Abstract
The autoimmune blistering diseases (AIBDs) are a group of heterogeneous skin diseases with autoantibodies directed against structural proteins in the skin. A new interest in the female bias towards autoimmune diseases in general has led to our attention to focus on how and why this female bias manifests in AIBD. The authors aim to review and explore the various aspects of AIBD affecting females more than males, including the higher prevalence, worse quality of life, and complex management issues such as pregnancy and lactation.
Keywords: Autoimmune blistering diseases, Epidemiology, Epidermolysis bullosa acquisita, Female, Pemphigoid, Pemphigus, Quality of life, Toxic epidermal necrolysis, Treatment
Capsule summary
What is already known on this topic?
-
•
Echoing autoimmune diseases in general, most autoimmune blistering diseases (AIBDs) have a female predominance, but the exact level of predominance is unknown.
-
•
Pregnancy raises several complicated management issues for females with an AIBD.
What does this article add to our knowledge?
-
•
Review of sex-specific epidemiology and etiology of each AIBD.
-
•
Exploration and explanation of the key factors underlying the detrimental impacts of AIBD on women’s quality of life (QOL).
-
•
Discussion of management issues in pregnancy and lactation for females with an AIBD.
How does this information impact clinical practice and/or change patient care?
-
•
An awareness and understanding of the female predominance in AIBDs will ensure more appropriate diagnosis, evaluation, and future research.
-
•
Emphasizing holistic care targeting the debilitating effects of AIBDs on women’s QOL.
-
•
Informing the reader of optimal, yet safe interventions for pregnant women with an AIBD.
Introduction
The autoimmune blistering diseases (AIBDs) are characterized by pathogenic autoantibodies directed at target antigens, whose function is epidermal cell-to-cell or epithelium-to-dermis adhesion. Echoing autoimmune diseases in general, there is an overall female predominance in the majority of AIBDs, including the pemphigus group and most of the pemphigoid group. Also, similar to many other skin diseases, the quality of life (QOL) of female patients with AIBDs is affected more than their male counterparts, secondary to factors such as social misconceptions, physical disability, and poor mental health. Furthermore, there are multiple management issues that particularly affect females with AIBD, including pregnancy and lactation. An awareness and understanding of the female sex bias in AIBDs will ensure more appropriate diagnosis, evaluation, intervention, and future research.
Generally, data available about autoimmune bullous diseases are limited, and sex-specific data is even rarer. Available studies are almost invariably limited by small patient size, selection biases, unclear diagnostic criteria, or unreliable outcome measures. This highlights the importance of maintaining AIBD registries and conducting well-designed epidemiological and QOL studies. In Australia, there is currently an AIBD registry being developed and expanded with ongoing research into the epidemiology and QOL of patients with AIBD (Daniel et al., 2011).
In this article, the sex-specific epidemiology and etiology of autoimmune diseases will be reviewed as a whole, followed by each AIBD in turn, as the sex bias in each AIBD varies. Then, the key factors underlying the detrimental impacts of AIBDs on women’s QOL will be explored and explained. Subsequently, various management issues in pregnancy and lactation will be discussed, with the focus on disease course and treatment safety.
Epidemiology and etiology
Sex bias in autoimmune diseases
The autoimmune diseases include over 70 chronic disorders, and are well known to be generally more prevalent in women (Whitacre, 2001). Certain autoimmune diseases show an enormous sex bias, with females representing over 85% of cases of Addison’s disease, systemic lupus erythematosus (SLE), Sjogren’s syndrome, and thyroiditis. Meanwhile, other autoimmune diseases show a relatively weaker sex bias, with females representing 60–75% of patients, such as rheumatoid arthritis (RA) and multiple sclerosis (MS) (Whitacre et al., 1999). As patients with one autoimmune disease are more likely to have another autoimmune disease, the female predominance of autoimmune diseases is likely further multiplied (Humbert et al., 1989). In addition to disease incidence, the age of onset for many autoimmune diseases, including SLE and MS, is earlier in females (Markle and Fish, 2014, McCarty et al., 1995).
Hormonal, immunological, microbiomial, and epigenetic theories may explain sex-specific autoimmunity. Sex hormones are reported to affect the susceptibility to autoimmune diseases. Clinically this is evidenced by sex hormone fluctuations accompanying the menstrual cycle, pregnancy, and menopause correlating to varied incidences of autoimmune diseases in females. Some preexisting autoimmune diseases, such as RA and MS, tend to remit in pregnancy and relapse in the postpartum period (Markle and Fish, 2014). Studies have shown that testosterone has a protective effect and estrogen has a stimulatory effect on the immune system (Ahmed and Penhale, 1982, Bebo et al., 1998, Harbuz et al., 1995, Voskuhl et al., 1996). These effects may have been secondary to sex hormone–dependent modulation of plasmacytoid dendritic cells’ function, evident by in vivo mice models (Calippe et al., 2008, Seillet et al., 2012). Another explanation proposed was that estrogen may directly stimulate macrophages through ERα, and thereby augmenting the immune response (Calippe et al., 2008). Furthermore, higher natural killer T-cell numbers were observed in women as compared with men, with the effects of androgen and ERα exposure on its functioning elucidated by mice models (Gourdy et al., 2005, Sandberg et al., 2003).
Hormonal status of the host may also be shaped by microbiome composition, which then reciprocally exerts influence over sex hormone levels (Markle and Fish, 2014, Yatsunenko et al., 2012). Furthermore, the X chromosome has been shown to contain a large quantity of immune-related genes, leading to theories suggesting sex-specific epigenetic mechanisms for autoimmunity (Markle and Fish, 2014).
Pemphigus
Pemphigus is an uncommon group of AIBDs with an overall female predominance. Its main types include pemphigus vulgaris (PV), accounting for 70% of total pemphigus cases, and pemphigus foliaceus (PF), accounting for 15–20% of total pemphigus cases. Other, less common types include paraneoplastic pemphigus, drug-induced pemphigus, pemphigus herpetiformis, and pemphigus erythematosus. Immunopathologically, it is characterized by autoantibodies of mainly IgG4 subclass targeted at the epidermal intercellular adhesion glycoproteins, desmoglein 1 (Dsg1), and desmoglein 3 (Dsg3). Clinically, it is manifested in patients aged 20 to 40 years and is characterized by flaccid blisters and erosions of the skin and mucous membranes (Fig. 1, Fig. 2).
Fig. 1.
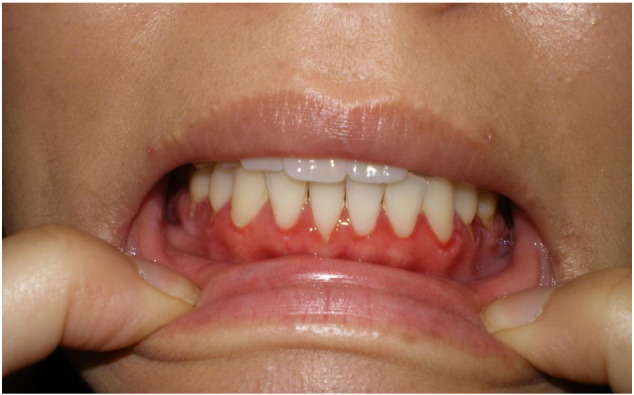
Debilitating shallow oral erosions in a female patient with pemphigus vulgaris.
Fig. 2.
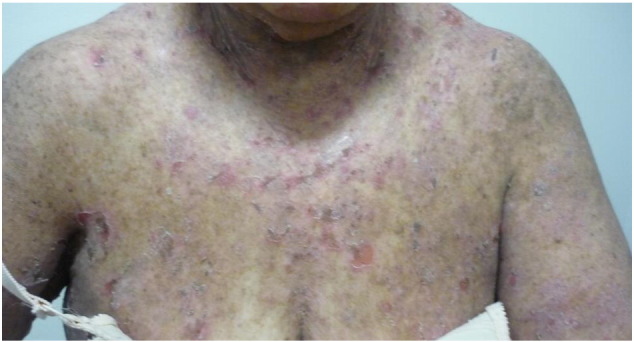
Exfoliative scales and erosions on the chest of a female patient with pemphigus foliaceus.
Multiple medium-to-large epidemiological studies have been conducted for pemphigus. Epidemiological data from the United Kingdom, Macedonia, Greece, Croatia, Italy, Bulgaria, France, Finland, South Africa, Tunisia, Mali, China, Taiwan, Turkey, Iran, Korea, and Kuwait have shown that pemphigus tends to bias females (Table 1) (Aboobaker et al., 2001, Alsaleh et al., 1999, Bastuji-Garin et al., 1995, Hietanen and Salo, 1982, Huang et al., 2012, Langan et al., 2008, Ljubojevic et al., 2002, Mahe et al., 1996, McPherson and Venning, 2011, Micali et al., 1998, Michailidou et al., 2007, Seo et al., 2003, Tsankov et al., 2000, Uzun et al., 2006, Zhu et al., 2014). The only exception was a relatively small study from the United States showing a female-to-male ratio of 1.0 (Woldegiorgis and Swerlick, 2001). When compared among continents, there were consistent female-to-male ratios ranging between 1.1 and 2.3 in studies conducted in Europe and Asia, and PV was almost always the dominant form in these studies. The ratios were much higher in studies conducted in Africa; for example, Mali and Tunisia had female-to-male ratios of 4.0 and 4.1, respectively (Bastuji-Garin et al., 1995, Mahe et al., 1996). These were likely influenced by the high incidence of endemic PF in these countries, which may have a much higher prevalence in young women (Bastuji-Garin et al., 1995). Limitations to our current knowledge of pemphigus sex ratios include its epidemiology in Oceania and North America. Also, no conclusion can be drawn regarding age-specific sex ratios of pemphigus given the limited number of identified cases to date (Marazza et al., 2009).
Table 1.
Reported female-to-male ratios of pemphigus in worldwide representative studies.
| Country | Cases | Dominant form | Years | Female-to-male ratio |
|---|---|---|---|---|
| Europe | ||||
| United Kingdom (Langan et al., 2008) | 138 | PV | 1996–2006 | 1.93 |
| Macedonia (McPherson and Venning, 2011) | 133 | PV | 1990–2004 | 1.33 |
| Greece (Northern region) (Michailidou et al., 2007) | 129 | PV | 1985–2004 | 2.25 |
| Croatia (Ljubojevic et al., 2002) | 159 | PV | 1996–1998 | 2.0 |
| Italy (Sicily) (Micali et al., 1998) | 84 | PV | 1982–1996 | 1.6 |
| Bulgaria (Sofia) (Tsankov et al., 2000) | 74 | PV | 1980–1995 | 1.11 |
| France (Bastuji-Garin et al., 1995) | 87 | PV | 1985–1990 | 1.2 |
| Finland (Hietanen and Salo, 1982) | 44 | PF | 1969–1978 | 1.1 |
| Africa | ||||
| South Africa (Aboobaker et al., 2001) | 112 | PF | 1987–1999 | 1.4 |
| Tunisia (Bastuji-Garin et al., 1995) | 198 | PF | 1986–1991 | 4.1 |
| Mali (Mahe et al., 1996) | 30 | PF | - | 4.0 |
| Asia | ||||
| China (Northeast region) (Zhu et al., 2014) | 221 | PV | 2001–2010 | 1.4 |
| Taiwan (Huang et al., 2012) | 853 | PV | 2002–2009 | 1.3 |
| Turkey (Mediterranean region) (Uzun et al., 2006) | 148 | PV | 1998–2004 | 1.35 |
| Iran (Chams-Davatchi et al., 2005) | 1209 | PV | 1984–2003 | 1.5 |
| Korea (Seo et al., 2003) | 51 | PV | 1993–2001 | 1.3 |
| Kuwait (Alsaleh et al., 1999) | 45 | PV | 1981–1996 | 2.0 |
| Israel (Pisanti et al., 1974) | 76 | PV | 1952–1972 | 1.62 |
| America | ||||
| United States (Southeast region) (Woldegiorgis and Swerlick, 2001) | 30 | PV | 1992–1999 | 1.0 |
Pemphigus vulgaris
Pemphigus vulgaris has a female predominance, evidenced by female-to-male ratios of 1.4, 2.3 and 1.62, respectively, in PV-only epidemiological studies conducted in China, Greece, and Israel (McPherson and Venning, 2011, Michailidou et al., 2007, Pisanti et al., 1974). Pemphigus vulgaris also echoes the pattern that autoimmune diseases occur more frequently in patients with another autoimmune disease and has associations with the female-predominant thyroid diseases and rheumatoid arthritis (Ameri et al., 2013, Gupta et al., 2011, Ljubojevic and Lipozencic, 2012, Svecova et al., 2014). Worsened outcome of PV has also been associated with females, as one recent analysis showed that the HLA alleles DRB1*04:02 and DQB1*03:02 were associated with severe PV, and DQB1*03:02 was found more frequently in female patients than in males (Svecova et al., 2014). However, no epidemiological studies to date have evidenced the association of sex with this genetic predisposition.
Pemphigus foliaceus
There is a characteristic female predominance found in the Tunisian subtype of PF, especially amongst young women. Pemphigus foliaceus is categorized into sporadic PF and endemic PF. The latter is also known as fogo selvagem, which includes Tunisian, Brazilian, Peruvian-Amazon, and Columbian pemphigus. Tunisian pemphigus was found to have a female-to-male ratio of 4.1; an overall earlier onset, with the mean age of onset at 28 years; and an overall increased incidence as compared with other forms of pemphigus, at 4 cases per million (Bastuji-Garin et al., 1995, Bastuji-Garin et al., 2002, Morini et al., 1993). A case–control study of 68 female pemphigus patients in Tunisia revealed the development of Tunisian pemphigus is significantly associated with traditional Tunisian lifestyle, including contact with ruminants, cutting up raw poultry, Turkish baths, and cosmetics (Bastuji-Garin et al., 2002). Another case series of 23 Tunisian pemphigus patients suspected the provoking role of pregnancy in disease development, as Tunisia is a country where young women have a high fecundity rate (Morini et al., 1993). On the other hand, Brazilian pemphigus was found to have no sex predisposition, and Columbian as well as Peruvian-Amazon pemphigus were both found to have a contrasting male predominance (Abreu-Velez et al., 2003, Ramos et al., 2012). The sex predisposition in sporadic PF has been unclear due to limited epidemiological data.
Pemphigoid
Pemphigoid diseases are the commonest group of AIBDs; they have an overall female predominance, the extent of which varies amongst its types. Pemphigoid diseases are defined by the presence of autoantibodies against distinct structural components of the dermal–epidermal junction. Within the pemphigoid group are bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), pemphigoid gestationis (PG), linear IgA diseases (LADs), and epidermolysis bullosa acquisita (EBA).
Bullous pemphigoid
Bullous pemphigoid (BP) is the commonest pemphigoid disease and has a slight female predominance. It is immunopathologically characterized by autoantibodies targeting BP180 and BP230, which are molecular constituents of the stratified epithelial hemidesmosomes (Liu et al., 1993, Stanley et al., 1981). Clinically, it is manifested by pruritic urticarial papules and plaques, progressing to form vesicles and tense subepidermal bullae (Fig. 3). Elderly patients after the seventh decade are typically affected. Medium-to-large epidemiological studies in Poland, United Kingdom, Switzerland, Scotland, Germany, Italy, France, Kuwait and Singapore have shown BP’s female-to-male ratios to range between 1.04 and 1.6 (Table 2) (Bernard et al., 1995, Gudi et al., 2005, Jung et al., 1999, Langan et al., 2008); Marazza et al., 2009, Nanda et al., 2006, Serwin et al., 2007, Wong and Chua, 2002). This female predominance may partly be explained by the fact that female patients are more likely than males to live to the elderly age in which BP develops, given their higher life expectancy. Interestingly, however, exceptionally higher female-to-male ratios of 5.1 and 1.91 are reported in Kuwait and Poland. Both of these studies have patient mean ages below 70 years, whereas other studies mostly have patient mean ages above 70 years. This trend recurred in the Switzerland study, which had also evidenced the incidence of BP to be higher in women until the age of 70 years, but thereafter the incidence is higher in men (Marazza et al., 2009). The reason for this age-specific trend in sex difference is unexplained. Although the female sex has been associated with a slightly increased incidence of BP, studies have not discovered the female sex to be associated with mortality rate or relapse rates of BP (Cai et al., 2014, Fichel et al., 2014). Future research regarding age-specific sex predominance of BP, as well as its epidemiology in America, Africa, and Oceania, may be implicated.
Fig. 3.
Urticarial papules and plaques in a female patient with newly diagnosed bullous pemphigoid.
Table 2.
Reported female-to-male ratios of bullous pemphigoid in worldwide representative studies.
| Country | Cases | Mean Age | Years | Female-to-male ratio |
|---|---|---|---|---|
| Europe | ||||
| Poland (Serwin et al., 2007) | 35 | 68.9F, 67.3M | 2000–2006 | 1.91 |
| United Kingdom (Langan et al., 2008) | 869 | 80 | 1996–2006 | 1.59 |
| Switzerland (Marazza et al., 2009) | 140 | 77.2 | 2001–2002 | 1.3 |
| Scotland (Gudi et al., 2005) | 83 | 79.2 | 1991–2001 | 1.5 |
| Germany (Jung et al., 1999) | 94 | 79.3F, 76.1M | 1989–1997 | 1.01 |
| Italy (Cozzani et al., 2001) | 32 | 74 | 1996–1997 | 1.46 |
| France (Bernard et al., 1995) | 69 | 82.4 | 1989–1994 | 1.48 |
| Asia | ||||
| Kuwait (Nanda et al., 2006) | 43 | 65.2 | 1991–2005 | 5.1 |
| Singapore (Wong and Chua, 2002) | 59 | 77 | 1998–1999 | 2.0 |
Mucous membrane pemphigoid
Mucous membrane pemphigoid, formally known as cicatricial pemphigoid, is a female-predominant and rare subtype of pemphigoid. It is immunopathologically characterized by autoantibodies against BP180, as well as BP230, laminin 332, α6β4 integrin, and type VII collagen (Oyama et al., 2006, Schmidt and Zillikens, 2013). Clinically, it is manifested by subepidermal blisters of the mucous membranes (Fig. 4). The female-to-male ratio of mucous membrane pemphigoid is reported to be almost 2.0 (Schifter et al., 2010, Woo and Greenberg, 2008). However, epidemiological studies evidencing this female predominance have not been performed.
Fig. 4.
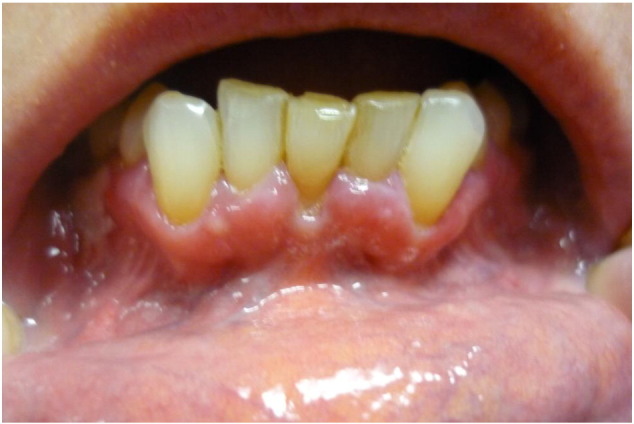
Vesicles and bullae in the gingiva of a female patient with mucous membrane pemphigoid.
Pemphigoid gestationis
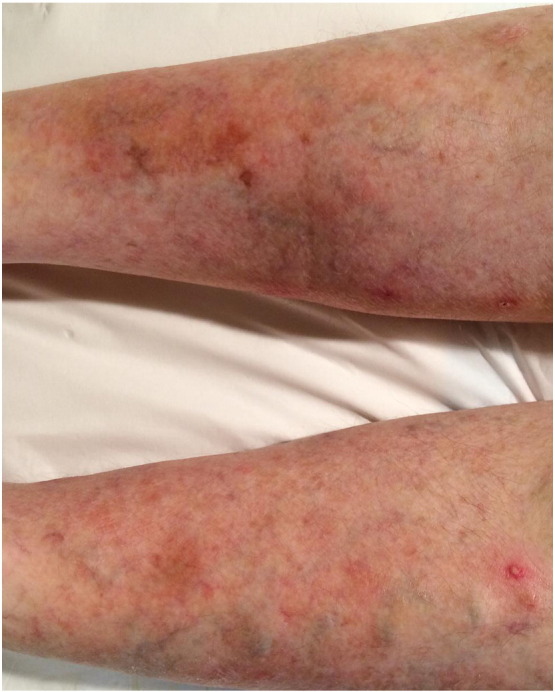
Pemphigoid gestationis (PG) is an uncommon female-exclusive pemphigoid disease, with autoantibodies directed against BP180 NC16A. It was formerly known as herpes gestationis, a term coined by Dr. John Milton in 1872 to describe a 45-year-old woman with a herpetiform rash in four out of nine pregnancies (Black, 2003). Clinically, PG is manifested by intensely pruritic and urticarial-alike red papules and plaques that progress to large, tense, fluid-filled blisters in two to four weeks’ time (Fig. 5). Its other names include dermatitis multiformis gestationis and gestational pemphigoid. Recent advances have shown PG to be immunopathologically and clinically similar to the pemphigoid group of diseases; hence it was renamed as pemphigoid gestationis. Since then, there have been several medium-sized case series on PG, although large epidemiology studies have been limited.
Fig. 5.
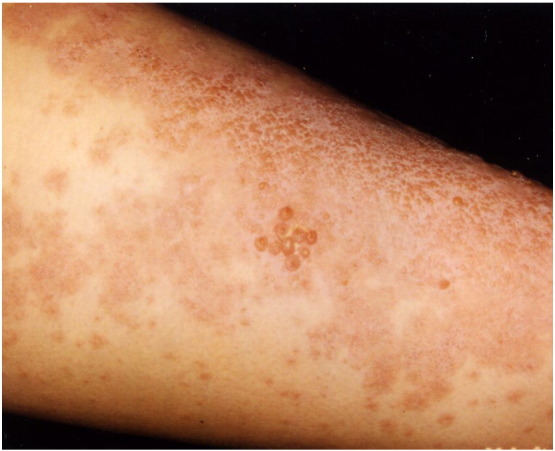
Urticarial plaques and grouped vesicles on the lower leg of a pregnant woman with pemphigoid gestationis.
Pemphigoid gestationis has an incidence of 1 case in 50,000 to 60,000 pregnancies (Al-Fouzan et al., 2006, Cobo et al., 2009). A recent review found it may be the second-most-common AIBD overall after BP, accounting for 9.75% of 41 AIBD cases in one study (Bertram et al., 2009). Almost one half of the cases develop in primigravida women. Pemphigoid gestationis commonly presents in the second or third trimester of pregnancy, but 10% of PG cases may occur in women within 4 weeks of giving birth (Ambros-Rudolph et al., 2006, Jenkins et al., 1999). In a study of 505 women with pregnancy-related dermatoses, 21 had PG; of these, 71% occurred in the third trimester and 29% in the second semester. Although PG most commonly develops in pregnancy, case reports also have shown it occurring in women with trophoblastic tumors, hydatidiform mole, or choriocarcinoma (Jenkins et al., 1999). Women with PG are also more likely to have other female-predominant autoimmune diseases, including Graves’ disease, Hashimoto’s thyroiditis, and pernicious anemia. This can be partially explained by the presence of HLA-DR3 and DR4 antigens in PG, as these were found to be independently associated with other autoimmune diseases including autoimmune thyroiditis and pernicious anemia (Shornick and Black, 1992).
Female sex hormones likely play a key role in PG disease course. Pemphigoid gestationis has been reported to flare after the use of oral contraceptives and during menstruation. Progesterone, a hormone that is raised at the end of pregnancy but plummets after the delivery of the baby, depresses antibodies production (Da Silva, 1999). This could explain the predelivery improvement in PG and its postpartum flare. On the other hand, estrogen, a hormone in oral contraceptives, enhances antibody production, which could explain the flare after administration of oral contraceptives (Intong and Murrell, 2011).
Epidermolysis bullosa acquisita
Epidermolysis bullosa acquisita is a rare, female-predominant, and phenotypically heterogeneous subtype of pemphigoid characterized by autoantibodies against type VII collagen. Three studies so far have specifically studied epidermolysis bullosa acquisita, and combined data with a total of 83 cases revealed that its female-to-male ratio is 1.56 (Buijsrogge et al., 2011, Kim et al., 2011, Zumelzu et al., 2011).
Linear IgA disease
Linear IgA disease is a very rare subtype of pemphigoid with scarce epidemiological data regarding sex-bias in its incidence. It is immunopathologically characterized by linear binding IgA mainly affecting BP180. Clinically, it is characterized by either annular blisters or targetoid plaques with studded vesicles and bullae (Fig. 6). Four large epidemiological studies conducted in Kuwait, Iran, Singapore, and Tunisia with 1,711 AIBD patients in total had 27 cases of linear IgA disease. Out of these, 14 cases were male and 13 were female, equaling a male-to-female ratio of 1.07 (Daneshpazhooh et al., 2012, Nanda et al., 2004, Wong and Chua, 2002, Zaraa et al., 2011).
Fig. 6.
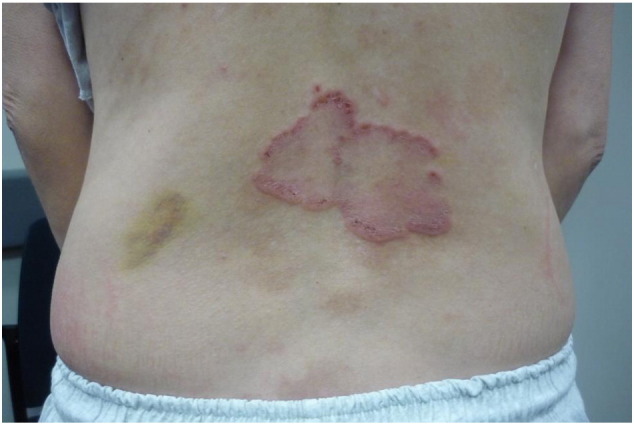
Annular plaques with raised and eroded margins in a female patient with linear IgA disease.
Dermatitis herpetiformis
In contrast to pemphigus and pemphigoid diseases, the evidence for sex predominance in dermatitis herpetiformis (DH) has been conflicting. Dermatitis herpetiformis has been shown to associate closely with celiac disease, as both conditions are mediated by IgA autoantibodies targeting the autoantigen transglutaminase. Although a small study has reported that DH has a male-to-female ratio ranging from 1.5 to 2, the opposite of this was found in a larger study of celiac disease, which has a female-to-male ratio ranging from 2 to 4.1 (Llorente-Alonso et al., 2006). The conflicting findings from the two studies prompt further research regarding DH’s epidemiology.
Stevens–Johnson syndrome/toxic epidermal necrolysis spectrum
Toxic epidermal necrosis (TEN), Stevens–Johnson Syndrome (SJS), and SJS/TEN overlap are life-threatening, typically drug-induced blistering skin reactions affecting > 30%, < 10%, and 10–30% of total body surface area, respectively. It has been proposed that there are autoimmune mechanisms underlying its pathogenesis, with antigen-induced T-cell activation leading to keratinocyte apoptosis (Schwartz et al., 2013). Toxic epidermal necrosis has been shown to be more female-predominant, though SJS may require more epidemiological studies to confirm if there is a sex predilection. A large study in France involving 253 TEN patients has found TEN to have a female-to-male ratio of 1.58 (Roujeau et al., 1990). This female predominance is confirmed by medium-size studies in India (25 patients) and Germany (40 patients) showing female-to-male ratios of 2.57 and 1.22, respectively (Sanmarkan et al., 2011, Weinand et al., 2013). However, regarding SJS, its female-to-male ratios have been inconsistent in the limited numbers of SJS-only studies performed so far, varying from 0.61 to 1.37 (Limpawattana et al., 2014, Sanmarkan et al., 2011). The female predominance in TEN, but not in SJS, has been unexplained so far.
Quality of life
AIBDs can have significant impacts on the patient’s QOL. Like many other skin diseases, the QOL of females with AIBD was found to be affected more than the QOL of males in several studies to date (Paradisi et al., 2009, Paradisi et al., 2012, Sampogna et al., 2006). The significant impacts of AIBDs on QOL are due to factors such as time spent on treatment, financial burden, social misconceptions, physical discomfit and limitations, and poor mental health (Chee and Murrell, 2011, Sebaratnam et al., 2012). Out of those, the last three factors were found to affect women much more than men in various studies. Between the two main groups of AIBDs, pemphigus has been shown to lead to a worse QOL with a Dermatology Life Quality Index (DLQI) score of 10 (compared to pemphigoid with a DLQI score of 6.92), possibly due to the rarity of mucosal involvement in pemphigoid (Mayrshofer et al., 2005). Overall, pemphigus QOL studies have been more frequently conducted than pemphigoid studies. This may be partially explained by the fact that pemphigus is associated with a worse QOL. Another explanation of the dearth of QOL studies in pemphigoid could be that QOL questionnaires are more difficult to administer in bullous pemphigoid patients, given their advanced age.
Measurement of QOL in AIBDs is of utmost importance, with significant recent efforts made into its research and developments. When conducting most QOL studies in AIBD patients, generic QOL measures such as the Medical Outcome Study 36-item Short-form Survey (SF-36) or skin-disease generic QOL measures, such as the Skindex-17, Skindex-29, or DLQI, have been used. In some studies, oral-health generic QOL measures such as Chronic Oral Mucosal Diseases Questionnaire (COMDQ) or psychiatric generic measures such as the 12-item General Health Questionnaire (GHQ-12) have also been used. However, none of those measures were disease-specific until the development of the Autoimmune Bullous Disease Quality of Life (ABQOL) questionnaire and the Treatment Autoimmune Bullous Disease Quality of Life (TABQOL) questionnaire by the Murrell group (Sebaratnam et al., 2013, Tjokrowidjaja et al., 2013). The ABQOL and TABQOL have both been shown as valid and reliable instruments for measuring QOL and treatment burden, respectively, in AIBDs.
Physical and mental factors are key contributors to the worse QOL of females with AIBDs as compared with men. A study by Paradisi et al. (2009) was conducted using the Skindex-29, the GHQ-12, and the SF-36 to evaluate the QOL in 139 pemphigus patients in Italy. The study found that women had worse physical and mental domain scores than men in the Skindex-29 and the GHQ-12. For the Skindex-29, women scored 41, 41, and 36 compared to men’s 31, 31, and 29 in its three components of symptoms (p = 0.01), emotions (p = 0.02), and social functioning (p > 0.05), respectively. For the GHQ-12, which measured psychological distress, 42.9% of women scoring ≥ 4, compared to only 34.7% of men. No statistical significance analysis was reported for the GHQ-12 results. This finding was confirmed in other diseases such as psoriasis and heart failure, where women also reported more physical disability, worse pain, more symptoms, and more psychological issues than men with the same disease severity (Emery et al., 2004, Sampogna et al., 2006).
The poorer physical scores in female pemphigus patients compared with their male counterparts may be partly explained by the oral lesions in pemphigus. A QOL study by Rajan et al. (2014) was conducted using the COMDQ in 70 patients with chronic oral mucosal lesions, which included 9 pemphigus patients. The study showed that women scored significantly worse in pain and functional limitation: 21.44 for women versus 16.14 for men. The pain and functional limitations may have significant subsequent implications for women, as this domain has been found to relate to poor eating habits, interpersonal relationships, appearance, and self-image (Slade et al., 2004).
Due to symptomatic and psychosocial factors, the QOL of females was found to be more affected than males even when their disease activity was quiescent (Tabolli et al., 2014). A study was conducted by Tabolli et al. (2014) using the Skindex-17 and the GHQ-12 in 203 pemphigus patients. The authors compared the QOL of 156 patients with active disease and 47 patients with quiescent disease. The 47 patients with quiescent disease had no active bullae or erosions, and had Physical Global Assessment scores as well as Ikeda scores of 0. The study found that females in the active and quiescent disease groups both scored higher than males in the symptoms and psychosocial components of the Skindex-17 and the GHQ-12. More than 20% of the patients in the quiescent disease group believed they “often/always” suffered from pemphigus. The item of Skindex-17 with smallest difference between patients with active and quiescent disease was that of “I tend to do things by myself.” Hence, it is likely that pemphigus had not only caused patients to do things by themselves when it was active, but also caused them to continue having the same behavioral pattern after remission. This may have affected females more given their worse QOL scores.
Quality of life is a multifactorial construct consisting of physical factors as well as personality, expectations, socioeconomic and marital status, and religious experience (Both et al., 2007). The worse QOL of females with AIBDs has also been found to be due to social misconceptions, poor self-image, and limited marriage prospects (Terrab et al., 2005). A study by Terrab et al. (2005) was conducted using the SF-36 as well as questions about the impact of pemphigus on self-perception, social relationships, and behavior to explore the QOL of 30 patients with pemphigus in Morocco. The study revealed that in Morocco, pemphigus was believed to be linked to cultural taboos such as poor hygiene and unconventional sexual practices, and would also be deemed incurable if drug-resistant. Young women were particularly affected by limited marriage prospects, which may further debilitate their psychosocial functioning.
In SJS/TEN, the QOL of females is likely compromised by its well-known, long-standing ocular and gynecological complications, which are likely underestimated in its prevalence. A study by Haber et al. (2005) was conducted using the SF-36 and DLQI in 13 patients admitted with TEN, 38 ± 27 months after their hospital discharge. The study found the TEN patients' SF-36 scores were significantly worse than the normal population, although no sex-specific analysis was performed on the small study population. Ocular complications occurred in 77% of patients, with the most common symptoms being chronic sensitivity and dry eyes. Gynecological complications, which included vaginal adhesions and dyspareunia, were less frequently observed than ocular complications, and occurred in two out of eight female patients. Despite its lower prevalence, the significant impact of the TEN-related gynecological sequelae on QOL was explored in another retrospective study conducted by Meneux et al. (1998) in 40 female patients with TEN. The study found five patients had chronic gynecological complications, and all of them had their sexual activities affected. Two patients with exclusive vulva sequelae, both of whom were younger than 30 years of age and nulliparous, completely avoided intercourse, which may subsequently compromise their future relationships.
Management issues
Pregnancy
Pregnancy and giving birth is a pivotal part of a woman’s life. However, for women with an AIBD, pregnancy raises several complicated management issues including fluctuating disease course, prudency required with investigations, treatment limitations, and the preference for a vaginal delivery.
In a fertile woman with an established AIBD, pregnancy planning is believed to improve pregnancy outcomes due to the above issues. Preconception counseling should be initially conducted in close collaboration with the patient’s obstetrician and family doctor, highlighting the difficulties and importance of achieving disease control during pregnancy. This should then be frequently revisited with any affected fertile woman (Braunstein and Werth, 2013). Pregnancy should be planned to occur at times of low disease activity so that certain contraindicated medications, such as mycophenolate or rituximab, can be stopped or replaced prior to conception with safer options such as dapsone, cyclosporine, IVIG, and azathioprine (McPherson and Venning, 2011). Shorter period follow-up appointments should be made throughout the pregnancy planning. Disease activity should be monitored clinically with a validated outcome measure such as the Autoimmune Bullous Disorder Intensity Score (ABSIS), Pemphigus Disease Area Index (PDAI), or Bullous Pemphigoid Disease Area Index (BPDAI), and serologically with autoantibody titers, when available (Murrell et al., 2012, Pfutze et al., 2007, Rosenbach et al., 2009).
An AIBD’s disease course may fluctuate during pregnancy. This is because pregnancy itself is a specific immune state with dramatic hormonal changes in not only estrogen and progesterone, but also cortisol, norepinephrine and dehydroepiandrosterone (McPherson and Venning, 2011). Partly under the influence of such hormonal changes, immunological transformations occur in the mother to accommodate the genetically different fetal tissue, leading to varied AIBD severity. Some AIBDs, such as pemphigoid gestationis, have their initial onset associated with pregnancy, while other preexisting AIBDs may improve or flare during pregnancy or postpartum (Table 3). Pemphigus vulgaris tends to improve during pregnancy but not until the third trimester, and then flares postpartum (Kardos et al., 2009). Associated neonatal PV may occur, but is transient and easily treated (Campo-Voegeli et al., 2002, Chowdhury and Natarajan, 1998). Pemphigus foliaceus tends to have a variable course and associated neonatal PF is extremely rare. On the other hand, BP is very rare in pregnancy, as it mainly affects the elderly population. Meanwhile, LAD usually improves in pregnancy and typically remits during the second trimester, but may later relapse during the postpartum period (Collier et al., 1994).
Table 3.
The disease course of various more common autoimmune blistering diseases and associated adverse outcomes during pregnancy.
| AIBD type | Disease course in pregnancy | Adverse outcomes | Neonatal blistering disease |
|---|---|---|---|
| Pemphigus vulgaris | May improve in the third trimester of pregnancy but flares postpartum | Adverse outcome in up to 10% of cases in one case series, associated with poor disease control. Risk for preterm birth in severe disease. |
Transient and easily treatable |
| Pemphigus foliaceus | Variable course | Minimal | Extremely rare |
| Bullous pemphigoid | Rarely seen in pregnancy | Not reported | Not reported |
| Linear IgA disease | May improve in the second trimester but flares postpartum | Minimal | Not reported |
| Pemphigoid gestationis | Typically occurs in second to third trimester | Increased risks for preterm birth, small for gestational age, and low birth weight | Extremely rare |
Abbreviation: AIBD, autoimmune blistering disease
Investigations including skin biopsies should be performed with prudency in the pregnant woman. While in established AIBD there is minimal need for a skin biopsy, in a newly presented woman, skin biopsies for histopathology and direct immunofluorescence are traditionally needed for diagnosis. In this case, it is important to ensure previous investigation results have been sought and to consider the individual’s circumstance prior to biopsying. This is because it is believed that there may be a small risk of prilocaine fetal methemoglobinemia and uterine blood flow disruption that could happen secondary to local anesthetics use during the skin biopsy (Hrgovic, 1990, Nau, 1985, Voorbrood et al., 1982). Meanwhile, newer and safer diagnostic methods, including enzyme-linked immunosorbent assay (ELISA) of BP180 and desmoglein 1 and 3 autoantibodies, indirect immunofluorescence, and biochip immunofluorescence microscopy, should be considered as potential alternatives to biopsying (Russo et al., 2014, Sardy et al., 2013, Tampoia et al., 2012).
Treatment for AIBD in pregnancy is often required, but choice of medication presents a challenge due to safety concerns. In general, scientific evidence is limited due to the rarity of AIBDs and the exclusion of pregnant woman from most drug trials. The current guidelines from the United States Food and Drug Administration (FDA) and the Australian Drug Evaluation Committee (ADEC) for common AIBD treatments’ safety in pregnancy are summarized in Table 4, with relevant recommendations from various studies (Murase et al., 2014).
Table 4.
Guidelines and recommendations for the use of autoimmune blistering disease treatments during pregnancy and lactation.
| Dermatologic Medications Commonly Used in AIBD | Pregnancy |
Lactation |
|||
|---|---|---|---|---|---|
| FDA1 | ADEC2 | Recommendations (Evidence level3) | Recommendations (Evidence level3) | ||
| Corticosteroids | Topical | C | A, B3, C | Use mild to moderate over potent strengths (IB) | Ok for use on nipples, except for Class I (IV) |
| Oral | C | A | May increase the risk of oral clefts in first trimester (IV) | Use < 3 weeks (IV) | |
| Anti-inflammatory | Dapsone | C | B2 | Associated with hyperbilirubinemia/hemolytic anemia (IV) | Avoid in G6PD/hyperbilirubinemia (IV) |
| IVIG | C | - | Limited evidence for safety (IV) | Can be used safely (III) | |
| Systemic immunosuppressives | Mycophenolate mofetil | D | D | Contraindicated (III) | Avoid, likely enters milk (IV) |
| Cyclosporin | C | C | Relatively safe in studies on transplants patients (III) | Evidence limited (IV) | |
| Azathioprine | D | D | Not recommended (III) | Monitor infant health (IV) | |
| Rituximab | C | - | Not recommended (III) | Minimal data, avoid (IV) | |
| Omalizumab | B | B1 | Limited evidence for safety (IV) | Evidence limited (IV) | |
1United States Food and Drug Administration (FDA) drug risk classifications
A: Clinical data show no evidence of risk to the fetus
B: Clinical data are limited or not available, but animal studies show no evidence of risk to the fetus, or clinical data show no evidence of risk to the fetus, but animal studies show adverse effects to the fetus
C: Clinical data are not available and animal studies are not available, or clinical data are not available, but animal studies show adverse effects to the fetus
D: Positive evidence of risk to the fetus from clinical data
X: Contraindicated based on animal studies or clinical data
2Australian Drug Evaluation Committee (ADEC) drug risk classifications
A: Extensive clinical experience in pregnant women and women of childbearing age has shown no increase in the frequency of malformations or other harmful effects on the fetus
B: Human data are lacking or inadequate. Limited use in pregnant women and women of childbearing age has shown no increase in the frequency of malformation or other harmful effects on the human fetus.
B1: Animal experiments have not given evidence of an increased incidence of fetal damage; similar to FDA category B
B2: Animal experiments are inadequate; similar to FDA category C
B3: Reproduction toxicity studies in animals have revealed an increased incidence of fetal damage, the significance of which is considered uncertain in humans; similar to FDA category C
C: Drugs which, owing to their pharmacologic effects, have caused or may be suspected of causing, harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible.
D: Drugs which have caused, are suspected to have caused, or may be expected to cause, an increased incidence of human fetal malformations or irreversible damage.
X: Contraindicated in pregnancy
3Evidence levels
IA: Meta-analysis of randomized controlled trials
IB: Randomized controlled trials
II: Nonrandomized controlled studies and any quasi-experimental study
III: Comparative, correlational, case–control
IV: Expert reports/opinions or clinical reports
Adapted from Murase et al., 2014
Topical corticosteroids have been reviewed in multiple large studies, including a Cochrane review, and have not been shown to link to severe adverse outcomes in pregnancy (Chi et al., 2009, Chi et al., 2010). However, very potent topical corticosteroids may be associated with fetal growth restrictions. Oral corticosteroids are relatively safe but may lead to serious adverse outcomes in larger doses. Studies have shown oral corticosteroid use to lead to a three-fold increased risk of orofacial clefts in the third trimester, as well as possible associations with premature delivery, premature membrane, intrauterine growth retardation, gestational diabetes, hypertension, preeclampsia, and eclampsia (Carmichael et al., 2007, Park-Wyllie et al., 2000). As such, experts recommend clinicians to avoid using oral corticosteroids over 20 mg per day in pregnant patients (Murase et al., 2014). In this case, other anti-inflammatory or immunosuppressive therapies may be considered as an alternative.
Out of the other anti-inflammatory and immunosuppressive therapies for AIBD, the relatively safer options in pregnancy would be dapsone, cyclosporine, IVIG, and omalizumab, although conclusive evidence has been limited (Braunstein and Werth, 2013, McPherson and Venning, 2011, Murase et al., 2014, Yu et al., 2014). These safer options would be useful for refractory AIBD in pregnancy. Azathioprine is considered the best second-line alternative to corticosteroids, although it has been reported to occasionally associate with preterm and low-birth-weight infants. On the other hand, mycophenolate mofetil should be avoided, as it has been shown to potentially increase the risk of various adverse outcomes including miscarriage, microtia, and various organ system abnormalities (Kim et al., 2013). Caution should also apply to rituximab, as it is potentially associated with hemolytic abnormalities (Murase et al., 2014).
At the end of the pregnancy, the delivery method in the pregnant AIBD woman should be vaginal, as slow wound healing may occur with caesarean delivery associated with corticosteroid use. This would need to be discussed with the patient’s obstetrician (Clay and Pandya, 2011).
Lactation
Lactating women with an AIBD also face the issue of drug safety. Although most medications are relatively safe in lactation period, it is important to be aware of mycophenolate mofetil, which is contraindicated in lactating women (Table 4). It has been shown that mycophenolate mofetil can be transferred into the milk of lactating rats. This exposure could lead to adverse effects on neonatal health, increasing the risks of infection and lymphoma (Butler et al., 2014). Also, potent topical steroids should be avoided around the nipple area and rituximab should be avoided in infants at risk of hyperbilirubinemia or glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Summary
AIBDs have a female bias and are associated with higher disease incidence, worse QOL, and multiple management issues in pregnancy and lactation. It is crucial to be aware of pemphigoid gestationis, which occurs exclusively in females, as well as many of the pemphigus and pemphigoid group diseases, which are female-predominant. The QOL of female patients, especially those with pemphigus, is worse than their male counterparts due to social, mental, and disease factors. As clinicians it is important to screen for and then intervene on a more holistic level to improve the female AIBD patient’s QOL. Management issues in pregnancy and lactation also pose several challenges including variations in disease course and postpartum exacerbations, prudency required with investigations, and safety issues limiting treatment options. No medication is safe beyond doubt, but low-dose corticosteroids, dapsone, cyclosporine, and IVIG are some of the safer options.
Footnotes
Funding: The authors state no funding has been received for the writing of this paper.
Conflict of interest: The authors state no conflict of interest with the content of the paper.
References
- Aboobaker J., Morar N., Ramdial P.K., Hammond M.G. Pemphigus in South Africa. Int J Dermatol. 2001;40(2):115–119. doi: 10.1046/j.1365-4362.2001.01124.x. [DOI] [PubMed] [Google Scholar]
- Abreu-Velez A.M., Hashimoto T., Bollag W.B., Tobon Arroyave S., Abreu-Velez C.E., Londono M.L. A unique form of endemic pemphigus in northern Colombia. J Am Acad Dermatol. 2003;49(4):599–608. doi: 10.1067/s0190-9622(03)00851-x. [DOI] [PubMed] [Google Scholar]
- Ahmed A.R., Gurcan H.M. Use of intravenous immunoglobulin therapy during pregnancy in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2011;25(9):1073–1079. doi: 10.1111/j.1468-3083.2010.03925.x. [DOI] [PubMed] [Google Scholar]
- Ahmed S.A., Penhale W.J. The influence of testosterone on the development of autoimmune thyroiditis in thymectomized and irradiated rats. Clin Exp Immunol. 1982;48(2):367–374. [PMC free article] [PubMed] [Google Scholar]
- Al-Fouzan A.W., Galadari I., Oumeish I., Oumeish O.Y. Herpes gestationis (Pemphigoid gestationis) Clin Dermatol. 2006;24(2):109–112. doi: 10.1016/j.clindermatol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Alsaleh Q.A., Nanda A., Al-Baghli N.M., Dvorak R. Pemphigus in Kuwait. Int J Dermatol. 1999;38(5):351–356. doi: 10.1046/j.1365-4362.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- Ambros-Rudolph C.M., Mullegger R.R., Vaughan-Jones S.A., Kerl H., Black M.M. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395–404. doi: 10.1016/j.jaad.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Ameri P., Cinotti E., Mussap M., Murialdo G., Parodi A., Cozzani E. Association of pemphigus and bullous pemphigoid with thyroid autoimmunity in Caucasian patients. J Am Acad Dermatol. 2013;68(4):687–689. doi: 10.1016/j.jaad.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Armenti V.T., Ahlswede K.M., Ahlswede B.A., Jarrell B.E., Moritz M.J., Burke J.F. National transplantation Pregnancy Registry–outcomes of 154 pregnancies in cyclosporine-treated female kidney transplant recipients. Transplantation. 1994;57(4):502–506. [PubMed] [Google Scholar]
- Bastuji-Garin S., Souissi R., Blum L., Turki H., Nouira R., Jomaa B. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol. 1995;104(2):302–305. doi: 10.1111/1523-1747.ep12612836. [DOI] [PubMed] [Google Scholar]
- Bastuji-Garin S., Turki H., Mokhtar I., Nouira R., Fazaa B., Jomaa B. Possible relation of Tunisian pemphigus with traditional cosmetics: a multicenter case–control study. Am J Epidemiol. 2002;155(3):249–256. doi: 10.1093/aje/155.3.249. [DOI] [PubMed] [Google Scholar]
- Bebo B.F., Jr., Schuster J.C., Vandenbark A.A., Offner H. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J Neurosci Res. 1998;52(4):420–426. doi: 10.1002/(SICI)1097-4547(19980515)52:4<420::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bernard P., Vaillant L., Labeille B., Bedane C., Arbeille B., Denoeux J.P. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131(1):48–52. [PubMed] [Google Scholar]
- Bertram F., Brocker E.B., Zillikens D., Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7(5):434–440. doi: 10.1111/j.1610-0387.2008.06976.x. [DOI] [PubMed] [Google Scholar]
- Black M.M. The Neil Smith Memorial Lecture: John Laws Milton. The Founder of St John's Hospital for Diseases of the Skin. Clin Exp Dermatol. 2003;28(1):89–91. doi: 10.1046/j.1365-2230.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- Both H., Essink-Bot M.L., Busschbach J., Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127(12):2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- Braunstein I., Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26(4):354–363. doi: 10.1111/dth.12076. [DOI] [PubMed] [Google Scholar]
- Buijsrogge J.J., Diercks G.F., Pas H.H., Jonkman M.F. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165(1):92–98. doi: 10.1111/j.1365-2133.2011.10346.x. [DOI] [PubMed] [Google Scholar]
- Butler D.C., Heller M.M., Murase J.E. Safety of dermatologic medications in pregnancy and lactation: Part II. Lactation. 2014;70(3):417e1–417e10. doi: 10.1016/j.jaad.2013.09.009. [quiz 27] [DOI] [PubMed] [Google Scholar]
- Cai S.C., Allen J.C., Lim Y.L., Chua S.H., Tan S.H., Tang M.B. Mortality of bullous pemphigoid in Singapore: risk factors and causes of death in 359 patients seen at the National Skin Centre. Br J Dermatol. 2014;170(6):1319–1326. doi: 10.1111/bjd.12806. [DOI] [PubMed] [Google Scholar]
- Calippe B., Douin-Echinard V., Laffargue M. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180(12):7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- Campo-Voegeli A., Muniz F., Mascaro J.M., Garcia F., Casals M., Arimany J.L. Neonatal pemphigus vulgaris with extensive mucocutaneous lesions from a mother with oral pemphigus vulgaris. Br J Dermatol. 2002;147(4):801–805. doi: 10.1046/j.1365-2133.2002.04969.x. [DOI] [PubMed] [Google Scholar]
- Carmichael S.L., Shaw G.M., Ma C., Werler M.M., Rasmussen S.A., Lammer E.J. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol. 2007;197(6):585e1–585e7. doi: 10.1016/j.ajog.2007.05.046. [discussion 683–4, e1–7] [DOI] [PubMed] [Google Scholar]
- Chams-Davatchi C., Valikhani M., Daneshpazhooh M., Esmaili N., Balighi K., Hallaji Z. Pemphigus: analysis of 1209 cases. Int J Dermatol. 2005;44(6):470–476. doi: 10.1111/j.1365-4632.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Chee S.N., Murrell D.F. Pemphigus and quality of life. Dermatol Clin. 2011;29(3):521–525. doi: 10.1016/j.det.2011.03.009. 3. [DOI] [PubMed] [Google Scholar]
- Chi C.C., Lee C.W., Wojnarowska F., Kirtschig G. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2009;3:CD007346. doi: 10.1002/14651858.CD007346.pub2. [DOI] [PubMed] [Google Scholar]
- Chi C.C., Wang S.H., Kirtschig G., Wojnarowska F. Systematic review of the safety of topical corticosteroids in pregnancy. J Am Acad Dermatol. 2010;62(4):694–705. doi: 10.1016/j.jaad.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Chowdhury M.M., Natarajan S. Neonatal pemphigus vulgaris associated with mild oral pemphigus vulgaris in the mother during pregnancy. Br J Dermatol. 1998;139(3):500–503. doi: 10.1046/j.1365-2133.1998.02418.x. [DOI] [PubMed] [Google Scholar]
- Clay T., Pandya A.G. Minimizing complications in autoimmune blistering diseases. Dermatol Clin. 2011;29(4):577–583. doi: 10.1016/j.det.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Cobo M.F., Santi C.G., Maruta C.W., Aoki V. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics (Sao Paulo) 2009;64(11):1043–1047. doi: 10.1590/S1807-59322009001100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier P.M., Kelly S.E., Wojnarowska F. Linear IgA disease and pregnancy. J Am Acad Dermatol. 1994;30(3):407–411. doi: 10.1016/s0190-9622(94)70047-8. [DOI] [PubMed] [Google Scholar]
- Cozzani E., Parodi A., Rebora A., Delmonte S., Barile M., Nigro A. Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatol Venereol. 2001;15(4):317–319. [PubMed] [Google Scholar]
- Da Silva J.A. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–117. doi: 10.1111/j.1749-6632.1999.tb07628.x. [discussion 17–8] [DOI] [PubMed] [Google Scholar]
- Daneshpazhooh M., Chams-Davatchi C., Payandemehr P., Nassiri S., Valikhani M., Safai-Naraghi Z. Spectrum of autoimmune bullous diseases in Iran: a 10-year review. Int J Dermatol. 2012;51(1):35–41. doi: 10.1111/j.1365-4632.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- Daniel B.S., Dermawan A., Murrell D.F. The autoimmune blistering diseases in Australia: status and services. Dermatol Clin. 2011;29(4):687–690. doi: 10.1016/j.det.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Emery C.F., Frid D.J., Engebretson T.O., Alonzo A.A., Fish A., Ferketich A.K. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;66(2):190–197. doi: 10.1097/01.psy.0000116775.98593.f4. [DOI] [PubMed] [Google Scholar]
- Fichel F., Barbe C., Joly P., Bedane C., Vabres P., Truchetet F. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150(1):25–33. doi: 10.1001/jamadermatol.2013.5757. [DOI] [PubMed] [Google Scholar]
- Goldberg N.S., DeFeo C., Kirshenbaum N. Pemphigus vulgaris and pregnancy: risk factors and recommendations. J Am Acad Dermatol. 1993;28(5 Pt 2):877–879. doi: 10.1016/0190-9622(93)70123-b. [DOI] [PubMed] [Google Scholar]
- Gourdy P., Araujo L.M., Zhu R., Garmy-Susini B., Diem S., Laurell H. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood. 2005;105(6):2415–2420. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
- Gudi V.S., White M.I., Cruickshank N., Herriot R., Edwards S.L., Nimmo F. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol. 2005;153(2):424–427. doi: 10.1111/j.1365-2133.2005.06662.x. [DOI] [PubMed] [Google Scholar]
- Gupta V.K., Kelbel T.E., Nguyen D., Melonakos K.C., Murrell D.F., Xie Y. A globally available internet-based patient survey of pemphigus vulgaris: epidemiology and disease characteristics. Dermatol Clin. 2011;29(3):393–404. doi: 10.1016/j.det.2011.03.016. 3. [DOI] [PubMed] [Google Scholar]
- Haber J., Hopman W., Gomez M., Cartotto R. Late outcomes in adult survivors of toxic epidermal necrolysis after treatment in a burn center. J Burn Care Rehabil. 2005;26(1):33–41. doi: 10.1097/01.bcr.0000150215.78220.79. [DOI] [PubMed] [Google Scholar]
- Harbuz M.S., Perveen-Gill Z., Lightman S.L., Jessop D.S. A protective role for testosterone in adjuvant-induced arthritis. Rheumatology. 1995;34(12):1117–1122. doi: 10.1093/rheumatology/34.12.1117. [DOI] [PubMed] [Google Scholar]
- Hietanen J., Salo O.P. Pemphigus: an epidemiological study of patients treated in Finnish hospitals between 1969 and 1978. Acta Derm Venereol. 1982;62(6):491–496. [PubMed] [Google Scholar]
- Hocking D.R. Neonatal haemolytic disease due to dapsone. Med J Aust. 1968;1(26):1130–1131. doi: 10.5694/j.1326-5377.1968.tb29209.x. [DOI] [PubMed] [Google Scholar]
- Hrgovic Z. Methemoglobinemia in a newborn infant following pudendal anesthesia in labor with prilocaine. A case report. Anasth Intensivther Notfallmed. 1990;25(2):172–174. [PubMed] [Google Scholar]
- Huang Y.H., Kuo C.F., Chen Y.H., Yang Y.W. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population-based study. J Invest Dermatol. 2012;132(1):92–97. doi: 10.1038/jid.2011.249. [DOI] [PubMed] [Google Scholar]
- Humbert P., Dupond J.L., Vuitton D., Agache P. Dermatological autoimmune diseases and the multiple autoimmune syndromes. Acta Derm Venereol Suppl. 1989;148:1–8. [PubMed] [Google Scholar]
- Intong L.R., Murrell D.F. Pemphigoid gestationis: pathogenesis and clinical features. Dermatol Clin. 2011;29(3):447–452. doi: 10.1016/j.det.2011.03.002. 3. [DOI] [PubMed] [Google Scholar]
- Jenkins R.E., Hern S., Black M.M. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24(4):255–259. doi: 10.1046/j.1365-2230.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Jung M., Kippes W., Messer G., Zillikens D., Rzany B. Increased risk of bullous pemphigoid in male and very old patients: A population-based study on incidence. J Am Acad Dermatol. 1999;41(2 Pt 1):266–268. doi: 10.1016/s0190-9622(99)70061-7. [DOI] [PubMed] [Google Scholar]
- Kardos M., Levine D., Gurcan H.M., Ahmed R.A. Pemphigus vulgaris in pregnancy: analysis of current data on the management and outcomes. Obstet Gynecol Surv. 2009;64(11):739–749. doi: 10.1097/OGX.0b013e3181bea089. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim Y.H., Kim S.C. Epidermolysis bullosa acquisita: a retrospective clinical analysis of 30 cases. Acta Derm Venereol. 2011;91(3):307–312. doi: 10.2340/00015555-1065. [DOI] [PubMed] [Google Scholar]
- Kim M., Rostas S., Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant. 2013;13(6):1383–1389. doi: 10.1111/ajt.12238. [DOI] [PubMed] [Google Scholar]
- Langan S.M., Smeeth L., Hubbard R., Fleming K.M., Smith C.J., West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpawattana P., Choonhakarn C., Kongbunkiat K. Clinical profiles of Stevens–Johnson syndrome among Thai patients. J Dermatol. 2014;41(7):634–637. doi: 10.1111/1346-8138.12499. [DOI] [PubMed] [Google Scholar]
- Liu Z., Diaz L.A., Troy J.L., Taylor A.F., Emergy D.J., Fairley J.A. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92(5):2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojevic S., Lipozencic J. Autoimmune bullous diseases associations. Clin Dermatol. 2012;30(1):17–33. doi: 10.1016/j.clindermatol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Ljubojevic S., Lipozencic J., Brenner S., Budimcic D. Pemphigus vulgaris: a review of treatment over a 19-year period. J Eur Acad Dermatol Venereol. 2002;16(6):599–603. doi: 10.1046/j.1468-3083.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- Llorente-Alonso M.J., Fernandez-Acenero M.J., Sebastian M. Gluten intolerance: sex and age-related features. Can J Gastroenterol. 2006;20(11):719–722. doi: 10.1155/2006/470273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe A., Flageul B., Cisse I., Keita S., Bobin P. Pemphigus in Mali: a study of 30 cases. Br J Dermatol. 1996;134(1):114–119. [PubMed] [Google Scholar]
- Marazza G., Pham H.C., Scharer L., Pedrazzetti P.P., Hunziker T., Trüeb R.M. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. 2009;161(4):861–868. doi: 10.1111/j.1365-2133.2009.09300.x. [DOI] [PubMed] [Google Scholar]
- Markle J.G., Fish E.N. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Mayrshofer F., Hertl M., Sinkgraven R., Sticherling M., Pfeiffer C., Zillikens D. Significant decrease in quality of life in patients with pemphigus vulgaris. Results from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3(6):431–435. doi: 10.1111/j.1610-0387.2005.05722.x. [DOI] [PubMed] [Google Scholar]
- McCarty D.J., Manzi S., Medsger T.A., Jr., Ramsey-Goldman R., LaPorte R.E., Kwoh C.K. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38(9):1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- McPherson T., Venning V.V. Management of autoimmune blistering diseases in pregnancy. Dermatol Clin. 2011;29(4):585–590. doi: 10.1016/j.det.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Meneux E., Wolkenstein P., Haddad B., Roujeau J.C., Revuz J., Paniel B.J. Vulvovaginal involvement in toxic epidermal necrolysis: a retrospective study of 40 cases. Obstet Gynecol. 1998;91(2):283–287. doi: 10.1016/s0029-7844(97)00596-6. [DOI] [PubMed] [Google Scholar]
- Micali G., Musumeci M.L., Nasca M.R. Epidemiologic analysis and clinical course of 84 consecutive cases of pemphigus in eastern Sicily. Int J Dermatol. 1998;37(3):197–200. doi: 10.1046/j.1365-4362.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- Michailidou E.Z., Belazi M.A., Markopoulos A.K., Tsatsos M.I., Mourellou O.N., Antoniades D.Z. Epidemiologic survey of pemphigus vulgaris with oral manifestations in northern Greece: retrospective study of 129 patients. Int J Dermatol. 2007;46(4):356–361. doi: 10.1111/j.1365-4632.2006.03044.x. [DOI] [PubMed] [Google Scholar]
- Morini J.P., Jomaa B., Gorgi Y., Saguem M.H., Nouira R., Roujeau J.C. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch Dermatol. 1993;129(1):69–73. doi: 10.1001/archderm.129.1.69. [DOI] [PubMed] [Google Scholar]
- Murase J.E., Heller M.M., Butler D.C., Am Acad Dermatol J. Safety of dermatologic medications in pregnancy and lactation: Part I. Pregnancy. J Am Acad Dermatol. 2014;70(3):401e1–401e14. doi: 10.1016/j.jaad.2013.09.010. [quiz 15] [DOI] [PubMed] [Google Scholar]
- Murrell D.F., Daniel B.S., Joly P., Borradori L., Amagai M., Hashimoto T. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3):479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Dvorak R., Al-Saeed K., Al-Sabah H., Alsaleh Q.A. Spectrum of autoimmune bullous diseases in Kuwait. Int J Dermatol. 2004;43(12):876–881. doi: 10.1111/j.1365-4632.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- Nanda A., Al-Saeid K., Al-Sabah H., Dvorak R., Alsaleh Q.A. Clinicoepidemiological features and course of 43 cases of bullous pemphigoid in Kuwait. Clin Exp Dermatol. 2006;31(3):339–342. doi: 10.1111/j.1365-2230.2005.02040.x. [DOI] [PubMed] [Google Scholar]
- Nau H. Clinical pharmacokinetics in pregnancy and perinatology. I. Placental transfer and fetal side effects of local anaesthetic agents. Dev Pharmacol Ther. 1985;8(3):149–181. doi: 10.1159/000457034. [DOI] [PubMed] [Google Scholar]
- Osadchy A., Koren G. Cyclosporine and lactation: when the mother is willing to breastfeed. Ther Drug Monit. 2011;33(2):147–148. doi: 10.1097/FTD.0b013e318208e3a4. [DOI] [PubMed] [Google Scholar]
- Oyama N., Setterfield J.F., Powell A.M., Sakuma-Oyama Y., Albert S., Bhogal B.S. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol. 2006;154(1):90–98. doi: 10.1111/j.1365-2133.2005.06998.x. [DOI] [PubMed] [Google Scholar]
- Paradisi A., Sampogna F., Di Pietro C., Cianchini G., Didona B., Ferri R. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60(2):261–269. doi: 10.1016/j.jaad.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Paradisi A., Cianchini G., Lupi F., Di Pietro C., Sampogna F., Didona B. Quality of life in patients with pemphigus receiving adjuvant therapy. Clin Exp Dermatol. 2012;37(6):626–630. doi: 10.1111/j.1365-2230.2011.04282.x. [DOI] [PubMed] [Google Scholar]
- Park-Wyllie L., Mazzotta P., Pastuszak A., Moretti M.E., Beique L., Hunnisett L. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62(6):385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Pfutze M., Niedermeier A., Hertl M., Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17(1):4–11. doi: 10.1684/ejd.2007.0090. [DOI] [PubMed] [Google Scholar]
- Pisanti S., Sharav Y., Kaufman E., Posner L.N. Pemphigus vulgaris: incidence in Jews of different ethnic groups, according to age, sex, and initial lesion. Oral Surg Oral Med Oral Pathol. 1974;38(3):382–387. doi: 10.1016/0030-4220(74)90365-x. [DOI] [PubMed] [Google Scholar]
- Rajan B., Ahmed J., Shenoy N., Denny C., Ongole R., Binnal A. Assessment of quality of life in patients with chronic oral mucosal diseases: a questionnaire-based study. Perm J. 2014;18(1):e123–e127. doi: 10.7812/TPP/13-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos W., Chacon G.R., Galarza C., Gutierrez E.L., Smith M.E., Ortega-Loayza A.G. Endemic pemphigus in the Peruvian Amazon: epidemiology and risk factors for the development of complications during treatment. Ann Bras Dermatol. 2012;87(6):838–845. doi: 10.1590/S0365-05962012000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbach M., Murrell D.F., Bystryn J.C., Dulay S., Dick S., Fakharzadeh S. Reliability and convergent validity of two outcome instruments for pemphigus. J Invest Dermatol. 2009;129(10):2404–2410. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roujeau J.C., Guillaume J.C., Fabre J.P., Penso D., Flechet M.L., Girre J.P. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990;126(1):37–42. doi: 10.1001/archderm.126.1.37. [DOI] [PubMed] [Google Scholar]
- Russo I., Saponeri A., Peserico A., Alaibac M. The use of biochip immunofluorescence microscopy for the diagnosis of Pemphigus vulgaris. Acta Histochem. 2014;116(5):713–716. doi: 10.1016/j.acthis.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Sampogna F., Chren M.M., Melchi C.F., Pasquini P., Tabolli S., Abeni D. Age, gender, quality of life and psychological distress in patients hospitalized with psoriasis. Br J Dermatol. 2006;154(2):325–331. doi: 10.1111/j.1365-2133.2005.06909.x. [DOI] [PubMed] [Google Scholar]
- Sandberg J.K., Bhardwaj N., Nixon D.F. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NKT cell compartment. Eur J Immunol. 2003;33(3):588–596. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- Sanmarkan A.D., Sori T., Thappa D.M., Jaisankar T.J. Retrospective analysis of Stevens–Johnson syndrome and toxic epidermal necrolysis over a period of 10 years. Indian J Dermatol. 2011;56(1):25–29. doi: 10.4103/0019-5154.77546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardy M., Kostaki D., Varga R., Peris K., Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69(5):748–753. doi: 10.1016/j.jaad.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Schifter M., Yeoh S.C., Coleman H., Georgiou A. Oral mucosal diseases: the inflammatory dermatoses. Aust Dent J. 2010;55(Suppl. 1):23–38. doi: 10.1111/j.1834-7819.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Schwartz R.A., McDonough P.H., Lee B.W. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173e1–173e13. doi: 10.1016/j.jaad.2013.05.003. [quiz 85–6] [DOI] [PubMed] [Google Scholar]
- Sebaratnam D.F., McMillan J.R., Werth V.P., Murrell D.F. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30(1):103–107. doi: 10.1016/j.clindermatol.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaratnam D.F., Hanna A.M., Chee S.N., Frew J.W., Venugopal S.S., Daniel B.S. Development of a quality-of-life instrument for autoimmune bullous disease: the Autoimmune Bullous Disease Quality of Life questionnaire. JAMA Dermatol. 2013;149(10):1186–1191. doi: 10.1001/jamadermatol.2013.4972. [DOI] [PubMed] [Google Scholar]
- Seillet C., Laffont S., Tremollieres F. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119(2):454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- Seo P.G., Choi W.W., Chung J.H. Pemphigus in Korea: clinical manifestations and treatment protocol. J Dermatol. 2003;30(11):782–788. doi: 10.1111/j.1346-8138.2003.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Serwin A.B., Bokiniec E., Piascik M., Masny D., Chodynicka B. Epidemiological and clinical analysis of pemphigoid patients in northeastern Poland in 2000–2005. Med Sci Monit. 2007;13(8):CR360–CR364. [PubMed] [Google Scholar]
- Shornick J.K., Black M.M. Secondary autoimmune diseases in herpes gestationis (pemphigoid gestationis) J Am Acad Dermatol. 1992;26(4):563–566. doi: 10.1016/0190-9622(92)70081-p. [DOI] [PubMed] [Google Scholar]
- Slade G.D., Foy S.P., Shugars D.A., Phillips C., White R.P., Jr. The impact of third molar symptoms, pain, and swelling on oral health-related quality of life. J Oral Maxillofac Surg. 2004;62(9):1118–1124. doi: 10.1016/j.joms.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Stanley J.R., Hawley-Nelson P., Yuspa S.H., Shevach E.M., Katz S.I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24(3):897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Svecova D., Parnicka Z., Pastyrikova L., Urbancek S., Luha J., Buc M. HLA DRB1* and DQB1* alleles are associated with disease severity in patients with pemphigus vulgaris. Int J Dermatol. 2014;54(2):168–173. doi: 10.1111/ijd.12418. [DOI] [PubMed] [Google Scholar]
- Tabolli S., Pagliarello C., Paradisi A., Cianchini G., Giannantoni P., Abeni D. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170(5):1087–1091. doi: 10.1111/bjd.12836. [DOI] [PubMed] [Google Scholar]
- Tampoia M., Giavarina D., Di Giorgio C., Bizzaro N. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering skin diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12(2):121–126. doi: 10.1016/j.autrev.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Terrab Z., Benchikhi H., Maaroufi A., Hassoune S., Amine M., Lakhdar H. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132(4):321–328. doi: 10.1016/s0151-9638(05)79276-0. [DOI] [PubMed] [Google Scholar]
- Thornton Y.S., Bowe E.T. Neonatal hyperbilirubinemia after treatment of maternal leprosy. South Med J. 1989;82(5):668. doi: 10.1097/00007611-198905000-00037. [DOI] [PubMed] [Google Scholar]
- Tjokrowidjaja A., Daniel B.S., Frew J.W., Sebarantnam D.F., Hanna A.M., Chee S. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease. Br J Dermatol. 2013;169(5):1000–1006. doi: 10.1111/bjd.12623. [DOI] [PubMed] [Google Scholar]
- Tsankov N., Vassileva S., Kamarashev J., Kazandjieva J., Kuzeva V. Epidemiology of pemphigus in Sofia, Bulgaria. A 16-year retrospective study (1980–1995) Int J Dermatol. 2000;39(2):104–108. doi: 10.1046/j.1365-4362.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- Uzun S., Durdu M., Akman A., Gunasti S., Uslular C., Memisoglu H.R. Pemphigus in the Mediterranean region of Turkey: a study of 148 cases. Int J Dermatol. 2006;45(5):523–528. doi: 10.1111/j.1365-4632.2004.02533.x. [DOI] [PubMed] [Google Scholar]
- Voorbrood B.S., Monnens L.A., Boon J.M. Methemoglobinemia in 2 newborn infants as a result of the use of prilocaine (Citanest) Ned Tijdschr Geneeskd. 1982;126(15):682–684. [PubMed] [Google Scholar]
- Voskuhl R.R., Pitchekian-Halabi H., MacKenzie-Graham A., McFarland H.F., Raine C.S. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996;39(6):724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- Weinand C., Xu W., Perbix W., Lefering R., Maegele M., Rathert M. 27 years of a single burn centre experience with Stevens–Johnson syndrome and toxic epidermal necrolysis: analysis of mortality risk for causative agents. Burns. 2013;39(7):1449–1455. doi: 10.1016/j.burns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Whitacre C.C. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Whitacre C.C., Reingold S.C., O'Looney P.A. A gender gap in autoimmunity. Science. 1999;283(5406):1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis S., Swerlick R.A. Pemphigus in the southeastern United States. South Med J. 2001;94(7):694–698. [PubMed] [Google Scholar]
- Wong S.N., Chua S.H. Spectrum of subepidermal immunobullous disorders seen at the National Skin Centre, Singapore: a 2-year review. Br J Dermatol. 2002;147(3):476–480. doi: 10.1046/j.1365-2133.2002.04919.x. [DOI] [PubMed] [Google Scholar]
- Woo S.B., Greenberg M.S. Ulcerative, vesicular, and bullous lesions. In: Woo S.B., Greenberg M.S., Ship J.A., editors. Burket’s oral medicine: diagnosis and treatment. 11th ed. B.C. Decker Inc.; Hamilton (Ontario): 2008. p. 67. [Google Scholar]
- Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bell M.G., Contreras M. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.K., Crew A.B., Messingham K.A., Fairley J.A., Woodley D.T. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71(3):468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraa I., Kerkeni N., Ishak F., Zribi H., El Euch D., Mokni M. Spectrum of autoimmune blistering dermatoses in Tunisia: an 11-year study and a review of the literature. Int J Dermatol. 2011;50(8):939–944. doi: 10.1111/j.1365-4632.2010.04801.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Pan J., Yu Z., Wang Y., Cai L., Zheng S. Epidemiology of pemphigus vulgaris in the Northeast China: a 10-year retrospective study. J Dermatol. 2014;41(1):70–75. doi: 10.1111/1346-8138.12286. [DOI] [PubMed] [Google Scholar]
- Zumelzu C., Le Roux-Villet C., Loiseau P., Busson M., Heller M., Aucouturier F. Black patients of African descent and HLA-DRB1*15:03 frequency overrepresented in epidermolysis bullosa acquisita. J Invest Dermatol. 2011;131(12):2386–2393. doi: 10.1038/jid.2011.231. [DOI] [PubMed] [Google Scholar]


