (at 0.5 ms) | 0.7 V invasive, 0.75 V with programmer | 0.75 V |
| Pacing impedance | 800 Ω | 850 Ω |
Discussion
Currently no alternative leads labeled for use in the LV are available on the market that would have allowed us to pace in the predetermined targeted location in this unusual anatomy (very large target vein). Wedging a quadripolar lead would have been an option, but because of the very large diameter of the vein we did not believe that any shape of lead would have been stable in the proximal/medium segment of the vein, and the most proximal electrode likely would not have been able to pace basal enough due to poor contact with the myocardium. Using an Attain Starfix (Medtronic), which contains an extendable lobe active fixation mechanism, would have an option, but in our opinion this lead would not have been stable in this vein because of the soft lobes. Moreover, the Starfix lead is a unipolar lead. There have been some reports of stenting leads into place,4 but because of the extreme diameter of this vein and discomfort with using stents on LV leads, we did not believe this to be an acceptable option. Referring the patient for epicardial lead placement also would have been a less appropriate option given the cost of an additional procedure and the challenges in placing the lead at the site of latest mechanical activation. This novel lead made it possible to achieve easy and stable placement in the targeted location despite the large diameter of the vein.
References
- 1.Khan F.Z., Virdee M.S., Palmer C.R. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 2.Saba S., Marek J., Schwartzman D. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region Trial. Circ Heart Fail. 2013;6:427–434. doi: 10.1161/CIRCHEARTFAILURE.112.000078. [DOI] [PubMed] [Google Scholar]
- 3.Yee R., Gadler F., Hussin A. Novel active fixation mechanism permits precise placement of a left ventricular lead: early results from a multicenter clinical study. Heart Rhythm. 2014;11:1150–1155. doi: 10.1016/j.hrthm.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Szilágyi S., Merkely B., Roka A. Stabilization of the coronary sinus electrode position with coronary stent implantation to prevent and treat dislocation. J Cardiovasc Electrophysiol. 2007;18:303–307. doi: 10.1111/j.1540-8167.2007.00722.x. [DOI] [PubMed] [Google Scholar]