Abstract
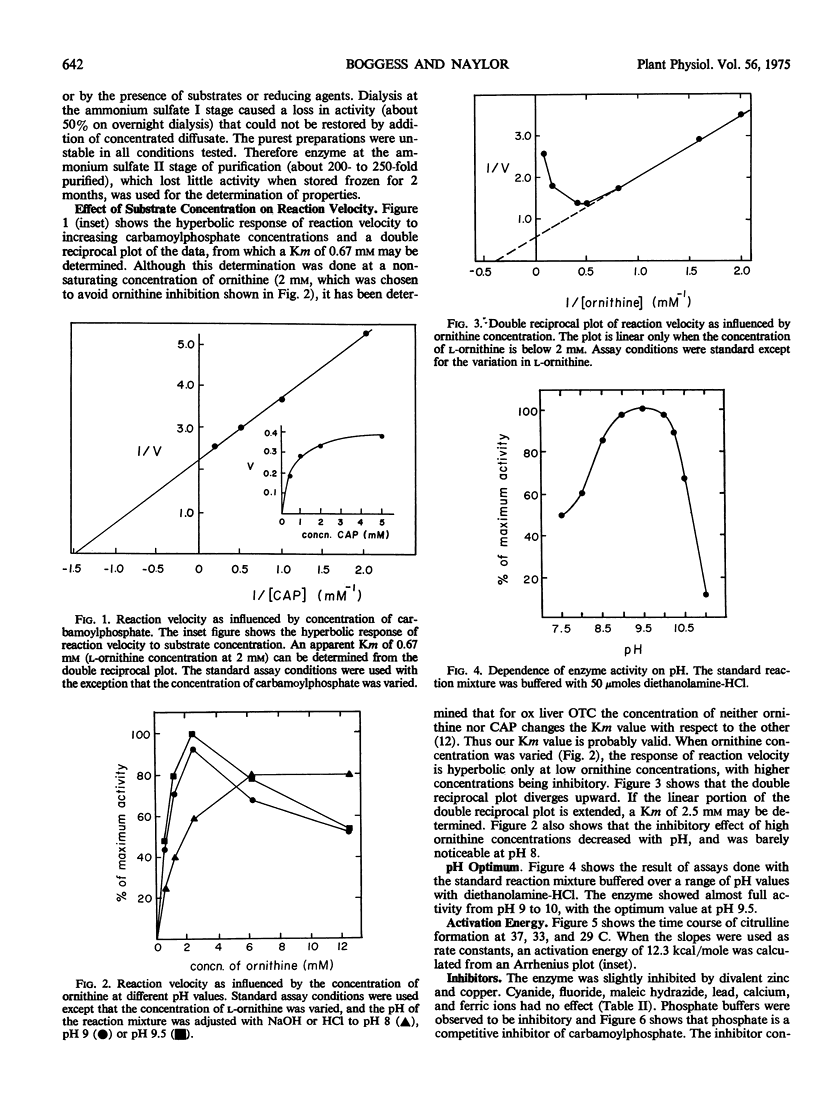
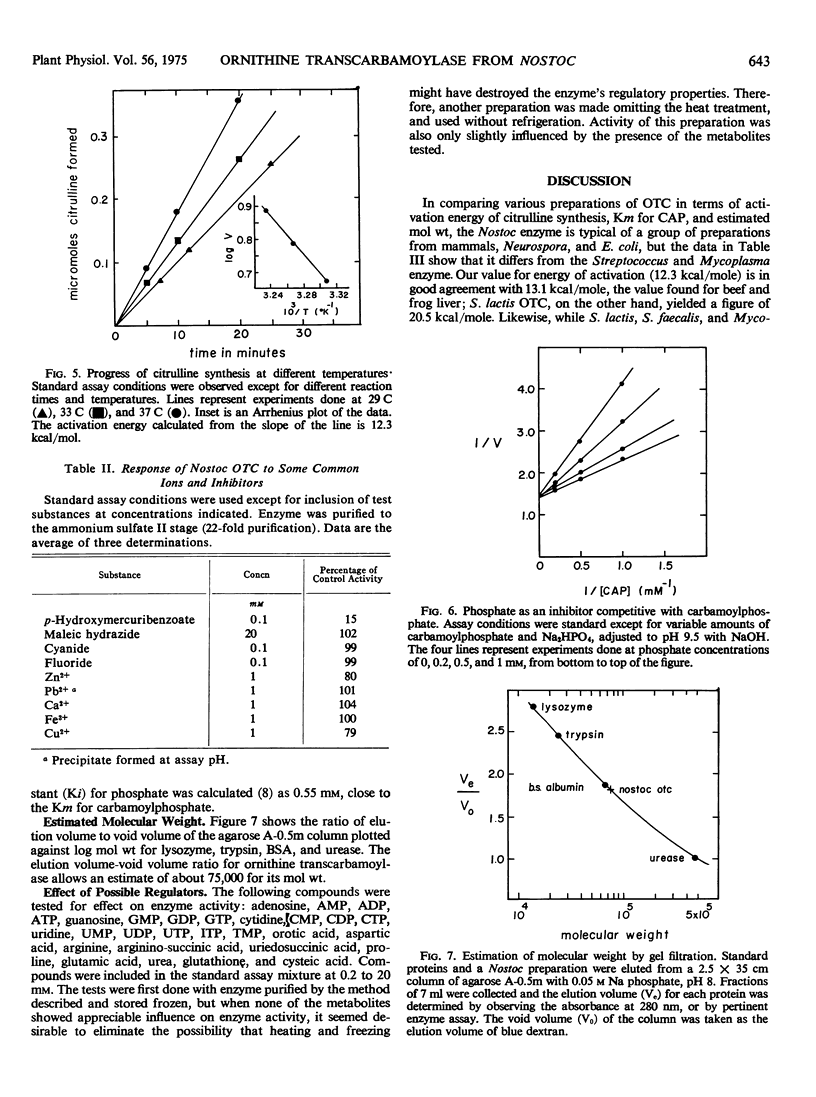
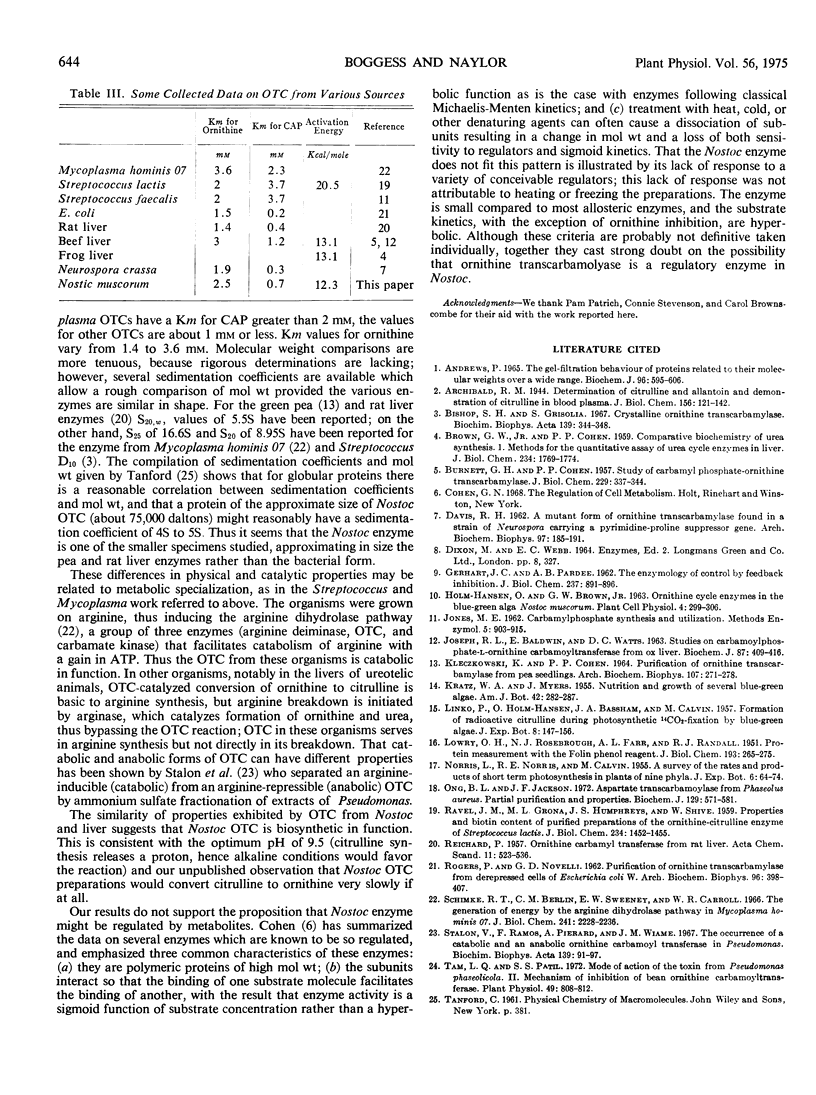
Ornithine transcarbamoylase (carbamoyl phosphate:l-ornithine carbamoyltransferase, EC 2.1.3.3) has been partially purified from the blue-green alga Nostoc muscorum Kützing, an organism in which the enzyme seems to be involved in a bicarbonate-fixing pathway leading to citrulline. Pertinent to possible regulation of this pathway, the enzyme shows hyperbolic substrate kinetics, has a molecular weight estimated at 75,000 daltons, and its catalytic capability is little influenced by a selection of metabolites that might conceivably act as regulators in vivo. Thus it seems unlikely that this enzyme is the control point for bicarbonate fixation. In terms of energy of activation (12.3 kcal/mole), size and Km for carbamoylphosphate, the Nostoc enzyme resembled preparations from liver and higher plants more than preparations from Streptococcus and Mycoplasma. The enzymes from Streptococcus and Mycoplasma are probably specialized for citrulline breakdown rather than citrulline synthesis. The Km for ornithine was 2.5 mm at a saturating concentration of carbamoylphosphate and the Km for carbamoylphosphate was 0.7 mm at an ornithine concentration of 2 mm. Ornithine was inhibitory at concentrations greater than 2 mm. Phosphate was a competitive inhibitor with respect to carbamoylphosphate. The pH optimum for citrulline synthesis was 9.5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. W., Jr, COHEN P. P. Comparative biochemistry of urea synthesis. I. Methods for the quantitative assay of urea cycle enzymes in liver. J Biol Chem. 1959 Jul;234(7):1769–1774. [PubMed] [Google Scholar]
- BURNETT G. H., COHEN P. P. Study of carbamyl phosphate-ornithine transcarbamylase. J Biol Chem. 1957 Nov;229(1):337–344. [PubMed] [Google Scholar]
- Bishop S. H., Grisolia S. Crystalline ornithine transcarbamylase. Biochim Biophys Acta. 1967 Jul 11;139(2):344–348. doi: 10.1016/0005-2744(67)90037-x. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- JOSEPH R. L., BALDWIN E., WATTS D. C. Studies on carbamoyl phosphate-L-ornithine carbamoyltransferease from ox liver. Biochem J. 1963 May;87:409–416. doi: 10.1042/bj0870409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLECZKOWSKI K., COHEN P. P. PURIFICATION OF ORNITHINE TRANSCARBAMYLASE FROM PEA SEEDLINGS. Arch Biochem Biophys. 1964 Aug;107:271–278. doi: 10.1016/0003-9861(64)90329-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Aspartate transcarbamoylase from Phaseolus aureus. Partial purification and properties. Biochem J. 1972 Sep;129(3):571–581. doi: 10.1042/bj1290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVEL J. M., GRONA M. L., HUMPHREYS J. S., SHIVE W. Properties and biotin content of purified preparations of the ornithinecitrulline enzyme of Streptococcus lactis. J Biol Chem. 1959 Jun;234(6):1452–1455. [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Purification of ornithine transcarbamylase from derepressed cells of Escherichia coli W. Arch Biochem Biophys. 1962 Feb;96:398–407. doi: 10.1016/0003-9861(62)90426-5. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. The occurrence of a catabolic and an anabolic ornithine carbamoyltransferase in Pseudomonas. Biochim Biophys Acta. 1967 May 16;139(1):91–97. doi: 10.1016/0005-2744(67)90115-5. [DOI] [PubMed] [Google Scholar]


