Abstract
Background:
Numerous surgical options exist to treat chondral lesions in the knee, including microfracture (MFx), osteochondral autograft transplantation (OAT), first-generation autologous chondrocyte implantation (ACI-1), and next-generation ACI (ACI-2).
Purpose:
To compare the cost-effectiveness of MFx, OAT, and ACI-1. The secondary purpose of this study was to compare the functional outcomes of MFx, OAT, ACI-1, and ACI-2.
Study Design:
Systematic review; Level of evidence, 2.
Methods:
Two independent reviewers conducted an online literature search of 2 databases for level 1 and 2 studies using the Lysholm, International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS), and/or Hospital for Special Surgery (HSS) Knee Score. A weighted mean difference in pre- to postoperative functional outcome score was calculated for each treatment. The mean per-patient costs associated with MFx, OAT, and ACI-1 were determined from a recent publication based on review of a national private insurance database. The cost for each procedure was then divided by the weighted mean difference in functional outcome score to give the cost-per-point change in outcome score.
Results:
A total of 12 studies (6 level 1, 6 level 2) met the inclusion criteria for the functional outcome analysis, including 730 knees (MFx, n = 300; OAT, n = 90; ACI-1, n = 68; ACI-2, n = 272). The mean follow-up was not significantly different between groups (MFx, 29.4 months; OAT, 38.3 months; ACI-1, 19.0 months; ACI-2, 26.7 months). The mean increase in functional outcome score was 23 for MFx, 19 for OAT, 20 for ACI-1, and 35 for ACI-2. The change in functional outcome score was significantly greater for ACI-2 when compared with all other treatments (P < .0001). The cost-per-point change in functional outcome score was $200.59 for MFx, $313.84 for OAT, and $536.59 for ACI-1.
Conclusion:
MFx, OAT, ACI-1, and ACI-2 are effective surgical procedures for the treatment of cartilage defects in the knee. All 4 treatments led to an increase in functional outcome scores postoperatively with a short-term follow-up. ACI-2 had a statistically greater improvement in functional outcome scores as compared with the other 3 procedures. MFx was found to be the most cost-effective treatment option and ACI-1 the least cost-effective.
Keywords: cost-effectiveness, knee, microfracture, osteochondral autograft transplantation, autologous chondrocyte implantation
Articular cartilage lesions in the knee occur through degenerative processes, acute injury, or chronic repetitive overload and often fail to heal spontaneously due to their avascularity and minimal chondrocyte migration and propagation.20 These lesions are associated with pain and loss of function and can eventually progress to osteoarthritis.10,27 They occur in approximately 12% of the population, and some degree of articular cartilage pathology has been identified in more than 60% of arthroscopic knee procedures.1,2,25 These lesions are most common in the medial compartment followed by the patellofemoral compartment.5 There have been multiple treatments proposed for focal knee chondral lesions, including microfracture (MFx), osteochondral autograft transplantation (OAT), osteochondral allograft transplantation, first-generation autologous chondrocyte implantation (ACI-1), and next-generation ACI (ACI-2).21
First-generation ACI involves embedding chondrocytes into the lesion under a periosteal patch. Newer ACI procedures (ACI-2) embed chondrocytes within biological or engineered matrices. Because of the presence of focal cartilage lesions and the economic burden associated with different treatment modalities, this injury is an immense socioeconomic issue.25 No studies have sought to determine the costs associated with an untreated knee articular cartilage lesion. Another concern—the cost of return to sport—has not yet been distinguished because of the variability of the length of physical therapy between patients.25
There is an increased emphasis on containing health care costs and improving health care efficiency. As such, the primary purpose of this study was to compare the cost-effectiveness of 3 common procedures for treating knee chondral lesions: MFx, OAT, and ACI-1. The secondary purpose was to compare the functional outcomes of MFx, OAT, ACI-1, and ACI-2. It was hypothesized that there would be no significant difference in the cost-effectiveness or functional outcomes of the different surgical treatment options for chondral lesions of the knee.
Methods
Literature Search
Two independent reviewers conducted an online literature search of PubMed and the Cochrane Library through February 2016 for level 1 and 2 studies evaluating outcomes of surgical treatment for chondral defects of the knee. The following subject headings and keywords were used to retrieve articles: knee, microfracture, osteochondral autograft transplantation, mosaicplasty, autologous chondrocyte implantation, KOOS, IKDC, Lysholm, and HSS. All potentially relevant articles and those with questionable eligibility were retrieved and reviewed as well as pertinent manuscripts cited in these articles. Article eligibility and discrepancies were discussed and resolved by 2 authors (J.B.S. and D.A.H.).
Inclusion and Exclusion Criteria
Inclusion criteria were level 1 or 2 studies that measured functional outcomes with either the Lysholm,26 International Knee Documentation Committee (IKDC) Subjective Knee form,10,12 Knee injury and Osteoarthritis Outcome Score (KOOS),22,23 and/or the Hospital for Special Surgery (HSS) Knee Score.11 Studies included were required to be written in English, to include both pre- and postoperative patient-reported outcome scores, and to directly compare patient-reported outcomes between at least 2 of the following treatments: MFx, OAT, ACI-1, and ACI-2. Articles that did not report standard deviations for outcome scores were excluded. Patients in all included studies were required to have International Cartilage Repair Society (ICRS) grade 3 or 4 isolated cartilage lesions of the femoral condyles or trochlea.
The Lysholm, IKDC, KOOS, and HSS Knee Score are each graded on a 0- to 100-point scale. These scores were grouped together to achieve a large sample size for analysis, as described previously.20 A weighted mean difference in preoperative to postoperative functional outcome score was calculated for each treatment. In each included study, functional outcome tests were administered at final follow-up.
Two analyses were performed. The first was an analysis of functional outcome scores after MFx, OAT, ACI-1, and ACI-2 procedures. The second analysis calculated a cost-per-point change in functional outcome score. The total costs associated with MFx, OAT, and ACI-1 were determined from a recent publication based on review of a national private insurance database.32 The charges for each procedure, including for the 1-year preoperative and 1-year postoperative periods, were calculated for each patient. Charges included in this analysis were imaging, outpatient visits, rehabilitation, joint injections, and repeat procedures for postoperative complications.32 Then, a per-patient mean charge was calculated. The cost for each procedure was then divided by the weighted mean difference in functional outcome score to give the cost-per-point change in outcome score. A comprehensive cost analysis of ACI-2 was not available, and there were not enough pre- and postoperative costs associated with ACI-2 in the literature to accurately estimate these costs ourselves. Therefore, ACI-2 was excluded from the cost-effectiveness analysis. Vendors of ACI-2 scaffolds utilized in the studies included in this analysis were contacted in an effort to provide readers with the range of costs associated with this technology, although contacts either did not respond to email or were not able to provide the information requested.
Surgical Techniques
Patients in all studies were required to have ICRS grade 3 or 4 isolated cartilage lesions of the femoral condyles or trochlea. Inclusion criteria were similar within all studies. Patients aged 18 to 50 years,2,4,25,29 18 to 55 years,6,7,24 16 to 50 years,31 and 18 to 45 years16 were included. One study analyzed outcomes in athletes younger than 40 years.9 Chondral defect size thresholds are shown in Table 1.
TABLE 1.
Characteristics of Studies Analyzing Treatments for Chondral Lesions of the Kneea
| Study | Level of Evidence | Treatment | Outcome Scores | Defect Size, cm2, Range |
|---|---|---|---|---|
| Basad et al2 | 1 | MFx/ACI-2 | Lysholm | 4-10 |
| Clavé et al4 | 1 | OAT/ACI-2 | IKDC | 2.5-7.5 |
| Cole et al6 | 2 | MFx/ACI-2 | KOOS/IKDC | 1-10 |
| Crawford et al7 | 2 | MFx/ACI-2 | KOOS/IKDC | Not listed |
| Gudas et al9 | 1 | MFx/OAT | HSS | 1-4 |
| Knutsen et al14 | 1 | MFx/ACI-1 | Lysholm | 2-10 |
| Kon et al16 | 2 | MFx/ACI-2 | IKDC | >1 |
| Lim et al18 | 2 | MFx/OAT/ACI-1 | Lysholm | 1-4 |
| Saris et al24 | 1 | MFx/ACI-2 | KOOS | 1-5 |
| Saris et al25 | 1 | MFx/ACI-2 | KOOS/IKDC | >3 |
| Ulstein et al29 | 2 | MFx/OAT | Lysholm/KOOS | 2-6 |
| Zeifang et al31 | 2 | ACI-1/ACI-2 | Lysholm/IKDC | 2.5-6 |
aACI-1, first-generation autologous chondrocyte implantation; ACI-2, next-generation autologous chondrocyte implantation; HSS, Hospital for Special Surgery Knee Score; IKDC, International Knee Documentation Committee Subjective Knee Form; KOOS, Knee injury and Osteoarthritis Outcome Score; MFx, microfracture; OAT, osteochondral autograft transplantation.
Microfracture
All studies performed arthroscopic microfracture according to the technique described by Steadman et al.28 First, debridement of all unstable and damaged cartilage around the lesion, as well as loose or marginally attached cartilage, was performed down to the subchondral bone plate, with removal of the calcified cartilage layer for a better clot adhesion.16 Then, multiple penetrations of the subchondral bone were completed using an arthroscopic awl. Lim et al18 started their hole punctures on the periphery of the lesion and continued toward the center of the lesion. Holes were placed far enough apart (3-4 mm) to avoid collapse of adjacent holes. After the holes were completed, the irrigation fluid pump pressure was lowered to visualize release of fat droplets and blood.29
Osteochondral Autograft Transplantation
OAT was performed after arthroscopic examination and debridement. Cylindrical osteochondral plugs were harvested from nonweightbearing articular regions of the knee and transplanted into the chondral lesion. Lim et al18 performed this operation arthroscopically. Ulstein et al29 performed the procedure through a medial parapatellar arthrotomy or a mini-arthrotomy, depending on the size and location of the lesion. Clavé et al4 utilized a parapatellar arthrotomy in all cases. Care was taken to match the lesion size and shape with the graft(s) to achieve a press-fit transplantation.4,9,18,29
First-Generation Autologous Chondrocyte Implantation
ACI-1 was performed in a 2-procedure process. In the first procedure, a normal cartilage sample was biopsied. Lim et al18 biopsied cartilage from the margin of the trochlea, while Knutsen et al14 harvested cartilage from outside the loaded area on the rim of the medial femoral condyle. This sample was then cultured and expanded in vitro. In the second procedure, the autologous chondrocytes were injected back into the chondral defect beneath a periosteal patch that was harvested from the proximal tibia or distal femur.14,18,31
Next-Generation Autologous Chondrocyte Implantation
ACI-2 techniques avoid the use of autogenous periosteal patches and instead use a biological or engineered scaffold/tissue. Basad et al2 implanted a chondrocyte-seeded collagen scaffold (MACI; Genzyme Biosurgery), while Crawford et al7 used an autologous cartilage tissue-engineered implant that combines a bovine type I collagen matrix scaffold with autogenous chondrocytes and bioreactor treatment (NeoCart; Histogenics). Kon et al16 seeded autogenous chondrocytes onto a hyaluronic acid–based scaffold (Hyaff 11; Fidia Advanced Biopolymers) and then implanted the bioengineered tissue Hyalograft C (Fidia Advanced Biopolymers). Saris et al25 used ChondroCelect (TiGenix), an autologous cell therapy product. Saris et al24 seeded chondrocytes onto a porcine-derived collagen type I/III membrane (ACI-Maix; Matricel GmbH), which was subsequently glued to the defect. Clavé et al4 used a solid agarose-alginate hydrogel scaffold capable of supporting autologous chondrocytes (Cartipatch; Tissue Bank of France). Cole et al6 used a unique device that minces cartilage into 1- to 2-mm pieces (DePuy Mitek). The fragments are then dispersed uniformly on a biodegradable scaffold (DePuy Mitek). Zeifang et al31 used the BioSeed-C scaffold to implant the chondrocytes (BioTissue Technologies).
Rehabilitation
One study utilized different rehabilitation protocols between treatment groups.2 In general, most studies utilized a period of partial weightbearing with crutches for 6 to 8 weeks, with gradual progression to full weightbearing after 8 to 10 weeks.2,4,6,7,9,14,18,25,29,31 Saris et al24 and Kon et al16 utilized a 4-stage rehabilitation program, with transition from one stage to the next determinant on patients reaching specific goals. Continuous passive motion was utilized in 7 studies.2,6,7,14,16,18,31 Basad et al2 used a dorsal plaster cast to prevent graft delamination in ACI-2 patients for 2 days. Saris et al25 used an unloader brace for 8 weeks postsurgery for all patients. Patients with a femoral condyle lesion were made nonweightbearing for 2 weeks in 1 study.6 All patients were nonweightbearing for 2 to 4 weeks in 4 studies.4,10,16,25
Statistical Analysis
Data extracted for this review included patient demographic information, pre- and postoperative functional outcome scores, follow-up duration, lesion size and location, and complications. Complications were events that led to an additional procedure(s), clinical failure as determined by individual study author(s), and/or adverse events that led to patients discontinuing the study. For continuous variables, a weighted mean and composite standard deviation was calculated for each group, as described previously.17 A 1-way analysis of variance (ANOVA) was used to compare changes in age, functional outcome scores, follow-up duration, lesion size, and complications between the 4 treatments. A Tukey post hoc analysis was performed in cases of P < .05.
The included studies were arranged according to surgical procedure (MFx, OAT, ACI-1, ACI-2). Since there were not enough studies reporting on the comparison of the same 2 procedures, the data were not stratified based on study design. Where possible, a mean change score (▵ score) was determined for subjective outcome scores, and 95% CIs were determined for the effect measures. The I2 statistic was calculated to quantify the degree of heterogeneity, and the Cochrane x2 was used to test for heterogeneity (significance set at P < .05). An I2 ≤ 40% represents an acceptable degree of heterogeneity.
Summary measures were estimated for each procedure using random-effects models, and these were included in multiple forest plots. A random-effects model was used due to some degree of anticipated heterogeneity among the eligible studies, and this model takes into account between-study variation. A meta-regression approach was not used because of the variability of procedures and subjective outcomes reported within these studies.
Meta-analyses, including tests for heterogeneity, the random-effects model, and generation of forest plots, were performed using the Metafor Package (A Meta-Analysis Package for R, http://www.metafor-project.org/).
Results
The initial literature search resulted in 273 articles. A title and abstract review resulted in 12 studies that met the inclusion and exclusion criteria (Figure 1).
Figure 1.
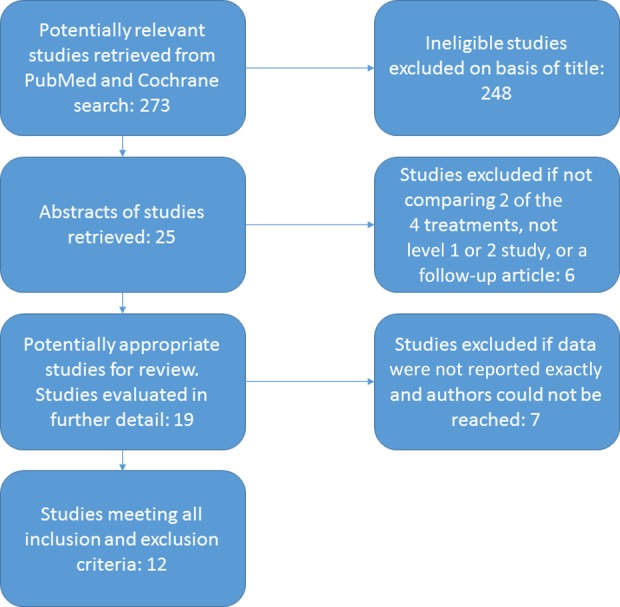
Flow diagram of article selection and review.
Six level 1 and 6 level 2 studies were included in the functional outcome score analysis (Table 1). A total of 730 knees were analyzed for functional outcomes (MFx, n = 300; OAT, n = 90; ACI-1, n = 68; ACI-2, n = 272) (Table 2). Weighted mean lesion size ranged from 1.9 to 4.7 cm2 in the MFx group, 2.8 to 3.6 cm2 in the OAT group, 2.8 to 5.1 cm2 in the ACI-1 group, and 2.1 to 4.9 cm2 in the ACI-2 group. Although lesions in the ACI-1 group were larger on average when compared with each of the other treatment groups, this was not statistically significant. The majority of lesions were present in the medial compartment in all treatment groups (MFx, 75%; OAT, 83%; ACI-1, 72%; ACI-2, 72%). Complications were events that led to an additional procedure(s), clinical failure as determined by individual study authors, and/or adverse events that led to patients discontinuing the study. The MFx group had significantly more complications when compared with ACI-1 and ACI-2 groups (both P < .05) and significantly fewer than the OAT group (P = .011). The OAT group had significantly more complications when compared with ACI-1 and ACI-2 groups (both P < .001), and patients were significantly younger than the MFx group (P = .019) and the ACI-2 group (P < .01).
TABLE 2.
Cost-Effectiveness of Surgical Treatments of Chondral Lesions of the Kneea
| MFx | OAT | ACI-1 | ACI-2 | |
|---|---|---|---|---|
| Studies, n | 10 | 4 | 3 | 8 |
| Knees, n | 300 | 90 | 68 | 272 |
| Mean age, y | 32.1 ± 9.0 | 28.3 ± 8.1b | 30.6 ± 11.0 | 33.0 ± 9.9 |
| % male | 67 | 66 | 65 | 68 |
| Mean follow-up, mo | 29.4 | 38.3 | 19.0 | 26.7 |
| Mean lesion size, cm2 | 3.4 ± 1.7 | 3.0 ± 1.4 | 4.4 ± 0.9 | 3.5 ± 2.1 |
| Lesion location, % | ||||
| Medial | 75 | 83 | 72 | 72 |
| Lateral | 20 | 12 | 28 | 18 |
| Patellofemoral | 5 | 0 | 0 | 10 |
| Complications | 2.7 ± 3.5 | 4.0 ± 2.2b | 1.7 ± 0.6 | 1.9 ± 4.2 |
| Cost, US$ | 3989.65 | 6110.46 | 10,195.16 | — |
| Mean change in functional outcome score | 19.9 | 19.5 | 19.0 | 34.5b |
| Cost-per-point change, US$c | 200.59 | 313.84 | 536.59 | — |
aContinuous data are given as a mean ± SD. No cost is listed for next-generation ACI because it was not included in the Zhang et al32 publication and there were not enough data on the pre- and postoperative costs associated with this procedure to accurately estimate these costs ourselves. ACI-1, first-generation autologous chondrocyte implantation; ACI-2, next-generation autologous chondrocyte implantation; MFx, microfracture; OAT, osteochondral autograft transplantation.
bIndicates statistical significance. MFx lesion size was calculated using 9 studies.6,7,9,14,16,18,24,25,29 MFx lesion location was calculated using 5 studies.8,16,18,24,29 OAT lesion location was calculated using 3 studies.8,18,29 ACI-1 lesion location was calculated using 1 study.18 ACI-2 lesion size was calculated using 7 studies,4,6,7,16,24,25,31 and lesion location was calculated using 2 studies.15,24 Twelve studies were used to determine complications.2,4,6,7,9,14,16,18,24,25,29,31
The overall weighted mean increase in functional outcome score for all studies included was 22.6 ± 22.6 for MFx, 18.6 ± 16.7 for OAT, 19.8 ± 23.2 for ACI-1, and 34.5 ± 21.0 for ACI-2. However, the mean change in functional outcome score for only studies included in the cost-effectiveness analysis is presented in Table 2. The change in functional outcome score was statistically greater for ACI-2 when compared with MFx, OAT, and ACI-1 (P < .0001), but no other significant differences were found between groups.
Procedural costs were calculated by Zhang et al32 in a retrospective review of a national private insurance database. The mean cost for each procedure was $3989.65, $6110.46, and $10,195.16 for MFx, OAT, and ACI-1, respectively.32 Diagnostic imaging represented the largest proportion of preoperative costs for all cartilage repair procedures, and rehabilitation was highest among postoperative costs.32 Mean costs for repeat procedures after complications were greatest for ACI-1 ($730.00) and lowest for MFx ($231.16). Repeat procedures in ACI-1 were primarily performed due to knee stiffness and cartilage hypertrophy, and no MFx patients had such additional procedures.32
As mentioned, a comprehensive cost analysis was not available for ACI-2. Thus, to perform a cost-effectiveness analysis, 8 studies were excluded.2,4,6,7,16,24,25,31 This left 109 knees undergoing MFx (mean follow-up, 32 months), 65 undergoing OAT (mean follow-up, 44 months), and 58 undergoing ACI-1 (mean follow-up, 18 months). The mean age at surgery was 30, 28, and 31 years among MFx, OAT, and ACI-1 patients, respectively. The mean lesion size was 3.4 cm2 for MFx, 2.8 cm2 for OAT, and 4.4 cm2 for ACI-1. The mean improvement in functional outcome score was 20 for MFx, 20 for OAT, and 19 for ACI-1. Using the aforementioned cost data, we calculated the cost-per-point change in functional outcome score as $200.59 for MFx, $313.84 for OAT, and $536.59 for ACI-1.
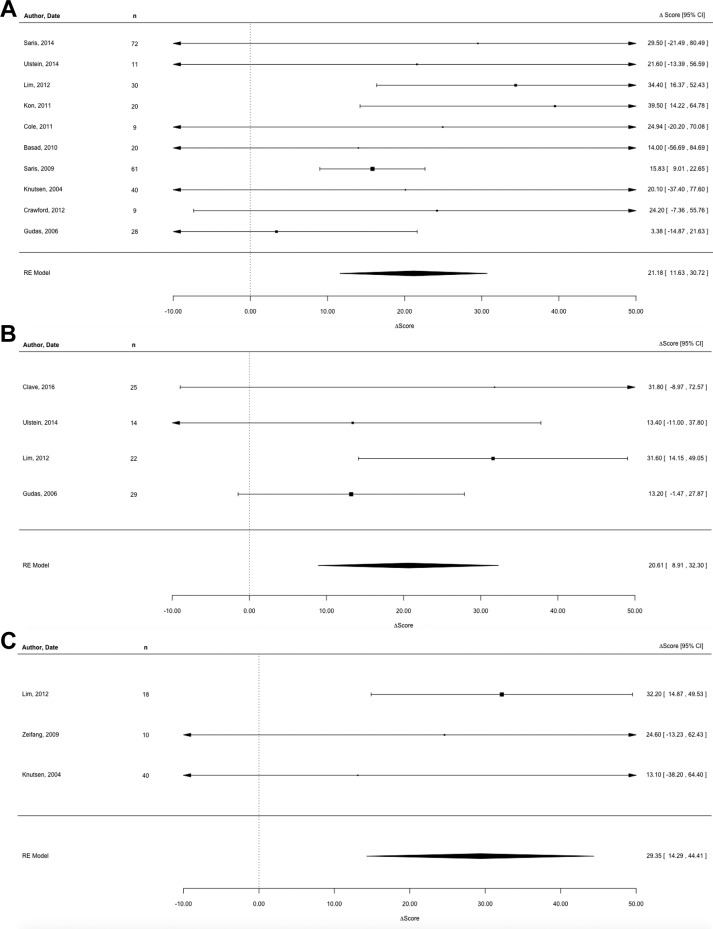
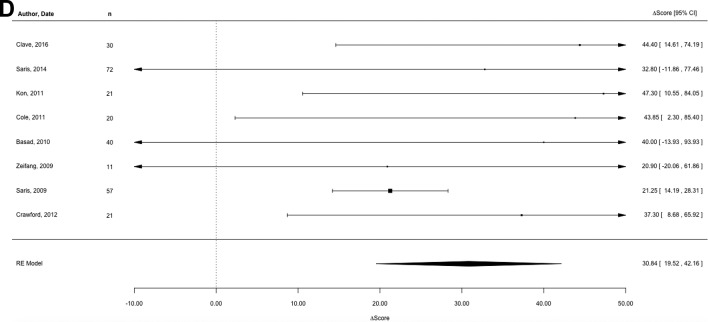
The 10 studies reporting the improvement of subjective outcome scores for MFx had a pooled estimate of 21.18 (95% CI, 11.63-30.72) (Figure 2A). Similarly, the pooled estimate from the 4 studies reporting the improvement of subjective outcome scores for OAT was 20.61 (95% CI, 8.91-32.30) (Figure 2B). The pooled estimate from the 3 studies reporting the improvement of subjective outcome scores for ACI-1 was 29.35 (95% CI, 14.29-44.41) (Figure 2C). The pooled estimate from the 8 studies reporting the improvement of subjective outcome scores for ACI-2 was 30.84 (95% CI, 19.52-42.16) (Figure 2D). Pooled analysis revealed no statistical difference between studies.
Figure 2.
Individual and pooled estimates of the improvement of subjective outcome scores for (A) microfracture, (B) osteochondral autograft transplantation, (C) first-generation autologous chondrocyte implantation, and (D) next-generation autologous chondrocyte implantation. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
Statistical assessment of heterogeneity found for MFx was I2 = 31.32% (95% CI, 0%-62.19%; P = .41), for OAT was I 2 = 21.08% (95% CI, 0%-72.76%; P = .37), for ACI-1 was I 2 = 0% (95% CI, 0%-25.16%; P = .76), and for ACI-2 was I 2 = 23.12% (95% CI, 0%-52.75%; P = .53). In some analyses, these outcomes may represent moderate heterogeneity; however, since there were a limited number of studies for each summary estimate, these statistics are largely underpowered, and a nonstatistically significant result must not be assumed to be evidence of no heterogeneity.
Discussion
This study measures the cost-effectiveness of 3 of the most common surgical procedures for treatment of focal knee cartilage lesions: MFx, OAT, and ACI. Next-generation ACI treatments provided significantly greater functional outcomes when compared with MFx, OAT, or ACI-1. ACI-1 was the most expensive procedure both in terms of total cost and cost-effectiveness.
Microfracture is a widely available, minimally invasive, arthroscopic technique associated with reasonable costs.2 This procedure is technically simple, although fibrocartilage replacing the native hyaline cartilage is not ideal and can lead to higher revision rates than those seen with OAT.21 Some authors believe that the MFx procedure fractures the subchondral bone, making the bone brittle and leading to worse results in future subsequent surgeries.3 Multiple close penetrations of the subchondral bone may contribute to subchondral bone cyst formation and subchondral plate disruption.13 One subchondral bone puncture, no matter how large the defect, would be adequate for leading to fibrin clot matrix formation.13
Osteochondral autograft transplantation involves harvesting cylindrical osteochondral plugs from nonweightbearing articular regions of the knee to be transplanted into the chondral lesion. The autograft is a viable living structure that provides excellent bony support to the overlying hyaline cartilage. The use of either a single or multiple grafts (mosaicplasty) to achieve congruent repair can cover the recipient defect.21 The OAT procedure restores a hyaline cartilage surface at the site of the lesion, though it requires longer operating times and more elaborate instrumentation than microfracture.21 The bony part of the transplanted graft usually heals completely with the surrounding bone, while the cartilage surface, though viable, may not fully heal to the surrounding cartilage.21
ACI-1 has been associated with limitations such as joint stiffness and arthrofibrosis after surgery, as well as periosteal hypertrophy that requires revision surgery in up to 42% of patients.15 In addition, chondrocytes tend to lose their ability to form matrix and produce hyaline cartilage, and it is unclear whether transplanted cells regain this function after transplantation.15 ACI-2 attempts to create cartilage-like tissue without the drawbacks associated with ACI-1.15
Miller et al19 studied the cost-effectiveness of microfracture and osteochondral autograft transplantation through a constructed cost model using surgical time, failure rates, revision surgeries, outcome scores, and return to athletics. The authors used cost data from their own institution and did not include preoperative costs or initial postoperative costs. In their study, MFx was more cost-effective when comparing Lysholm and HSS scores, but OAT was more cost-effective when comparing Tegner and ICRS scores. The authors also noted that there was a significantly lower cost for return to play in athletes after OAT compared with MFx. They concluded that MFx and OAT are comparable in terms of net cost and cost-effectiveness for the treatment of isolated articular cartilage lesions of the distal femur. The present study builds on this study by adding a new and increasingly used treatment, ACI, to a cost-effectiveness analysis. Our study also used pre- and postoperative costs, which may give a clearer picture of total costs associated with each procedure, as some procedures need more preoperative imaging and some can lead to more complications postoperatively.
There are several limitations to this study. Few studies listed standard deviations for age, follow-up time, and lesion size, limiting statistical comparisons of these demographics. Lesion size was greater in ACI-1 when compared with each of the other treatment options, although only 1 study listed standard deviations for this treatment, limiting the conclusions that can be drawn from this statistic. Lesion location was also not listed in many studies. Follow-up time varied between treatments, with patients undergoing OAT having a greater mean follow-up (38 months) compared with MFx (29 months), ACI-1 (19 months), and ACI-2 (27 months). In addition, microfracture is typically used as a short-term solution and has not shown promising long-term results.8 OAT groups were significantly younger when compared with MFx and ACI-2 groups, although some studies were not included in this analysis due to lack of standard deviations. Furthermore, there were significantly more complications in OAT patients when compared with all other groups, and significantly more in MFx patients when compared with patients undergoing ACI-1 and ACI-2. In this study, ACI-1 had the lowest mean increase in functional outcome score as well as the shortest mean follow-up. Thus, longer follow-up in these studies may have shown further improvement in functional outcomes. The mean lesion size for microfracture was 3.4 cm2, which is larger than the indicated size for this procedure.30 In addition, the publication used to obtain the costs associated with MFx, OAT, and ACI-1 only accounts for the costs up to 1 year postsurgery, so any required revisions after this period are unaccounted for.32 Furthermore, the article did not include costs associated with ACI-2, and there were not enough data on the pre- and postoperative costs associated with ACI-2 to accurately estimate these costs ourselves. Therefore, ACI-2 was excluded in our cost-effectiveness analysis. Finally, the heterogeneity of some procedures, especially ACI-2, represents another limitation of this study.
Conclusion
MFx, OAT, ACI-1, and ACI-2 are 4 effective surgical procedures for the treatment of cartilage defects in the knee at short-term follow-up. All 4 treatments led to an increase in functional outcome scores postoperatively. ACI-2 had a statistically greater improvement in functional outcome scores compared with the other 3 procedures. MFx was found to be the most cost-effective treatment option and ACI-1 the least cost-effective.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: E.C.M. receives royalties from Biomet and Elsevier; is a paid consultant for Biomet; and receives research support from Biomet, Mitek, Smith & Nephew, and Stryker.
References
- 1. Arøen A, Løken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. [DOI] [PubMed] [Google Scholar]
- 2. Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. [DOI] [PubMed] [Google Scholar]
- 3. Bert JM. Abandoning microfracture of the knee: has the time come? Arthroscopy. 2015;31:501–505. [DOI] [PubMed] [Google Scholar]
- 4. Clavé A, Potel JF, Servien E, Neyret P, Dubrana F, Stindel E. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res. 2016;34:658–665. [DOI] [PubMed] [Google Scholar]
- 5. Ciccotti MC, Kraeutler MJ, Austin LS, et al. The prevalence of articular cartilage changes in the knee joint in patients undergoing arthroscopy for meniscal pathology. Arthroscopy. 2012;28:1437–1444. [DOI] [PubMed] [Google Scholar]
- 6. Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170–1179. [DOI] [PubMed] [Google Scholar]
- 7. Crawford DC, DeBerardino TM, Williams RJ., 3rd Neocart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2010;94:979–989. [DOI] [PubMed] [Google Scholar]
- 8. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579–1588. [DOI] [PubMed] [Google Scholar]
- 9. Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:834–842. [DOI] [PubMed] [Google Scholar]
- 10. Higgins LD, Taylor MK, Park D, et al. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74:594–599. [DOI] [PubMed] [Google Scholar]
- 11. Insall JN. Total knee replacement In: Insall JN, ed. Surgery of the Knee. New York, NY: Churchill Livingstone; 1984:681. [Google Scholar]
- 12. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29:600–613. [DOI] [PubMed] [Google Scholar]
- 13. Johnson LL, Spector M. The new microfracture: all things considered. Arthroscopy. 2015;31:1028–1031. [DOI] [PubMed] [Google Scholar]
- 14. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–464. [DOI] [PubMed] [Google Scholar]
- 15. Kon E, Delcogliano M, Filardo G, Montaperto C, Marcacci M. Second generation issues in cartilage repair. Sports Med Arthrosc. 2008;16:221–229. [DOI] [PubMed] [Google Scholar]
- 16. Kon E, Filardo G, Berruto M, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549–2557. [DOI] [PubMed] [Google Scholar]
- 17. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41:2439–2448. [DOI] [PubMed] [Google Scholar]
- 18. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470:2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43:2175–2181. [DOI] [PubMed] [Google Scholar]
- 20. Mundi R, Bedi A, Chow L, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44:1888–1895. [DOI] [PubMed] [Google Scholar]
- 21. Patil S, Tapasvi SR. Osteochondral autografts. Curr Rev Musculoskelet Med. 2015;8:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;3:1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. [DOI] [PubMed] [Google Scholar]
- 24. Saris D, Price A, Widuchowski W, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384–1394. [DOI] [PubMed] [Google Scholar]
- 25. Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10S–19S. [DOI] [PubMed] [Google Scholar]
- 26. Smith HJ, Richardson JB, Tennant A. Modification and validation of the Lysholm Knee Scale to assess articular cartilage damage. Osteoarthritis Cartilage. 2009;17:53–58. [DOI] [PubMed] [Google Scholar]
- 27. Spahn G, Hofmann GO. Focal cartilage defects within the medial knee compartment. Predictors for osteoarthritis progression [in German]. Z Orthop Unfall. 2014;152:480–488. [DOI] [PubMed] [Google Scholar]
- 28. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. [DOI] [PubMed] [Google Scholar]
- 29. Ulstein S, Arøen A, Røtterud JH, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22:1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yanke AB, Wuerz T, Saltzman BM, Butty D, Cole BJ. Management of patellofemoral chondral injuries. Clin Sports Med. 2014;33:477–500. [DOI] [PubMed] [Google Scholar]
- 31. Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. [DOI] [PubMed] [Google Scholar]
- 32. Zhang JY, Cohen Y, Wang JC, McAllister DR, Petrigliano FA, Jones KJ. The costs associated with the perioperative management of articular cartilage lesions in the United States. Orthop J Sports Med. 2015;3(7 suppl 2):23259 67115S00119. [Google Scholar]