Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Elevated hematocrit promotes arterial thrombus formation.
During arterial thrombosis, elevated hematocrit enhances platelet accumulation at the site of vessel injury.
Abstract
Red blood cells (RBCs) demonstrate procoagulant properties in vitro, and elevated hematocrit is associated with reduced bleeding and increased thrombosis risk in humans. These observations suggest RBCs contribute to thrombus formation. However, effects of RBCs on thrombosis are difficult to assess because humans and mice with elevated hematocrit typically have coexisting pathologies. Using an experimental model of elevated hematocrit in healthy mice, we measured effects of hematocrit in 2 in vivo clot formation models. We also assessed thrombin generation, platelet-thrombus interactions, and platelet accumulation in thrombi ex vivo, in vitro, and in silico. Compared with controls, mice with elevated hematocrit (RBCHIGH) formed thrombi at a faster rate and had a shortened vessel occlusion time. Thrombi in control and RBCHIGH mice did not differ in size or fibrin content, and there was no difference in levels of circulating thrombin-antithrombin complexes. In vitro, increasing the hematocrit increased thrombin generation in the absence of platelets; however, this effect was reduced in the presence of platelets. In silico, direct numerical simulations of whole blood predicted elevated hematocrit increases the frequency and duration of interactions between platelets and a thrombus. When human whole blood was perfused over collagen at arterial shear rates, elevating the hematocrit increased the rate of platelet deposition and thrombus growth. These data suggest RBCs promote arterial thrombosis by enhancing platelet accumulation at the site of vessel injury. Maintaining a normal hematocrit may reduce arterial thrombosis risk in humans.
Introduction
Red blood cells (RBCs) are the most abundant cells in blood. Normal RBC numbers range from 4.2 to 6.1 × 109/mL in humans; men have slightly higher levels than women. RBCs are primarily known for their hemoglobin-mediated role in oxygen transport. However, a growing body of evidence suggests RBCs have biochemical and biophysical properties that may contribute to thrombosis. First, RBCs are the principal determinant of blood viscosity, an established risk factor for thrombosis.1 Blood viscosity increases nonlinearly with increased hematocrit.2,3 Consequently, elevated hematocrit, even within a clinically relevant range (40% to 60%), increases blood viscosity at both arterial and venous shear rates.4 Second, simulations and experimental studies using models of intact (uninjured) arteries suggest RBCs enrich the near-wall region with platelets (margination).5,6 Third, RBCs enhance platelet αIIbβ3 activation and P-selectin exposure in response to agonists (eg, collagen, thrombin).7-9 Fourth, platelet accumulation on excised subendothelial matrices increases with increasing hematocrit (10% to 70%) at arterial shear rates.10 Finally, RBCs and RBC-derived microvesicles have exposed phosphatidylserine on their outer membrane and can support thrombin generation in vitro.11-18 Overall, these studies support the hypothesis that elevated RBCs can directly enhance thrombosis. However, which, if any, of these pathways contribute to thrombosis in vivo is unclear. Determining the contribution of RBCs to coagulation in vivo is clinically important because elevated hematocrit is an independent risk factor for cardiovascular disease and cardiovascular-related deaths.19-24 Thus, increased understanding of the relationship between hematocrit and thrombosis is needed to guide clinical strategies.
Previous studies in animal models have examined the pathophysiologic effects of elevated hematocrit in JAK 2V617F–induced polycythemia vera (PV) or erythrocytosis mediated by endogenous overproduction of erythropoietin.25-28 Findings from these studies expose complex, and sometimes discordant, effects of hematocrit on coagulation and failed to reveal a clear relationship between hematocrit and thrombosis. For example, a mouse model of JAK 2V617F–induced PV exhibits a prothrombotic phenotype following FeCl3 injury to mesenteric vessels, but an apparent paradoxical increase in bleeding following tail transection.27 However, these mice are deficient in platelet glycoprotein VI and have reduced plasma von Willebrand factor multimers,27 making it difficult to assess the contribution of elevated hematocrit to thrombosis. Mice genetically engineered to overexpress human erythropoietin also show increased bleeding in a tail bleeding model.26 However, these animals have a markedly increased hematocrit (80% to 85%) not seen in humans, and the increased bleeding may be explained by a reduction in plasma volume.26 Erythropoietin-infused mice with less dramatically elevated hematocrit (60% ± 5.5%) do not differ from controls in a FeCl3-induced mesentery thrombosis model.27 However, because erythropoietin has downstream effects on multiple cell types,29-32 it is also difficult to assess specific effects of elevated hematocrit with this approach.
Herein, we analyzed the effect of RBCs on thrombus formation in an experimental model of elevated hematocrit in healthy mice. Compared with controls, increased hematocrit shortened the time to carotid artery occlusion and shortened the clotting time following tail transection. Ex vivo, in silico, and in vitro analyses suggest this effect did not stem from increased thrombin generation, but rather, was associated with increased frequency of platelet-thrombus interactions, leading to accelerated platelet accumulation and more rapid thrombus growth.
Methods
Proteins and materials are detailed in supplemental Materials, available on the Blood Web site.
Mouse model of elevated hematocrit
Procedures were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Whole blood was collected from healthy, anesthetized 6- to 8-week-old male and female C57Bl/6 mice via inferior vena cava venipuncture into 3.2% sodium citrate (10% vol/vol, final). Blood was centrifuged (150g, 10 minutes) to separate RBCs from platelet-rich plasma (PRP). RBCs were resuspended in sterile citrate glucose saline (1.29 mM sodium citrate, 3.33 mM glucose, 124 mM NaCl, pH 7.2) and washed by centrifugation (3× 400g, 5 minutes). Washed RBCs were resuspended in sterile HEPES-buffered saline (20 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid, 150 mM NaCl, pH 7.4) and centrifuged (400g, 10 minutes) to pack RBCs. RBCs were counted (HV950FS Hemavet cell counter; Drew Scientific, Dallas, TX), and the hematocrit was adjusted to 70%. Recipient 6- to 8-week-old male C57Bl/6 mice were anesthetized, injected with packed RBCs via retroorbital plexus (∼250 µL), and allowed to recover. After 24 hours, blood was drawn from the inferior vena cava for hematological analysis or animals were subjected to thrombosis models (separate mice). Methods for blood smears (Diff-Stain), flow cytometry, thrombin-antithrombin complex (TATc), and whole blood viscosity measurements are detailed in supplemental Materials.
Mouse models of clotting
The FeCl3 injury model was performed as described.33,34 Briefly, 6- to 8-week-old male C57Bl/6 mice were anesthetized. The right common carotid artery was exposed, dried, and treated with FeCl3 (10%, 0.5 × 0.5-mm filter paper, 2 minutes). Blood flow was monitored auditorily by Doppler ultrasonic flow probe. Time to vessel occlusion (TTO) was the time between FeCl3 administration and lack of flow for 1 minute. Doppler tracings recorded for a subset of mice were used to determine thrombus onset time (inflection point when blood flow began to decrease and resulted in vessel occlusion) and thrombus formation rate (maximum rate of decrease in blood flow [min−1] following the thrombus onset time). Mice that did not experience carotid occlusion were omitted from onset and rate analysis because they did not form defined thrombi.
Tail transection assays were performed as described in supplemental Materials.
Phlebotomy
Blood was drawn from healthy, consenting human donors in accordance with the Declaration of Helsinki and University of North Carolina at Chapel Hill and University of Colorado at Boulder Institutional Review Boards. Donors had not ingested aspirin for ≥5 days prior to phlebotomy. Blood was collected via antecubital venipuncture into 0.105 M sodium citrate, pH 5.5 (10% vol/vol, final).
Thrombin generation in reconstituted human whole blood
Thrombin generation was measured using whole blood–calibrated automated thrombography15 as detailed in supplemental Materials.
Computational modeling
Simulations of human whole blood flow driven by a pressure gradient through a 50-µm channel were conducted with a custom Lattice-Boltzmann Immersed Boundary method code.35-37 For each simulation, the channel was partially occluded by a preformed thrombus of specified shape and size. The pressure gradient was set to achieve a wall shear rate of ∼1100 s−1. At a sequence of times, the fluid velocity field was computed, accounting for mechanical forces generated by numerous deformable RBCs and a smaller number of less deformable platelets. Rheological properties of the RBC suspension emerge from the simulations. Trajectories through a space of 700 points on each RBC surface and 100 points on each platelet surface were tracked as functions of time, thus determining each cell’s location, orientation, and deformation. Simulations were conducted for hematocrits of 40% and 60%, for small (base, 44 µm; top surface, 34 µm; height, 7.2 µm) and large (base, 44 µm; top surface, 24 µm; height, 11.5 µm) thrombi. For each case, an RBC distribution at statistical equilibrium was precomputed and then 10 flow simulations were carried out starting with this RBC distribution and with a platelet placed at a randomly chosen position in the near-wall RBC-depleted zone 14 to 44 µm upstream of the thrombus. Simulation results were processed using custom MATLAB scripts.
Microfluidic model with reconstituted whole blood
Fibrillar collagen (500 µg/mL) was patterned in 200-µm spots onto clean glass, as described.38 Human whole blood was separated into PRP and RBCs by centrifugation (200g, 20 minutes) and reconstituted to 45% and 60% hematocrits. Reconstituted aliquots were labeled with 3,3′-dihexyloxacarbocyanine iodide and Alexa Fluor 555-labeled fibrinogen (1 µM and 56 µg/mL final concentrations, respectively) at 37°C for 15 minutes. Labeled blood was recalcified with buffer (75 mM CaCl2 and 37 mM MgCl2 in HEPES-buffered saline, 9:1 flow rate ratio) using a continuous microfluidic mixer39 upstream of a custom polydimethylsiloxane flow chamber (height, 51 µm; width, 500 µm), and perfused over collagen (750 s−1, 15 minutes). Final hematocrits were 41% and 54%. Total thrombus size, fibrin formation, and platelet accumulation were captured by relief contrast and epifluorescence microscopy (Olympus IX81, ×20; numerical aperature = 0.45, λexcitation/λemission 475/505, 545/580, at 6 frames per minute). Thrombus area fraction, fibrin formation, and platelet accumulation were measured as described.39
Statistical methods
Descriptive statistics (mean, median, standard deviation [SD]) were calculated. Groups were compared using Student t tests (normally distributed data using Lilliefors test for normality) or Wilcoxon-Mann-Whitney rank sum tests (nonnormally distributed data) in KaleidaGraph version 4.1.3 (Synergy Software, Reading, PA). For viscosity experiments, viscosity and shear rate were logarithmically transformed and then analyzed using a linear model. For microfluidics experiments, data were analyzed by paired Student t test.
Results
Experimental model of elevated hematocrit in mice
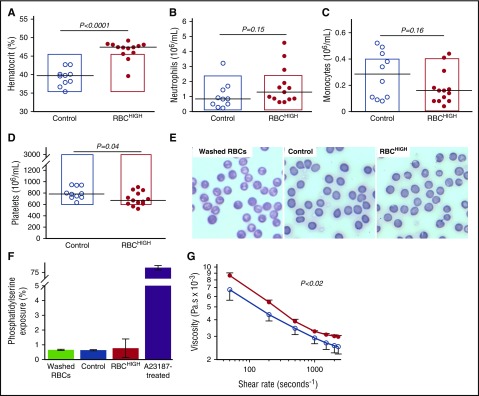
To study the effect of elevated hematocrit in vivo, we used an experimental model in which we raised the hematocrit in healthy mice with fresh, washed RBCs from healthy donor mice. Complete blood counts showed RBCHIGH mice had an elevated hematocrit compared with control mice (P < .0001; Figure 1A; Table 1). Levels of neutrophils and monocytes were similar in controls and RBCHIGH mice (Figure 1B-C; Table 1). Platelet levels were slightly lower in RBCHIGH mice compared with controls (Figure 1D; Table 1), but remained in the normal range. Blood smears showed normal morphology of washed RBCs (Figure 1E left) and RBCs from control mice (Figure 1E middle) and RBCHIGH mice (Figure 1E right). Consistent with prior reports,11,12,40,41 <1% of packed RBCs or circulating RBCs in control or RBCHIGH mice had exposed phosphatidylserine (Figure 1F). Plasmas isolated from control and RBCHIGH mice had similar circulating TATc (3.7 ± 0.5 and 2.2 ± 1.3 ng/mL, respectively, mean ± SD). As expected, whole blood from RBCHIGH mice was more viscous than whole blood from control mice over a range of shear rates (30-2300 s−1; Figure 1G), and blood flow was slightly, although nonsignificantly, slower in RBCHIGH mice vs controls (1457.9 ± 295.0 vs 2177.9 ± 1230.6 Hz, respectively, mean ± SD). Together, these data establish a model of elevated hematocrit in otherwise healthy mice.
Figure 1.
Model of elevated hematocrit in mice. RBCs from “donor” mice were injected into “recipient” mice (RBCHIGH) via retroorbital plexus. After 24 hours, blood was drawn from RBCHIGH and control (uninfused) mice. Complete cell counts indicate (A) elevated hematocrit, but normal (B) neutrophil, (C) monocyte, and (D) platelet numbers. In panels A-D, each dot is a separate mouse; lines indicate median values, and boxes represent the normal range. (E) Blood smears (Diff-stain kit [IMEB, San Marcos, CA], imaged on an Olympus BX71 at ×40 and digitally zoomed) of washed RBCs and whole blood from control and RBCHIGH mice indicate normal RBC morphology. (F) Flow cytometry indicates normal phosphatidylserine exposure on washed RBCs and RBCs isolated from control and RBCHIGH mice. Bars indicate mean ± SD, N = 3. (G) Viscosity measurements show RBCHIGH mice (closed circles) have significantly increased viscosity (Pascal-seconds, Pa.s) at low and high shear compared with control mice (open circles). Symbols show mean ± SD, N = 3-5 per group.
Table 1.
Complete blood counts in mice
| Normal range | Control | RBCHIGH | P value* | |
|---|---|---|---|---|
| Hematocrit, % | 35.1-45.4 | 39.3 ± 0.7 | 46.6 ± 0.7 | <.0001 |
| Neutrophils, ×106/mL | 0.1-2.4 | 1.1 ± 0.3 | 1.7 ± 0.4 | .15 |
| Monocytes, ×106/mL | 0.0-0.4 | 0.3 ± 0.1 | 0.2 ± 0.0 | .16 |
| Platelets, ×106/mL | 592-2972 | 802 ± 33 | 699 ± 33 | .04 |
Data show mean ± SD.
Student t test.
Compared with controls, RBCHIGH mice have a faster time to artery occlusion and shorter tail bleeding time
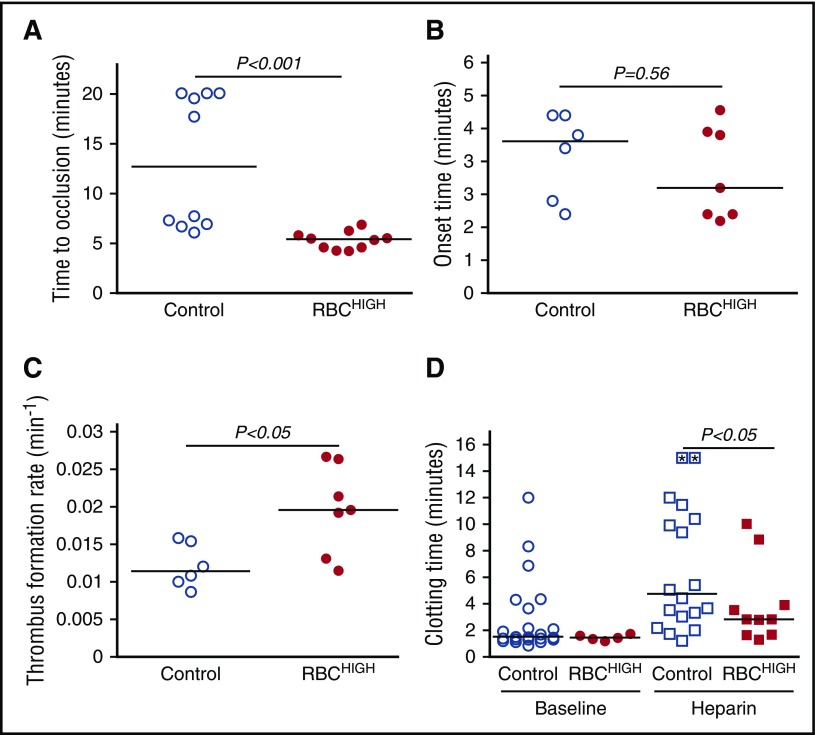
To determine the effect of elevated hematocrit on thrombosis, we first subjected control and RBCHIGH mice to the FeCl3/carotid artery thrombosis model. Compared with controls, RBCHIGH mice had a significantly shorter TTO (13.2 ± 6.6 vs 5.3 ± 0.9 minutes, respectively, mean ± SD; 12.7 [13.9] vs 5.4 [2.6] minutes, respectively, median [range], P < .001; Figure 2A). At the time when 50% of control mice had complete vessel occlusion, 100% of RBCHIGH mice had an occluded vessel. A separate experiment in which control mice were compared with mice injected with RBC wash supernatant (RBCwash) showed no difference in TTO (10.9 ± 7.1 vs 8.8 ± 5.7 minutes for control and RBCwash mice, respectively, mean ± SD; 7.1 [15] vs 7.3 [15.8] minutes, respectively, median [range], P = .87, N = 6/group), indicating the shortened TTO in RBCHIGH mice was not caused by RBC releasates during RBC preparation. Doppler tracings indicated the thrombus onset time for RBCHIGH mice was not significantly different from control mice (Figure 2B), but the thrombus formation rate was significantly faster (0.020 ± 0.006 vs 0.012 ± 0.003 min−1, respectively, mean ± SD, P < .05; Figure 2C).
Figure 2.
Compared with control mice, RBCHIGHmice have a shortened TTO. (A-D) Control and RBCHIGH mice were subjected to FeCl3-induced carotid artery thrombosis. (A) TTO. When vessels did not occlude, time to occlusion was recorded as 20 minutes (3 control mice). (B) Onset and (C) rate of thrombus formation in control (uninfused) and RBCHIGH mice. (D) Control and RBCHIGH mice were treated with saline (baseline) or heparin and then subjected to tail transection. Each dot or box is a separate mouse; boxes with asterisks represent mice that did not form clots. Lines indicate median values, Wilcoxon 1-tailed comparison.
To test whether elevated hematocrit promoted clotting in a second in vivo model, we subjected control and RBCHIGH mice to a tail transection assay. Elevated hematocrit did not shorten the clotting time under normal conditions, likely due to the already very short clotting time (median ∼88 seconds). However, when mice were infused with heparin to prolong the clotting time, RBCHIGH mice had a shorter clotting time than control mice (P < .05; Figure 2D). Together, data from these 2 models suggest elevated hematocrit accelerates clot formation in vivo.
Thrombi from control and RBCHIGH mice do not differ in size or fibrin content
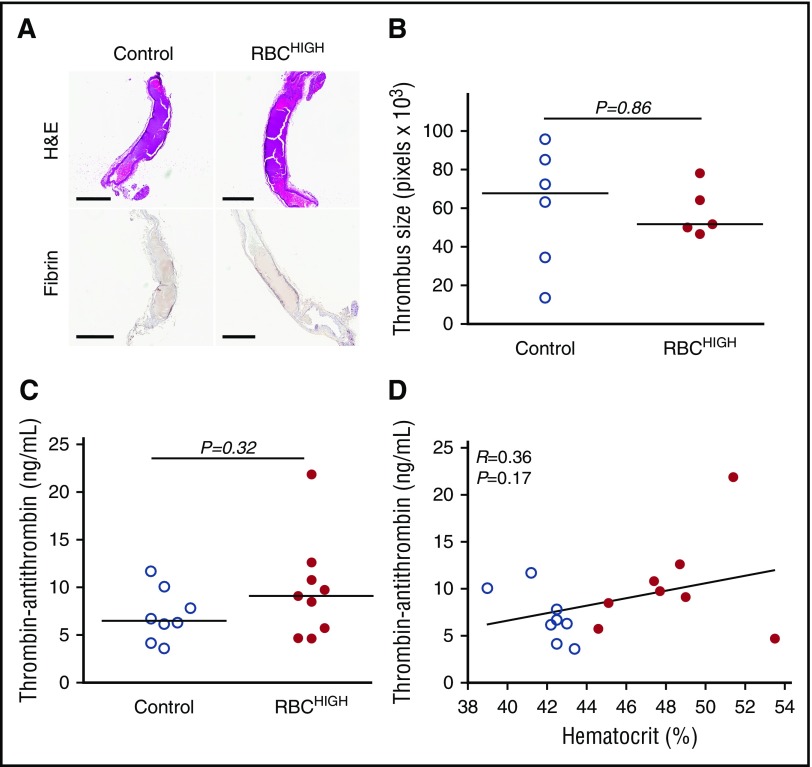
To determine how elevated hematocrit accelerates thrombosis, we first excised the occluded carotid artery from a subset of mice subjected to FeCl3 injury. Both control and RBCHIGH mice had fully occlusive thrombi that were similar in size (Figure 3A-B). Thrombi from both control and RBCHIGH mice were primarily composed of proteinaceous material with sparse, small islands of RBCs and showed similar fibrin staining (amount and intensity; Figure 3A). These data suggest elevated hematocrit does not increase thrombus size or fibrin content.
Figure 3.
Following FeCl3injury, control and RBCHIGHmice have similar thrombus morphology and similar levels of circulating TATc. (A-B) Thrombi from a subset of mice subjected to FeCl3-induced carotid artery thrombosis were excised, fixed in 10% formalin, transferred to 70% ethanol, and analyzed. (A) Hematoxylin and eosin (H&E) staining (upper panels) and immunohistochemistry for fibrin (brown staining; lower panels). Scale bars indicate 500 µm. (B) Thrombus size was determined by measuring pixel area of the thrombus within the vessel. (C) TATc were measured by enzyme-linked immunosorbent assay in plasmas from control (uninfused) and RBCHIGH mice following FeCl3-induced artery occlusion. (D) Relationship between hematocrit and TATc levels measured after FeCl3-induced artery occlusion in control (open circles) and RBCHIGH (closed circles) mice. In panels B-D, each dot is a separate mouse. In panels B-C, horizontal lines indicate medians.
Thrombin generation does not differ in control and RBCHIGH mice in vivo
We then tested the hypothesis that RBCHIGH mice had enhanced activation of coagulation following FeCl3 injury. We drew blood from mice 5 minutes after artery occlusion and measured TATc in plasma. TATc were similar in control and RBCHIGH mice (7.1 ± 2.8 and 9.8 ± 5.3 ng/mL, respectively, mean ± SD, P = .32; Figure 3C), and circulating TATc levels did not correlate with hematocrit (Figure 3D). These data suggest elevated levels of normal RBCs do not promote thrombus formation by increasing thrombin generation.
Effect of RBCs thrombin generation depends on the platelet concentration
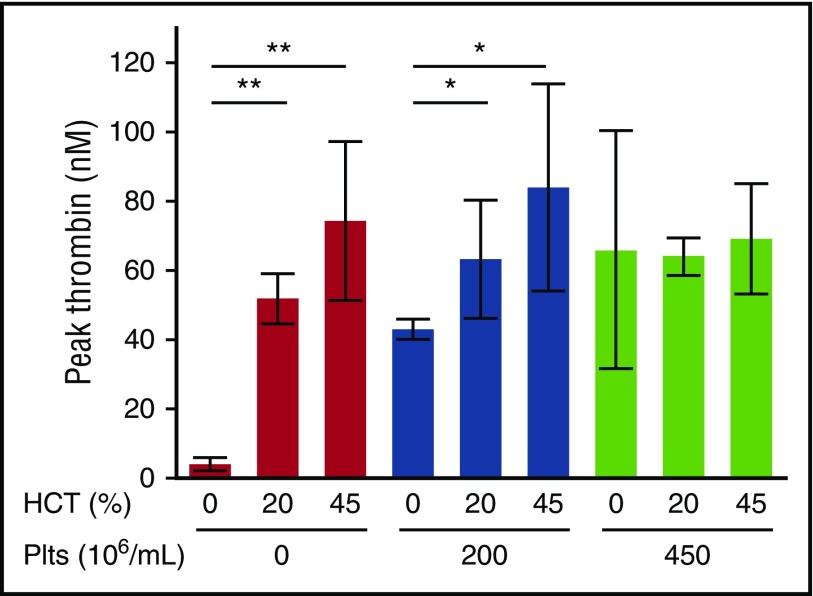
The observation that RBCHIGH mice did not have increased circulating TATc suggested elevated RBCs did not augment thrombin generation during arterial thrombosis in mice. To examine the relative contribution of RBCs and platelets to thrombin generation, we isolated and then reconstituted plasma, platelets, and RBCs from healthy humans to levels indicated in Figure 4. We then triggered coagulation with tissue factor and recalcification and measured thrombin generation by whole blood–calibrated automated thrombography. In the absence of platelets, increasing hematocrit increased the thrombin generation rate, peak, and endogenous thrombin potential (Figure 4; Table 2). This finding is consistent with prior studies12,13,15 and is thought to reflect prothrombin cleavage on the surface of phosphatidylserine-expressing RBCs.12 However, in reactions with 200 × 106 platelets/mL, hematocrit had less effect on thrombin generation (Figure 4; Table 2), and in reactions with 450 × 106 platelets/mL, there was no effect of hematocrit on thrombin generation (Figure 4; Table 2). Increased thrombin generation was detected in control reactions with elevated prothrombin (data not shown), indicating the lack of increased thrombin detected was not due to fluorogenic substrate consumption. Together with the lack of increased circulating TATc in RBCHIGH mice (Figure 3C), these data suggest that in mice and humans with normal or elevated platelet levels, normal RBCs do not enhance thrombin generation during arterial thrombus formation.
Figure 4.
The effect of RBCs on thrombin generation depends on the endogenous platelet concentration. Thrombin generation in reconstituted whole blood with varying hematocrit (HCT) and platelets (Plts) was analyzed by calibrated automated thrombography. Hematocrits >45% interfered with the thrombin generation assay and could not be measured reproducibly. N = 3-6 per condition; bars represent mean peak thrombin ± SD. *P < .05; **P < .005.
Table 2.
Thrombin generation in reconstituted human whole blood
| No platelets, % | 200 × 106/mL platelets, % | 450 × 106/mL platelets, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 20 | 45 | 0 | 20 | 45 | 0 | 20 | 45 | |
| Hematocrit | |||||||||
| Lag time, min | 9.1 ± 5.2 | 9.8 ± 2.0 | 12.9 ± 1.7 | 7.2 ± 1.0 | 7.3 ± 1.2 | 7.4 ± 0.6 | 7.4 ± 1.0 | 6.9 ± 0.8 | 7.2 ± 1.3 |
| Time to peak, min | 23.8 ± 10.0 | 14.2 ± 2.0 | 18.7 ± 2.7 | 13.2 ± 1.7 | 11.2 ± 1.6 | 11.2 ± 1.0 | 12.5 ± 1.4 | 10.9 ± 1.2 | 11.0 ± 1.7 |
| Rate, nM/min | 0.2 ± 0.1 | 4.7 ± 0.5* | 6.4 ± 1.1* | 3.0 ± 0.2 | 5.4 ± 1.0* | 8.5 ± 1.7* | 3.7 ± 1.9 | 6.1 ± 0.4 | 6.5 ± 1.1 |
| Peak, nM | 3.9 ± 2.0 | 51.8 ± 7.3* | 74.3 ± 23.0* | 42.9 ± 2.9 | 63.2 ± 17.1* | 84.0 ± 30.0* | 65.8 ± 34.9 | 64.1 ± 5.4 | 69.1 ± 15.9 |
| Endogenous thrombin potential, nM*min | 51.0 ± 50.5 | 382.3 ± 74.9* | 619.3 ± 209.9* | 418.8 ± 59.9 | 412.0 ± 149.5 | 525.2 ± 197.8 | 500.8 ± 261.1 | 414.0 ± 43.8 | 457.5 ± 122.2 |
Thrombin generation was measured by whole blood–calibrated automated thrombography. Data show mean ± SD. N = 3-6 separate experiments per condition.
Analysis of variance with Dunnett’s post-hoc test, P < .05 vs 0% hematocrit for each platelet concentration.
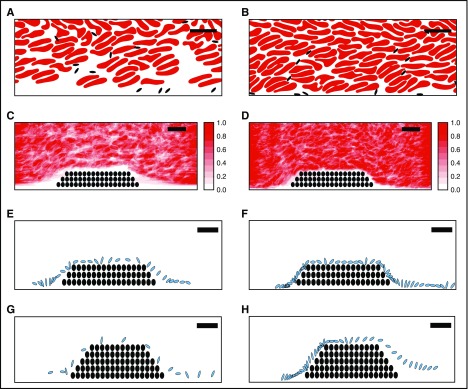
A computational model suggests elevated hematocrit increases the time that platelets spend in proximity to a thrombus
Direct numerical simulations of RBCs and platelets near a porous thrombus show RBCs strongly influence platelet motion and orientation.37 Because arterial thrombi are platelet rich, we then tested the hypothesis that the prothrombotic effect of elevated hematocrit was mediated by enhanced interactions of flowing platelets with the thrombus. We conducted in silico simulations of whole blood flow at 40% and 60% hematocrit, in both the absence and the presence of small and large porous thrombi. Comparisons with and without thrombi enabled us to evaluate effects of interstitial velocity on platelet behavior.
Figure 5A-D shows the instantaneous configuration and time-averaged distribution of RBCs and platelets resulting from simulations at 40% and 60% hematocrit. These data reveal a zone along a straight wall in which RBC concentrations were reduced and platelet concentrations were enhanced, and that this near-wall zone was narrower for 60% than 40% hematocrit (Figure 5A-B). The zone’s width was also smaller for 60% than 40% hematocrit both upstream of (1.0 vs 3.0 µm, respectively) and over (0.28 vs 1.28 µm, respectively) a small thrombus (Figure 5C-D). Results were similar in simulations with larger thrombi (data not shown). This decreased near-wall zone resulted from increased interstitial velocity within the porous thrombus vs the solid wall. Narrowing of this zone pushed platelets close to the thrombi, so that platelets spent more time in proximity to the thrombus than to a comparable segment of flat vessel wall; ie, at 40% hematocrit, for the same trajectory length (44 µm), platelets spent 11.5 milliseconds within 0.5 µm of the porous thrombus vs only 3.1 milliseconds near the solid wall.
Figure 5.
A computational model suggests elevated hematocrit increases the time that platelets spend in proximity to a thrombus. Simulations of whole blood flow with 40% (A, C, E, G) and 60% (B, D, F, H) hematocrit were conducted as described in “Methods.” (A-B) Snapshots showing instantaneous positions of RBCs (red) and platelets (black) for (A) 40% and (B) 60% hematocrit in flowing blood indicate RBCs are less prevalent close to vessel walls and platelets are more prevalent in this near-wall RBC-depleted zone. (C-D) Time-averaged RBC distribution for flow past a small thrombus (filled circles) in (C) 40% and (D) 60% hematocrit. The near-wall depleted zone is narrower in higher hematocrit. Scale indicates relative RBC distribution. (E-H) Time-dependent progression (2-millisecond intervals) of an individual platelet (blue) over a small or large thrombus (black circles): (E) 40% hematocrit, small thrombus, (F) 60% hematocrit, small thrombus, (G) 40% hematocrit, large thrombus, (H) 60% hematocrit, large thrombus. For both the small and the large thrombus, the platelet spends substantially more time near the thrombus for 60% hematocrit than for 40% hematocrit. Bars indicate 10 µm.
Figure 5E-H shows a sequence of snapshots every 2 milliseconds of a representative platelet's position near small (Figure 5E-F) and large (Figure 5G-H) thrombi. For the small thrombus, the average time the platelet spent within 0.5 µm of the thrombus was greater for 60% hematocrit than 40% hematocrit (29.5 ± 26.6 milliseconds vs 11.5 ± 10.0 milliseconds, respectively, mean ± SD). Trends for the larger thrombus were similar, but interactions were even longer and showed a greater difference between hematocrits (41.0 ± 39.4 vs 15.4 ± 9.5 milliseconds, mean ± SD). Consistent with previous studies,37 interactions in both cases were particularly frequent and prolonged near the upstream face of the thrombus. A linear fit of mean platelet interaction times with the small thrombus for 40% and 60% hematocrits predicts an increase of ∼55% in the mean interaction time for a 47% hematocrit. The analogous calculation for the large thrombus predicts an increase of 58%. These predicted values from the simulations agree well with the 67% increase in mean thrombus growth rate observed in vivo for 47% vs 40% hematocrit. Together, these data suggest elevated hematocrit enhances platelet contact with a thrombus in flowing blood, and that the frequency of these interactions increases with thrombus growth.
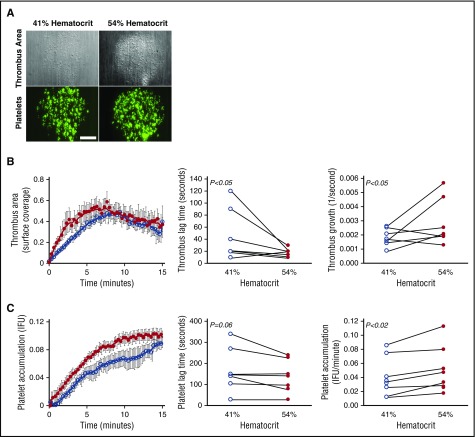
Elevated hematocrit increases the rate of platelet accumulation on collagen at an arterial shear rate
Finally, to directly compare the effect of hematocrit on fibrin formation and platelet accumulation, we used an in vitro microfluidic model to visualize and measure thrombus growth on 200-µm collagen spots at an arterial shear rate (750 s−1). Consistent with the in vivo findings and predictions from in silico simulations, elevated hematocrit significantly accelerated thrombus growth (Figure 6A-B), exposing a direct, prothrombotic effect of increased hematocrit on thrombus formation. The sensitivity of these in vitro experiments to very early thrombus formation also revealed a small, but significant, shortening of the lag time to thrombus initiation that was not detectable in the in vivo experiments. This reduction in lag time is consistent with in silico predictions of enhanced margination at higher hematocrit near a straight, nonporous wall (Figure 5A-B). There was no measurable effect of hematocrit on fibrin deposition (data not shown), consistent with in vivo and in vitro findings, indicating little to no effect of elevated hematocrit on thrombin generation or fibrin formation at normal platelet counts. Notably, however, Figure 6C illustrates a moderate, although significant, effect of hematocrit on the rate of platelet accumulation in the thrombus. This finding is consistent with predictions from the computational model, suggesting elevated hematocrit increases the frequency and duration of platelet-thrombus interactions, and therefore, the likelihood that these interactions will result in platelet accumulation in the thrombus. Collectively, these data suggest elevated hematocrit promotes platelet interactions with a forming thrombus, leading to faster platelet accumulation and accelerated thrombus growth.
Figure 6.
Elevated hematocrit increases the rate of platelet accumulation on collagen at an arterial shear rate. Reconstituted whole blood was perfused over type I collagen at 750 seconds−1. (A) Representative images of thrombus surface coverage and platelet fluorescence 1.5 minutes after initiation of flow. Scale bar indicates 50 µm. (B) Average thrombus area (normalized to collagen spot area as fractional surface coverage) and individual thrombus lag times and rates for all experiments. (C) Average platelet accumulation (integrated fluorescence intensity units [IFU] of DiOC6-labeled platelets) and individual platelet lag times and rates for all experiments. N = 7 independent donors per condition. Values show mean ± SD for 41% (open circles) and 54% (closed circles) hematocrit; lines indicate paired data for individual blood donors.
Discussion
Although RBCs have procoagulant properties in vitro, effects of RBCs on thrombosis in vivo are difficult to assess because humans and mice with elevated hematocrit typically have coexisting pathologies. Herein, we used an experimental model of elevated hematocrit in healthy mice and showed these mice have accelerated arterial thrombus formation and a shortened tail transection clotting time. Elevated hematocrit did not enhance thrombin generation or fibrin deposition in vivo or in vitro in the presence of normal numbers of platelets. However, elevated hematocrit increased the frequency of platelet interactions with thrombi in silico and accelerated the rate of platelet accumulation in thrombi in a microfluidic-based model of thrombus formation. Collectively, these findings suggest RBCs independently promote arterial thrombosis and show this occurs via a platelet-dependent increase in thrombus growth.
Epidemiologic studies of healthy humans associate elevated hematocrit with thrombosis and suggest increased risk exists at even moderately elevated baseline hemoglobin/hematocrit levels. In a large prospective cohort analysis (>8000 subjects), incidence of myocardial infarction (MI), coronary insufficiency, or coronary heart disease deaths was more than double in individuals with high hematocrit (>49%) compared with individuals with low hematocrit (<42%).19 Likewise, the Framingham study followed >5000 men and women (30-62 years old) over 34 years and showed that, compared with individuals in the mid–hematocrit quintile, young men and women in the highest quintile (men ≥49%, women ≥46%) had an increased risk of death from cardiovascular disease.21 Similarly, in the British Regional Heart Study involving >7700 men, risk of major ischemic heart disease events was increased by 30% at hematocrit >46%, compared with those below this cutoff.22 Additional longitudinal studies of young men showed that a high hematocrit at baseline was associated with 1.4- to 1.9-fold increased risk of MI during the follow-up period.20,24 In most of these studies, the association with thrombosis was independent of other cardiovascular risk factors, including smoking, itself a recognized cause of elevated hematocrit.
Increased hematocrit has also been implicated in thrombosis associated with a number of erythrocytotic disorders, including PV, Chuvash polycythemia, and erythropoietin-induced erythrocytosis. In PV, risk of cardiovascular death and major thrombosis is significantly reduced by maintaining the hematocrit <45%, compared with 45% to 50%.42 However, determining specific prothrombotic effects of hematocrit in these patients is complicated by the underlying disease pathophysiology.
Together with older studies, our findings provide biophysical mechanisms that may explain these observations: elevated hematocrit increases the duration and frequency of platelet-thrombus interactions. In contrast to most computational studies of margination in long, straight tubes,35,43-46 our model includes the perturbation of blood flow by a thrombus penetrating into the lumen. Importantly, like thrombi in vivo,47 the thrombi in our in silico model are porous, allowing for interstitial flow through the thrombus interior. The magnitude of interstitial flow increases with hematocrit, ultimately pushing platelets closer to the surface, while reducing tumbling. As a result, platelets are in close proximity to the thrombus for longer times, increasing the probability of receptor-ligand bond formation. Related in silico studies show that even at a hematocrit of 10%, RBCs enhance platelet deposition in stenosed vessels, in part by reducing the distance between a platelet and a nonporous thrombus.48 Effects of elevated hematocrit on platelet accumulation in our assays were significant but moderate, consistent with the moderately increased risk observed in epidemiologic studies.19-21,24 Notably, the premise that elevated hematocrit promotes thrombosis via platelet-dependent mechanisms is consistent with clinical findings that platelet antagonism reduces cardiovascular death, nonfatal MI, and stroke in patients with PV.49 Thus, these data may provide rationale for the efficacy of platelet inhibition strategies in patients with elevated hematocrit and increased thrombosis risk.
Because RBCs can support thrombin generation,12-15 the observation that elevated hematocrit did not increase circulating TATc was somewhat surprising. However, the dependence of arterial thrombosis on platelet function50 and scarcity of RBCs in the thrombus (Figure 3A) suggest phosphatidylserine expressed by normal RBCs does not substantively augment local thrombin generation during arterial thrombosis. However, RBCs may enhance thrombin generation in certain clinical situations. For example, patients with thrombocytopenia51 or increased numbers of circulating phosphatidylserine-positive RBCs (eg, sickle cell disease, β-thalassemia, or PV)40,52-55 may demonstrate clinically meaningful, RBC-mediated thrombin generation. Indeed, in patients with sickle cell disease, numbers of circulating phosphatidylserine-positive RBCs correlate with circulating prothrombin fragment 1.2.52 In addition, RBCs may support thrombin generation in venous thrombi, where the RBC content is higher than in arterial thrombi. Further studies are warranted to evaluate the effects of elevated hematocrit in these situations. Our approach to raise the hematocrit in mice with RBCs harvested from other mice is exploitable for investigating these situations as well as the effect of RBCs with biophysical and/or biochemical abnormalities, including sickled RBCs, in future studies.
This study has potential limitations. First, mice differ from humans in vascular dimensions, size of RBCs and platelets, number of platelets, and blood rheology. However, our data demonstrate consistent, prothrombotic effects of elevated hematocrit in both murine and human experimental systems. Second, although FeCl3 is a common agent for inducing thrombosis in animal models, ferric ions cause charge-suppression of plasma proteins and blood cells leading to initial adhesion of blood cells, including RBCs, to the endothelium by nonbiological mechanisms.56-60 However, subsequent propagation of thrombus into the vessel lumen, including platelet accumulation and thrombus growth, is still thought to recapitulate key aspects of arterial thrombus formation.57,60 Moreover, results were supported by independent experimental systems in vivo (tail transection), in silico (simulation), and in vitro (microfluidics). Third, our findings are limited to mildly elevated hematocrit. In future studies, it may be useful to examine the relationship between hematocrit and thrombosis across a broader range. Such studies may identify a nonlinear, and even J-shaped,21 response to hematocrit. For example, in situations where hematocrit elevation is extreme such as in patients with cyanotic congenital heart disease, hyperviscosity may paradoxically manifest as a bleeding tendency,61 an association that can also be seen in mice with extremely high hematocrits.26 Thus, the relationship between thrombosis and elevated hematocrit/hemoglobin may be complex and dependent on the mechanism and degree of erythrocytosis. Finally, our study does not address potential effects of chronically elevated hematocrit, in which additional systemic effects may also impact thrombosis.
In summary, our data suggest elevated hematocrit promotes arterial thrombosis. Effects are independent of thrombin generation, but associated with accelerated platelet accumulation within the thrombus. These findings suggest maintaining a normal hematocrit or reducing platelet function in patients with elevated hematocrit may reduce arterial thrombosis risk.
Acknowledgments
The authors thank Erica Sparkenbaugh for advice, James R. Byrnes for reading the manuscript, and Kenzie S. McConnell for assistance with data processing.
This study was supported by funding from the National Institutes of Health, National Heart, Lung, and Blood Institute, (R56HL094740 and R01HL126974) (A.S.W.), (R01HL120728) (K.B.N., A.L.F.), and (R01HL126864) (A.L.F.), (1UL1TR001111) (NC TraCS Institute/A.S.W.), (T32HL069768 and T32HL007149) (University of North Carolina), National Science Foundation CAREER Award (CBET-1351672) (K.B.N.) and grant DMS-1521748) (A.L.F.), American Heart Association (14GRNT20410094) (K.B.N.), and by the National Center for Advancing Translational Sciences.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.L.W. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; M.L., T.S., L.A.H., J.D.B., J.A.C., M.J.M., A.R.W., and B.C.C. performed experiments and analyzed and interpreted the data; R.P. designed experiments; J.W.H. and M.R.F. analyzed and interpreted the data; N.S.K., A.L.F., K.B.N., and A.S.W. designed the research, analyzed and interpreted the data, and wrote the manuscript. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 819 Brinkhous-Bullitt Building, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.
References
- 1.Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997;96(1):168-173. [DOI] [PubMed] [Google Scholar]
- 2.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263(6 Pt 2):H1770-H1778. [DOI] [PubMed] [Google Scholar]
- 3.Errill EW. Rheology of blood. Physiol Rev. 1969;49(4):863-888. [DOI] [PubMed] [Google Scholar]
- 4.Kwaan HC, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost. 2003;29(5):451-458. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski EF, Yam K, Gerace M. Evaluation of hemostasis in flowing blood. Am J Hematol. 2012;87(Suppl 1):S51-S55. [DOI] [PubMed] [Google Scholar]
- 6.Fogelson AL, Neeves KB. Fluid mechanics of blood clot formation. Annu Rev Fluid Mech. 2015;47:377-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvain J, Pena A, Cayla G, et al. . Impact of red blood cell transfusion on platelet activation and aggregation in healthy volunteers: results of the TRANSFUSION study. Eur Heart J. 2010;31(22):2816-2821. [DOI] [PubMed] [Google Scholar]
- 8.Santos MT, Valles J, Marcus AJ, et al. . Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991;87(2):571-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallés J, Santos MT, Aznar J, et al. . Platelet-erythrocyte interactions enhance alpha(IIb)beta(3) integrin receptor activation and P-selectin expression during platelet recruitment: down-regulation by aspirin ex vivo. Blood. 2002;99(11):3978-3984. [DOI] [PubMed] [Google Scholar]
- 10.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207(4430):541-543. [DOI] [PubMed] [Google Scholar]
- 11.Kuypers FA, Lewis RA, Hua M, et al. . Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87(3):1179-1187. [PubMed] [Google Scholar]
- 12.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120(18):3837-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyrou V, Lormeau JC, Hérault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81(3):400-406. [PubMed] [Google Scholar]
- 14.Horne MK III, Cullinane AM, Merryman PK, Hoddeson EK. The effect of red blood cells on thrombin generation. Br J Haematol. 2006;133(4):403-408. [DOI] [PubMed] [Google Scholar]
- 15.Ninivaggi M, Apitz-Castro R, Dargaud Y, de Laat B, Hemker HC, Lindhout T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin Chem. 2012;58(8):1252-1259. [DOI] [PubMed] [Google Scholar]
- 16.van Beers EJ, Schaap MC, Berckmans RJ, et al. ; CURAMA study group. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94(11):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin O, Delobel J, Prudent M, et al. . Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53(8):1744-1754. [DOI] [PubMed] [Google Scholar]
- 18.Mooberry MJ, Bradford R, Hobl EL, Lin FC, Jilma B, Key NS. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J Thromb Haemost. 2016;14(5):1031-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorlie PD, Garcia-Palmieri MR, Costas R Jr, Havlik RJ. Hematocrit and risk of coronary heart disease: the Puerto Rico Health Program. Am Heart J. 1981;101(4):456-461. [DOI] [PubMed] [Google Scholar]
- 20.Erikssen G, Thaulow E, Sandvik L, Stormorken H, Erikssen J. Haematocrit: a predictor of cardiovascular mortality? J Intern Med. 1993;234(5):493-499. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up. Am Heart J. 1994;127(3):674-682. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee G, Shaper AG, Whincup PH. Ischaemic heart disease: association with haematocrit in the British Regional Heart Study. J Epidemiol Community Health. 1994;48(2):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabatine MS, Morrow DA, Giugliano RP, et al. . Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111(16):2042-2049. [DOI] [PubMed] [Google Scholar]
- 24.Toss F, Nordström A, Nordström P. Association between hematocrit in late adolescence and subsequent myocardial infarction in Swedish men. Int J Cardiol. 2013;168(4):3588-3593. [DOI] [PubMed] [Google Scholar]
- 25.Paffett-Lugassy N, Hsia N, Fraenkel PG, et al. . Functional conservation of erythropoietin signaling in zebrafish. Blood. 2007;110(7):2718-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata J, Hasegawa J, Siemens HJ, et al. . Hemostasis and coagulation at a hematocrit level of 0.85: functional consequences of erythrocytosis. Blood. 2003;101(11):4416-4422. [DOI] [PubMed] [Google Scholar]
- 27.Lamrani L, Lacout C, Ollivier V, et al. . Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124(7):1136-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strassel C, Kubovcakova L, Mangin PH, et al. . Haemorrhagic and thrombotic diatheses in mouse models with thrombocytosis. Thromb Haemost. 2015;113(2):414-425. [DOI] [PubMed] [Google Scholar]
- 29.Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med. 2013;210(2):205-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70(2):246-250. [DOI] [PubMed] [Google Scholar]
- 31.Choi D, Schroer SA, Lu SY, et al. . Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med. 2010;207(13):2831-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hand CC, Brines M. Promises and pitfalls in erythopoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward? J Invest Med. 2011;59:1073-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117(18):4953-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walton BL, Getz TM, Bergmeier W, Lin F-C, Uitte de Willige S, Wolberg AS. The fibrinogen γA/γ′ isoform does not promote acute arterial thrombosis in mice. J Thromb Haemost. 2014;12(5):680-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowl L, Fogelson AL. Analysis of mechanisms for platelet near-wall excess under arterial blood flow conditions. J Fluid Mech. 2011;676:348-375. [Google Scholar]
- 36.Crowl LM, Fogelson AL. Computational model of whole blood exhibiting lateral platelet motion induced by red blood cells. Int J Numer Methods Biomed Eng. 2010;26(3-4):471-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skorczewski T, Erickson LC, Fogelson AL. Platelet motion near a vessel wall or thrombus surface in two-dimensional whole blood simulations. Biophys J. 2013;104(8):1764-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rana K, Timmer BJ, Neeves KB. A combined microfluidic-microstencil method for patterning biomolecules and cells. Biomicrofluidics. 2014;8(5):056502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann M, Wallbank AM, Dennis KA, et al. . On-chip recalcification of citrated whole blood using a microfluidic herringbone mixer. Biomicrofluidics. 2015;9(6):064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood BL, Gibson DF, Tait JF. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood. 1996;88(5):1873-1880. [PubMed] [Google Scholar]
- 41.Gilson CR, Kraus TS, Hod EA, et al. . A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49(8):1546-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchioli R, Finazzi G, Specchia G, et al. ; CYTO-PV Collaborative Group. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Graham MD. Mechanism of margination in confined flows of blood and other multicomponent suspensions. Phys Rev Lett. 2012;109(10):108102. [DOI] [PubMed] [Google Scholar]
- 44.Reasor DA Jr, Mehrabadi M, Ku DN, Aidun CK. Determination of critical parameters in platelet margination. Ann Biomed Eng. 2013;41(2):238-249. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Shaqfeh ES. Shear-induced platelet margination in a microchannel. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83(6 Pt 1):061924. [DOI] [PubMed] [Google Scholar]
- 46.Vahidkhah K, Diamond SL, Bagchi P. Platelet dynamics in three-dimensional simulation of whole blood. Biophys J. 2014;106(11):2529-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stalker TJ, Welsh JD, Tomaiuolo M, et al. . A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124(11):1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, Diacovo TG, Chen J, Freund JB, King MR. Simulation of platelet, thrombus and erythrocyte hydrodynamic interactions in a 3D arteriole with in vivo comparison. PLoS One. 2013;8(10):e76949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landolfi R, Marchioli R, Kutti J, et al. ; European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114-124. [DOI] [PubMed] [Google Scholar]
- 50.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227-1234. [DOI] [PubMed] [Google Scholar]
- 51.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setty BN, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98(12):3228-3233. [DOI] [PubMed] [Google Scholar]
- 53.Fujita H, Sakuma R, Tomiyama J, et al. . Increased phosphatidylserine exposure on the erythrocyte membrane in patients with polycythaemia vera. Br J Haematol. 2011;152(2):238-240. [DOI] [PubMed] [Google Scholar]
- 54.Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A. Phosphatidylserine in the outer leaflet of red blood cells from beta-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol. 1993;44(1):63-65. [DOI] [PubMed] [Google Scholar]
- 55.Yasin Z, Witting S, Palascak MB, Joiner CH, Rucknagel DL, Franco RS. Phosphatidylserine externalization in sickle red blood cells: associations with cell age, density, and hemoglobin F. Blood. 2003;102(1):365-370. [DOI] [PubMed] [Google Scholar]
- 56.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121(18):3733-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciciliano JC, Sakurai Y, Myers DR, et al. . Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood. 2015;126(6):817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woollard KJ, Sturgeon S, Chin-Dusting JP, Salem HH, Jackson SP. Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. J Biol Chem. 2009;284(19):13110-13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoenwaelder SM, Jackson SP. Ferric chloride thrombosis model: unraveling the vascular effects of a highly corrosive oxidant. Blood. 2015;126(24):2652-2653. [DOI] [PubMed] [Google Scholar]
- 60.Neeves KB. Physiochemical artifacts in FeCl3 thrombosis models. Blood. 2015;126(6):700-701. [DOI] [PubMed] [Google Scholar]
- 61.Jensen AS, Johansson PI, Idorn L, et al. . The haematocrit--an important factor causing impaired haemostasis in patients with cyanotic congenital heart disease. Int J Cardiol. 2013;167(4):1317-1321. [DOI] [PubMed] [Google Scholar]