Introduction
KEY TEACHING POINTS
|
Substrate-based radiofrequency (RF) ablation of scar-related ventricular tachycardia (VT) has become standard practice, often in addition to an intracardiac cardioverter-defibrillator.1, 2 VT procedures without identified substrate are particularly challenging cases, especially when VT is noninducible.3, 4, 5, 6
An intramural focus is often challenging to ablate using conventional RF ablation,7 and different options are or will be possible such as high-power unipolar ablation, bipolar ablation, irrigated needle ablation, or selective coronary ethanol injection.8, 9, 10, 11
The following case is used to illustrate a patient-tailored approach to choose between bipolar and ethanol ablation after a failed RF attempt, since needle ablation is not approved yet for human use.
Case report
We present the case of an 81-year-old man with an ischemic cardiomyopathy (decreased left ventricular ejection fraction of 30%) with 3-vessel disease, treated with coronary bypass surgery in 2004, and carrier of a dual-chamber pacemaker since 2002. The patient was in permanent atrial fibrillation with slow ventricular response. He entered the laboratory in incessant monomorphic VT (tachycardia cycle of 408 ms) for a procedure planned under local anesthesia and sedation. According to the baseline electrocardiographic characteristics, the ablation site was expected in the left ventricular outflow tract (LVOT) (compare the morphology of the VT with an inferior axis and a QS pattern in lead V1 in Figure 1). The procedure was performed using a 4-mm irrigated catheter (ThermoCool SF, Biosense Webster, Inc, Diamond Bar, CA) with a 3-dimensional electroanatomic mapping system (CARTO3, Biosense Webster, Inc), based on the earliest local activation, a 12/12 pace map, a QS pattern on the unipolar recording, and a presystolic electrogram signal before the initiating premature ventricular contraction (PVC) (Figure 2). Since the monomorphic tachycardia was well tolerated, entrainment attempts were performed but each attempt stopped the tachycardia. Ablation till 40 W in the septal LVOT region was only partially successful after a prolonged ablation time of 137 seconds with only transient suppression of the VT episodes during ablation, indicating a deep intramyocardial focus. Further activation mapping was performed: The great cardiac vein showed late activation, compared with the onset of the triggering PVC. Then, the aortic cusps were mapped and finally the right ventricular outflow tract (RVOT) was mapped. Ablation was performed on the opposing site of the LVOT at 40 W, and VT episodes shortened and disappeared during RF delivery, but quickly came back after ablation. Since an intramural septal focus was expected as a result of deductive extensive mapping in all surrounding chambers (left ventricle, right ventricle, and great cardiac vein; Figure 3), the question was—after 13 minutes of LVOT ablation and 3 minutes of RVOT ablation—whether to selectively inject ethanol into a distal coronary branch or to perform bipolar ablation.
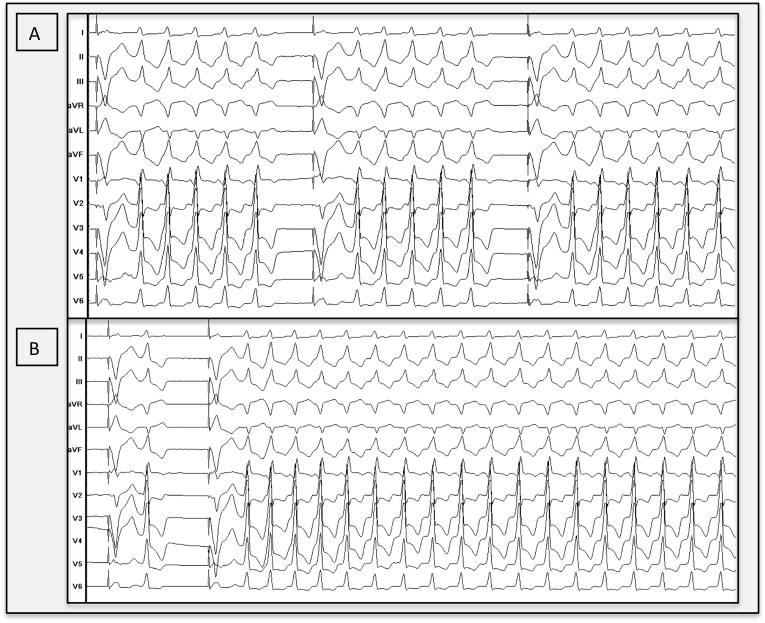
Figure 1.
Clinical ventricular tachycardia. A: Monomorphic clinical ventricular tachycardia (tachycardia cycle length 408 ms) with inferior axis and QS morphology in lead V1, suggesting an exit site at the aortomitral continuity. The patient is in permanent atrial fibrillation. B: In the electrophysiology laboratory, we observed poorly tolerated sustained ventricular tachycardia episodes.
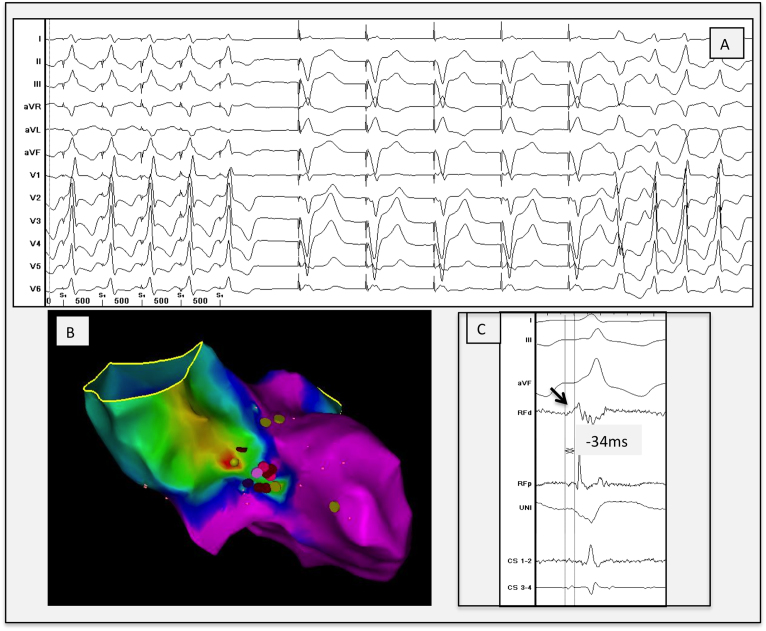
Figure 2.
A Perfect local pace map (first 5 paced beats) at the septal left ventricular outflow tract site illustrating a 12/12 pace-map match with the initiating premature ventricular contraction, followed by 5 paced beats from the right ventricular apex (device) followed by the initiating premature ventricular contraction and the clinical ventricular tachycardia. B: Anteroposterior view of the local activation map made by the CARTO3, demonstrating earliest activation (focal red spot) at the aortomitral continuity. C: Local electrogram at the ablation site, showing a low-voltage sharp electrogram 34 ms before QRS onset of the clinical ventricular tachycardia and an isoelectric phase followed by a QS morphology on the unipolar. CS = coronary sinus; d = distal; p = proximal; RF = radiofrequency; UNI = unipolar recording.
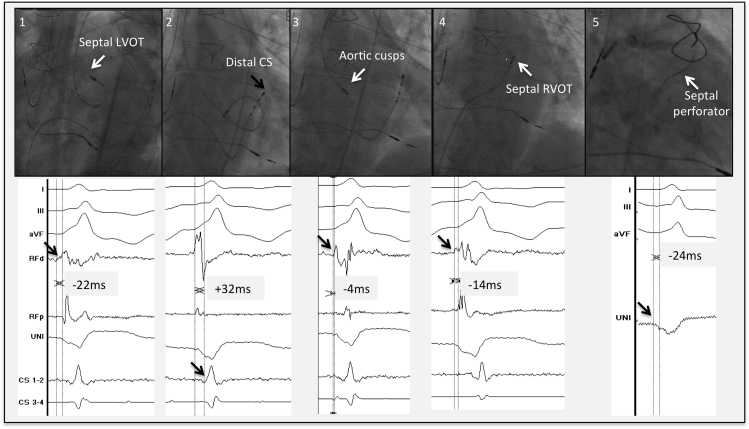
Figure 3.
Different regions are mapped in the outflow tract region: 1. Septal LVOT: small presystolic sharp signal at −22 ms from the onset of the QRS. 2. Great cardiac vein: local signal at +32 ms from the onset of the QRS. 3. Aortic cusps: presystolic local signal −4 ms from the onset of the QRS. 4. RVOT: local electrogram at −14 ms compared with the onset of the QRS. 5. Intraseptal: the unipolar signal of the guidewire shows a QS signal slightly before (±24 ms) the onset of the QRS. The exact onset of the unipolar signal was not easy to define in this case. CS = coronary sinus; d = distal; LVOT = left ventricular outflow tract; p = proximal; RF = radiofrequency; RVOT = right ventricular outflow tract.
In this particular patient, we chose to use ethanol ablation because of the reversible effect and the possibility to evaluate the effect of iced saline in incessant VT. A coronary angiogram was made, and a distal branch of the second septal perforator was selected for cannulation. The local unipolar recording of the guidewire shows an early activation (−24 ms) at this site with a QS pattern (Figure 3, point 5, lower panel). A reversible challenge with 1.5-cm3 sterile cold saline was performed after intracoronary balloon inflation and clearly affected VT episodes: directly after the injection, VT accelerated to finally terminate after 30 seconds (Figure 4). Ethanol (1.5 cm3) was injected, and the patient was monitored. Directly after the injection, shortening of the episodes to triplets and single PVCs occurred before the complete disappearance of VT episodes. Afterward, no PVC or VT was observed and no VT was inducible. After the procedure, the patient was informed about future possible therapeutic options and the patient renounced a possible upgrade to intracardiac cardioverter-defibrillator therapy. Troponine I levels raised to a peak value of 3.26 ng/mL 24 hours after the ethanol injection and declined rapidly. At 9-month follow-up, no new episodes of sustained or nonsustained VT were detected on his dual-chamber pacemaker.
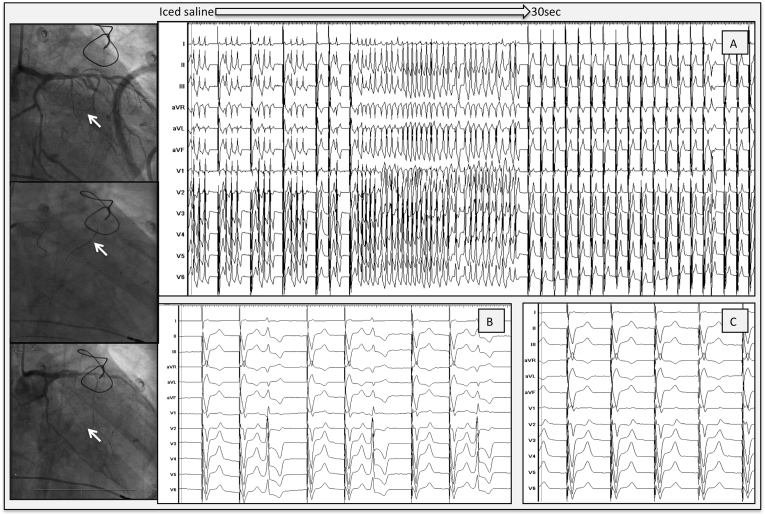
Figure 4.
Selective ethanol injection into a septal perforator. Left panel with fluoroscopic views: Upper panel: left coronary angiography before injection. Distal branch of septal perforator is the target to cannulate (arrow). Middle panel: left coronary angiography with 1 guidewire inside the first and 1 guidewire inside the second perforator (arrow). Lower panel: angiography after ethanol injection. A distal branch (arrow) of the second perforator is occluded. A: Injection of iced saline. Fast VT with disappearance of VT, after 30 seconds. B: Injection of ethanol. Single premature ventricular contraction, with no VT afterward. C: Final result. Complete eradication of VT and premature ventricular contraction after a couple of minutes. VT = ventricular tachycardia.
Discussion
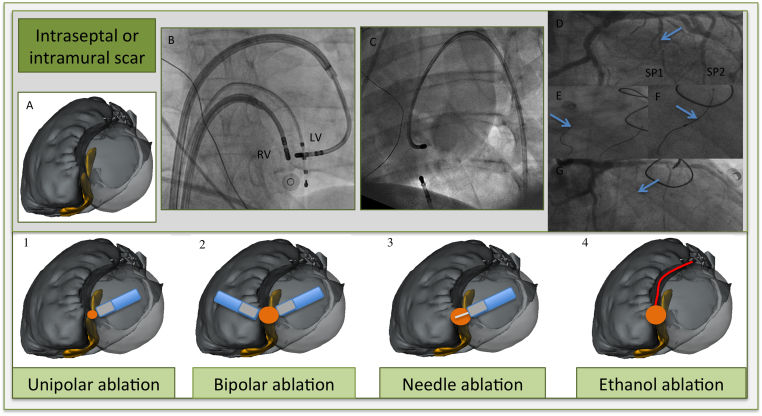
VT ablation of an intramural septal focus is particularly challenging, since conventional ablation strategies often fail to obtain transmural lesion formation. Different ablation techniques to create possible larger and (more) transmural lesions are shown in Figure 5.
Figure 5.
Different ablation techniques for intraseptal or intramural scar. A: Illustration of merged multidetector computed tomography/magnetic resonance imaging segmentation with postablation nontransmural scar regions (in yellow) on both sites of the septum (left anterior oblique view). Blue, ablation catheters; gray, tip; white, needle; read dot, ablation target; orange dot, expected ablation lesion; red, first septal perforator. 1. High output ablation (till 70 W) gives a slightly larger lesion; however, often not transmural and the risk of steam pop or catheter perforation increases. 2. Bipolar ablation. Two ablation catheters at opposing septum sites. (B: Anteroposterior view, 2 ablation catheters in steerable sheaths and an RV apex catheter for pacing purposes). 3. Irrigated needle catheter at LV septum. Needle extended (7–9 mm) inside the septum with staining of contrast. (C: fluoroscopic image with contrast staining at another basolateral site. Needle catheter in steerable sheath, and quadripolar catheter in coronary sinus and RV apex. Dual-chamber implantable cardioverter-defibrillator leads image). 4.Selective coronary ethanol injection (cf fluoroscopic sequence). D: Coronary angiogram shows septal perforators, with the first septal perforator as target (arrow). E: Guidewire cannulation of first (arrow) and second septal perforator. F: Balloon occlusion (arrow) with iced saline injection and later with 1.5-mL ethanol injection because of ventricular tachycardia termination. G: Occlusion of the first septal perforator (arrow). LV = left ventricular; RV = right ventricular.
In this case, we could clearly influence the exit site with conventional ablation (VT morphology), but we encountered an inability to penetrate in the true intramural focus. This explains why ablation was partially successful at the septal LVOT site (pace-map match of exit site) since episodes shortened, and shortly disappeared, but recurrence was quickly seen after the termination of RF.
In the case series of Sacher et al10 and the update of Tokuda et al,12 the transcoronary ethanol VT ablation technique performed after a failed RF attempt was demonstrated to be effective with a long-term VT-free survival in 67% at 24-month follow-up. Less data are available for bipolar ablation in human use and mostly small case series or case reports: data of bipolar ablation performed for RVOT, septal VT, and free-wall VT are found with short-term success ratios varying from 50% to 75%.9, 11, 13 In addition, the bipolar ablation technique was found to be more effective than unipolar ablation in a computational model, except in the situation of the epicardial catheter tip surrounded by air or placed over a fat tissue layer.14
The aim of this case report was not to present ethanol ablation as a novel technique—since this technique exists since 1987, and already became a validated method—but to explain how to take a patient-tailored approach between bipolar ablation and ethanol ablation after a previous failed RF attempt on the basis of the (dis)advantages of both strategies that are listed in Table 1.
Table 1.
(Dis)advantages of bipolar and ethanol ablation
| Ablation strategy | Advantages | Disadvantages |
|---|---|---|
| Bipolar ablation |
|
|
| Ethanol ablation |
|
|
In this case, the focus was expected to be quite basal, close to the main conduction system. Bipolar ablation is powerful, but it lacks the ability to identify the intramural activation time of the local electrogram and is irreversible, which is more dangerous close to the main conduction system. The main disadvantage of bipolar ablation is the lack of catheter tip visualization with current mapping systems and the irreversibility of the effect. Accurate position of both catheters at the correct opposing sites based on different fluoroscopic angles alone can be challenging. Nowadays, one has to change the NAV catheter (magnetic visualization on CARTO3, Biosense Webster, Inc) version for 2 standard ablation catheters, which significantly increases the cost of the procedure and suppresses the contact force and vector orientation of the tip, both often valuable to evaluate septal contact. Since this leads to loss of all information of the mapping system, no distance measurement between the 2 catheter tips is possible. Ideally, the smallest distance between both tips would be looked for.
Ethanol ablation—initially used as a chemical method to perform septal myectomy in hypertrophic cardiomyopathy—is limited by the anatomy of the coronary artery system and most often used in septal perforators of the left anterior descending and distal branches of the left circumflex artery.10 It can be guided by the QS morphology and precocity of the unipolar local electrogram to choose the best distal coronary branch, and injection of iced saline can be used as a reversible challenge and for the evaluation of the effect (possible effect on the conduction system and termination of the VT when applied during VT) before ethanol injection.15 Probably, the history of coronary artery bypass graft and having a left main trunk protected by a functional bypass is also important since there is always the risk of having an ethanol leak and thrombosis of the left main trunk. These arguments led to the decision to perform ethanol ablation in this patient.
In the future, irrigated needle ablation could be a valuable and apparently safe alternative since unipolar intramural recording from the needle tip is also feasible and larger intramural lesions can be created, without the anatomical restriction of the coronary artery system. However, this ablation technique is not reversible. More data for human use are still needed.8
Conclusion
This case illustrates the patient-tailored approach for an intramural VT focus based on the choice between bipolar and ethanol ablation.
Footnotes
Dr Berte has received an educational European Heart Rhythm Association grant. Dr Jaïs, Dr Haïssaguerre, Dr Derval, and Dr Sacher have received lecture fees from Biosense Webster and St Jude Medical.
References
- 1.Aliot E.M., Stevenson W.G., Almendral-Garrote J.M. European Heart Rhythm Association (EHRA); Registered Branch of the European Society of Cardiology (ESC); Heart Rhythm Society (HRS); American College of Cardiology (ACC); American Heart Association (AHA). EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Europace. 2009;11:771–817. doi: 10.1093/europace/eup098. [DOI] [PubMed] [Google Scholar]
- 2.Jais P., Maury P., Khairy P. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 3.Desjardins B., Yokokawa M., Good E., Crawford T., Latchamsetty R., Jongnarangsin K., Ghanbari H., Oral H., Pelosi F., Chugh A., Morady F., Bogun F. Characteristics of intramural scar in patients with non-ischemic cardiomyopathy and relation to intramural ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2013;6:891–897. doi: 10.1161/CIRCEP.113.000073. [DOI] [PubMed] [Google Scholar]
- 4.Tokuda M., Kojodjojo P., Tung S., Tedrow U.B., Nof E., Inada K., Koplan B.A., Michaud G.F., John R.M., Epstein L.M., Stevenson W.G. Acute failure of catheter ablation for ventricular tachycardia due to structural heart disease: causes and significance. J Am Heart Assoc. 2013;2:e000072. doi: 10.1161/JAHA.113.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida K., Yokokawa M., Desjardins B., Good E., Oral H., Chugh A., Pelosi F., Morady F., Bogun F. Septal involvement in patients with post-infarction ventricular tachycardia: implications for mapping and radiofrequency ablation. J Am Coll Cardiol. 2011;58:2491–2500. doi: 10.1016/j.jacc.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haqqani H.M., Tschabrunn C.M., Tzou W.S. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8:1169–1176. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Sapp J.L., Cooper J.M., Zei P., Stevenson W.G. Large radiofrequency ablation lesions can be created with a retractable infusion-needle catheter. J Cardiovasc Electrophysiol. 2006;17:657–661. doi: 10.1111/j.1540-8167.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 8.Sapp J.L., Beeckler C., Pike R., Parkash R., Gray C.J., Zeppenfeld K., Kuriachan V., Stevenson W.G. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289–2295. doi: 10.1161/CIRCULATIONAHA.113.003423. [DOI] [PubMed] [Google Scholar]
- 9.Koruth J.S., Dukkipati S., Miller Ma, Neuzil P., d’Avila A., Reddy V.Y. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9:1932–1941. doi: 10.1016/j.hrthm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Sacher F., Sobieszczyk P., Tedrow U., Eisenhauer A.C., Field M.E., Selwyn A., Raymond J.M., Koplan B., Epstein L.M., Stevenson W.G. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008;5:62–68. doi: 10.1016/j.hrthm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Berte B., Sacher F., Mahida S., Yamashita S., Lim H.S., Denis A., Derval N., Hocini M., Haissaguerre M., Cochet H., Jais P. Impact of septal radiofrequency ventricular tachycardia ablation: insights from magnetic resonance imaging. Circulation. 2014;130:716–718. doi: 10.1161/CIRCULATIONAHA.114.010175. [DOI] [PubMed] [Google Scholar]
- 12.Tokuda M., Sobieszczyk P., Eisenhauer A.C., Kojodjojo P., Inada K., Koplan B.A., Michaud G.F., John R.M., Epstein L.M., Sacher F., Stevenson W.G., Tedrow U.B. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011;4:889–896. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 13.Teh A.W., Reddy V.Y., Koruth J.S., Miller M.A., Choudry S., D’Avila A., Dukkipati S.R. Bipolar radiofrequency catheter ablation for refractory ventricular outflow tract arrhythmias. J Cardiovasc Electrophysiol. 2014;25:1093–1099. doi: 10.1111/jce.12460. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Suarez A., Trujillo M., Koruth J., d’Avila A., Berjano E. Radiofrequency cardiac ablation with catheters placed on opposing sides of the ventricular wall: computer modelling comparing bipolar and unipolar modes. Int J Hyperthermia. 2014;30:372–384. doi: 10.3109/02656736.2014.949878. [DOI] [PubMed] [Google Scholar]
- 15.Roten L., Derval N., Pascale P., Jais P., Sacher F. What next after failed septal ventricular tachycardia ablation? Indian Pacing Electrophysiol J. 2012;12:180–185. doi: 10.1016/s0972-6292(16)30524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]