Abstract
Background
Ewing’s sarcoma (ES) and primitive neuroectodermal tumors (PNET) are closely related tumors. Although soft tissue ES/PNET are common in clinical practice, they are rare in the small intestine. Because of the absence of characteristic clinical symptoms, they are easily misdiagnosed as other benign or malignant diseases.
Case presentation
Here, we present the case of a 16-year-old female who complained of anemia and interval hematochezia. Her serum test results showed only a slight elevation of CA-125 and a low level of hemoglobin. Computer tomography and magnetic resonance imaging revealed a cystic and solid mass in the lower abdominal quadrant and pelvic region, which prompted suspicion of a malignant gastrointestinal stromal tumor of the small intestine. After resection, the tumor’s histology and immunohistochemistry (positive for CD99, vimentin and synaptophysin) results suggested ES/PNET. Fluorescent in situ hybridization tests proved the breakpoint rearrangement of the EWSR1 gene in chr 22.Ultrastructural analysis revealed neurosecretory and glycogen granules in the tumor cell cytoplasm.
Conclusions
Together, these data supported the diagnosis of a rare case of localized ES/PNET in the small intestine without adjuvant chemo- or radiotherapy. To our knowledge, this is the first report from China of a primary small bowel ES/PNET in the English-language literature. In addition, on the basis of findings from previous publications and the current case, the optimal treatment for localized gastrointestinal ES/PNET is discussed.
Keywords: Ewing’s sarcoma, Primitive neuroectodermal tumor, Extraosseous, Small intestine, FISH, EWS gene
Background
Ewing’s sarcoma (ES)/primitive neuroectodermal tumor (PNET) is a small round cell tumor with simple sarcoma-specific genetic alterations resulting in TET/FET family member and ETS family member fusion proteins [1]. Pathologists no longer categorize ES and PNET as different tumors because their genetic abnormalities overlap. Instead, they are termed the Ewing’s sarcoma family of tumors [2, 3], together with the Askin tumor. ES/PNET are most commonly seen in patients younger than 20 years of age and are derived mainly from bone [4]. The tumor has been discovered in most organs, including the pancreas, liver, adrenal gland, esophagus, and uterus [5–11]. However, ES/PNET is extremely rare in the small bowel. Although it has been reported previously in this location [12–17], none of these reports came from China. Here, we present the first reported case in China of primary ES/PNET in the ileum with EWS rearrangement.
Case presentation
Clinical history
A 16-year-old Chinese girl presented complaining of anemia and interval hematochezia. Her hemoglobin was 54 g/L on admission. Capsule endoscopy and double-balloon enteroscopy showed mucosal hyperemia, edema and mass protrusion on the ileal wall. Computed tomography (CT) scans and three-dimensional reconstruction revealed a 10.0 × 7.3 × 5.3 cm irregular mass that had developed from the ileal wall in the right lower quadrant (Fig. 1a-h). The lesion showed intense but inhomogeneous enhancement following contrast administration (Fig. 1e-h), particularly in the arterial phase. There was a small amount of effusion in the pelvic cavity. Pelvic magnetic resonance imaging (MRI) indicated a right ovarian cyst in addition to the above mass. Both CT and MRI prompted suspicion of malignant GIST of the small bowel. Her serum CA-125 was slightly increased (50.5 U/mL, standard 0–36 U/mL), but the other markers were within normal limits. The tumor and a loop of small intestine were resected through a right ventral midline incision. The patient recovered uneventfully. Postoperative bone scintigraphy proved that there was no lesion in the skeletal system (Fig. 1 d). Her chest CT scan and cerebral MRI were also unremarkable. Thus, the patient was classified as T2aN0M0 according to the 8th edition of the AJCC Cancer Staging Manual.
Fig. 1.
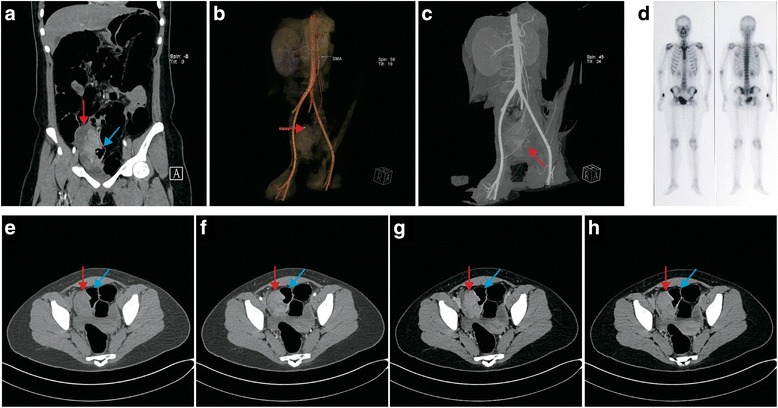
Abdominal and pelvic CT scan, 3D reconstruction and ECT demonstrating the tumor originating in the ileum of the patient. CT scan was performed immediately after enteroscopy. Thus, the patient’s intestine was dilated. a Coronal scan arterial phase reveals that the tumor was derived from ileal wall. b 3D reconstruction with volume rendering technique illustrates the supporting vasculature. c 3D reconstruction with maximum intensity projection demonstrates major vascular support of the tumor. d Postoperative bone scintigraphy proved that there was no lesion in her skeletal system. e Plain scan revealed pelvic a 10.0 × 7.6 × 5.3 cm mass with areas of necrosis. Most of the mass was had clear boundaries with surrounding tissues, although part of it obliterated the lumen of the terminal ileum. Workup for metastasis was negative. f-h Contrast-enhanced arterial, venous, and delayed phase pelvic CT scan revealed enhancement of the solid part of the tumor in all phases, with the peak in the arterial phase
Gross features
On laparotomy, a large cystic and solid mass 10.5 cm in diameter was found arising from the ileal wall. The cut tumor surface showed large central hemorrhagic and necrotic changes and pseudocystic degeneration. The tumor tissue was mostly light gray and solid, with some softer and more friable reddish congested areas (Fig. 2).
Fig. 2.
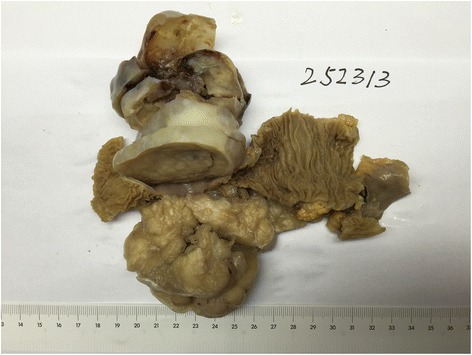
Gross features of the tumor with the resected ileum
Histological features
Cross sections revealed solid nests of small round tumor cells arising from the muscular layer and infiltrating all layers of the ileum wall. Cystic and hemorrhagic changes were seen on part of the sections, as were sharply demarcated borders that were frequently covered by intact serosa. No vascular tumor embolus or perineuronal invasion were observed. The serosal layer and the surgical margins of the specimen were free of disease. Under high-power view, tumor cells were round or elliptical, possessing scant eosinophilic cytoplasm and abortive pseudorosette formation. The tumor cell nuclei were round, with exquisite chromatin, ambiguous nucleoli, and 9/10 high-power-field pathological mitoses (Fig. 3a-c). Tumor cells showed positive immunoreactivity for Vimentin and CD99 (Fig. 3 d, j) and moderate staining for Cam5.2, Syn and PR (Fig. 3 f, g, k). Results were negative for CKpan, LCA, S-100, HMB45, Melan-A, CD31, CD34, NSE, P53, CD56, CgA, SMA, Desmin, CD117, Dog-1, ER, Bcl-2, and alpha-inhibin.
Fig. 3.
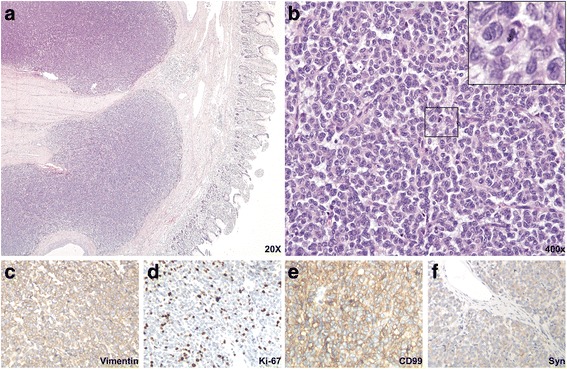
Histological and immunohistochemical features of the intestinal tumor. a Low-power view with HE staining indicates sheets of tumor cells invading the myometrium and submucosa. b High-power HE view suggests that the tumor cells are small, round and form Homer-Wright structures. The boxed region is amplified in the upper right corner and is used to show pathological mitosis. c-f The tumor is positively stained for Vimentin, Syn, CD99, and the Ki-67 index is high (~40%). All immunohistochemistry images were taken under 200× magnification
FISH
Dual color break-apart probe FISH examination showed that 90% of the cells (100 counted cells per slide) exhibited 1 yellow and 1 red signal (1F1R) and that 6% of the cells exhibited 1 yellow, 1 red and 1 green (break-apart) signal (1F1G1R). However, only 4% cell had two yellow signals, which proved a break of the EWSR1 locus (2 F) (Fig. 4a-c).
Fig. 4.
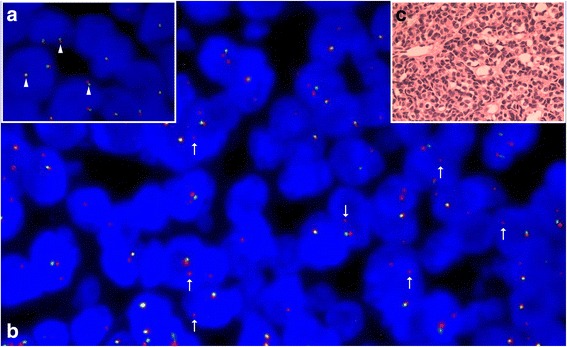
Dual color (red/green) break-apart probe FISH test of the tumor. a Normal karyotype cells have two yellow (red/green merged) signals (arrowheads). b Most tumor cells (90%) had one yellow (red/green merged) signal and one red signal (arrows). C. Consecutive sections were HE-stained
EM
Transmission electron microscopy revealed dense clusters of tumor cells, interspersed with a few interstitial cells (Fig. 5 a). The tumor cells were small and irregular, with scant cytoplasm and organelles, and significant nuclear atypia (Fig. 5 a). Some cells had small nucleoli (Fig. 5 a). Occasionally, gap junctions between the cells were observed (Fig. 5 b), but neuroendocrine granules in the cytoplasm were rarely seen (Fig. 5 c). Most cells had glycogen particles attached to the endoplasmic reticulum (Fig. 5 d).
Fig. 5.
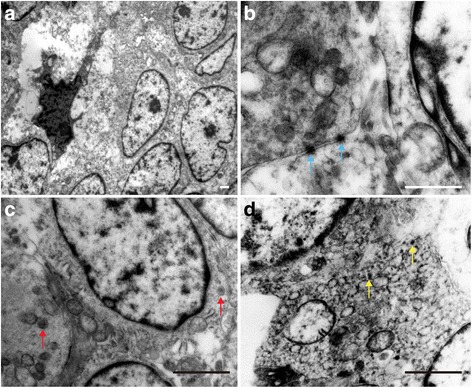
Ultrastructure analysis of the tumor. a At lower magnification, EM shows the general tumor ultrastructure. b Cell-cell gap junctions (blue arrow) were observed in some areas. c Neurosecretory granules (red arrow) were rarely seen in the tumor cytoplasm. d Glycogen granules (yellow arrow) existed in most tumor cells. All bars = 2000 nm
Treatments and outcome
The patient underwent an exploratory laparotomy, and tumor resection was performed along with 60 cm of ileum. The patient refused chemotherapy and/or radiotherapy as adjuvant treatments. She is currently alive (10 months after the surgery) without any signs of recurrence.
Discussion
ES/PNET belongs to a family of tumors that harbor the EWSR1-ETS fusion protein, according to recent studies [18]. It is the second most common pediatric sarcoma of bone. It most commonly arises from bone but can develop in extraskeletal sites [19]. The EWSR1 gene, together with several other genes, forms the TET family [20]. Their motif of RNA binding activity enables the EWSR1-ETS fusion protein to regulate target genes as transcription factors [21, 22]. Previous research provided evidence that mesenchymal stem cells may be candidate cells from which ES/PNET originate and that EWSR1–FLI1 may be the sole initiating factor in the pathogenesis of these tumors [20, 23]. Such expression results in cell transformation, with the subsequent emergence of tumors bearing the morphological and gene expression hallmarks of Ewing’s sarcoma [24].
Gastroenterological ES/PNET is extremely rare. Here, we have summarized all previous publications of gastrointestinal ES/PNET in Table 1 [7, 12, 13, 17, 25–49]. Among the 36 cases, 3 cases were derived from the esophagus, 9 from the stomach, 5 were of colorectal origin, and 19 arose from the small intestine. The patient gender ratio (female/male) was 22/14, and the ages ranged from 9 to 68 years. Thirty-one of 32 cases were positive for CD99 immunoreactivity. Fluorescent in situ hybridization or real-time PCR tests confirmed that most cases had the EWSR1-ETS fusion protein. Intriguingly, however, only 4 non-metastatic gastrointestinal ES/PNET cases were treated only by resection of the tumor. Follow-up of these cases suggested that the patients were relatively younger and had up to 20 months of disease-free survival. In the current case, the young patient also refused to take adjuvant chemo- or radiotherapy. To our delight, after the 10-month follow-up examination, the patient is currently alive and well, without any sign of recurrence.
Table 1.
Review of reported cases of gastrointestinal ES/PNET
| Tumor site | Age | Sex | CgA | Syn | CK | CD99 | CD117 | FLI1 | FISH break-apart EWSR1 | RT–PCR EWS–FLI1 | Metastasis at diagnosis | Treatments | Follow-up | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esophagus | 44 | F | - | - | - | + | - | + | + | + | - | Cx | ND | Johnson AD et al. |
| Esophagus | 56 | M | - | ND | - | + | ND | ND | ND | + | Lymph nodes | Sx + ImCx | ND | Maesawa C et al. |
| Esophagus | 21 | M | ND | ND | - | - | - | ND | + | ND | - | Sx + Rx | ND | Kim SB et al. |
| Gastric | 31 | F | ND | ND | - | + | ND | + | - | + | - | Sx + Rx | 3 years DFS | Khuri S et al. |
| Gastric | 19 | M | ND | ND | ND | + | + | ND | ND | ND | - | Cx | ND | Aras M et al. |
| Gastric | 41 | F | + | + | ND | + | + | ND | ND | + | Intra-peritoneal | Sx + Cx + Rx | Died 110 months after surgery | Inoue M et al. |
| Gastric | 30 | M | - | - | - | + | ND | ND | ND | ND | - | Sx | 6 month DFS | Ankouz A er al |
| Gastric | 14 | M | - | - | - | + | + | ND | ND | + | Liver | Sx + Cx | 24 months DFS | Czekalla R et al. |
| Gastric | 55 | M | + | + | - | + | + | - | ND | + | Lymph nodes | Sx | 13 months DFS | Song JM et al. |
| Gastric | 68 | M | - | ND | - | + | + | ND | ND | + | Liver | Sx + Cx | Died 13 months after diagnosis | Rafailidis S et al. |
| Peri-gastric | 44 | F | - | - | - | + | + | ND | + | ND | - | Sx | 20 months DFS | Colovic RB et al. |
| Gastric | 63 | F | ND | ND | ND | ND | ND | ND | + | + | - | Sx + Cx | ND | Maxwell AM er al |
| Colorectal | 59 | M | ND | + | - | + | - | ND | ND | + | Peritoneal dissemination | Sx | Died 7 months after diagnosis | Kuwabara K et al. |
| Colorectal | 24 | F | - | - | - | + | ND | ND | ND | + | - | Sx | 20 months DFS | Tokudome N et al. |
| Colorectal | 17 | M | - | - | - | + | ND | ND | ND | + | - | Sx + Cx | 1 years DFS | Drut R er al |
| Colorectal | 34 | F | ND | ND | - | + | ND | ND | ND | + | Liver | Sx + StemCx | 7 years DFS | Aboumarzouk OM et al. |
| Colorectal | 53 | M | - | ND | - | + | ND | ND | ND | ND | - | Sx + Cx + Rx | Died 2 years after diagnosis | Vardy J et al. |
| Small bowel | 21 | F | ND | - | + | + | ND | ND | ND | ND | - | Sx + Cx | 10 months DFS | Adair et al. |
| Small bowel | 20 | F | ND | ND | ND | + | ND | ND | + | - | - | Sx + Cx | 18 months DFS | Kie et al. |
| Small bowel | 13 | M | ND | - | + | + | ND | ND | + | ND | - | Sx | 1 years DFS | Sarangarajan etal |
| Small bowel | 40 | M | ND | + | - | + | ND | ND | + | ND | Intra-peritoneal | Sx + Cx | Died with recurrence 5 months after diagnosis | Horie and Kato |
| Small bowel | 14 | M | ND | - | + | + | ND | ND | + | + | - | Sx + Cx | 10 month DFS | Graham et al. |
| Small bowel | 9 | F | - | - | + | ND | ND | ND | + | + | - | Sx + Cx | Died 25 months after diagnosis | Shek et al. |
| Small bowel | 53 | F | ND | ND | ND | + | ND | ND | ND | ND | - | Sx | ND | Balasubram-anina et al. |
| Small bowel | 63 | M | ND | ND | ND | + | + | ND | ND | ND | Adrenal glands + lymph nodes | Sx + Cx | ND | Kim et al. |
| Small bowel | 44 | M | ND | ND | - | + | ND | ND | ND | ND | Intra-peritoneal | Sx + Cx | Died 13 months after diagnosis | Sethi and Smith |
| Small bowel | 32 | M | ND | ND | ND | + | ND | + | + | ND | - | Sx + Cx | 6 months DFS | Rodarte Shade et al. |
| Small bowel | 15 | F | ND | ND | ND | ND | ND | ND | + | + | - | Sx + Cx | ND | Vignail et al. |
| Small bowel | 18 | M | ND | ND | ND | ND | ND | ND | ND | ND | - | Sx + Cx | ND | Boehm et al. |
| Small bowel | 18 | M | + | + | + | + | + | + | + | + | Liver | Sx | Died 8 months after diagnosis | Milione M et al. |
| Small bowel | 20 | M | + | + | + | + | + | + | + | + | Liver | Sx + Cx | Died 28 months after diagnosis | Milione M et al. |
| Small bowel | 42 | M | + | + | + | + | + | + | + | + | - | Sx + Cx | Died 11 months after diagnosis | Milione M et al. |
| Small bowel | 45 | M | + | + | + | + | + | + | + | + | - | Sx + Cx | Died 13 months after diagnosis | Milione M et al. |
| Small bowel | 15 | F | + | + | + | + | + | + | + | + | - | Sx + Cx + Rx | 28 months DFS | Milione M et al. |
| Small bowel | 57 | M | + | + | + | + | + | + | + | + | - | Lost | Lost | Milione M et al. |
| Small bowel | 28 | F | + | + | + | + | - | - | + | + | Liver | Sx + Cx | 204 months DFS | Milione M et al. |
F Female, M Male, ND Not done, Sx Surgery, Cx Chemotherapy, ImCx Immuno chemotherapy, StemCx Stem cell based chemotherapy, Rx Radiotherapy, DFS Disease free survival
To date, the 5-year survival rate of localized ES/PNET is relatively high (65%-75%). However, the outcome for metastatic patients is usually poor (<30%), despite the use of chemo- and/or radiotherapy [50]. Several studies have indicated that localized extraskeletal ES/PNET has a more favorable outcome than skeletal tumors [51, 52]. The optimal management for localized ES/PNET is still debated. The National Comprehensive Cancer Network guidelines recommend that any ES/PNET should be treated with local treatment (surgery and/or radiotherapy) plus chemotherapy [53]. Nevertheless, consistent with our findings in Table 1, others have suggested that complete surgery, if feasible, may be a better option for local disease considering the late side effects of high-dose radiotherapy especially for children [52, 54]. Because small bowel ES/PNET is extremely rare and difficult to cure, our case will contribute to the understanding of the prognosis and determination of optimal management.
In the current case, the 16-year-old female patient was initially misdiagnosed with malignant GIST because of the clinical symptoms and imaging results. To differentiate among ES/PNET, malignant GIST, clear-cell sarcoma, and synovial sarcoma, immunohistochemistry, ultrastructure analysis and FISH tests were performed. Malignant GIST usually expresses CD117, Dog-1 and CD34, which were all negative in this case. Although both synovial sarcoma and ES/PNET could have genetic rearrangements, the regions of these translocations are quite different. In ES/PNET, Chr22 EWS-FLI or EWS-FEV translocations are commonly reported [16]. However, in synovial sarcoma, SYT-SSX translocation is frequently observed [55]. Clear-cell sarcoma could be ruled out by negative immunohistochemistry for HMB45, S-100 and Melan A. A previous study also indicated the necessity of distinguishing from an intraabdominal desmoplastic small round cell tumor (IDSRCT) by histological and immunohistochemical characteristics when ES/PNET occurs in the abdominal cavity [13].
Previous demographic research has suggested that Ewing’s sarcoma is far less frequent in China than in the United States Caucasian population [56]. However, whether this finding is related to genetic background differences remains to be studied. Two recent publications noted a difference in Ewing’s sarcoma occurrence between Caucasian and Hispanic populations [57, 58]. However, they did not include a reason to explain this differences.
Conclusions
In conclusion, we have described for the first time a rare case of localized ES/PNET occurring in the small intestine in the Chinese population, as confirmed by ultrastructure and genetic analyses. This case, together with previous reports, has expanded the spectrum of tumors in the small intestine.
Acknowledgments
The authors thank Dr. Na Jia from the Department of Pathology, Beijing Cancer Hospital, for his expert technical assistance performing FISH analyses.
Funding
This study was supported by a grant-in-aid for Scientific Research from the General Hospital of the Air Force, PLA to Ren Li and from the Junior Scientists Fund (16QNP025) to Li Teng.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
T Li performed histopathological evaluations and drafted the manuscript. L Ren conceived the study and participated in the design and preparation of the manuscript. F Zhang collected, evaluated and interpreted the clinical and surgical data. YR Cao evaluated the FISH and electron microscopic findings. SB Ning performed the gastroenterological examination and provided related data. YM Bi examined the patient with CT, MRI, and bone scintigraphy and provided related data. WC Xue discussed the pathological diagnosis and supported the FISH test. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Ethical approval and consent to participate
The ethical approval and documentation for the participation and publication of this case report were waived with approval of the Institutional Review Board at the General Hospital of the Air Force, PLA.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Bcl-2
B cell lymphoma 2
- CA-125
Cancer antigen- 125
- CD
Cluster of differentiation
- CgA
Glycoprotein hormone alpha chain
- CKpan
Pan-cytokeratin
- CT
Computed tomography
- ER
Estrogen hormone receptor
- ES
Ewing’s sarcoma
- GIST
Gastrointestinal stromal tumor
- LCA
Lymphocyte common antigen
- MRI
Magnetic resonance imaging
- NSE
Neuron-specific enolase
- PNET
Primitive neuroectodermal tumor
- SMA
Alpha smooth muscle actin
Contributor Information
Teng Li, Email: jacksonlt@163.com.
Fang Zhang, Email: zhangfang2017@126.com.
Yarui Cao, Email: cyrmyt918@163.com.
Shoubin Ning, Email: ning-shoubin@163.com.
Yongmin Bi, Email: beeym@126.com.
Weicheng Xue, Email: xuewc2004@aliyun.com.
Li Ren, Email: renlifei2000@sina.com.
References
- 1.Kim SK, Park YK. Ewing sarcoma: a chronicle of molecular pathogenesis. Hum Pathol. 2016;55:91–100. doi: 10.1016/j.humpath.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Dehner LP. Primitive neuroectodermal tumor and Ewing’s sarcoma. Am J Surg Pathol. 1993;17(1):1–13. doi: 10.1097/00000478-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dehner LP. The evolution of the diagnosis and understanding of primitive and embryonic neoplasms in children: living through an epoch. Mod Pathol. 1998;11(7):669–85. [PubMed] [Google Scholar]
- 4.Collini P, Mezzelani A, Modena P, Dagrada P, Tamborini E, Luksch R, et al. Evidence of neural differentiation in a case of post-therapy primitive neuroectodermal tumor/Ewing sarcoma of bone. Am J Surg Pathol. 2003;27(8):1161–6. doi: 10.1097/00000478-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez RE, Folpe AL, Lapham RL, Ro JY, O’Shea PA, Weiss SW, et al. Primary Ewing’s sarcoma/primitive neuroectodermal tumor of the kidney: a clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol. 2002;26(3):320–7. doi: 10.1097/00000478-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Harimaya K, Oda Y, Matsuda S, Tanaka K, Chuman H, Iwamoto Y. Primitive neuroectodermal tumor and extraskeletal Ewing sarcoma arising primarily around the spinal column: report of four cases and a review of the literature. Spine (Phila Pa 1976) 2003;28(19):E408–12. doi: 10.1097/01.BRS.0000085099.47800.DF. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AD, Pambuccian SE, Andrade RS, Dolan MM, Aslan DL. Ewing sarcoma and primitive neuroectodermal tumor of the esophagus: report of a case and review of literature. Int J Surg Pathol. 2010;18(5):388–93. doi: 10.1177/1066896908316903. [DOI] [PubMed] [Google Scholar]
- 8.Cambruzzi E, Guerra EE, Hilgert HC, Schmitz HJ, Silva VL, Milani DM, et al. Primitive neuroectodermal tumor of the liver: a case report. Case Rep Med. 2011;2011:748194. doi: 10.1155/2011/748194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren YL, Tang XY, Li T. Ewing sarcoma-primitive neuroectodermal tumor of the uterus: a clinicopathologic, immunohistochemical and ultrastructural study of one case. Arch Gynecol Obstet. 2011;283(5):1139–43. doi: 10.1007/s00404-010-1557-3. [DOI] [PubMed] [Google Scholar]
- 10.Bose P, Murugan P, Gillies E, Holter JL. Extraosseous Ewing’s sarcoma of the pancreas. Int J Clin Oncol. 2012;17(4):399–406. doi: 10.1007/s10147-011-0311-6. [DOI] [PubMed] [Google Scholar]
- 11.Abi-Raad R, Manetti GJ, Colberg JW, Hornick JL, Shah JG, Prasad ML. Ewing sarcoma/primitive neuroectodermal tumor arising in the adrenal gland. Pathol Int. 2013;63(5):283–6. doi: 10.1111/pin.12063. [DOI] [PubMed] [Google Scholar]
- 12.Adair A, Harris SA, Coppen MJ, Hurley PR. Extraskeletal Ewings sarcoma of the small bowel: case report and literature review. J R Coll Surg Edinb. 2001;46(6):372–4. [PubMed] [Google Scholar]
- 13.Shek TW, Chan GC, Khong PL, Chung LP, Cheung AN. Ewing sarcoma of the small intestine. J Pediatr Hematol Oncol. 2001;23(8):530–2. doi: 10.1097/00043426-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Batziou C, Stathopoulos GP, Petraki K, Papadimitriou C, Rigatos SK, Kondopodis E, et al. Primitive neurectodermal tumors: a case of extraosseous Ewing’s sarcoma of the small intestine and review of the literature. J BUON. 2006;11(4):519–22. [PubMed] [Google Scholar]
- 15.Prasertvit S, Stoikes N. A rare case of ewing’s sarcoma of the small intestine. Am Surg. 2013;79(2):E78–9. [PubMed] [Google Scholar]
- 16.Milione M, Gasparini P, Sozzi G, Mazzaferro V, Ferrari A, Casali PG, et al. Ewing sarcoma of the small bowel: a study of seven cases, including one with the uncommonly reported EWSR1-FEV translocation. Histopathology. 2014;64(7):1014–26. doi: 10.1111/his.12350. [DOI] [PubMed] [Google Scholar]
- 17.Vignali M, Zacche MM, Messori P, Natale A, Busacca M. Ewing’s sarcoma of the small intestine misdiagnosed as a voluminous pedunculated uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):234–5. doi: 10.1016/j.ejogrb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LD. Ewing sarcoma and primitive neuroectodermal tumor. Ear Nose Throat J. 2007;86(2):79–80. [PubMed] [Google Scholar]
- 19.Sandberg AA, Bridge JA. Updates on cytogenetics and molecular genetics of bone and soft tissue tumors: Ewing sarcoma and peripheral primitive neuroectodermal tumors. Cancer Genet Cytogenet. 2000;123(1):1–26. doi: 10.1016/S0165-4608(00)00295-8. [DOI] [PubMed] [Google Scholar]
- 20.Riggi N, Cironi L, Suva ML, Stamenkovic I. Sarcomas: genetics, signalling, and cellular origins. Part 1: The fellowship of TET. J Pathol. 2007;213(1):4–20. doi: 10.1002/path.2209. [DOI] [PubMed] [Google Scholar]
- 21.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363(6430):640–4. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 22.Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, Reddy ES. The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994;9(10):3087–97. [PubMed] [Google Scholar]
- 23.Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, et al. EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008;68(7):2176–85. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 24.Castillero-Trejo Y, Eliazer S, Xiang L, Richardson JA, Ilaria RL., Jr Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells Results in EWS/FLI-1-dependent, ewing sarcoma-like tumors. Cancer Res. 2005;65(19):8698–705. doi: 10.1158/0008-5472.CAN-05-1704. [DOI] [PubMed] [Google Scholar]
- 25.Horie Y, Kato M. Peripheral primitive neuroectodermal tumor of the small bowel mesentery: a case showing perforation at onset. Pathol Int. 2000;50(5):398–403. doi: 10.1046/j.1440-1827.2000.01045.x. [DOI] [PubMed] [Google Scholar]
- 26.Sarangarajan R, Hill DA, Humphrey PA, Hitchcock MG, Dehner LP, Pfeifer JD. Primitive neuroectodermal tumors of the biliary and gastrointestinal tracts: clinicopathologic and molecular diagnostic study of two cases. Pediatr Dev Pathol. 2001;4(2):185–91. doi: 10.1007/s100240010141. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian B, Dinakarababu E, Molyneux AJ. Primary primitive neuroectodermal tumour of the small bowel mesentery: case report. Eur J Surg Oncol. 2002;28(2):197–8. doi: 10.1053/ejso.2001.1155. [DOI] [PubMed] [Google Scholar]
- 28.Graham DK, Stork LC, Wei Q, Ingram JD, Karrer FM, Mierau GW, et al. Molecular genetic analysis of a small bowel primitive neuroectodermal tumor. Pediatr Dev Pathol. 2002;5(1):86–90. doi: 10.1007/s10024-001-0192-1. [DOI] [PubMed] [Google Scholar]
- 29.Maesawa C, Iijima S, Sato N, Yoshinori N, Suzuki M, Tarusawa M, et al. Esophageal extraskeletal Ewing’s sarcoma. Hum Pathol. 2002;33(1):130–2. doi: 10.1053/hupa.2002.30219. [DOI] [PubMed] [Google Scholar]
- 30.Tokudome N, Tanaka K, Kai MH, Sueyoshi K, Matsukita S, Setoguchi T. Primitive neuroectodermal tumor of the transverse colonic mesentery defined by the presence of EWS-FLI1 chimeric mRNA in a Japanese woman. J Gastroenterol. 2002;37(7):543–9. doi: 10.1007/s005350200084. [DOI] [PubMed] [Google Scholar]
- 31.Drut R, Drut M, Muller C, Marron A. Rectal primitive neuroectodermal tumor. Pediatr Pathol Mol Med. 2003;22(5):391–8. doi: 10.1080/pdp.22.5.391.398. [DOI] [PubMed] [Google Scholar]
- 32.Czekalla R, Fuchs M, Stolzle A, Nerlich A, Poremba C, Schaefer KL, et al. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol. 2004;16(12):1391–400. doi: 10.1097/00042737-200412000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Soulard R, Claude V, Camparo P, Dufau JP, Saint-Blancard P, Gros P. Primitive neuroectodermal tumor of the stomach. Arch Pathol Lab Med. 2005;129(1):107–10. doi: 10.5858/2005-129-107-PNTOTS. [DOI] [PubMed] [Google Scholar]
- 34.Vardy J, Joshua AM, Clarke SJ, Yarrow PM, Lin BP. Small blue cell tumors of the rectum. Case 1. Ewing’s sarcoma of the rectum. J Clin Oncol. 2005;23(4):910–2. doi: 10.1200/JCO.2005.03.096. [DOI] [PubMed] [Google Scholar]
- 35.Kuwabara K, Ishida H, Shirakawa K, Yokoyama M, Nakada H, Hayashi Y, et al. Primitive neuroectodermal tumor arising in the colon: report of a case. Surg Today. 2006;36(2):193–7. doi: 10.1007/s00595-005-3104-6. [DOI] [PubMed] [Google Scholar]
- 36.Kim DW, Chang HJ, Jeong JY, Lim SB, Lee JS, Hong EK, et al. Ewing’s sarcoma/primitive neuroectodermal tumor (ES/PNET) of the small bowel: a rare cause of intestinal obstruction. Int J Colorectal Dis. 2007;22(9):1137–8. doi: 10.1007/s00384-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 37.Sethi B, Smith GT. Primary primitive neuroectodermal tumour arising in the small bowel. Histopathology. 2007;50(5):665–6. doi: 10.1111/j.1365-2559.2007.02631.x. [DOI] [PubMed] [Google Scholar]
- 38.Colovic RB, Grubor NM, Micev MT, Matic SV, Atkinson HD, Latincic SM. Perigastric extraskeletal Ewing’s sarcoma: a case report. World J Gastroenterol. 2009;15(2):245–7. doi: 10.3748/wjg.15.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafailidis S, Ballas K, Psarras K, Pavlidis T, Symeonidis N, Marakis G, et al. Primary Ewing sarcoma of the stomach--a newly described entity. Eur Surg Res. 2009;42(1):17–20. doi: 10.1159/000166166. [DOI] [PubMed] [Google Scholar]
- 40.Ankouz A, Elbouhadouti H, Lamrani J, Bouassria A, Louchi A, Taleb KA. [Peripheral primitive neuroectodermal tumor with gastric primary location: about a new case] Pan Afr Med J. 2010;6:15. [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, Wakai T, Korita PV, Sakata J, Kurosaki R, Ogose A, et al. Gastric Ewing sarcoma/primitive neuroectodermal tumor: A case report. Oncol Lett. 2011;2(2):207–10. doi: 10.3892/ol.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodarte-Shade M, Palomo-Hoil R, Vazquez J, Ancer A, Vilches N, Flores-Gutierrez JP, et al. Primitive Neuroectodermal Tumor (PNET) of the Small Bowel in a Young Adult with Lower Gastrointestinal Bleeding. J Gastrointest Cancer. 2012;43(Suppl 1):S243–5. doi: 10.1007/s12029-012-9409-y. [DOI] [PubMed] [Google Scholar]
- 43.Aras M, Dede F, Dane F, Aktas B, Turoglu HT. FDG PET/CT appearance of portal vein tumor thrombus in the gastric primitive neuroectodermal tumor: uncommon primary tumor site with rare finding. Clin Nucl Med. 2013;38(1):47–9. doi: 10.1097/RLU.0b013e3182708530. [DOI] [PubMed] [Google Scholar]
- 44.Insabato L, Guadagno E, Natella V, Somma A, Bihl M, Pizzolorusso A, et al. An unusual association of malignant gastrointestinal neuroectodermal tumor (clear cell sarcoma-like) and Ewing sarcoma. Pathol Res Pract. 2015;211(9):688–92. doi: 10.1016/j.prp.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Kim SB, Lee SH, Gu MJ. Esophageal subepithelial lesion diagnosed as malignant gastrointestinal neuroectodermal tumor. World J Gastroenterol. 2015;21(18):5739–43. doi: 10.3748/wjg.v21.i18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boland JM, Folpe AL. Oncocytic variant of malignant gastrointestinal neuroectodermal tumor: a potential diagnostic pitfall. Hum Pathol. 2016;57:13–6. doi: 10.1016/j.humpath.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Khuri S, Gilshtein H, Sayidaa S, Bishara B, Kluger Y. Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Stomach. Case Rep Oncol. 2016;9(3):666–71. doi: 10.1159/000449126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maxwell AW, Wood S, Dupuy DE. Primary extraskeletal Ewing sarcoma of the stomach: a rare disease in an uncommon location. Clin Imaging. 2016;40(5):843–5. doi: 10.1016/j.clinimag.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Song MJ, An S, Lee SS, Kim BS, Kim J. Primitive Neuroectodermal Tumor of the Stomach: A Case Report. Int J Surg Pathol. 2016;24(6):543–7. doi: 10.1177/1066896916639371. [DOI] [PubMed] [Google Scholar]
- 50.Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015;33(27):3036–46. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 51.Cash T, McIlvaine E, Krailo MD, Lessnick SL, Lawlor ER, Laack N, et al. Comparison of clinical features and outcomes in patients with extraskeletal versus skeletal localized Ewing sarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2016;63(10):1771–9. doi: 10.1002/pbc.26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galyfos G, Karantzikos GA, Kavouras N, Sianou A, Palogos K, Filis K. Extraosseous Ewing Sarcoma: Diagnosis, Prognosis and Optimal Management. Indian J Surg. 2016;78(1):49–53. doi: 10.1007/s12262-015-1399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biermann JS. Updates in the treatment of bone cancer. J Natl Compr Canc Netw. 2013;11(5 Suppl):681–3. doi: 10.6004/jnccn.2013.0200. [DOI] [PubMed] [Google Scholar]
- 54.Qureshi SS, Laskar S, Kembhavi S, Talole S, Chinnaswamy G, Vora T, et al. Extraskeletal Ewing sarcoma in children and adolescents: impact of narrow but negative surgical margin. Pediatr Surg Int. 2013;29(12):1303–9. doi: 10.1007/s00383-013-3409-2. [DOI] [PubMed] [Google Scholar]
- 55.Machado I, Navarro L, Pellin A, Navarro S, Agaimy A, Tardio JC, et al. Defining Ewing and Ewing-like small round cell tumors (SRCT): The need for molecular techniques in their categorization and differential diagnosis. A study of 200 cases. Ann Diagn Pathol. 2016;22:25–32. doi: 10.1016/j.anndiagpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Li FP, Tu JT, Liu FS, Shiang EL. Rarity of Ewing’s sarcoma in China. Lancet. 1980;1(8180):1255. doi: 10.1016/S0140-6736(80)91719-5. [DOI] [PubMed] [Google Scholar]
- 57.Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116(8):1964–73. doi: 10.1002/cncr.24937. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs AJ, Fishbein J, Levy CF, Glick RD. Chest wall Ewing sarcoma: a population-based analysis. J Surg Res. 2016;204(2):475–80. doi: 10.1016/j.jss.2016.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


