Abstract
Background:
Glycaemic control is suboptimal in a large proportion of people with type 2 diabetes who are consequently at an increased and avoidable risk of potentially severe complications. We sought to explore attitudes and practices among healthcare professionals that may contribute to suboptimal glycaemic control through a review of recent relevant publications in the scientific literature.
Methods:
An electronic search of the PubMed database was performed to identify relevant publications from January 2011 to July 2015. The electronic search was complemented by a manual search of abstracts from key diabetes conferences in 2014/2015 available online.
Results:
Recently published data indicate that glycaemic control is suboptimal in a substantial proportion (typically 40%–60%) of people with diabetes. This is the case across geographic regions and in both low- and higher-income countries. Therapeutic inertia appears to be an important contributor to poor glycaemic control in up to half of people with type 2 diabetes. In particular, prescribers are often willing to tolerate extended periods of ‘mild’ hyperglycaemia as well as having low expectations for their patients. There are often delays of 3 years or longer in initiating or intensifying glucose-lowering therapy when needed.
Conclusion:
Many people with type 2 diabetes are failed by current management, with approximately half not achieving or maintaining appropriate target blood glucose levels, leaving these patients at increased and avoidable risk of serious complications.
Review criteria:
The methodology of this review article is detailed in the ‘Methods’ section.
Keywords: Diabetes mellitus, type 2, glycaemic control, review, surveys, clinical inertia
Introduction
The importance of early and intensive control of blood glucose levels in improving outcomes for people with type 2 diabetes mellitus (T2DM) was established in the UK Prospective Diabetes Study (UKPDS).1 Despite differences in glucose levels between the intensive and standard treatment groups disappearing soon after completion of the active study intervention, the benefits of earlier intensive glycaemic control in reducing diabetic complications were found to persist in long-term (10-year) follow-up.2 Combined with evidence that even short periods (i.e. weeks) of hyperglycaemia increase the risk for developing diabetic complications,3,4 this highlights the importance of trying to establish good glycaemic control as early as possible.5
Subsequent large-scale intervention studies have confirmed the benefit of intensive glycaemic control in reducing microvascular risk,6–8 but the findings regarding cardiovascular disease and overall mortality have been less conclusive.8–15
Of note, what constitutes intensive therapy has evolved over the two decades since the UKPDS as new antihyperglycaemic agents have become available. The newer agents, particularly those associated with a low risk of hypoglycaemia compared with sulphonylureas and insulin, offer new options for combination therapy aimed at achieving better glycaemic control.
The Global Partnership for Effective Diabetes Management was an early advocate for adoption of tailored targets and treatment in T2DM,16 and subsequent guidelines have evolved to reflect the evidence, with universal glycaemic targets and treatment algorithms replaced with recommendations to individualize targets and treatment.17,18 While it seems clear that individualized targets are needed to optimize care, it is critical that good glycaemic control, as defined by individualized HbA1c targets, is pursued without delay and with commitment. However, it is widely recognized that glucose levels are not well controlled in many people with T2DM. Through a review of recently published data, we aimed to summarize current standards of glycaemic control globally. We also sought to identify the factors that may contribute to poor rates of glycaemic control.
Methods
Searches were performed, last updated in July 2015, in the US National Library of Medicine/National Institutes of Health PubMed database. The search algorithm used the term ‘diabetes’ in combination with (‘AND’) ‘survey’, ‘therapeutic inertia’, ‘clinical inertia’, ‘target’, ‘barriers’ and ‘obstacles’ and combinations thereof. Abstracts of the publications identified by the searches were reviewed manually and included in the review if they reported (1) original data related to proportions of people with T2DM achieving glycaemic control or (2) obstacles or barriers contributing to suboptimal glycaemic control. Publications were excluded from the review if they were (1) published before 2011 or (2) published in a language other than English. Manuscripts written originally in a language other than English but where an English translation is available were included.
The search of the PubMed database was complemented by a manual search of online abstracts from recent (2013–2015) diabetes conferences including those from the European Association for the Study of Diabetes and the American Diabetes Association annual meetings. Terms used in the PubMed search were also entered into the Google search engine, and the results were reviewed manually to identify any additional relevant data from credible sources that might inform the analysis and review. A meta-analysis was not performed owing to the diverse nature of the data collected.
Results and discussion
Information sources
A total of 45 relevant publications were identified by the search strategy and included in the literature review and analysis. Details of the publications are included in Appendix 1.
Standards of glycaemic control
The results of the literature review suggest that suboptimal glycaemic control is a common and widespread problem. The proportion of patients not achieving HbA1c targets in 12 studies19–32 published between 2011 and 2015 inclusive is summarized in Table 1. Even in the study reporting the best rate of control, one in every three patients was above the HbA1c target.32 A multi-centre, cross-sectional study of ~5800 people with T2DM from nine European countries25 found that HbA1c exceeded the target of ≤7.0% (≤53 mmol/mol) in 37% of cases, with rates for individual countries ranging from 26% in the Netherlands up to 52% in Turkey.
Table 1.
Summary of studies reporting rates for people not achieving targets for HbA1c.
| Country/region | N | Population/setting | Proportion not at target (%)a | Reference |
|---|---|---|---|---|
| Australia | 613 | T1DM or T2DM, with/without retinopathy | Retinopathy: 76 No retinopathy: 49 |
Lamoureux et al.19 |
| Canada | 3002 | T2DM registry | 47 | Braga et al.20 |
| Canada | 5123 | Survey of primary care physicians | 50 | Leiter et al.21 |
| Canada | 10,590 | T2DM in specialist clinics | 62 | Aronson et al.22 |
| China | 13,790 | T2DM in tertiary hospitals | 65 | Lu et al.23 |
| China | 25,817 | T2DM as hospital outpatients | 52 | Ji et al.24 |
| Europeb | 5817 | T2DM in primary care | 37 | Pablos-Velasco et al.25 |
| France | 2109 | T2DM in primary care | 41 | Halimi et al.26 |
| India | 300 | T2DM in a rural college | 87 | Kahlon and Pathak27 |
| Puerto Rico | 600 | T1DM or T2DM | 63 | Rodriguez-Vigil et al.28 |
| Spain | 2783 | T2DM in primary care | 36 | Mata-Cases et al.29 |
| Taiwan | 215,679 | T1DM or T2DM | 64 | Huang et al.30 |
| UK | 1,835,634 | T2DM | ≤6.5: 74 ≤7.5: 35 |
The Health and Social Care Information Centre (HSCIC)31 |
| USA | 1373 | T1DM or T2DM | 35 | Laiteerapong et al.32 |
TIDM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
Target HbA1c ≤ 7.0% (≤53 mmol/mol), other than for the study of Laiteerapong et al. which used individualized targets of HbA1c < 6.5%, <7.0% or <8.0% (<48 mmol/mol, <53 mmol/mol or <64 mmol/mol) based on patient characteristics.
Nine European countries: Belgium, France, Germany, Greece, Italy, The Netherlands, Spain, Turkey and the United Kingdom.
Other publications show that HbA1c levels are markedly above target in a large proportion of people with T2DM. In a registry-based study of >10,000 people undergoing care for T2DM in specialist clinics in Canada, 62% were not at target [HbA1c ≤ 7.0% (≤53 mmol/mol)] and 15% had an HbA1c level of 9.0% (75 mmol/mol) or above.22 A Puerto Rican study of 600 adults with type 1 or type 2 diabetes reported that just 37% were at the recommended HbA1c target.28 A survey of >200,000 people with diabetes in Taiwan30 found HbA1c > 9.0% (75 mmol/mol) in nearly one in every four people and 30% of outpatients with T2DM attending a medical college in rural India had HbA1c ≥ 9.5% (≥80 mmol/mol).27
There was some evidence to suggest that suboptimal control occurred more frequently in low- and middle-income countries compared with wealthier countries, with the highest rate of 87% for non-achievement of HbA1c target reported in rural India.27 However, high proportions of people in high-income, resource-rich settings also had inadequate control of hyperglycaemia.
Suboptimal glycaemic control is often accompanied by poor control of other cardiometabolic risk factors. In a prospective cohort study of more than 3000 people with T2DM in Canada,20 the proportion of people with HbA1c, blood pressure and low-density lipoprotein (LDL) cholesterol at target on study entry was 47%, 46% and 36%, respectively. All three risk factors were controlled to target in just 19% of people, and only 7% maintained good control of glycaemia, blood pressure and LDL cholesterol throughout the 12-month study. A Canadian study of more than 5000 people with T2DM seen by 479 primary care physicians21 found that the proportions achieving targets were 50% for HbA1c, 36% for blood pressure and 57% for LDL cholesterol, with only 13% reaching all three targets. Similar results were found in more than 25,000 outpatients with T2DM in China,24 with the proportions of patients achieving targets for HbA1c, blood pressure and lipids of 48%, 28% and 36%, respectively.
Importantly, nearly all of the studies reviewed assessed the quality of glycaemic control against a single target of HbA1c ≤ 7.0% (≤53 mmol/mol). Within the context of individualized targets now recommended in guidelines, a somewhat higher target [e.g. HbA1c ≤ 7.5% (≤59 mmol/mol)] may have represented optimal management based on factors such as age, presence of cardiovascular comorbidities and duration of diabetes. This is unlikely to account for the large proportion of patients not controlled to HbA1c ≤ 7.0% (≤53 mmol/mol) and certainly should not justify the sizeable proportion of people with HbA1c levels exceeding 9.0% (75 mmol/mol). However, it highlights that a single target is unlikely to represent optimal management for all people.
The review provides strong evidence that in all regions of the world, many people with T2DM are not achieving good glycaemic control. Although there is considerable heterogeneity in the studies and the data generated, rates of uncontrolled glycaemia are typically in the range of 40%–60%. Consequently, many people are left at increased risk of diabetic complications and the associated burden of illness and mortality, as well as consequent economic costs.
Barriers to effective glycaemic control
Recent studies examining barriers to the achievement of good glycaemic control are summarized in Table 2.33–48 The barriers include patient-, healthcare system–, and prescriber-level factors.
Table 2.
Summary of studies reporting barriers to achievement of good glycaemic control.
| Country/region | N | Methodology | Key findings | Reference |
|---|---|---|---|---|
| Australia | 854 | Survey of primary care physicians in rural Australia | Nearly half of the primary care physicians reported learning needs related to pharmacological management of T2DM. Many lacked confidence in providing effective insulin treatment. | Thepwongsa et al.33 |
| Europe | – | Review of guidelines and national plans across Europe | While most countries have guidelines and national plans for the management of diabetes, they are often not rigorously monitored and/or are not comprehensive | European Coalition for Diabetes34 |
| International | 4785 | Online survey of HCPs | In total, 60% reported need for a major improvement in diabetes self-management education. Up to one in three HCPs reported receiving no formal diabetes education. | Holt et al.35 |
| Ireland | 31 | Semi-structured interviews with primary care physicians (n = 29) and practice nurses (n = 2) | Barriers noted included lack of value placed on chronic disease management and lack of coordination between primary and secondary care. Lack of resources for primary care seen as at odds with shift of routine diabetes care into primary care. | Mc Hugh et al.36 |
| Ireland | 66 | Focus groups with primary care physicians (n = 55) and practice nurses (n = 11) | Most frequently cited barriers to transfer of diabetes care to general practice included lack of financial incentive, lack of access to secondary resources, lack of staff/increased workload and time constraints. | O’Connor et al.37 |
| Ireland | – | Review of practice in 19 hospitals in Republic of Ireland caring for children with T1DM | Wide variability in the support available for transition from paediatric to adult care across hospitals in the Republic of Ireland. | Letshwiti et al.38 |
| Netherlands | 18 | Semi-structured interviews with randomly selected HCPs | Funding issues, lack of motivation among patients and lack of awareness of lifestyle/prevention initiatives among HCPs raised as major barriers to optimal care. | Raaijmakers et al.39 |
| United Arab Emirates | 9 | Semi-structured interviews with HCPs | Barriers identified included heavy workloads, lack of coordinated care, poor patient awareness and adherence and cultural attitudes and beliefs about diabetes. | Alhyas et al.40 |
| UK | 1261 | Case review of 128 people with T2DM and HbA1c ≥ 10% (≥86 mmol/mol) attending primary health centres | Leading reasons for poor glycaemic control included poor adherence with lifestyle measures and medication, side effects of therapy, lack of insulin titration and infrequent clinic attendance. | Khan et al.41 |
| UK | 2149 | Online survey of trainee doctors | Only 35% of respondents felt that their postgraduate training had prepared them adequately to optimize treatment of diabetes and less than half would generally take the initiative to optimize glycaemic control. | George et al.42 |
| USA | 252 | Survey of primary care providers linked to healthcare records | Resistance to lifestyle interventions and taking insulin, poor adherence to pharmacotherapy and psychosocial issues identified as main barriers to optimal glycaemic control. | LeBlanc et al.43 |
| USA | 25 | Focus group discussions with physicians | Barriers identified included a persistent orientation towards acute care, inability to provide adequate self-management education and lack of public health support. | Elliott et al.44 |
| USA | 185 | Review of case records of adolescents with T1DM | Adolescents transitioned to adult care were 2.5 times as likely to have poor glycaemic control as those who continued in paediatric care. | Lotstein et al.45 |
| USA | 118 | Prospective study of youth with T1DM transitioning to adult care | Early transition from paediatric to adult care was associated with worse self-care behaviour and worse glycaemic control. | Helgeson et al.46 |
| USA | 258 | Survey of young adults transitioning to adult care for T1DM | Less than half of the young adults received a recommendation for an adult care provider and <15% reported having a transition preparation visit or receiving written transition materials. | Garvey et al.47 |
| USA | – | Focus group discussions tailored to different groups of HCPs | Most HCPs lack confidence in using complex insulin regimens and all need education on T2DM management guidelines and how to intensify therapy for patients not reaching glycaemic goals. | Williamson et al.48 |
TIDM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; HCPs: healthcare professionals.
Patient-level barriers
Prescribers often identify poor patient motivation and self-management as leading barriers to achieving glycaemic targets. The second Diabetes, Attitudes, Wishes and Needs (DAWN2) study,35 included an online survey of 4785 healthcare professionals (HCPs) (~40% primary care clinicians, 30% diabetes specialists, 20% nurses and 10% dieticians) from 17 countries across four continents. The majority of the respondents (60%) indicated a need for improvements in self-management by people with diabetes, particularly in the area of diet and exercise. Resistance to lifestyle measures was also identified as a leading barrier by 252 primary care physicians, nurse practitioners and physician assistants in the United States,43 with 52% of HCPs indicating that it was often or almost always a factor contributing to chronic lack of glycaemic control. Other leading patient-related barriers identified by the HCPs included psychosocial issues (34%), reluctance to use insulin (31%) and non-compliance (30%). A study in the United Kingdom examining the reasons for very poor glycaemic control [HbA1c ≥ 10% (≥86 mmol/mol)] in 128 people with T2DM attending three primary care centres41 found poor adherence to lifestyle measures to be most frequently involved. Other factors included infrequent clinic attendance (16%), side effects (16%), poor compliance with pharmacotherapy (14%), lack of knowledge of diabetes (14%), lack of titration of insulin (13%) and insulin refusal (12%). Poor patient motivation and adherence were also identified as barriers to good glycaemic control in qualitative studies with HCPs.39,40 Interviews with a small number (n = 9) of HCPs in the United Arab Emirates revealed a tendency to view patient attitudes and behaviours as the origin of poor glycaemic control and to believe that if patients were more knowledgeable about diabetes, cooperated with HCPs and were adherent with treatment, then they could achieve their glycaemic targets.40
Healthcare system–related barriers
The studies reviewed also revealed that a number of healthcare system-level factors are often seen as important barriers to good glycaemic control. The DAWN2 study, for example, found that only 30% of the HCPs surveyed believed that healthcare was well organized for the management of chronic conditions.35 Participants from Western European countries and China were more likely to feel that healthcare was well organized than those from North or South America, Eastern Europe, India or Japan (Figure 1). Approximately half of the participants considered the healthcare remuneration system to be a barrier and most (~60%) indicated a need for better access to qualified nurse educators/specialist diabetes nurses and psychologists/psychiatrists.
Figure 1.
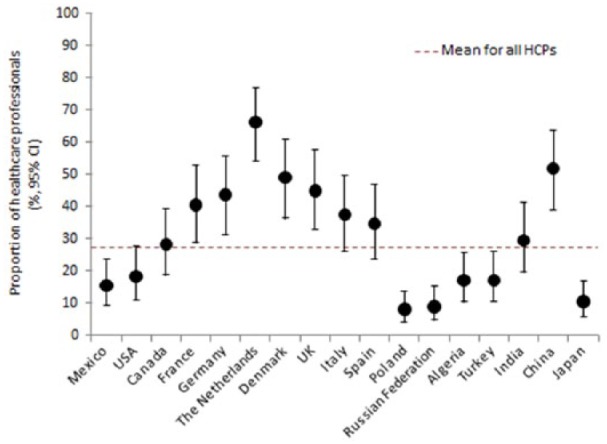
Proportion of healthcare professionals reporting that healthcare in their country is well organized for the management of chronic conditions, including debates.
Source: Adapted from Holt et al.35
A survey of 47 European countries found that while all had guidelines in place for managing diabetes, only 7 (15%) had protocols for monitoring their implementation.34 The majority (83%) of countries had or were developing national plans for diabetes, but these were often not comprehensive. There was substantial variability in the provision of multidisciplinary care. While 80% reported that nurses play an important role in providing education for self-management, diabetes specialist nursing was recognized as a speciality in only 19 countries (40%). Despite the widespread belief that patients need to be more engaged and effective in self-management, only 25% of the countries recommended continuous education for all people with diabetes.34 Lack of effective self-management education was also identified as a barrier in a qualitative study involving focus group discussions with 25 primary care clinicians in the United States, along with a persistent focus on acute care, poor integration of diabetes care, lack of clinical information and inadequate public health support.44 Lack of coordination in care and limited access to secondary resources were also identified as important barriers by studies in The Netherlands39 and Ireland.36,37
Limitations on the time available to manage patients are often identified as an obstacle to good glycaemic control. In the survey of 252 US primary care physicians, lack of time for treating complex patients was reported as often or always a factor in failure to achieve good glycaemic control by 41% of the physicians. Treatment costs were much less frequently seen as a barrier, with 25% of the HCPs indicating that it made no contribution and most (58%) saying that it was a factor in only a minority (20%–40%) of cases of poor glycaemic control.43 Other studies have also suggested that HCPs in primary care often feel that they lack the time or other resources needed to manage diabetes effectively.37 This raises particular concerns, given that health policy in some countries involves shifting more of the responsibility for diabetes management onto primary care.
Physician-level barriers
Compared with patient- and system-related barriers to effective glycaemic control, there is limited information available regarding physician-related barriers. However, there is evidence to suggest that a lack of diabetes-focused education may be contributing to failure to achieve therapeutic targets. For example, one in five of the HCPs surveyed in the DAWN2 study indicated that they had received no formal postgraduate diabetes training,35 and in a UK survey, only 35% of 2149 trainee doctors felt that their postgraduate training had prepared them adequately to optimize treatment of diabetes and only 41% reported that they would take the initiative to optimize glycaemic control.42 A survey of 209 primary care clinicians in Australia identified gaps in knowledge relating to the medical management of T2DM, with 46% self-reporting a need for education on pharmacological management.33 It appears that prescribers often lack confidence in the use of insulin, particularly with more complex regimens.33,48
Barriers to effective intensification of treatment
People with diabetes often continue in poor glycaemic control for extended periods of time without appropriate action being taken.26,29,30,49–59 A retrospective cohort study of >80,000 people with T2DM in the United Kingdom revealed substantial delays in intensifying treatment when glycaemic control was suboptimal. For people who were inadequately controlled on one oral glucose–lowering agent, the median time from HbA1c being above a threshold of ≥7.0% (≥53 mmol/mol) to treatment intensification with an additional oral agent was nearly 3 years, and even when HbA1c was ≥8.0% (≥64 mmol/mol), intensification was delayed by a median of 1.6 years. There were even longer delays (median of ~6–7 years) in intensifying treatment with the addition of a third oral agent or the initiation of insulin. Mean HbA1c at intensification ranged from 8.7%–9.7% (72–83 mmol/mol).55 In a study of nearly 20,000 people with T2DM initiated on basal insulin at hospitals across China, mean HbA1c at the time of insulin initiation was 9.6%.51
A study of primary care records of >17,000 patients with T2DM being treated with oral glucose–lowering agents in France found that >3000 of the patients were not at glycaemic target and required treatment intensification. However, treatment was actually intensified in only 39% of the patients requiring it and the majority (~60%) of changes were delayed by 6 months or more and a substantial proportion (~40%) by more than a year.53 The DIAttitude study (Table 3) also looked at therapeutic inertia in the care of >2000 people with T2DM by 236 primary care physicians26 and found that among patients with uncontrolled glycaemia, representing 41% of all the patients studied, only 7% had their treatment intensified. The most common (60%) reason given for not intensifying treatment was that the patient’s HbA1c was satisfactory. Other leading reasons given included that lifestyle advice was more of a priority than changing medication (20%), the decision was postponed until the next clinic visit (11%) and that HbA1c had decreased since the previous clinic visit (7%), although in the majority (58%) of these cases, an HbA1c reduction was not confirmed by the available records. The problem appears to be common and widespread, with further studies in Bahrain,49 Croatia,52 Spain,29 Taiwan30 and the United States57 reporting high rates (typically 30%–60%) of clinical inertia when managing elevated glucose levels in people with diabetes.
Table 3.
Summary of studies reporting rates of and factors contributing to therapeutic inertia in the management of type 2 diabetes.
| Country/region | N | Methodology | Key findings | Reference |
|---|---|---|---|---|
| Bahrain | 334 | Prevalence over 30 months in a random sample of people attending a diabetes clinic | Clinical inertia in managing glycaemia occurred in 29% of consultations, compared with 80% for LDL cholesterol and 68% for systolic blood pressure. | Whitford et al.49 |
| Belgium | 114 | Focus group discussions with primary care physicians | Primary care physicians acknowledged existence of clinical inertia, but some found it insulting. The risk of inertia was linked to feeling overwhelmed/disempowered due to patient- or health system–level factors. | Aujoulat et al.50 |
| China | 19,894 | Observational registry of people with T2DM who initiated basal insulin at 209 hospitals across China | Before initiation of basal insulin, the mean HbA1c was 9.6%. The proportions of patients using 1, 2 or >2 oral agents before insulin initiation were 48%, 43% and 9%, respectively. | Ji et al.51 |
| Croatia | 10,275 | Observational, cross-sectional study in primary care using data provided by physicians | Clinical inertia occurred in 56% of consultations. Factors associated with clinical inertia were higher HbA1c, treatment initiated by a diabetologist, physical inactivity and administration of drugs other than oral antidiabetics. | Bralic Lang et al.52 |
| France | 17,493 | Analysis of data from primary care electronic records | Treatment was intensified in only a minority (39%) of the patients requiring it (18% of all patients). Intensification was delayed by >1 year in 40% of patients. | Balkau et al.53 |
| France | 2109 | Analysis of primary care records from 236 primary care physicians | In total, 41% of the patients required intensification according to guidelines, but only in 7% was treatment intensified. Leading reason for not intensifying therapy was that HbA1c was satisfactory. | Halimi et al.26 |
| France | 1933 | Online survey of adults with T2DM | Early (versus late) initiation of insulin therapy was nearly 10 times more likely to be prescribed by an endocrinologist/diabetologist than by a primary care physician. Younger age and current smoking were associated with early versus late insulin initiation. | Reach et al.54 |
| Spain | 2783 | Retrospective, multi-centre cross-sectional study of randomly selected patients in primary care centres | Clinical inertia present in 33% of T2DM cases, ranging from 37% for HbA1c of 7.1%–8% (54–64 mmol/mol) to 27% for HbA1c of ≥9% (≥75 mmol/mol). Greatest inertia in people treated with lifestyle only or monotherapy. | Mata-Cases et al.29 |
| Taiwan | 168,876 | Retrospective, cohort study of people with T2DM participating in a diabetes payment programme | Estimated prevalence of therapeutic inertia was 39%. Inertia was more likely among people treated in primary care compared with diabetes clinics and by cardiologists versus endocrinologists. | Huang et al.30 |
| UK | 81,573 | Retrospective cohort study of records in clinical practice database | Substantial delays in intensifying pharmacological therapy [median 3 year delay before adding second agent when HbA1c ≥ 7.0% (≥53 mmol/mol)]. Mean HbA1c at intensification of 8.7%–9.7% (72–83 mmol/mol). | Khunti et al.55 |
| UK | 20 | Semi-structured interviews with primary care HCPs | HCPs generally accept a degree of responsibility for clinical inertia but sought to lessen their own accountability by highlighting patient- and system-level barriers. | Zafar et al.56 |
| USA | 7654 | Retrospective analysis of administrative data from a large health insurer | Clinical inertia detected in >75% of people with T2DM and elevated HbA1c. An HbA1c increase in ≥1% (≥11 mmol/mol) led to a change in treatment in just 19% of patients with a baseline HbA1c of 7%–8% (53−64 mmol/mol) and 28% of patients with baseline HbA1c ≥ 9% (≥75 mmol/mol). | Davis et al.57 |
| USA | 770 | Online survey of 508 primary care physicians providing clinical data for 770 patients | First-ranked reasons for not initiating glucose-lowering therapy included diet and exercise treatment (58%), mild hyperglycaemia (24%) patient concerns (13%), concerns about antihyperglycaemic agents (3%) and comorbidities/polypharmacy (2%). | Marrett et al.58 |
| USA | 83 | Structured interviews with primary care providers | Barriers to insulin initiation identified by the providers included patient resistance (64%) and problems with patient self-management (43%). | Ratanawongsa et al.59 |
LDL: low-density lipoprotein; T2DM: type 2 diabetes mellitus; HCPs: healthcare professionals.
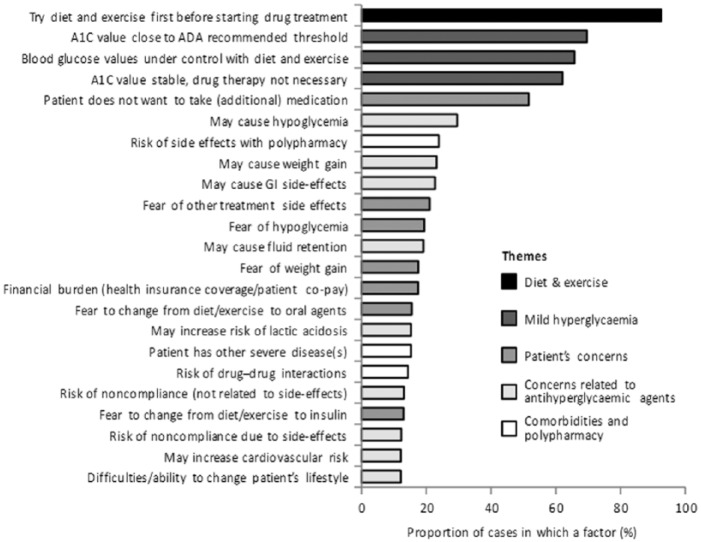
A number of studies have attempted to explore the factors that may act as obstacles to action in addressing inadequate glycaemic control. In an online survey completed by 508 primary care physicians who provided data on 770 elderly patients (>65 years),58 the leading reason for not initiating glucose-lowering medication, given as the first-ranked reason for 58% of the patients and a contributory factor in nearly all patients, was that they were treated with diet and exercise, and presumably, this was believed to be adequate management (Figure 2). However, 33% of the patients were not at target HbA1c, suggesting substantial clinical inertia in this population. Other reasons given for not starting drug treatment included that hyperglycaemia was mild (84%), patient concerns (61%), physician concerns about glucose-lowering agents (49%) and presence of comorbidities and polypharmacy (37%). When asked to identify a threshold for initiating treatment with glucose-lowering agents in the elderly population, the mean HbA1c threshold value stated was 7.1% (54 mmol/mol). In approximately 20% of the cases, the physicians indicated that they were planning to initiate glucose-lowering therapy within the next month, but more than half (54%) of these patients already had an HbA1c level above the physician-stated threshold.
Figure 2.
Leading reasons for not initiating glucose-lowering therapy. Results from an online survey of 508 US primary care physicians providing clinical date for 770 patients.
Source: Adapted from Marrett et al.58
A qualitative study of 20 HCPs in the United Kingdom found a pattern of seeking to lessen their own sense of accountability for clinical inertia by emphasizing patient-level barriers such as comorbidities, motivation and self-management capabilities as well as health system–level barriers, especially time constraints. There was also a tendency for the HCPs to overestimate the achievement of targets in their primary care centres.56
Factors contributing to therapeutic inertia in the initiation of insulin treatment for T2DM were investigated in the Translating Research into Action for Diabetes (TRIAD) project using structured interviews with primary care HCPs in the United States.59 The majority of the 83 clinicians interviewed supported guideline recommendations on glycaemic targets, with 69% choosing an HbA1c < 7% (<53 mmol/mol) as the ideal target for good glycaemic control. At the same time, approximately half (54%) indicated that they individualized targets. The most frequently cited reasons for setting higher individualized HbA1c targets included advanced age or short life expectancy (54%), poor self-management capacity due to poor cognitive abilities (35%), presence of comorbidities (34%), low educational level or poor health literacy (34%) and patients’ unwillingness to self-manage their diabetes (33%). The most frequent reasons cited by the clinicians for not initiating insulin treatment were patient refusal or resistance (64%) and lack of patient self-management skills (43%).
Factors determining initiation of insulin were also investigated using data for 1933 people with T2DM from the French National Health and Wellness Survey, an annual Internet-based survey among French adults.54 Early initiation of insulin therapy was almost 10 times more likely to be prescribed by an endocrinologist or diabetologist than by a primary care physician. Early versus late insulin initiation was also more likely in patients who were younger, had diabetes-related complications or smoked. Insulin-treated patients were more likely to be adherent, and there was no apparent deterioration in quality of life associated with insulin use.
In focus group discussions with 114 primary care physicians in Belgium,50 many of the physicians found the concept of clinical inertia interesting, but some also found it insulting and felt it implied that they were passive in their work. They also noted that it was important to differentiate between cases where there was a conscious decision to not pursue lower targets based on a consideration of the individual patient factors and those where there was genuine inertia. It was acknowledged that genuine clinical inertia was a real risk in primary care, but there was a tendency to ascribe this risk to patient- and system-level barriers rather than physician-level behaviours and practices.
In summary, recent studies suggest that clinical inertia is a common and widespread problem, typically affecting the care of 30%–50% of people with T2DM. The causes are diverse and complex, but surveys of HCPs tend to emphasize patient-level barriers to treatment intensification, such as patient reluctance to start insulin therapy, or system-level barriers, such as disconnects between what is recommended in guidelines and what is reimbursed in practice. However, it also appears that HCPs sometimes delay taking necessary action and may be willing to tolerate periods of ‘mild’ hyperglycaemia. It also appears that many HCPs, while recognizing the importance of good glycaemic control, have low expectations for their patients.
The issue of clinical inertia needs to be better understood if it is to be addressed effectively, particularly regarding the patient-, HCP- and system-level factors that underlie it. This is challenging as clinical inertia as defined in studies typically captures a range of behaviours, some of which may not reflect suboptimal care. In particular, the importance of individualizing glycaemic targets rather than following a ‘one-size-fits-all’ approach is now captured in guidelines. However, studies of clinical inertia often apply a single glycaemic threshold [typically HbA1c = 7% (53 mmol/mol)] across a broad range of patients.
Potential benefits of early, intensive intervention
Guidelines for management of T2DM have traditionally advocated a stepwise approach to management, in which treatment is started with diet and exercise, followed by the addition of oral antihyperglycaemic monotherapy, then combination therapy and eventually insulin. However, when applied systematically without considering individual factors such as the patient’s current HbA1C level, it can lead to treatment futility with people receiving therapy that has little chance of lowering glucose levels sufficiently to achieve their glycaemic target.
Several recent publications have indicated potential benefits of early, intensive intervention with pharmacotherapy, including combinations of oral glucose–lowering agents and/or insulin.60–65 The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study compared metformin alone, metformin plus a thiazolidinedione and metformin plus intensive lifestyle intervention as initial therapy for new-onset T2DM in people aged 10–17 years and with obesity. The proportion of people who maintained glycaemic control over 5 years was significantly higher with the combination pharmacotherapy than either metformin alone or metformin plus intensive lifestyle measures. The combination pharmacotherapy was also associated with greater improvements in insulin sensitivity and β-cell function compared with the other two approaches. The results lend support to early, intensive intervention with combination therapy, at least in this population of young people with new-onset T2DM.60,61
Early intensive therapy was also studied in older (mean age = 45 years) people with newly diagnosed T2DM who started treatment for 3 months with metformin plus insulin and were then randomized to either continuing metformin plus insulin therapy or triple oral glucose–lowering therapy with metformin, glyburide and pioglitazone. Good glycaemic control was maintained for over 6 years with no significant difference between the groups (end of study HbA1c = 7.3% for metformin plus insulin and 6.4 for triple oral combination; p = 0.4).62
A review of the health records for nearly 3000 people with T2DM in the United States found that early initiation of metformin (within 6 months of diagnosis) significantly reduced HbA1c (−0.36%; p < 0.001) and BMI (−0.46 kg/m2; p < 0.001) compared with when introduction of pharmacotherapy with metformin was delayed. The likelihood of achieving HbA1c ≤ 7% was doubled and the likelihood of requiring therapy intensification was reduced by 28% with early versus delayed initiation of metformin.63 Another study in the United States looking at medical records for 5870 people with T2DM found a similar pattern for early introduction of combination oral glucose–lowering therapy. Early intensification was defined as addition of a second oral drug to metformin therapy within 3 months of treatment failure (HbA1c ≥ 7.5%) and late intensification as the introduction of combination therapy 10–15 months after loss of glycaemic control. People who received early intensification were 38% more likely to achieve good glycaemic control at 1.5–2 years than those with late intensification.64 In the ADVANCE trial of 11,140 patients with T2DM, therapeutic intensification with addition of an oral glucose–lowering agent doubled the chance of achieving good glycaemic control, and with intensification with insulin, the odds were increased 2.5-fold.65
Conclusion
Recently published evidence highlights that many people with T2DM do not achieve good control of blood glucose leaving them at increased and avoidable risk of serious complications. A tendency to focus on patient-related obstacles may fail to consider issues arising from the attitudes, perceptions and behaviours of healthcare providers. In particular, there are often delays in the implementation of appropriate interventions to achieve glycaemic targets due to a complex range of negative factors that exacerbate the situation. An improved understanding of these factors would better inform strategies to assist HCPs in more timely treatment of inadequately controlled glycaemia.
Take home message for the clinician
Despite a wide range of therapeutic options available, insufficient numbers of people with type 2 diabetes are reaching their glycaemic targets and large numbers are exposed to prolonged periods of hyperglycaemia. A number of obstacles exist globally that must be addressed and overcome if we are to improve the outcomes for patients.
Acknowledgments
The Global Partnership for Effective Diabetes Management includes the following members: Pablo Aschner (Bogotá, Colombia), Clifford Bailey (Birmingham, UK), Lawrence Blonde (New Orleans, USA), Stefano Del Prato (Pisa, Italy), Anne-Marie Felton (London, UK), Ramon Gomis (Barcelona, Spain), Edward Horton (Boston, USA), Linong Ji (Beijing, China), James LaSalle (Excelsior Springs, USA), Lawrence Leiter (Toronto, Canada), Stephan Matthaei (Quakenbrück, Germany), Margaret McGill (Sydney, Australia), Neil Munro (London, UK), Richard Nesto (Burlington, USA), Jay Skyler (Miami, USA) and Paul Zimmet (Melbourne, Australia). The authors would like to thank Ian Faulkner of International Medical Press for editorial support including implementation of the literature search and assistance with drafting and revision of the manuscript.
Appendix
Appendix 1.
Publications identified in the review and included in the literature analysis.
| Citation | Year |
|---|---|
| Alhyas et al.40 | 2013 |
| Aronson et al.22 | 2015 |
| Aujoulat et al.50 | 2015 |
| Balkau et al.53 | 2012 |
| Braga et al.20 | 2012 |
| Bralic Lang et al.52 | 2015 |
| Davis et al.57 | 2014 |
| Elliott et al.44 | 2011 |
| European Coalition for Diabetes34 | 2014 |
| Garvey et al.47 | 2013 |
| George et al.42 | 2011 |
| Halimi et al.26 | 2012 |
| Harrison et al.62 | 2014 |
| The Health and Social Care Information Centre (HSCIC)31 | 2014 |
| Helgeson et al.46 | 2013 |
| Holt et al.35 | 2013 |
| Huang et al.30 | 2015 |
| Ji et al.24 | 2013 |
| Ji et al.51 | 2015 |
| Kahlon and Pathak27 | 2011 |
| Khan et al.41 | 2011 |
| Khunti et al.55 | 2013 |
| Laiteerapong et al.32 | 2015 |
| Lamoureux et al.19 | 2012 |
| LeBlanc et al.43 | 2015 |
| Leiter et al.21 | 2013 |
| Letshwiti et al.38 | 2015 |
| Lotstein et al.45 | 2013 |
| Lu et al.23 | 2014 |
| Marrett et al.58 | 2012 |
| Mata-Cases et al.29 | 2013 |
| Mc Hugh et al.36 | 2013 |
| Narasimhan and Weinstock60 | 2014 |
| O’Connor et al.37 | 2013 |
| Pablos-Velasco et al.25 | 2014 |
| Raaijmakers et al.39 | 2013 |
| Ratanawongsa et al.59 | 2012 |
| Reach et al.54 | 2013 |
| Rodriguez-Vigil et al.28 | 2014 |
| Romanelli et al.63 | 2015 |
| Thepwongsa et al.33 | 2014 |
| Whitford et al.49 | 2014 |
| Williamson et al.48 | 2014 |
| Zafar et al.56 | 2015 |
| Zeitler et al.61 | 2012 |
Footnotes
Author contribution: All authors contributed to the conception and design of the review and the analysis and interpretation of the data gathered, were involved in the drafting and/or revision of the article and had final approval of the version to be published.
Declaration of conflicting interests: Larry Blonde has received research support from Eli Lilly and Company, Novo Nordisk and Sanofi and has received speaker/consultant honoraria from Amylin Pharmaceuticals, Johnson & Johnson – Janssen, Johnson & Johnson Diabetes Institute, Merck & Co., Inc., Pfizer Inc, Novo Nordisk, Sanofi and Santarus. Dr Blonde’s late spouse’s estate contains shares of Pfizer. Pablo Aschner is a member of the Global Partnership for Effective Diabetes Management and has received honoraria from GlaxoSmithKline for his participation in board meetings and other related scientific activities. He has also participated in advisory panels and provided ad hoc consultancy to AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp & Dohme, Novartis and Sanofi-Aventis. Clifford Bailey has received research support from AstraZeneca, Sanofi-Aventis and has received presentation support from, has participated in advisory panels and provided ad hoc consultancy to, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Novo Nordisk and Takeda. Linong Ji has received fees for lectures and consulting from Abbott, AstraZeneca, Bristol-Myers Squibb, Merck, Metabasis, Novartis, Eli Lilly, Roche, Sanofi-Aventis and Takeda. Lawrence Leiter has received research funding and has acted as a consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, Servier and Takeda. Stephan Matthaei has received research support from Sanofi-Aventis and Novo Nordisk and honoraria for advisory boards and/or speaker engagements from AstraZeneca, Bayer, Bristol-Myers Squibb, Disetronic, Eli Lilly, GlaxoSmithKline, LifeScan, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi-Aventis and Takeda.
Funding: The literature search was supported by an unrestricted educational grant from AstraZeneca (grant ID #69680).
References
- 1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 3. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003; 290: 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348: 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aschner PJ, Ruiz AJ. Metabolic memory for vascular disease in diabetes. Diabetes Technol Ther 2012; 14(Suppl. 1): S68–S74. [DOI] [PubMed] [Google Scholar]
- 6. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 8. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 9. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 10. Duckworth WC, Abraira C, Moritz TE, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011; 25: 355–361. [DOI] [PubMed] [Google Scholar]
- 11. Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 6: CD008143. [DOI] [PubMed] [Google Scholar]
- 13. Mannucci E, Monami M, Lamanna C, et al. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2009; 19: 604–612. [DOI] [PubMed] [Google Scholar]
- 14. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52: 2288–2298. [DOI] [PubMed] [Google Scholar]
- 15. Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010; 33: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Prato S, LaSalle J, Matthaei S, et al. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010; 64: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 18. International Diabetes Federation. Global guidelines for type 2 diabetes. 6th ed. Brussels: International Diabetes Federation, 2012. [Google Scholar]
- 19. Lamoureux EL, Fenwick E, Xie J, et al. Methodology and early findings of the Diabetes Management Project: a cohort study investigating the barriers to optimal diabetes care in diabetic patients with and without diabetic retinopathy. Clin Exp Ophthalmol 2012; 40: 73–82. [DOI] [PubMed] [Google Scholar]
- 20. Braga MF, Casanova A, Teoh H, et al. Poor achievement of guidelines-recommended targets in type 2 diabetes: findings from a contemporary prospective cohort study. Int J Clin Pract 2012; 66: 457–464. [DOI] [PubMed] [Google Scholar]
- 21. Leiter LA, Berard L, Bowering CK, et al. Type 2 diabetes mellitus management in Canada: is it improving? Can J Diabetes 2013; 37: 82–89. [DOI] [PubMed] [Google Scholar]
- 22. Aronson R, Orzech N, Ye C, et al. Specialist-led diabetes registries and predictors of poor glycemic control in type 2 diabetes: insights into the functionally refractory patient from the LMC Diabetes Registry database. J Diabetes. Epub ahead of print 24 March 2015. DOI: 10.1111/1753-0407. [DOI] [PubMed] [Google Scholar]
- 23. Lu J, Weng J, Gu W, et al. Non-pharmaceutical factors for poor glycemic control in 13,970 Chinese women with drug-treated type 2 diabetes: a cross-sectional survey in 77 tertiary hospitals in four Chinese cities. Patient Prefer Adherence 2014; 8: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med 2013; 126: 925.e11–925.e22. [DOI] [PubMed] [Google Scholar]
- 25. Pablos-Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol 2014; 80: 47–56. [DOI] [PubMed] [Google Scholar]
- 26. Halimi S, Balkau B, Attali C, et al. Therapeutic management of orally treated type 2 diabetic patients, by French general practitioners in 2010: the DIAttitude Study. Diabetes Metab 2012; 38(Suppl. 3): S36–S46. [DOI] [PubMed] [Google Scholar]
- 27. Kahlon AS, Pathak R. Patterns of glycemic control using glycosylated hemoglobin in diabetics. J Pharm Bioallied Sci 2011; 3: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez-Vigil E, Rodriguez-Chacon M, Trabanco C, et al. Achievement of national clinical practice recommendations among those in the Puerto Rican population with diabetes mellitus. P R Health Sci J 2014; 33: 157–162. [PubMed] [Google Scholar]
- 29. Mata-Cases M, Benito-Badorrey B, Roura-Olmeda P, et al. Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Curr Med Res Opin 2013; 29: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 30. Huang LY, Shau WY, Yeh HL, et al. A model measuring therapeutic inertia and the associated factors among diabetes patients: a nationwide population-based study in Taiwan. J Clin Pharmacol 2015; 55: 17–24. [DOI] [PubMed] [Google Scholar]
- 31. The Health and Social Care Information Centre (HSCIC). National diabetes audit 2012–2013, 2014, http://www.hscic.gov.uk/catalogue/PUB14970/nati-diab-audi-12-13-care-proc-rep.pdf
- 32. Laiteerapong N, Fairchild PC, Chou CH, et al. Revisiting disparities in quality of care among US adults with diabetes in the era of individualized care, NHANES 2007-2010. Med Care 2015; 53: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thepwongsa I, Kirby C, Paul C, et al. Management of type 2 diabetes: Australian rural and remote general practitioners’ knowledge, attitudes, and practices. Rural Remote Health 2014; 14: 2499. [PubMed] [Google Scholar]
- 34. European Coalition for Diabetes. Diabetes in Europe policy puzzle: the state we are in. European Coalition for Diabetes, 2014, https://www.idf.org/sites/default/files/youngleaders/ECD-PP4finalweb_march2015.pdf
- 35. Holt RI, Nicolucci A, Kovacs BK, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national comparisons on barriers and resources for optimal care – healthcare professional perspective. Diabet Med 2013; 30: 789–798. [DOI] [PubMed] [Google Scholar]
- 36. Mc Hugh S, O’Mullane M, Perry IJ, et al. Barriers to, and facilitators in, introducing integrated diabetes care in Ireland: a qualitative study of views in general practice. BMJ Open 2013; 3: e003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Connor R, Mannix M, Mullen J, et al. Structured care of diabetes in general practice: a qualitative study of the barriers and facilitators. Ir Med J 2013; 106: 77–80. [PubMed] [Google Scholar]
- 38. Letshwiti JB, Scully PC, Quinn A, et al. Transition to adult care for adolescents with diabetes – a national survey. Ir Med J 2015; 108: 118–119. [PubMed] [Google Scholar]
- 39. Raaijmakers LG, Hamers FJ, Martens MK, et al. Perceived facilitators and barriers in diabetes care: a qualitative study among health care professionals in the Netherlands. BMC Fam Pract 2013; 14: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alhyas L, Nielsen JD, Dawoud D, et al. Factors affecting the motivation of healthcare professionals providing care to Emiratis with type 2 diabetes. JRSM Short Rep 2013; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan H, Lasker SS, Chowdhury TA. Exploring reasons for very poor glycaemic control in patients with Type 2 diabetes. Prim Care Diabetes 2011; 5: 251–255. [DOI] [PubMed] [Google Scholar]
- 42. George JT, Warriner D, McGrane DJ, et al. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. QJM 2011; 104: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LeBlanc ES, Rosales AG, Kachroo S, et al. Provider beliefs about diabetes treatment have little impact on glycemic control of their patients with diabetes. BMJ Open Diabetes Res Care 2015; 3: e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elliott DJ, Robinson EJ, Sanford M, et al. Systemic barriers to diabetes management in primary care: a qualitative analysis of Delaware physicians. Am J Med Qual 2011; 26: 284–290. [DOI] [PubMed] [Google Scholar]
- 45. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics 2013; 131: e1062–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Helgeson VS, Reynolds KA, Snyder PR, et al. Characterizing the transition from paediatric to adult care among emerging adults with Type 1 diabetes. Diabet Med 2013; 30:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garvey KC, Wolpert HA, Laffel LM, et al. Health care transition in young adults with type 1 diabetes: barriers to timely establishment of adult diabetes care. Endocr Pract 2013; 19: 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williamson C, Glauser TA, Burton BS, et al. Health care provider management of patients with type 2 diabetes mellitus: analysis of trends in attitudes and practices. Postgrad Med 2014; 126: 145–160. [DOI] [PubMed] [Google Scholar]
- 49. Whitford DL, Al Anjawi HA, Al Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes 2014; 8: 133–138. [DOI] [PubMed] [Google Scholar]
- 50. Aujoulat I, Jacquemin P, Hermans MP, et al. Clinical inertia in general practice, a matter of debate: a qualitative study with 114 general practitioners in Belgium. BMC Fam Pract 2015; 16: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ji L, Zhang P, Weng J, et al. Observational Registry of Basal Insulin Treatment (ORBIT) in patients with type 2 diabetes uncontrolled by oral hypoglycemic agents in China-study design and baseline characteristics. Diabetes Technol Ther 2015; 17: 735–744. [DOI] [PubMed] [Google Scholar]
- 52. Bralic Lang V, Bergman MB, Kranjcevic K. Family physician clinical inertia in glycemic control among patients with type 2 diabetes. Med Sci Monit 2015; 21: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balkau B, Bouee S, Avignon A, et al. Type 2 diabetes treatment intensification in general practice in France in 2008-2009: the DIAttitude Study. Diabetes Metab 2012; 38(Suppl. 3): S29–S35. [DOI] [PubMed] [Google Scholar]
- 54. Reach G, Le Pautremat V, Gupta S. Determinants and consequences of insulin initiation for type 2 diabetes in France: analysis of the National Health and Wellness Survey. Patient Prefer Adherence 2013; 7: 1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zafar A, Stone MA, Davies MJ, et al. Acknowledging and allocating responsibility for clinical inertia in the management of Type 2 diabetes in primary care: a qualitative study. Diabet Med 2015; 32: 407–413. [DOI] [PubMed] [Google Scholar]
- 57. Davis J, Chavez B, Juarez DT. Adjustments to diabetes medications in response to increases in hemoglobin a1c: an epidemiologic study. Ann Pharmacother 2014; 48: 41–47. [DOI] [PubMed] [Google Scholar]
- 58. Marrett E, Zhang Q, Kanitscheider C, et al. Physician reasons for nonpharmacologic treatment of hyperglycemia in older patients newly diagnosed with type 2 diabetes mellitus. Diabetes Ther 2012; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ratanawongsa N, Crosson JC, Schillinger D, et al. Getting under the skin of clinical inertia in insulin initiation: the Translating Research Into Action for Diabetes (TRIAD) Insulin Starts Project. Diabetes Educ 2012; 38: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clin Proc 2014; 89: 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harrison LB, Adams-Huet B, Li X, et al. Intensive therapy in newly diagnosed type 2 diabetes: results of a 6-year randomized trial. J Investig Med 2014; 62: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romanelli RJ, Chung S, Pu J, et al. Comparative effectiveness of early versus delayed metformin in the treatment of type 2 diabetes. Diabetes Res Clin Pract 2015; 108: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rajpathak SN, Rajgopalan S, Engel SS. Impact of time to treatment intensification on glycemic goal attainment among patients with type 2 diabetes failing metformin monotherapy. J Diabetes Complications 2014; 28: 831–835. [DOI] [PubMed] [Google Scholar]
- 65. Van Dieren S, Kengne AP, Chalmers J, et al. Intensification of medication and glycaemic control among patients with type 2 diabetes – the ADVANCE trial. Diabetes Obes Metab 2014; 16: 426–432. [DOI] [PubMed] [Google Scholar]