Abstract
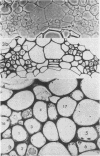
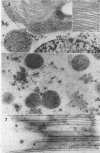
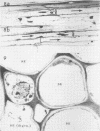
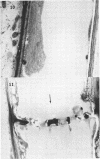
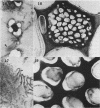
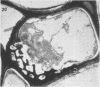
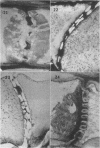
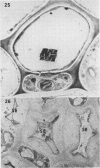
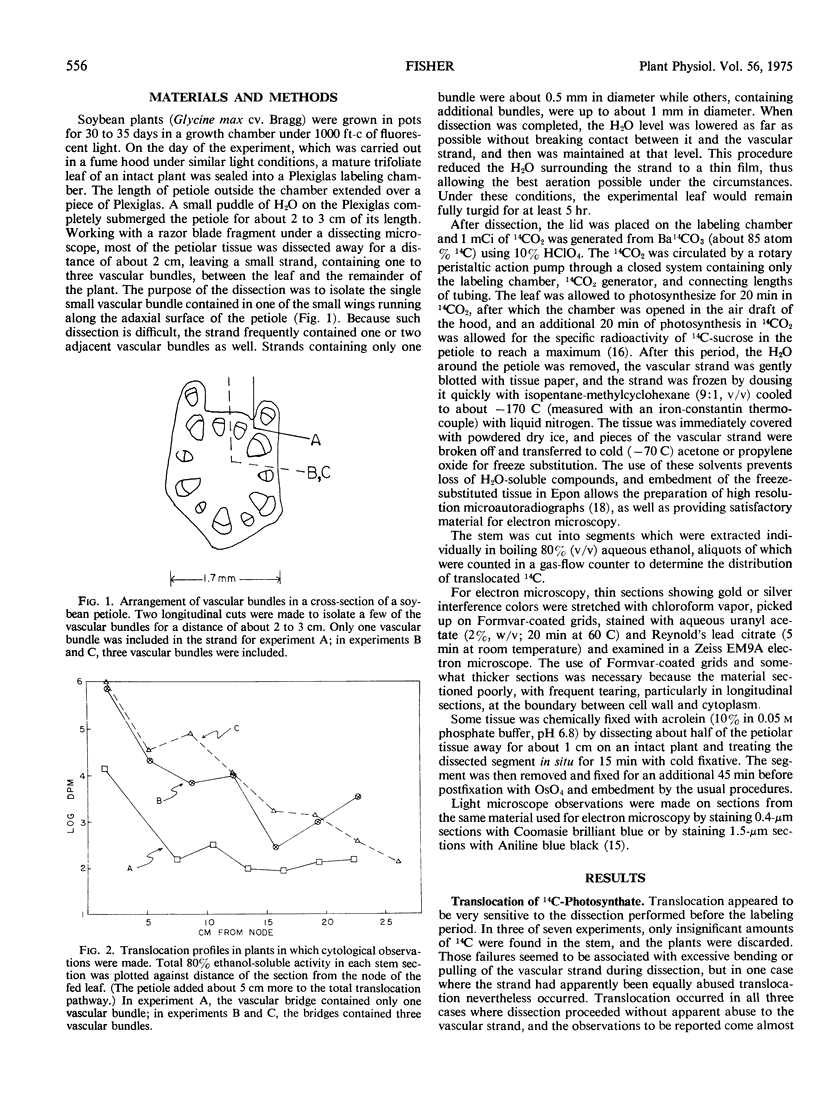
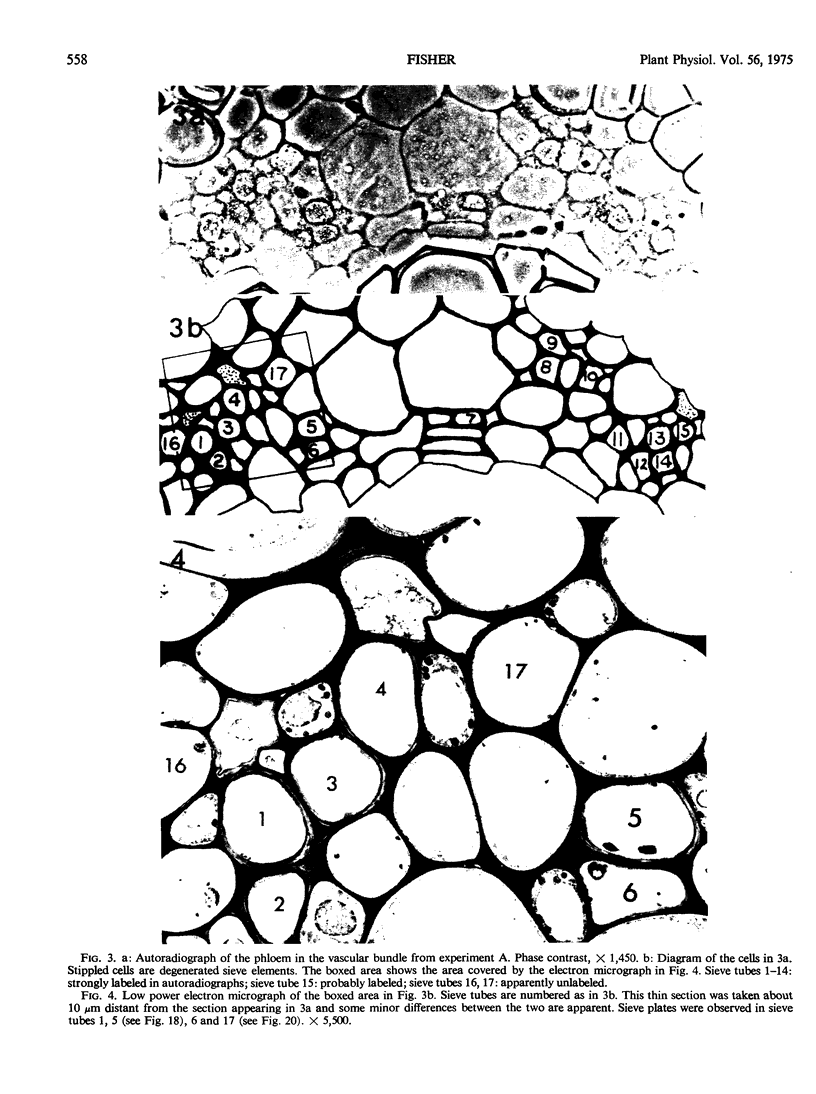
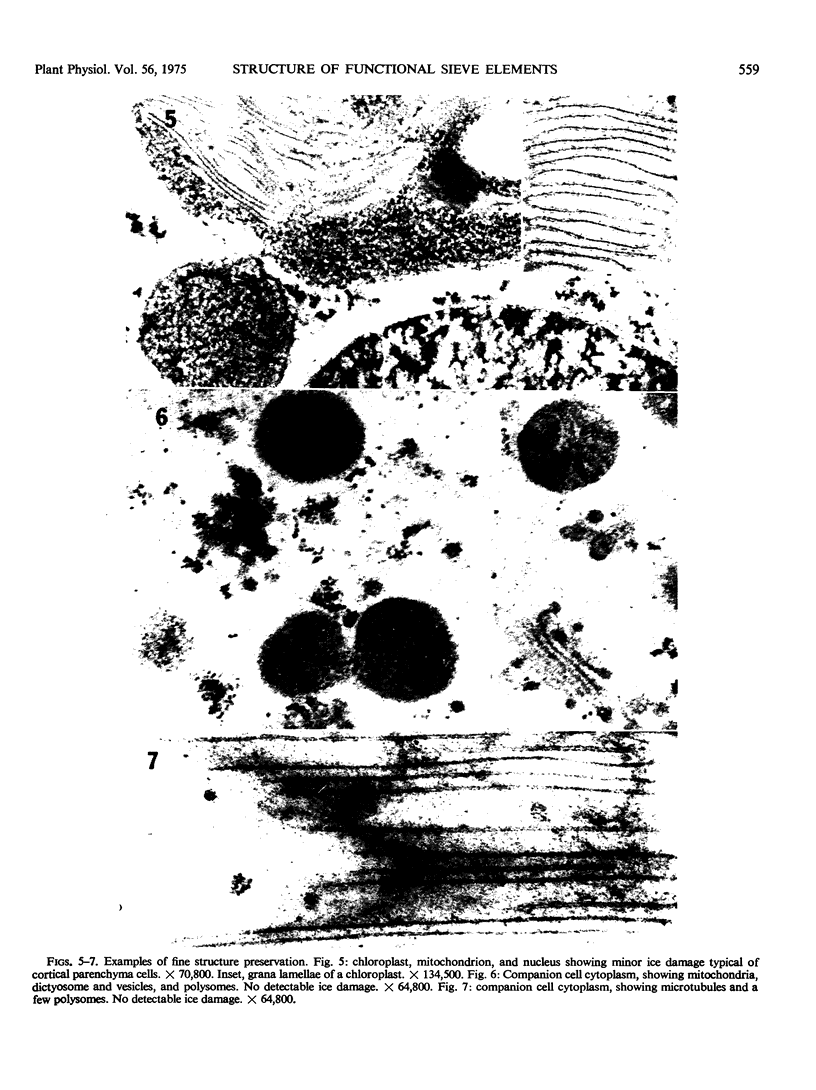
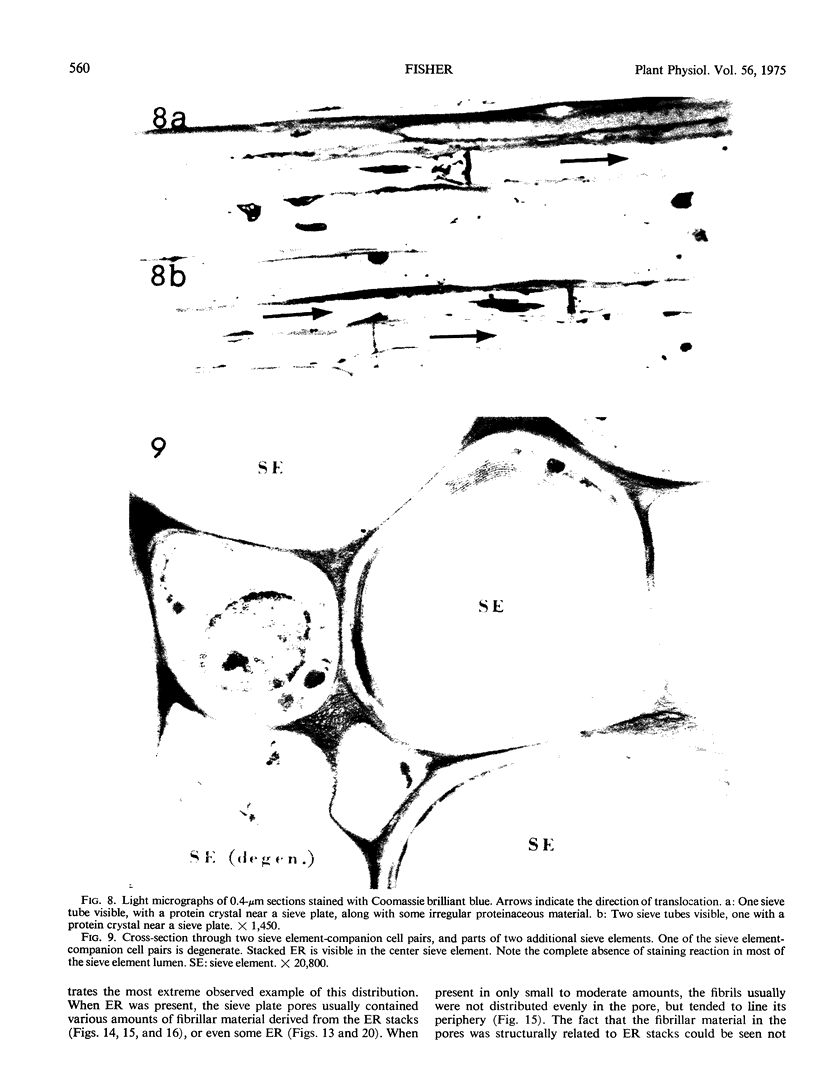
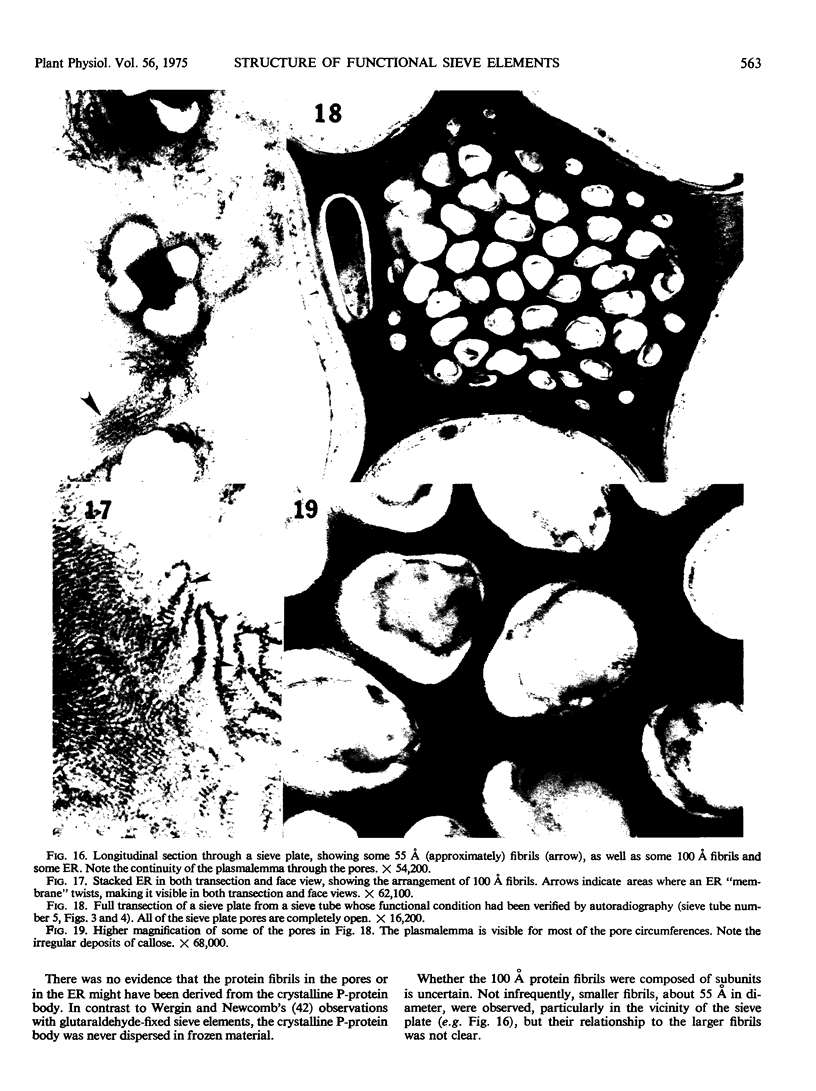
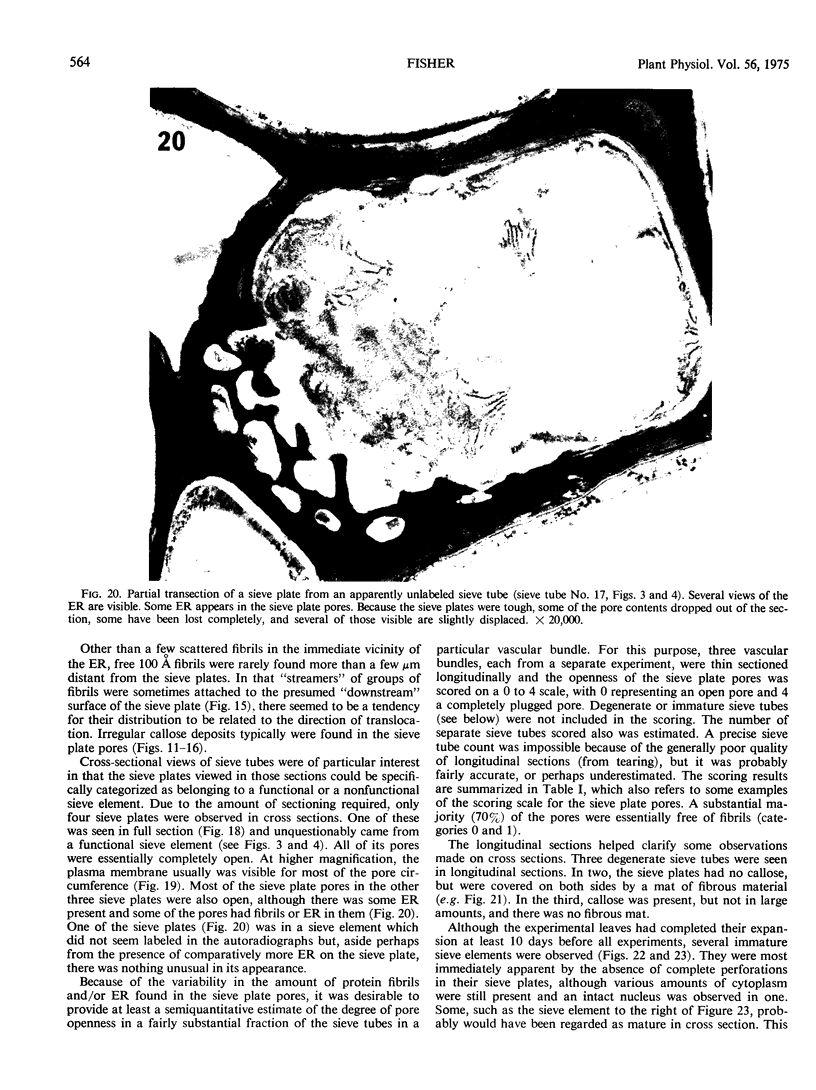
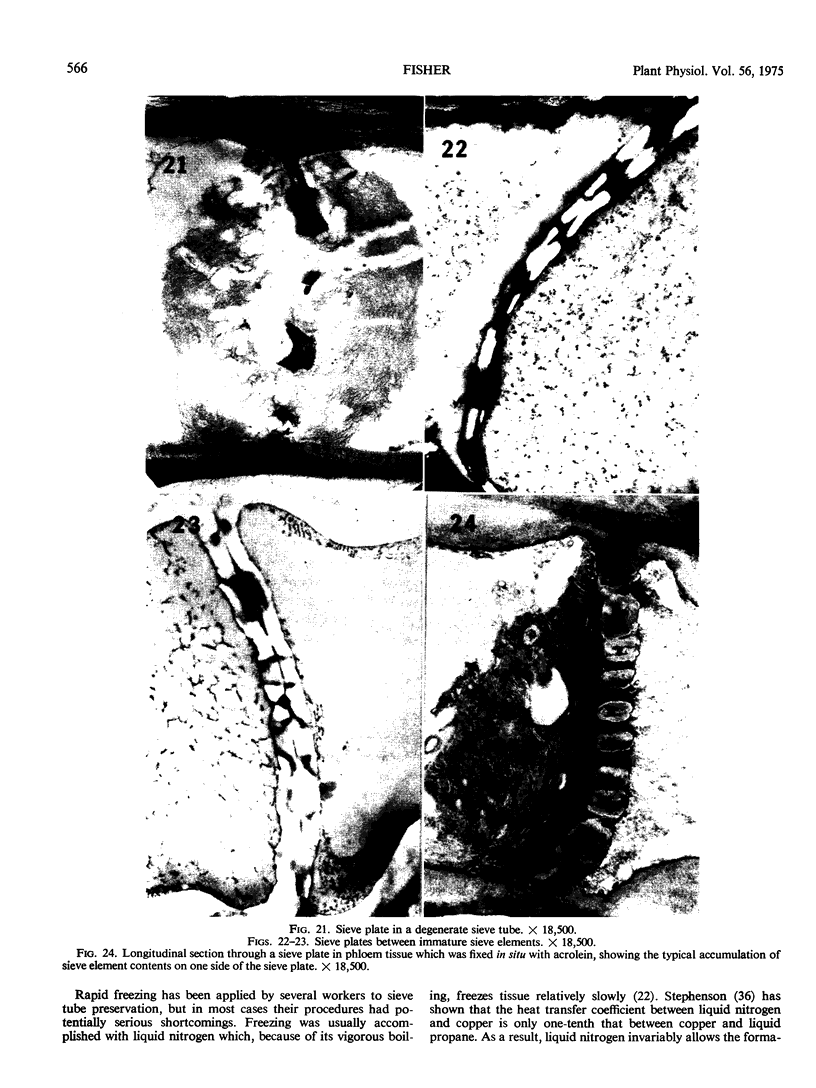
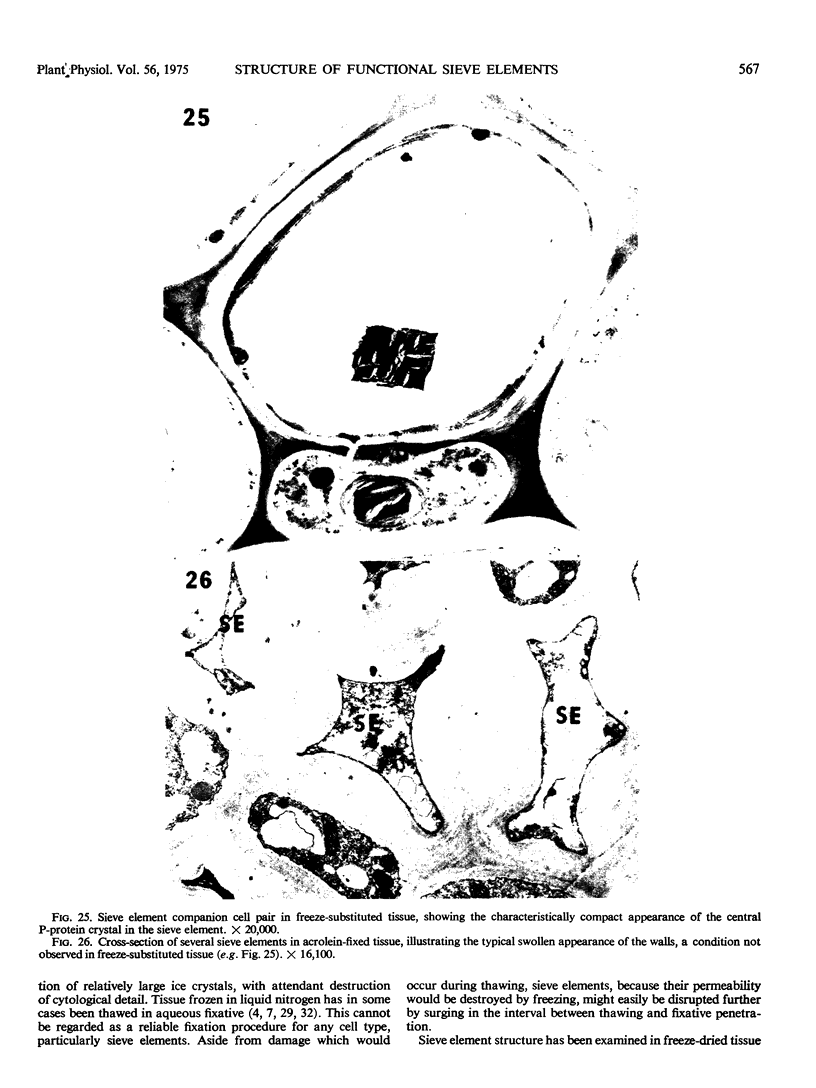
Soybean (Glycine max cv. Bragg) petiolar tissue containing translocated 14C-sucrose was quick frozen, freeze-substituted in acetone or propylene oxide and embedded in Epon. This procedure allowed cytological observations on sieve elements whose functional condition could be verified by microautoradiography. Sieve elements and companion cells were essentially free of ice damage. Aside from a P-protein crystal, the central portion of the sieve tube lumen was devoid of stainable content except in the vicinity of sieve plates. Various sized clumps of stacked endoplasmic reticulum (ER) lined the wall. Superficially, the ER “membranes” seemed to consist of parallel arrays of 100 Å protein fibrils. Although that possibility could not be excluded, it seemed more likely that the fibrils were actually between ER cisternae and that the lipoprotein ER membrane could not be detected readily due to the loss of lipids during tissue preparation. The amount and distribution of proteinaceous material in the vicinity of sieve plates was variable but, when present, still consisted almost entirely of 100 Å fibrils organized into membrane-like arrays. Stacks of ER in various degrees of disorganization and a few 100 Å fibrils were found near sieve plates, with some fibrils extending through the pores. However, most (70%) of the sieve plate pores were essentially free from obstruction. The observations favor an osmotically generated pressure flow mechanism of translocation in soybean.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Cronshaw J. Sieve element pores in Nicotiana pith culture. J Ultrastruct Res. 1970 Sep;32(5):458–471. doi: 10.1016/s0022-5320(70)80022-3. [DOI] [PubMed] [Google Scholar]
- Cronshaw J., Anderson R. Phloem differentiation in tobacco pith culture. J Ultrastruct Res. 1971 Feb;34(3):244–259. doi: 10.1016/s0022-5320(71)80070-9. [DOI] [PubMed] [Google Scholar]
- Cronshaw J., Anderson R. Sieve plate pores of Nicotiana. J Ultrastruct Res. 1969 Apr;27(1):134–148. [PubMed] [Google Scholar]
- Doebbler G. F. Cryoprotective compounds. Review and discussion of structure and function. Cryobiology. 1966 Jul-Aug;3(1):2–11. doi: 10.1016/s0011-2240(66)80144-x. [DOI] [PubMed] [Google Scholar]
- Esau K., Gill R. H. Aggregation of endoplasmic reticulum and its relation to the nucleus in a differentiating sieve element. J Ultrastruct Res. 1971 Jan;34(1):144–158. doi: 10.1016/s0022-5320(71)90010-4. [DOI] [PubMed] [Google Scholar]
- Fisher D. B. Artifacts in the Embedment of Water-soluble Compounds for Light Microscopy. Plant Physiol. 1972 Feb;49(2):161–165. doi: 10.1104/pp.49.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Housley T. L. The Retention of Water-soluble Compounds during Freeze-Substitution and Microautoradiography. Plant Physiol. 1972 Feb;49(2):166–171. doi: 10.1104/pp.49.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 translocation in soybean: I. Kinetics in the stem. Plant Physiol. 1970 Feb;45(2):107–113. doi: 10.1104/pp.45.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16(1):92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T., Geiger D. R. Mechanism of inhibition of translocation by localized chilling. Plant Physiol. 1973 Feb;51(2):372–377. doi: 10.1104/pp.51.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder J., Cronshaw J. A biochemical and cytochemical study of adenosine triphosphatase activity in the phloem of Nicotiana tabacum. J Cell Biol. 1974 Jan;60(1):221–235. doi: 10.1083/jcb.60.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig H., Dörr I., Kollmann R. Vinblastine-induced precipitation of phloem proteins in vitro. Protoplasma. 1971;73(2):293–302. doi: 10.1007/BF01275601. [DOI] [PubMed] [Google Scholar]
- Weatherley P. E., Johnson R. P. The form and function of the sieve tube: a problem in reconciliation. Int Rev Cytol. 1968;24:149–192. doi: 10.1016/s0074-7696(08)61399-6. [DOI] [PubMed] [Google Scholar]